Abstract
Myeloid derived suppressor cells (MDSCs) are a diverse collection of immune cells that suppress anti-tumor immune responses. Decreasing MDSCs accumulation in the tumor microenvironment could improve the anti-tumor immune response and improve immunotherapy. Here, we examine the impact of physiologically relevant thermal treatments on the accumulation of MDSCs in tumors in mice. We found that different temperature-based protocols, including 1) weekly whole-body hyperthermia, 2) housing mice at their thermoneutral temperature (TT,~30°C), and 3) housing mice at a subthermoneutral temperature (ST,~22°C) while providing a localized heat source, each resulted in a reduction in MDSC accumulation and improved tumor growth control compared to control mice housed at ST, which is the standard, mandated housing temperature for laboratory mice. Additionally, we found that low dose β-adrenergic receptor blocker (propranolol) therapy reduced MDSC accumulation and improved tumor growth control to a similar degree as the models that relieved cold stress. These results show that thermal treatments can decrease MDSC accumulation and tumor growth comparable to propranolol therapy.
Keywords: Myeloid derived suppressor cells, thermal stress, hyperthermia, β-adrenergic signaling, β-adrenergic receptor blockers
1. Introduction
Myeloid Derived Suppressor Cells (MDSCs) are a collection of immune cells that play a key role in suppressing immune responses [1, 2]. This is potentially beneficial during tissue remodeling, wound healing, and prevention of auto-immunity [3-5]. However, these cells are also recruited to the tumor microenvironment when malignancies develop [6, 7]. In this context, the immunosuppressive effects of MDSCs lead to impairment of anti-tumor immunity, contributing to cancer progression and treatment resistance. Although many avenues of therapy are being investigated as methods to mitigate the negative impact of MDSCs [8], past work from our laboratory and others has shown limiting exposure to chronic stress could be one such mechanism [9, 10].
The human neuroendocrine response to chronic stress is partially mediated by catecholamines from the sympathetic nervous system signaling through beta-adrenergic receptors (β-AR) [11]. Interestingly, mice and other mammals that possess large amounts of brown adipose tissue use the sympathetic nervous system and the same neuroendocrine mediators, such as norepinephrine, to stimulate heat generation and prevent hypothermia when exposed to cold stress [12]. Our lab observed that increased production of norepinephrine occurs (needed for thermogenesis) not only during acute cold stress, but also when mice are housed at mildly subthermoneutral temperatures [13], which is approximately 22°C [14]. Because the IACUC mandated standard temperature (ST) for housing mice is typically 22-23°C, mice housed under these temperatures experience low levels of chronic cold stress. Past work from our laboratory and that of others has revealed that this mild chronic cold stress, which increases adrenergic receptor signaling throughout the body, can have effects on many immune cells, including effector T cells [15-18], regulatory T cells [19, 20], dendritic cells [21, 22], natural killer cells [23], and MDSCs [9]. Taken together, the impact of cold stress on these immune cells culminates in more rapid tumor growth; however, the relative contributions of changes in each of these cell types is not clear.
We have previously determined that the increase in systemic adrenergic signaling mediated by cold stress leads to an increase in MDSC accumulation and immunosuppressive function, which could be mitigated by housing mice at TT or blocking β-AR signaling with clinically available β-blockers [24]. The goal of this study was to investigate whether other methods of manipulating caging temperature and/or adrenergic signaling could be implemented to limit MDSC activity. We observed that allowing mice access to a simple cage heating system with a localized heat source [25], or weekly whole-body hyperthermia (WBH) treatments [26], also limited MDSC accumulation in tumors and tumor growth. This inhibition of MDSC accumulation was similar to that achieved using even very low doses of the pan β-adrenergic receptor antagonist, propranolol, in mice housed at ST. Together these data support the notion that the modulation of ambient temperature, and/or the mitigation of adrenergic stress signaling, are potential methods for mitigating MDSC mediated immune suppression.
2. Methods
Animals.
BALB/c (H-2d) mice were purchased from Charles River. All mice were maintained under standard housing conditions unless otherwise specified, and were approximately 8 weeks of age when tumor implantation occurred. All experiments were performed in accordance with the animal care guidelines at Roswell Park Comprehensive Cancer Center, and all protocols used were approved by the institutional animal care and use committee (IACUC).
Tumor models.
4T1 mammary carcinoma tumor cells were purchased from ATCC (ATCC, catalog CRL-2539), and are tested for mycoplasma yearly with the Mycoplasma Plus PCR Primer Set (Agilent Technologies, catalog 302008). Cells were cultured in RPMI 1640 (Corning), with 10% FBS, 1% l-glutamine, 1% penicillin/streptomycin, and 5% ambient CO2 with 95% air. 105 cells in 100 μL of PBS were orthotopically injected into the 4th mammary fat pad, and were always passed twice in culture after thawing before use. Tumor size was measured by the same individual using the same set of calipers throughout the experiments, and tumor volume was calculated by the equation: 2S × L / 2, where S is the small dimension and L is the large dimension.
Ambient temperature manipulation.
