Abstract
Introduction
Postoperative delirium (POD) is a common neurological complication after hip fracture surgery and is associated with high morbidity and mortality in elderly patients. Although the specific mechanism of POD remains unclear, circadian rhythm disruptions have recently drawn increased attention. To date, only limited postoperative time points of plasma melatonin level measurements were recorded in previous studies, and such data cannot represent a comprehensive melatonin rhythm. The process of anaesthesia (either general anaesthesia (GA) or regional anaesthesia (RA)) is known to influence the melatonin rhythm. However, how these two anaesthesia methods differently affect the postoperative melatonin rhythm is still unknown. Therefore, we hypothesise that RA may attenuate the disruption of the melatonin rhythm, which might decrease the incidence of POD in elderly patients undergoing hip surgery.
Methods and analysis
In this prospective cohort clinical trial, 138 patients scheduled for hip fracture surgery will be divided into two groups to receive either GA or RA. The primary aim is to compare the circadian rhythm of melatonin secretion between the two groups and explore its association with the incidence of POD.
Ethics and dissemination
The study has been approved by the Medical Science Research Ethics Committees of Beijing Jishuitan Hospital (JLKS201901-04). The results of the study will be published in peer-reviewed international journals.
Trial registration number
ChiCTR1900027393.
Keywords: anaesthesia in orthopaedics, delirium & cognitive disorders, adult anaesthesia
Strengths and limitations of this study.
A major strength of this study is that to our knowledge, this is the first trial to evaluate the effects of different anaesthesia methods (regional anaesthesia and general anaesthesia) on the circadian rhythm of melatonin secretion and explore their association with postoperative delirium in elderly patients undergoing hip fracture surgery.
Both objective and subjective sleep quality are included in the current study. This can provide a better and more comprehensive understanding of the connection between circadian rhythms and anaesthesia.
The limitation of the trial is that melatonin is the optimal peripheral biomarker of circadian timing; more biomarkers such as cytokines and genes (peripheral clock gene) should be included.
Although we will record both subjective and objective sleep quality, such measurements are incomplete, for example, polysomnography is the gold standard for diagnosis of sleep disorders.
Our study will be conducted until discharge from the hospital, which might be insufficient for delirium screening.
Introduction
Postoperative delirium (POD), a common neurological complication in elderly patients undergoing hip fracture surgery (range: 4%–53%), is usually associated with increased morbidity and mortality rates, long-term cognitive decline and a heavier healthcare economic burden.1–6 Many studies have indicated that preoperative brain dysfunction (frail brain) in elderly patients is a predisposing factor for POD,7 8 while the perioperative change of neuroinflammatory markers (eg, C reactive protein (CRP) and interleukin-6 (IL-6)), oxidative stressor markers (cortisol) and the crucial hormone (melatonin) within elderly frail brain are the precipitating factors for POD.8 9 However, the specific mechanism of POD remains unclear.9 10
Circadian rhythm disruptions have recently drawn increased attention as a possible mechanism of POD.10 11 Circadian rhythms refer to the behavioural and physiological cycles that occur within approximately 24 hours, including the sleep–wake cycles, hormones secretion and core body temperature.12 Among these indicators, plasma melatonin is considered to be the best peripheral marker of endogenous circadian rhythms.13–15 Previous studies have revealed potential correlations between plasma melatonin secretion disruption and POD.10 16–20 Nevertheless, only limited postoperative time points (eg, once a day) of plasma melatonin level measurements were recorded in those studies, and such data cannot represent a comprehensive melatonin rhythm. Moreover, one study indicated that the urinary excretion of 6-sulfatoxymelatonin (6-SMT), the main metabolite of melatonin, during delirium was associated with clinical subtypes of delirium (higher in hypoactive and lower in hyperactive delirium).21 However, measuring the melatonin level in a urine sample instead of a plasma sample at only one time point is insufficient to describe the real pattern of the melatonin rhythm in the acute phase of delirium. Therefore, the exact association between the melatonin rhythm and delirium requires further exploration.