As described previously [27], rat cages with a false floor were used to house mice in groups of 5 for all standard temperature (ST, ~22°C), thermoneutral temperature (TT, ~30°C), and local heat source (~22°C with hand warmer) experiments. For the local heat source treatment group, cages were kept at ST, and newly opened hand warmers were exchanged daily at approximately 8 am. For mice at ST and TT conditions, cages were placed in rooms maintained to their appropriate temperatures, and these cages were also manipulated daily in the same way that local heat source treatment cages were. Briefly, the hand warmers produce heat as they undergo an exothermic reaction converting iron to iron oxide in the presence of oxygen in the air [24]. In both local heat and TT conditions the core body temperature did not change compared to the baseline.
Propranolol treatments (β-blocker).
For studies in which the pan-β-adrenergic receptor antagonist, propranolol, was used to assess the impact of adrenergic signaling on tumor growth and MDSC accumulation, 4T1 tumor-bearing mice were housed at ST. Daily treatment with propranolol (P0884, Sigma-Aldrich) began 4 days prior to tumor implantation and continued throughout the course of the experiment. Mice received a dose of 10.0, 1.0, or 0.1 mg/kg (200.0, 20.0, or 2.0 μg respectively in 200 μL of PBS) by intraperitoneal injection.
Whole body hyperthermia treatments.
Similar to as was described previously [28], mice received whole body hyperthermia treatments weekly for the duration of the tumor growth experiments. Once mice were received from Charles River, a temperature probe/transponder from the Electronic Laboratory Animal Monitoring System from Biomedic Data Systems (Maywood, NJ) was implanted subcutaneously into the dorsal thoracic region of one mouse from each cage, and mice were given at least 2 weeks to heal/acclimate to their housing conditions. Immediately prior to treatment, mice received 1.0 mL of PBS via an intraperitoneal injection for hydration. WBH mice were then transferred to a cage that was pre-heated to 38.5°C and placed within the environmental chamber (Memmert model BE500; Memmert, East Troy, WI). Core body temperatures were measured every 30 minutes, and the temperature of the chamber was increased by 1°C every half hour until the core body temperature of WBH mice reached 39.0°C. That temperature was maintained, and an average core temperature was held constant between 39.0°C and 40.0°C for 6 hours. Control mice were subjected to the same conditions and temperature readings as well. It is worth noting that in the hyperthermia protocol core body temperature raised above baseline but the core body temperature did not change in local heat or TT conditions.
Flow cytometry.
For spleens, after dissection, mechanical disruption and filtration through a 70 μm filter (Corning) was done to create single cell suspensions. AKC lysis buffer (Gibco) was used to lyse red blood cells prior to staining. For tumors, after dissection and removal, a scalpel was used to mechanically break down the tissue. Collagenase/hyaluronidase (Stem Cell Technologies, 07912) was then used following the manufacturer’s instructions, and samples were filtered through 70 μm filters prior to staining. 106 live cells per spleen and tumor samples were used for staining. Cells were first washed in flow running buffer (0.1% BSA in PBS) and incubated with anti-CD16/32 (Fc receptor blocker, 1:200) at 4°C for 10 minutes. Live/dead aqua (ThermoFisher Scientific) was used to gate out dead cells, and the following antibodies were used to identify MDSC populations: CD45 (clone 30F11), CD11b (clone M1/70), Ly6G (clone 1A8), Ly6C (clone HK1.4). All flow cytometry data were collected on the LSR Fortessa flow cytometer (BD Biosciences) and analyzed with FlowJo v7 software (Tree Star, Inc.). Absolute numbers of cells in tissues were determined by multiplying percentage of live CD45+ CD11b+ Ly6G+ Ly6C− (PMN-MDSC) and live CD45+ CD11b+ Ly6C+ Ly6G− (M-MDSC) by the cell numbers of the sample, divided by the sample mass in mg.
Statistics.
The Student’s t test was used to compare data between 2 groups, and 2-way ANOVA with Tukey’s post hoc analysis was used to generate tumor growth statistics using GraphPad Prism. All tumor growth data are presented as mean ± SEM, and all other data are presented as median ± minimum to maximum.
Study approval.
Generation of the mice and all mice studies were reviewed and approved by the Roswell Park Comprehensive Cancer Center IACUC (protocol numbers 757M and 1038M).
3. Results:
3.1. Self-selected temperature modulation decreases tumor growth and MDSC accumulation.
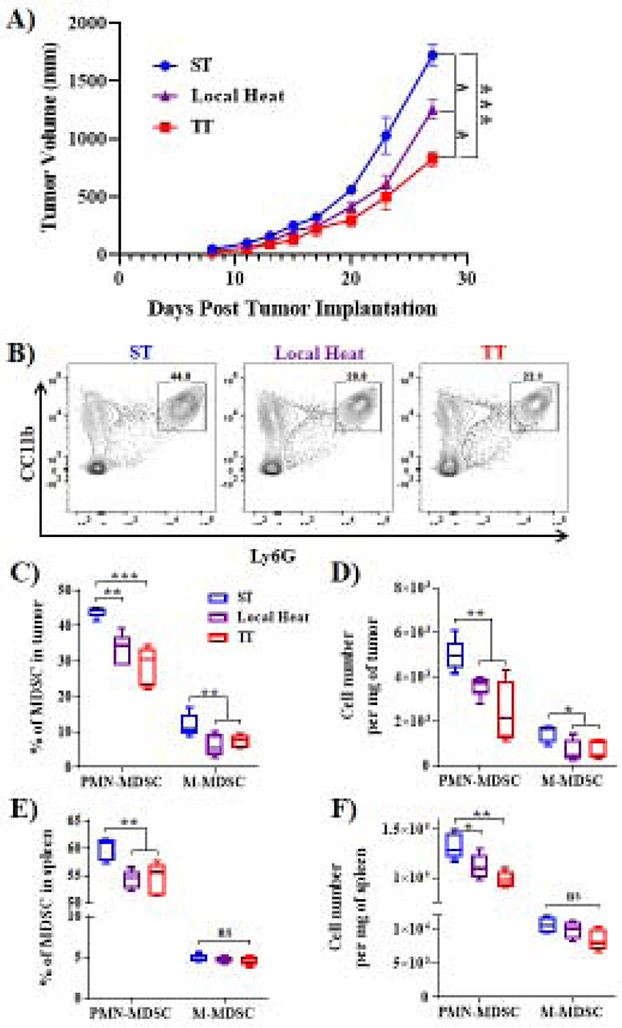
Previous data has shown that housing mice at TT (30°C) results in a decrease in MDSC accumulation compared to that seen in mice housed under the subthermoneutral temperatures required for laboratory mice [9]. We implemented a new murine housing cage system that allows for the insertion of exothermic heating packets, colloquially known as hand warmers, which can heat specific regions of a cage [27]. This allows mice to select warmer regions of the cage whenever they desire. This model is referred to here as the local heat model.
Using this system, and our previously described ST and TT models [9] in which mice are housed in rooms kept at either 22°C or 30°C, mice were allowed to acclimate to their particular housing condition for 2 weeks. Then, 4T1 tumor cells were orthotopically implanted, housing conditions were maintained, and tumor growth was monitored. We determined that TT housing conditions allowed for the greatest control of tumor growth, while our local heat source model also significantly decreased tumor growth when compared to ST housing conditions (Fig. 1A). Importantly, using flow cytometry (Fig. 1B), we determined that the local heat source housing condition suppressed the accumulation of MDSCs in tumors (Fig. 1C & D) and spleens (Fig. 1E & F) to a similar degree as TT housing. Importantly, it is possible that the difference in tumor volumes at the endpoint of these experiments was due to effects of local heat and TT on other cell types, and that the decrease in MDSC accumulation is actually a reflection of a change in tumor volume. To determine whether this was the case, analysis of tumors from mice at ST and TT was done at day 15 when tumor volumes were equivalent and at day 25. We found that MDSC populations were decreased in tumors from mice housed in TT conditions at both time points (Sup. Fig. 1A-D). Additionally, we have previously shown that mice housed at ST and TT have equivalent numbers of MDSCs prior to tumor implantation [9]. Taken together, these data indicate that cold stress by itself does not increase MDSC accumulation in healthy mice, but it does increase accumulation in tumor bearing mice.
Figure 1). Housing temperature can decrease tumor growth and MDSC accumulation.
(A). 4T1 tumor growth kinetics in WT BALB/c mice housed under ST (22°C), TT (30°C), or local heat source conditions (n=5 mice per group). (B) Representative flow cytometry data assessing intratumoral MDSC populations. (C and D) Percentage of PMN-MDSC and M-MDSC subpopulations, as well as absolute numbers per mg of tumor. (E and F) Percentage of PMN-MDSC and M-MDSC subpopulations, as well as absolute numbers per mg of spleen. Tumor growth curves are presented as the mean ± SEM, and MDSC data are presented as median ± minimum to maximum. Two-way ANOVA was used to analyze statistical significance among tumor growth in different groups, and One-way ANOVA was used to analyze statistical significance among MDSC populations. In all panels, *P < 0.05, **P < 0.01., and ***P < 0.01. A P value less than 0.05 was considered significant.