The process of anaesthesia (either general anaesthesia (GA) or regional anaesthesia (RA)) is known to influence the circadian rhythms.22–24 On one hand, GA is associated with unconsciousness similar to a dreamless sleep, which may strongly alter circadian rhythms through the use of drugs such as N-methyl-D-aspartate receptor antagonists or gamma-aminobutyric acid receptor agonists.22 25 26 On the other hand, RA is more helpful in the relief of sleep disturbances after surgery than GA,27 which may result from better maintenance of the melatonin rhythm postoperatively. However, the effects of these two anaesthesia methods on the postoperative melatonin rhythm have not been compared. Therefore, we hypothesise that RA may attenuate the disruption of the melatonin rhythm, which might decrease the incidence of POD in elderly patients undergoing hip surgery. In this report, we present the design of a prospective cohort clinical trial to compare the effects of GA versus RA on the circadian rhythm of melatonin secretion and the incidence of POD in elderly patients undergoing hip fracture surgery.
Methods and analysis
Trial design
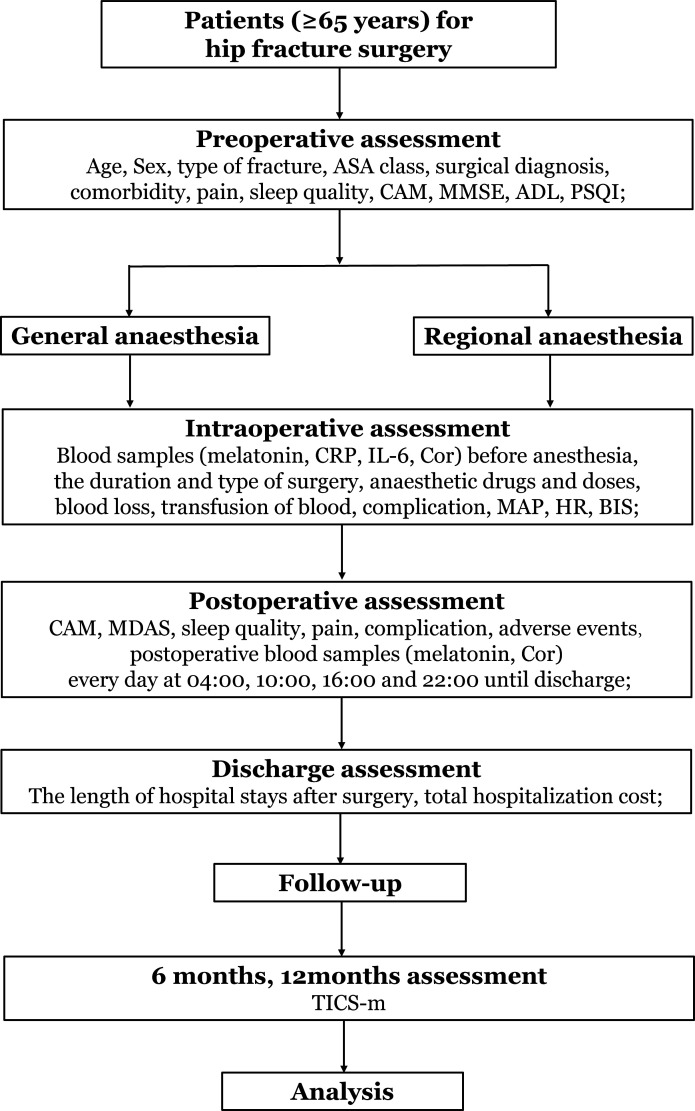
This prospective single-centre, 1:1 matched cohort clinical trial is designed to compare the effects of GA and RA on the melatonin rhythm and incidence of POD. We aim to recruit 138 patients with hip fracture (≥65 years of age) who will be assigned to either the GA group (n=69) or the RA group (n=69) depending on their situation and personal willingness as well as the preference of the anesthesiologists and surgeons. We will conduct the trial at the Beijing Jishuitan Hospital in China from November 2019 until the target sample size is achieved. Figure 1 shows the flow chart of this study.
Figure 1.
Flow chart of the trial. ADL, Activities of Daily Living; ASA, American Society of Anesthesiologists; BIS, bispectral index; CAM, Confusion Assessment Method; Cor, cortisol; CRP, C reactive protein; HR, heart rate; IL, interleukin; MAP, mean arterial pressure; MDAS, Memorial Delirium Assessment Scale; MMSE, Mini-Mental State Examination; PSQI, Pittsburgh Sleep Quality Index; TICS-m, Modified Telephone Interview for Cognitive Status.
Primary outcome
The primary outcome is to compare the circadian rhythm of melatonin secretion between the GA and RA groups and explore its association with the incidence of POD until discharge from the hospital.
Secondary outcomes
The secondary outcomes are to compare the subtype and severity of POD, plasma cortisol levels, CRP, IL-6, sleep quality and pain until discharge from the hospital, and postoperative cognitive function at 6 months and 1 year after surgery between the GA and RA groups.