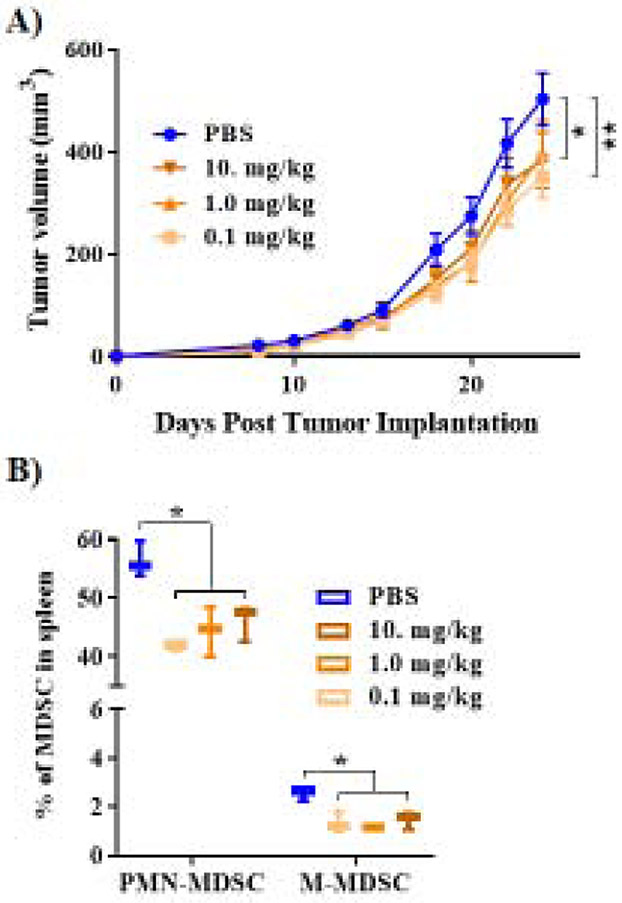
3.2. Limiting adrenergic stress with a low dose β-blocker decreases tumor growth and MDSC accumulation.
Previous results using several murine tumor models indicate that daily treatment with propranolol at a dose of 10mg/kg (in PBS) is another intervention capable of minimizing the effects of ST housing induced cold stress, limiting MDSC activity, and improving tumor control. Here, we utilized BALB/c mice bearing orthotopically implanted 4T1 mammary carcinoma tumors, and found that even a 100-fold decrease in propranolol dose (daily i.p. injections of 0.1mg/kg) was capable of limiting tumor growth rates (Fig. 2A). Additionally, single cell suspensions of splenic tissue were prepared and stained for flow cytometry to assess levels of both major MDSC subtypes, M-MDSC and PMN-MDSC. We found that all 3 doses of propranolol: 10.0, 1.0, and 0.1 mg/kg suppressed M-MDSC and PMN-MDSC numbers to a similar degree (Fig. 2B). These data suggest that blocking stress mediated adrenergic signaling delays tumor growth, partially by decreasing MDSC accumulation. We compared this effect to that achieved by thermal manipulation of housing temperature.
Figure 2). Low dose β-blocker treatment decreases tumor growth and MDSC accumulation.
(A). 4T1 tumor growth kinetics in WT BALB/c mice treated with propranolol at doses of 10.0, 1.0, or 0.1 mg/kg (n=6 mice per group), housed at ST (22°C). (B) MDSC populations measured by flow cytometry in splenic tissue of mice bearing 4T1 tumors, treated with propranolol at doses of 10.0, 1.0, or 0.1 mg/kg (n=6 mice per group). Tumor growth curves are presented as the mean ± SEM, and MDSC data are presented as median ± minimum to maximum. Two-way ANOVA was used to analyze statistical significance among tumor growth in different groups, and One-way ANOVA was used to analyze statistical significance among MDSC populations. In all panels, *P < 0.05 and **P < 0.01. A P value less than 0.05 was considered significant.
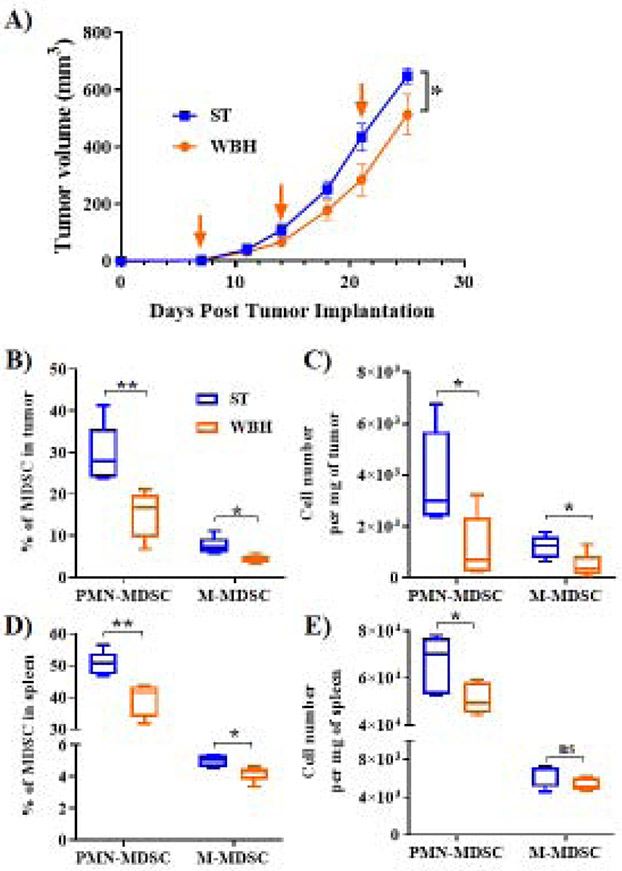
3.3. Weekly whole-body hyperthermia decreases tumor growth and MDSC accumulation.
In the experiments conducted above, body temperature remains stable. To test the impact of actually increasing the core body temperature of mice on MDSC accumulation, whole body hyperthermia (WBH) experiments were carried out. BALB/c mice were orthotopically implanted with 4T1 tumor cells, and all mice were housed at ST. Once a week, one group of mice received a WBH treatment during which they were temporarily housed at temperatures between 38.5°C and 39.5°C, which allows their core temperatures to increase [28]. The WBH treatment concluded once the core temperature of a surrogate mouse reached an average of 39.0°C or greater for six hours. We found that tumor growth was delayed significantly in WBH treated mice compared to control ST mice (Fig 3A). Additionally, flow cytometry of tumors (Fig 3B & C) and spleens (Fig 3D & E) indicated that levels of both subtypes of MDSCs were decreased as well. These effects were similar in magnitude to that achieved by using propranolol or TT housing, although the mechanism(s) of action driving WBH mediated changes may be unique because this is the only model in which the core temperature of treated mice is increased. Taken together, these data support the notion that changes in housing conditions and temperature-based therapies can significantly alter the immunosuppressive environment within the tumor by decreasing MDSC accumulation.
Figure 3). Whole body hyperthermia decreases tumor growth and MDSC accumulation.
(A). 4T1 tumor growth kinetics in WT BALB/c mice housed at ST (22°C) and treated with whole body hyperthermia (WBH), or control ST treatment (n=5 mice per group). Arrows indicate days on which WBH or control treatments were administered. (B and C) Percentage of PMN-MDSC and M-MDSC subpopulations, as well as absolute numbers per mg of tumor. (D and E) Percentage of PMN-MDSC and M-MDSC subpopulations, as well as absolute numbers per mg of spleen. Tumor growth curves are presented as the mean ± SEM, and MDSC data are presented as median ± minimum to maximum. Two-way ANOVA was used to analyze statistical significance among tumor growth in different groups, and One-way ANOVA was used to analyze statistical significance among MDSC populations. In all panels, *P < 0.05 and **P < 01. A P value less than 0.05 was considered significant.
4. Discussion
Without a potent co-incident anti-tumor immune response, cancer therapies are less effective [29]. MDSC populations are capable of shifting the delicate balance of this immune response from anti-tumor to pro-tumor [2], and it is likely that decreasing MDSC accumulation allows for improved therapeutic efficacy along with enhanced immune cell killing of tumor cells [30, 31]. MDSCs suppress the anti-tumor immune response by inhibiting T cell and natural killer cell activity, promoting a premetastatic niche, and contributing to resistance to immunotherapy [32]. We have previously shown that housing temperature induced cold stress, and the adrenergic signaling induced by this cold stress, significantly increases MDSC accumulation and immunosuppressive functions through β2-AR signaling [9]. Here, we have compared the effects of three temperature-based protocols on tumor growth and MDSC accumulation, including 1) weekly whole-body hyperthermia (WBH), 2) housing mice at TT (~30°C), and 3) housing mice at ST (~22°C) while providing them with access to a local heat source. We also compared these protocols to mice housed under ST conditions, but given daily treatments of different doses of propranolol. These treatment protocols exert their effects through unique mechanisms as described below.
By permanently changing housing temperatures to TT, or by using our local heat source model, mice do not require adaptive thermogenesis to maintain their core body temperature (approximately 37°C) [33]. As a result, systemic levels of norepinephrine are lower; thus β2-AR signaling in MDSCs is reduced. In propranolol studies, all mice are housed at ST and experience cold stress, which drives an increase in systemic norepinephrine levels to drive thermogenesis in order to maintain normal core body temperature. However, propranolol treatments inhibit signaling through β-ARs expressed by many cells throughout the body, including MDSCs, and mitigate the effects of elevated norepinephrine levels. Interestingly, our β-AR antagonist studies show that even a low dose of propranolol (0.1mg/kg) is sufficient to reduce MDSC accumulation, compared to the previously used dose of 10.0mg/kg [9]. This emphasizes how responsive this immunosuppressive pathway is to β-AR signaling.
In comparison, mice in our hyperthermia studies experience chronic cold stress throughout the week, as they are all housed at ST. Then, the hyperthermia treatment they receive every seven days temporarily increases their core body temperature from ~37°C to above 39°C for six hours and briefly interrupts the cold stress they are experiencing. Although we have shown that these weekly hyperthermia treatments reduce MDSC accumulation and tumor growth, it is unknown whether this is due to reduced levels of cold stress, or whether this treatment reduces MDSC accumulation through some other mechanism. It is possible that the decrease in MDSC accumulation due to hyperthermia occurred as a result of direct changes in MDSC biology, such as a decrease in MDSC survival in the tumor microenvironment or a decrease in MDSC expansion at the level of the bone marrow. Although these cold stress limiting treatments are different, it is evident that the processes driving MDSC accumulation are sensitive to each of these models.
It is important to appreciate that many cell types throughout the body, including other immune cells, are likely affected by each of the treatments in this study, in addition to MDSCs. We know that immune cells, including T cells [19], dendritic cells [21], and natural killer cells [23] express functional β-ARs, and blocking β-adrenergic signaling likely directly affects them, independent of changes in MDSC mediated immunosuppression. For example, in the past we have shown that cold stress and β-AR signaling impairs CD8+ T cell function and anti-PD-1 immunotherapy [15-17, 34], increases regulatory T cell activity in models of graft vs. host disease to limit severity and mortality [19], and limits effector T cell responses to radiation therapy [34, 35]. We have also shown that hyperthermia can have effects on Langerhans cells and lymphocytes [28]. Although the effects of cold stress, adrenergic signaling, and hyperthermia on these cell types are important to consider, these current studies specifically focus on how these experimental models affect MDSC accumulation. Still, even within this focused scope of investigation, there are many other potential mechanisms that could be responsible for the observed changes in MDSCs accumulation.
For example, tumor associated factors released into the tumor microenvironment are thought to generally be responsible for the expansion of MDSC populations [36], and it is possible that the responses to therapy we observed could in part be due to effects on other cell types, such as T cells [37, 38] or tumor cells, which could alter the tumor microenvironment and lead to the production of less tumor associated factors. However, this was in part controlled for because 4T1 tumor cells have been shown to lack functional β2-ARs [39], so changes in adrenergic signaling directly on tumor cells is unlikely to be responsible for the observed phenotype.