Eligibility criteria
Inclusion criteria
Participants will be included if they meet the following criteria: age of ≥65 years, hospital admission for surgical treatment of hip fracture and American Society of Anesthesiologists (ASA) physical status classification of I to III.
Exclusion criteria
Patients who meet any of the following criteria will be excluded: Parkinson’s disease, dementia (including dementia caused by Parkinson’s disease, Alzheimer’s disease or Lewy body dementia), a stroke within the prior 6 months or any other central nervous system disease, alcohol-related disorders, multiple trauma, preoperative delirium, severe deafness or vision problems, communication difficulties, night shift duty, taking medication related to melatonin, a plan to be transferred to the intensive care unit postoperatively and refusal to participate in the study or unexpected discharge.
Preoperative baseline data collection and evaluation
Trained investigators who are blind to the patient grouping will evaluate the patients and their medical charts to screen out potential participants according to the inclusion and exclusion criteria. Baseline data will be collected, including demographic data (eg, age, sex, race, education level, height and weight), surgical diagnosis, comorbidities, medication history, surgical history, smoking and drinking status, and the results of physical, laboratory and instrumental examinations. We will also record the ASA physical status classification. Specifically, preoperative cognitive scans will be performed using the Mini-Mental State Examination (MMSE)28; preoperative delirium will be diagnosed with the Confusion Assessment Method (CAM)29 30; activities of daily living and instrumental activities of daily living will be assessed with the Katz Index of Independence in Activities of Daily Living (ADL)31; sleep quality will be assessed by the Pittsburgh Sleep Quality Index (PSQI)32; and pain intensity will be assessed using the numerical rating scale (NRS).33
Perioperative management
All individuals will be placed in a standard private room with a consistent light–dark cycle and room temperature during the study period. The lights will be turned off between 20:00 and 06:00 even at the time of blood sample collection, and light exposure (controlled to be <10 lux) will be measured during the night-time sleep period. The participants will be requested to refrain from alcohol, caffeine and chocolate. Their activities will be restricted from 20:00 to 06:00, during which time they will be free from interference factors including noise, television and mobile phones during the study period.
Anaesthesia and analgesia
All patients will receive an ultrasound-guided fascia iliac block (a single injection of 30 mL of 0.33% ropivacaine) after admission to the operating room. During the operation, the patients’ eyes will be carefully covered with eye patches to avoid light effects. Sedatives (benzodiazepine and dexmedetomidine) or anticholinergics will be avoided during the perioperative phase.
In the RA group, ropivacaine will be used for spinal anaesthesia or combined spinal and epidural anaesthesia at the L2-3 or L3-4 levels, and the sensory block will be controlled at T8-T10. If RA fails, the patients will receive surgery under GA and will withdraw from this trial to avoid the influence of lumbar puncture. In the GA group, standard GA will be administered to the patients. Anaesthesia will be intravenously induced with 0.5–1.5 mg/kg propofol, 0.2 mg/kg etomidate, 0.3 µg/kg sufentanil and 0.15 mg/kg cisatracurium. Anaesthesia will be maintained with sevoflurane, propofol and remifentanil, and the bispectral (BIS) value will be maintained between 40 and 60. Mechanical ventilation will be continued to keep the end-tidal carbon dioxide concentration at 35–40 mm Hg in the GA group. A Vigileo/FloTrac device will be used for measuring stroke volume variation and cardiac index to guide fluid therapy. Fluids or vasoactive substances will be administrated to maintain mean arterial pressure >70 mm Hg, heart rate <100 beat per min (bpm) and urine output >0.5 mL/kg/hour. Meanwhile, all patients will be warmed with forced air and intravenous fluids. On the day of surgery, intraoperative parameters will be recorded, including the mean arterial pressure, heart rate, BIS value, start time of anaesthesia and surgery, duration and type of anaesthesia and surgery, anaesthetic drugs and doses, sedative doses, blood loss, fluid balance and transfusion of blood or clotting products, complications and adverse events.
For postoperative analgesia management, the patients will receive intravenous patient-controlled analgesia (100 µg sufentanil and 8 mg ondansetron mixed with normal saline to a total volume of 100 mL). Intramuscular injection of pethidine (50 mg) or oral use of oxycodone (5 mg)/acetaminophen (325 mg) will be made as remedy analgesia.
Postoperative management and follow-up
All patients will be followed up twice a day (8:00 and 20:00) until discharge from the hospital. The investigators blind to grouping will record the total hospitalisation cost, the in-hospital length of stay and the occurrence of postoperative complications during hospitalisation. Long-term cognitive function will be assessed for 1 year after surgery.
-
Delirium assessment
Delirium will be diagnosed using the CAM, and the subtype and severity of delirium will be assessed by using the Memorial Delirium Assessment Scale.34 Surveillance will involve twice-daily visits (08:00 and 20:00) by a trained geriatrician. The duration of delirium will be measured as the number of days from onset of delirium symptoms to resolution of symptoms. If the researcher is in doubt regarding this assessment, the diagnosis of POD will be referred to an external expert.
-
Sleep assessment
All participants will continuously wear a sleep tracker (Fitbit Charge 3; Fitbit, Inc, San Francisco, California, USA), which is an available and inexpensive method to quantify sleep patterns.35 The investigators will provide instructions on wearing the sleep tracker, a mobile device capable of downloading Fitbit Connect software and completing the corresponding sleep logs (bedtime, wake time and nap times). The frequency of syncing will be checked by the research staff weekly, and the Fitbit Charge proprietary algorithm will process the sensor data to automate the sleep variables.36 All patients will wear sleep trackers until discharge from the hospital. Based on the data from the sleep tracker, sleep logs and direct observation by a third party, we will derive the following data: total sleep time (min), sleep onset latency (min), wake time after sleep onset (min), time in rapid eye movement sleep and time spent in ‘light sleep’ and ‘deep sleep’ (according to Fitbit Inc). Subjective sleep quality will be assessed with an NRS (0=worst sleep and 10=best sleep).37
The intensity of postoperative pain both at rest and with movement will be evaluated twice daily (08:00 and 20:00) with the NRS.
Cognitive function will be assessed by the same geriatrician at 6 months and 1 year after surgery. The Modified Telephone Interview for Cognitive Status will be used to test the patient’s cognitive function in non-face-to-face interviews.37
Blinding
An investigator will be assigned to preserve and distribute results. Investigators will be not blinded to the study group assignment, but the trained geriatrician responsible for postoperative evaluation including POD diagnosis, as well as PSQI, ADLs and NRS scores are blinded to the grouping.
Light exposure
Considering that light suppresses melatonin secretion except in life-threatening situations, the only illumination from a penlight will be used during these interventions.15 19 Blood sample collection and nursing interventions at night will be performed with a penlight to avoid light exposure. Light intensity readings will be obtained with a digital metre to ensure that light exposure does not exceed 10 lux.
Sample collection
Usually, the melatonin concentration is low throughout the day and then rises to a peak between 02:00 and 04:00.15 To establish the circadian rhythm, the plasma concentration of melatonin will be measured at least four times a day.15 19 Postoperative blood samples will be dynamically collected every day at 04:00, 10:00, 16:00 and 22:00 until the patient is discharged through venipuncture (2 mL per sample).16 19 All blood samples will be collected in the heparinised tubes. The samples will be centrifuged at 3000 rpm for 10 min and will be stored at −80°C until further analysis. We will measure plasma melatonin, cortisol, CRP and IL-6. Laboratory technicians will be blinded to the results of all clinical data. The concentration of melatonin will be measured with the enzyme immunoassay method (cat no. RE 54021; IBL International GmbH, Hamburg, Germany) with a limit of detection of 1.6 pg/mL.38 Plasma cortisol levels will be analysed using an electrochemiluminescence immunoassay method (Roche Diagnostics GmbH, Germany). To measure inflammation, we will measure plasma CRP and IL-6 using immunoturbidimetry (Beckman Coulter, Inc, USA) and electrochemiluminescence immunoassay method (Roche, Germany), respectively.
Sample size estimation
Melatonin is secreted in a biphasic pattern in which night-time secretion increases from 20:00 to 22:00 with a peak at 02:00 to 04:00, and daytime secretion decreases from 06:00 to 08:00 with a nadir at 12:00.14 39 Cronin et al15 researched the night-time melatonin secretion of adult patients for 3 days after gynaecological surgery and found that the lowest level occurred on the first night postoperatively. In another study of elderly patients undergoing major abdominal surgery, Shigeta et al40 found that the postoperative amplitude of night-time melatonin secretion was significantly lower than the preoperative amplitude. These findings indicate that the peak of melatonin secretion is suppressed to the greatest degree in the first postoperative night, possibly leading to a melatonin rhythm shift. Therefore, for the present study, we will choose 04:00 on the first postoperative night as the time point for comparing the melatonin concentration between the groups. Our pilot study showed that the mean±SD of melatonin was 10.48±4.04 and 13.40±6.85 pg/mL in the GA and RA groups (n=15 in each group), respectively. Using a two-sided α value of 0.05% and 85% power and considering a 10% dropout rate, we plan to include 138 patients in this study, with 69 patients in each group (1:1).