Additionally, the thermogenic process that occurs in brown adipose tissue as a response to cold stress is driven by β3-AR signaling [40]. This signaling triggers the conversion of the chemical energy trapped within lipids into heat, rather than adenosine triphosphate molecules [41]. As a consequence, thermogenesis requires a great deal of metabolic energy, and interestingly, mice housed at even colder temperatures, such as 4°C, can maintain their core body temperature if they are able to consume significantly more dietary calories to use for thermogenesis [42, 43]. Considering that a non-trivial portion of the body’s energy consumption is typically allocated to the immune system [44], it is possible that this decrease in available energy could also contribute to impairment of the anti-tumor immune response. However, we have shown that when ST and TT tumor growth experiments have been carried out in β2-AR knockout mice and SCID mice, there is no change in tumor growth [16]. This suggests that while mice experiencing cold stress may burn more calories and consume more food, these changes do not significantly affect tumor growth, which is primarily driven by β2-AR signaling in immune cells in these models.
When pharmacologically inhibiting β-adrenergic signaling, it is important to note that because propranolol is a pan-β-antagonist, β1-ARs and γ3-ARs are antagonized under this treatment, in addition to γ2-ARs. In this case, it is possible that other systemic metabolic changes, such as an increase in plasma fatty acids, glycerol, and/or insulin, and a reduction in blood glucose driven by β3-AR inhibition [45] could lead to changes in MDSC accumulation or other relevant immune populations. In addition, the cardiovascular effects of propranolol mediated by β1-AR inhibition have been thoroughly described, and these effects could potentially result in changes in tumor growth or production of systemic tumor associated factors that expand MDSC populations by some undiscovered mechanism.
In a previous study, we determined that as tumor volumes increase, mice seek warmer environments. When allowed to move freely between cages at different temperatures, tumor bearing mice spend more time at temperatures as warm as 38°C [17]. In this study, the hand warmers heat the floor above where they are placed to ~35°C, but this heat begins to taper off after 6 hours and eventually drops to ~24°C by the time they are replaced the following morning [27]. This is still warmer than ST (~22°C), but it is possible that a more constant and/or warmer local heat source could provide even greater tumor control. This could provide insight into why our local heat model was not as effective at reducing tumor growth as constant TT housing (~30°C) was. Although we do not have data describing how often the tumor bearing mice in this local heat model are physically on the heat source, it has been shown that non-tumor bearing mice spend significantly more time on the false floor when it is heated rather than at ST [46].
This desire for tumor bearing mice to seek warmth also supports the notion of using WBH treatments to increase core body temperatures and relieve this cold stress. In these experiments, WBH was administered once a week, however more regular treatments may provide an even greater decrease in tumor growth rates. Although these findings in mouse models are important, the most vital aspect of these studies is their potential implications for human patients that could receive hyperthermia treatments for breast cancer or other malignancies. We know that breast cancer patients commonly report feeling cold [47, 48], yet little research has been done to determine how this symptom of feeling cold affects outcomes, or whether it impacts important processes such as MDSC accumulation, macrophage accumulation, or T cell function. Further, it is unknown whether a temperature-based therapy, such as hyperthermia, might relieve these symptoms of feeling cold, skew the tumor microenvironment to a more anti-tumor state, and improve clinical survival rates.
In summary, we showed that by implementing several different methods of limiting chronic cold stress in mice, tumor growth rates and MDSC accumulation could be reduced. This study provides rationale to re-think experimental design, and consider how the environment, especially housing temperature, affects in vivo experiments. This also gives credence to the importance of the nervous system’s role in modulating the anti-tumor immune response, indicating that more investigations of the central nervous system-immune response axis are necessary. Finally, we hope that these provocative findings will encourage the investigation of potential clinical treatment regimens that utilize the human body’s response to changes in temperature to improve patient outcomes.
Supplementary Material
Highlights:
MDSC accumulation in tumors is highly sensitive to various manipulations of housing temperature, which can affect their physiological response to adrenergic stress.
Providing a local heat source within the cage of tumor bearing mice limits cold stress, is associated with diminished intra-tumoral MDSC accumulation, and improves tumor growth control.
Intermittent whole body hyperthermia treatments given to mice housed under standard cool temperatures is also associated with diminished intratumoral MDSC accumulation and improved tumor growth control.
Local heating, housing at thermoneutrality, and intermittent whole body hyperthermia provide similar tumor growth control and MDSC reduction as that seen with various doses of the pan β-adrenergic receptor antagonist, propranolol.