Circadian melatonin rhythm data
Circadian markers of the melatonin rhythm, including the mesor (mean concentration) and amplitude (peak value minus the mean value), and acrophase time (peak time), will be calculated by Origin (OriginLab Corp, USA) based on cosinor regression y = a+b×cos(x×π/12 - c×π/12), in which a, b and c represent mesor, amplitude and acrophase, respectively.41 The data will be subsequently smoothed with the cosinor method curve-fitting procedure (GraphPad Prism 8; GraphPad Software, Inc).41 42
Adverse events
In the study, the intervention measures of both groups are anaesthesia methods currently being used. Therefore, there will be no additional risk to participants. The safety of patients will be monitored, and patients will receive study information including explicit details on whom to contact and will be told to withdraw from the trial and get the corresponding compensation in case of an adverse event situation.
Data management and storage
At the first meeting, researchers will make appointments for the following dates to improve adherence to the study. All the data will be collected recorded in the case report form (CRF). After the data are recorded, the data will be stored in a locked cabinet. Data entry will be performed by two investigators with the EpiData3.10 database system. Personal information of participants will be confidentially kept, and all data will be identified by a name acronym and a study identification number in the CRF. The data analysis will be performed by the primary investigator and designated teammates.
Ethics and declarations
During the study period, we will follow the Declaration of Helsinki and Chinese guidelines of Good Clinical Practice to guarantee the right of the patients. The study protocol has been approved by the Medical Science Research Ethics Committees of Beijing Jishuitan Hospital (JLKS201901-04). This study has been registered at the Chinese Clinical Trial Registry (ChiCTR1900027393). To inform the purpose and risks of the study, each enrolled patient accompanied by at least one family member or proxy will sign a written informed consent. Besides, participants will sign the additional consent provisions for collection and use of participant data and biological specimens in ancillary studies, and all participants and investigators will keep the written informed consent. The primary investigator will regularly report on the progress and changes of the study to the local ethics committee. The results of the study will be published in peer-reviewed international journals and will be presented at national and international conferences and symposiums.
Data safety monitoring
The data monitoring committee (DMC) comprised of two specialists outside the trial will be monitoring the progress and safety of the trial, and the DMC can give suggestions on safety issues and even pause the trial.
Statistical analysis
Demographic data and information on pain scores, delirium and sleep assessment data, and adverse events during and after surgery will be recorded. Data will be presented as mean and SD, median and IQR (25th–75th percentile) or frequency and proportion depending on the variable type and distribution. Categorical variables will be assessed with the χ2 test or Fisher’s exact test. Differences in continuous variables between the groups will be assessed with a parametric t-test, the Wilcoxon rank-sum test or the Kruskal-Wallis test. The Mann-Whitney U test will be used to analyse non-normal variables. Results will be presented with 95% CIs. The association between melatonin parameters and sleep quality as well as POD will be also validated by Pearson or Spearman analysis and linear regression analysis. Multiple imputation methods, that is, mean completer and regression, will be used to handle missing data. A p value of <0.05 will be considered statistically significant. All statistical analyses will be performed with SPSS software, V.21.0 (IBM Corp, Armonk, New York, USA).
Discussion
This clinical trial is designed to test whether RA can relieve perioperative melatonin rhythm disruption compared with GA and decrease the incidence of POD in elderly patients with hip fracture.
Although some previous studies have shown connections between melatonin secretion disruption and delirium, few have elucidated the real melatonin rhythm pattern.43–45 For example, a prospective observational study by Yoshitaka et al46 showed that the delta plasma melatonin concentration (calculated based on the preoperative and postoperative values at selected time points) at 1 hour after surgery was significantly lower in patients with than without delirium. Melatonin secretion exhibits a biphasic pattern in which daytime secretion is low (5 pg/mL on average), and night-time secretion is relatively high (50–100 pg/mL).14 39 However, the sample collection time points of melatonin in the above-mentioned study (before the operation, 1 hour after the operation and at 08:00 on postoperative days 1 and 2) represented either night-time secretion on the operation day or daytime secretion on the postoperative days; thus, the measurements do not represent the comprehensive rhythm of melatonin. In contrast, in the current study the blood samples will be taken before anaesthesia, postoperative blood samples (melatonin) every day at 04:00, 10:00, 16:00 and 22:00 until discharge. These measurements are adequate to establish a melatonin rhythm curve. They will show the individual postoperative melatonin concentrations and allow us to analyse the irregular pattern of melatonin secretion in patients with delirium.
Previous trials have shown that GA may influence postoperative melatonin secretion.22–24 First, in patients who received combined intravenous and inhaled anaesthesia, the melatonin levels decreased the first night after surgery.24 47 Second, GA by inhalation was associated with a significant decrease in the melatonin concentration on the night of surgery, and this decrease was 350% greater than that on the third night after surgery.15 Third, total intravenous anaesthesia induced a phase delay in the 6-SMT rhythm; the peak time was late by about 1 hour, and the amplitude of the melatonin rhythm decreased by 85% on the night of surgery.12 In contrast, another study demonstrated that RA was helpful to relieve sleep disturbance after surgery,27 which may result from better maintenance of the melatonin rhythm postoperatively. However, little is known about how the two anaesthesia methods differently affect the melatonin rhythm. Kärkelä et al24 conducted a similar clinical study and found that anaesthesia and surgery disturbed the circadian melatonin rhythm, but there was no significant difference in the melatonin secretion between the spinal anaesthesia and GA groups for minor orthopaedic operations.24 We do not consider that these results are especially robust and generalisable because of several limitations of the trial, such as the small sample size (40 participants), measurement of only the saliva and urine concentrations of melatonin (no plasma or serum concentrations) and insufficient melatonin measurement timing (measurement of only night-time secretion levels). We hypothesise that RA can maintain an almost normal melatonin rhythm for the following two reasons. First, RA may significantly reduce the types and amounts of general anaesthetics used compared with GA, which can strongly alter melatonin secretion through different signal pathways. Second, RA allows better pain control and improved sleep quality compared with GA, which further facilitates a decrease in the severity of melatonin rhythm disruption.
Conclusion
In conclusion, to our knowledge, this is the first study to compare the circadian rhythm of melatonin secretion between GA and RA groups and to explore the potential association between circadian rhythm disruptions and the development of delirium. Our findings will promote a greater understanding of delirium pathophysiology and provide clinical evidence for the optimal anaesthesia method in elderly patients undergoing hip fracture surgery.
Supplementary Material
Footnotes
YY and YS contributed equally.
WZ and ZL contributed equally.
Contributors: ZL and XG obtained funding. ZL, WZ, GW and XG contributed to the study design. YY, YS, YJ and YZ performed the trial. XM, XJ and YH contributed to data collection. CL, YL, CS and XW analysed the data. YS and YY drafted the manuscript. ZL and WZ carefully reviewed the manuscript. All authors have read and approved the final manuscript.
Funding: This research is supported by the National Natural Science Foundation of China (81971012 and 81873726), Key Clinical Projects of Peking University Third Hospital (BYSYZD2019027), and Peking University 'Clinical Medicine plus X' Youth Project (PKU2020LCXQ016).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Sieber FE, Neufeld KJ, Gottschalk A, et al. . Effect of depth of sedation in older patients undergoing hip fracture repair on postoperative delirium: the STRIDE randomized clinical trial. JAMA Surg 2018;153:987–95. 10.1001/jamasurg.2018.2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruce AJ, Ritchie CW, Blizard R, et al. . The incidence of delirium associated with orthopedic surgery: a meta-analytic review. Int Psychogeriatr 2007;19:197–214. 10.1017/S104161020600425X [DOI] [PubMed] [Google Scholar]
- 3.Su X, Meng Z-T, Wu X-H, et al. . Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet 2016;388:1893–902. 10.1016/S0140-6736(16)30580-3 [DOI] [PubMed] [Google Scholar]