Acknowledgments
The authors thank Jeanne M. Prendergast for technical assistance, the Genomics Shared Resource (Roswell Park), and the Roswell Park Flow Cytometry Core Facility for expert support. This project was supported by National Institutes of Health (NIH) grants, R01 CA205246, and R01 CA099326 (to E.R.), and F32 CA239356 (to H.M.) and the Roswell Park Alliance Foundation, Breast Cancer Coalition of Rochester and NCI grant P30CA016056.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Talmadge JE, Gabrilovich DI, History of myeloid-derived suppressor cells, Nat. Rev. Cancer, 13 (2013) 739–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ostrand-Rosenberg S, Fenselau C, Myeloid-derived suppressor cells: immune-suppressive cells that impair antitumor immunity and are sculpted by their environment, J. Immunol, 200 (2018) 422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Boros P, Ochando J, Zeher M, Myeloid derived suppressor cells and autoimmunity, Hum. Immunol, 77 (2016) 631–636. [DOI] [PubMed] [Google Scholar]
- [4].Millrud CR, Bergenfelz C, Leandersson K, On the origin of myeloid-derived suppressor cells, Oncotarget, 8 (2017) 3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Veglia F, Perego M, Gabrilovich D, Myeloid-derived suppressor cells coming of age, Nat. Immunol, 19(2018) 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Anani W, Shurin MR, Targeting myeloid-derived suppressor cells in cancer, Tumor Immune Microenvironment in Cancer Progression and Cancer Therapy, Springer; 2017, pp. 105–128. [DOI] [PubMed] [Google Scholar]
- [7].Tcyganov E, Mastio J, Chen E, Gabrilovich DI, Plasticity of myeloid-derived suppressor cells in cancer, Curr. Opin. Immunol, 51 (2018) 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu Y, Wei G, Cheng WA, Dong Z, Sun H, Lee VY, Cha S-C, Smith DL, Kwak LW, Qin H, Targeting myeloid-derived suppressor cells for cancer immunotherapy, Cancer Immunol. Immunother, 67 (2018) 1181–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mohammadpour H, MacDonald CR, Qiao G, Chen M, Dong B, Hylander BL, McCarthy PL, Abrams SI, Repasky EA, β2 adrenergic receptor–mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells, J. Clin. Invest, 129 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schmidt D, Peterlik D, Reber SO, Lechner A, Männel DN, Induction of suppressor cells and increased tumor growth following chronic psychosocial stress in male mice, PLoS One, 11 (2016)e0159059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yaribeygi H, Panahi Y, Sahraei H, Johnston TP, Sahebkar A, The impact of stress on body function: A review, EXCLI journal, 16 (2017) 1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cannon B, Nedergaard J, Brown adipose tissue: function and physiological significance, Physiol. Rev, 84 (2004) 277–359. [DOI] [PubMed] [Google Scholar]
- [13].Eng JW-L, Reed CB, Kokolus KM, Pitoniak R, Utley A, Bucsek MJ, Ma WW, Repasky EA, Hylander BL, Housing temperature-induced stress drives therapeutic resistance in murine tumour models through β 2-adrenergic receptor activation, Nature communications, 6 (2015) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].N.R. Council, Environment, Housing, and Management, Guide for the Care and Use of Laboratory Animals. 8th edition, National Academies Press (US)2011. [Google Scholar]
- [15].Hylander BL, Gordon CJ, Repasky EA, Manipulation of ambient housing temperature to study the impact of chronic stress on immunity and cancer in mice, J. Immunol, 202 (2019) 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bucsek MJ, Qiao G, MacDonald CR, Giridharan T, Evans L, Niedzwecki B, Liu H, Kokolus KM, Eng JW-L, Messmer MN, β-adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8+ T cells and undermines checkpoint inhibitor therapy, Cancer Res., 77 (2017) 5639–5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kokolus KM, Capitano ML, Lee C-T, Eng JW-L, Waight JD, Hylander BL, Sexton S, Hong C-C, Gordon CJ, Abrams SI, Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature, Proc. Natl. Acad. Sci, 110 (2013) 20176–20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Daher C, Vimeux L, Stoeva R, Peranzoni E, Bismuth G, Wieduwild E, Lucas B, Donnadieu E, Bercovici N, Trautmann A, Blockade of β-adrenergic receptors improves CD8+ T-cell priming and cancer vaccine efficacy, Cancer. Immunol. Res, (2019) canimm. 0833.2018. [DOI] [PubMed] [Google Scholar]
- [19].Mohammadpour H, Sarow JL, MacDonald CR, Chen GL, Qiu J, Sharma UC, Cao X, Herr MM, Hahn TE, Blazar BR, β2-adrenergic receptor activation on donor cells ameliorates acute GvHD, JCI Insight, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Guereschi MG, Araujo LP, Maricato JT, Takenaka MC, Nascimento VM, Vivanco BC, Reis VO, Keller AC, Brum PC, Basso AS, Beta2-adrenergic receptor signaling in CD 4+ Foxp3+ regulatory T cells enhances their suppressive function in a PKA-dependent manner, Eur. J. Immunol, 43 (2013) 1001–1012. [DOI] [PubMed] [Google Scholar]
- [21].Mohammadpour H, O’Neil R, Qiu J, McCarthy PL, Repasky EA, Cao X, Blockade of host β2-adrenergic receptor enhances graft-versus-tumor effect through modulating APCs, J. Immunol, 200 (2018) 2479–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Takenaka MC, Araujo LP, Maricato JT, Nascimento VM, Guereschi MG, Rezende RM, Quintana FJ, Basso AS, Norepinephrine Controls Effector T Cell Differentiation through β2-Adrenergic Receptor–Mediated Inhibition of NF-κB and AP-1 in Dendritic Cells, J. Immunol, 196 (2016) 637–644. [DOI] [PubMed] [Google Scholar]