- 4.Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet 2014;383:911–22. 10.1016/S0140-6736(13)60688-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wildes TS, Mickle AM, Ben Abdallah A, et al. . Effect of Electroencephalography-Guided anesthetic administration on postoperative delirium among older adults undergoing major surgery: the engages randomized clinical trial. JAMA 2019;321:473–83. 10.1001/jama.2018.22005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leslie DL, Marcantonio ER, Zhang Y, et al. . One-Year health care costs associated with delirium in the elderly population. Arch Intern Med 2008;168:27–32. 10.1001/archinternmed.2007.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aldecoa C, Bettelli G, Bilotta F, et al. . European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol 2017;34:192–214. 10.1097/EJA.0000000000000594 [DOI] [PubMed] [Google Scholar]
- 8.Maldonado JR Delirium pathophysiology: an updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry 2018;33:1428–57. 10.1002/gps.4823 [DOI] [PubMed] [Google Scholar]
- 9.Marcantonio ER, Rudolph JL, Culley D, et al. . Serum biomarkers for delirium. J Gerontol A Biol Sci Med Sci 2006;61:1281–6. 10.1093/gerona/61.12.1281 [DOI] [PubMed] [Google Scholar]
- 10.Maldonado JR Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry 2013;21:1190–222. 10.1016/j.jagp.2013.09.005 [DOI] [PubMed] [Google Scholar]
- 11.Scott BK Disruption of circadian rhythms and sleep in critical illness and its impact on the development of delirium. Curr Pharm Des 2015;21:3443–52. 10.2174/1381612821666150706110656 [DOI] [PubMed] [Google Scholar]
- 12.Gögenur I, Middleton B, Kristiansen VB, et al. . Disturbances in melatonin and core body temperature circadian rhythms after minimal invasive surgery. Acta Anaesthesiol Scand 2007;51:1099–106. 10.1111/j.1399-6576.2007.01387.x [DOI] [PubMed] [Google Scholar]
- 13.Tordjman S, Chokron S, Delorme R, et al. . Melatonin: pharmacology, functions and therapeutic benefits. Curr Neuropharmacol 2017;15:434–43. 10.2174/1570159X14666161228122115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brzezinski A Melatonin in humans. N Engl J Med 1997;336:186–95. 10.1056/NEJM199701163360306 [DOI] [PubMed] [Google Scholar]
- 15.Cronin AJ, Keifer JC, Davies MF, et al. . Melatonin secretion after surgery. Lancet 2000;356:1244–5. 10.1016/S0140-6736(00)02795-1 [DOI] [PubMed] [Google Scholar]
- 16.Olofsson K, Alling C, Lundberg D, et al. . Abolished circadian rhythm of melatonin secretion in sedated and artificially ventilated intensive care patients. Acta Anaesthesiol Scand 2004;48:679–84. 10.1111/j.0001-5172.2004.00401.x [DOI] [PubMed] [Google Scholar]
- 17.Miyazaki T, Kuwano H, Kato H, et al. . Correlation between serum melatonin circadian rhythm and intensive care unit psychosis after thoracic esophagectomy. Surgery 2003;133:662–8. 10.1067/msy.2003.149 [DOI] [PubMed] [Google Scholar]
- 18.Scholtens RM, van Munster BC, van Faassen M, et al. . Plasma melatonin levels in hip fracture patients with and without delirium: a confirmation study. Mech Ageing Dev 2017;167:1–4. 10.1016/j.mad.2017.08.016 [DOI] [PubMed] [Google Scholar]
- 19.Piotrowicz K, Klich-Rączka A, Pac A, et al. . The diurnal profile of melatonin during delirium in elderly patients-preliminary results. Exp Gerontol 2015;72:45–9. 10.1016/j.exger.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald JM, Adamis D, Trzepacz PT, et al. . Delirium: a disturbance of circadian integrity? Med Hypotheses 2013;81:568–76. 10.1016/j.mehy.2013.06.032 [DOI] [PubMed] [Google Scholar]
- 21.Balan S, Leibovitz A, Zila SO, et al. . The relation between the clinical subtypes of delirium and the urinary level of 6-SMT. J Neuropsychiatry Clin Neurosci 2003;15:363–6. 10.1176/jnp.15.3.363 [DOI] [PubMed] [Google Scholar]
- 22.Coppola S, Caccioppola A, Chiumello D. Internal clock and the surgical ICU patient. Curr Opin Anaesthesiol 2020;33:177–84. 10.1097/ACO.0000000000000816 [DOI] [PubMed] [Google Scholar]
- 23.Poulsen RC, Warman GR, Sleigh J, et al. . How does general anaesthesia affect the circadian clock? Sleep Med Rev 2018;37:35–44. 10.1016/j.smrv.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 24.Kärkelä J, Vakkuri O, Kaukinen S, et al. . The influence of anaesthesia and surgery on the circadian rhythm of melatonin. Acta Anaesthesiol Scand 2002;46:30–6. 10.1034/j.1399-6576.2002.460106.x [DOI] [PubMed] [Google Scholar]
- 25.Dispersyn G, Pain L, Challet E, et al. . General anesthetics effects on circadian temporal structure: an update. Chronobiol Int 2008;25:835–50. 10.1080/07420520802551386 [DOI] [PubMed] [Google Scholar]