- [23].Diaz-Salazar C, Bou-Puerto R, Mujal AM, Lau CM, von Hoesslin M, Zehn D, Sun JC, Cell-intrinsic adrenergic signaling controls the adaptive NK cell response to viral infection, J. Exp. Med, 217 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sands WA, Kimmel WL, Wurtz BR, Stone MH, McNeal JR, Comparison of commercially available disposable chemical hand and foot warmers, Wilderness Environ. Med, 20 (2009) 33–38. [DOI] [PubMed] [Google Scholar]
- [25].Kang C, Jeong S-Y, Song SY, Choi EK, The emerging role of myeloid-derived suppressor cells in radiotherapy, Radiation Oncology Journal, 38 (2020) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].De Cicco P, Ercolano G, Ianaro A, The new era of cancer immunotherapy: targeting myeloid-derived suppressor cells to overcome immune evasion, Front. Immunol, 11 (2020) 1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gordon CJ, Puckett ET, Repasky ES, Johnstone AF, A device that allows rodents to behaviorally thermoregulate when housed in vivariums, Journal of the American Association for Laboratory Animal Science, 56 (2017) 173–176. [PMC free article] [PubMed] [Google Scholar]
- [28].Ostberg JR, Gellin C, Patel R, Repasky EA, Regulatory potential of fever-range whole body hyperthermia on Langerhans cells and lymphocytes in an antigen-dependent cellular immune response, J. Immunol, 167 (2001) 2666–2670. [DOI] [PubMed] [Google Scholar]
- [29].Yang Z, Guo J, Weng L, Tang W, Jin S, Ma W, Myeloid-derived suppressor cells—new and exciting players in lung cancer, J. Hematol. Oncol, 13 (2020) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bird L, MDSC metabolite stuns T cells, Nat. Rev. Immunol, 20 (2020) 352–353. [DOI] [PubMed] [Google Scholar]
- [31].Law AM, Valdes-Mora F, Gallego-Ortega D, Myeloid-Derived Suppressor Cells as a Therapeutic Target for Cancer, Cells, 9 (2020) 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Weber R, Fleming V, Hu X, Nagibin V, Groth C, Altevogt P, Utikal J, Umansky V, Myeloid-derived suppressor cells hinder the anti-cancer activity of immune checkpoint inhibitors, Front. Immunol, 9 (2018) 1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gordon C, Thermal physiology of laboratory mice: defining thermoneutrality, J. Therm. Biol, 37 (2012) 654–685. [Google Scholar]
- [34].Chen M, Qiao G, Hylander BL, Mohammadpour H, Wang X-Y, Subjeck JR, Singh AK, Repasky EA, Adrenergic stress constrains the development of anti-tumor immunity and abscopal responses following local radiation, Nature communications, 11 (2020) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].MacDonald CR, Bucsek MJ, Qiao G, Chen M, Evans L, Greenberg DJ, Uccello TP, Battaglia NG, Hylander BL, Singh AK, Adrenergic receptor signaling regulates the response of tumors to ionizing radiation, Radiat. Res, 191 (2019) 585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lee W-C, Hsu P-Y, Hsu H-Y, Stem cell factor produced by tumor cells expands myeloid-derived suppressor cells in mice, Sci. Rep, 10 (2020) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Qiao G, Bucsek MJ, Winder NM, Chen M, Giridharan T, Olejniczak SH, Hylander BL, Repasky EA, β-Adrenergic signaling blocks murine CD8+ T-cell metabolic reprogramming during activation: a mechanism for immunosuppression by adrenergic stress, Cancer Immunol. Immunother, 68 (2019) 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Skitzki JJ, Repasky EA, Evans SS, Hyperthermia as an immunotherapy strategy for cancer, Current opinion in investigational drugs (London, England: 2000), 10 (2009) 550. [PMC free article] [PubMed] [Google Scholar]
- [39].Szpunar MJ, Burke KA, Dawes RP, Brown EB, Madden KS, The antidepressant desipramine and α2-adrenergic receptor activation promote breast tumor progression in association with altered collagen structure, Cancer Prevention Research, 6 (2013) 1262–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cypess AM, Weiner LS, Roberts-Toler C, Elía EF, Kessler SH, Kahn PA, English J, Chatman K, Trauger SA, Doria A, Activation of human brown adipose tissue by a β3-adrenergic receptor agonist, Cell Metab., 21 (2015) 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Montanari T, Pošćić N, Colitti M, Factors involved in white-to-brown adipose tissue conversion and in thermogenesis: a review, Obes. Rev, 18 (2017) 495–513. [DOI] [PubMed] [Google Scholar]
- [42].Tokuyama K, Himms-Hagen J, Brown adipose tissue thermogenesis, torpor, and obesity of glutamate-treated mice, American Journal of Physiology-Endocrinology And Metabolism, 251 (1986) E407–E415. [DOI] [PubMed] [Google Scholar]
- [43].Sojka P, Griess R, Nielsen M, Locomotor activity and body temperature in selected mouse lines differing greatly in feed intake, J. Anim. Sci, 91 (2013) 3557–3563. [DOI] [PubMed] [Google Scholar]
- [44].Rauw WM, Immune response from a resource allocation perspective, Frontiers in genetics, 3 (2012) 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].MacPherson RE, Castellani L, Beaudoin M-S, Wright DC, Evidence for fatty acids mediating CL 316,243-induced reductions in blood glucose in mice, American Journal of Physiology-Endocrinology and Metabolism, 307 (2014) E563–E570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chan CE, Hare MT, Martin GW, Gordon CJ, Swoap SJ, The heat is on: A device that reduces cold stress-induced tachycardia in laboratory mice, J. Therm. Biol, 79 (2019) 149–154. [DOI] [PubMed] [Google Scholar]
- [47].Kokolus KM, Hong C-C, Repasky EA, Feeling too hot or cold after breast cancer: is it just a nuisance or a potentially important prognostic factor?, Int. J. Hyperthermia, 26 (2010) 662–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Golubnitschaja O, Feeling cold and other underestimated symptoms in breast cancer: anecdotes or individual profiles for advanced patient stratification?, EPMA Journal, 8 (2017) 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.