- 26.Guo X, Kuzumi E, Charman SC, et al. . Perioperative melatonin secretion in patients undergoing coronary artery bypass grafting. Anesth Analg 2002;94:1085–91. 10.1097/00000539-200205000-00006 [DOI] [PubMed] [Google Scholar]
- 27.Kjølhede P, Langström P, Nilsson P, et al. . The impact of quality of sleep on recovery from fast-track abdominal hysterectomy. J Clin Sleep Med 2012;8:395–402. 10.5664/jcsm.2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Girard TD, Thompson JL, Pandharipande PP, et al. . Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir Med 2018;6:213–22. 10.1016/S2213-2600(18)30062-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evered L, Silbert B, Knopman DS, et al. . Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth 2018;121:1005–12. 10.1016/j.bja.2017.11.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palanca BJA, Wildes TS, Ju YS, et al. . Electroencephalography and delirium in the postoperative period. Br J Anaesth 2017;119:294–307. 10.1093/bja/aew475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz S, Ford AB, Moskowitz RW, et al. . Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA 1963;185:914–9. 10.1001/jama.1963.03060120024016 [DOI] [PubMed] [Google Scholar]
- 32.Buysse DJ, Reynolds CF, Monk TH, et al. . The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 33.Bahreini M, Jalili M, Moradi-Lakeh M. A comparison of three self-report pain scales in adults with acute pain. J Emerg Med 2015;48:10–18. 10.1016/j.jemermed.2014.07.039 [DOI] [PubMed] [Google Scholar]
- 34.Gross AL, Tommet D, D'Aquila M, et al. . Harmonization of delirium severity instruments: a comparison of the DRS-R-98, MDAS, and CAM-S using item response theory. BMC Med Res Methodol 2018;18:92. 10.1186/s12874-018-0552-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Zambotti M, Goldstone A, Claudatos S, et al. . A validation study of Fitbit charge 2™ compared with polysomnography in adults. Chronobiol Int 2018;35:465–76. 10.1080/07420528.2017.1413578 [DOI] [PubMed] [Google Scholar]
- 36.Castner J, Mammen MJ, Jungquist CR, et al. . Validation of fitness tracker for sleep measures in women with asthma. J Asthma 2019;56:719–30. 10.1080/02770903.2018.1490753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Li H-J, Wang D-X, et al. . Impact of inhalational versus intravenous anaesthesia on early delirium and long-term survival in elderly patients after cancer surgery: study protocol of a multicentre, open-label, and randomised controlled trial. BMJ Open 2017;7:e018607. 10.1136/bmjopen-2017-018607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kennaway DJ A critical review of melatonin assays: past and present. J Pineal Res 2019;67:e12572. 10.1111/jpi.12572 [DOI] [PubMed] [Google Scholar]
- 39.Rzepka-Migut B, Paprocka J. Melatonin-Measurement methods and the factors modifying the results. A systematic review of the literature. Int J Environ Res Public Health 2020;17:1916. 10.3390/ijerph17061916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shigeta H, Yasui A, Nimura Y, et al. . Postoperative delirium and melatonin levels in elderly patients. Am J Surg 2001;182:449–54. 10.1016/S0002-9610(01)00761-9 [DOI] [PubMed] [Google Scholar]
- 41.Liu L, Wang Z, Cao J, et al. . Effect of melatonin on monochromatic light-induced changes in clock gene circadian expression in the chick liver. J Photochem Photobiol B 2019;197:111537. 10.1016/j.jphotobiol.2019.111537 [DOI] [PubMed] [Google Scholar]
- 42.Refinetti R, Lissen GC, Halberg F. Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res 2007;38:275–325. 10.1080/09291010600903692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu Y, Li Y-W, Wang L, et al. . Promoting sleep and circadian health may prevent postoperative delirium: a systematic review and meta-analysis of randomized clinical trials. Sleep Med Rev 2019;48:101207. 10.1016/j.smrv.2019.08.001 [DOI] [PubMed] [Google Scholar]
- 44.FitzGerald JM, O'Regan N, Adamis D, et al. . Sleep-Wake cycle disturbances in elderly acute general medical inpatients: longitudinal relationship to delirium and dementia. Alzheimers Dement 2017;7:61–8. 10.1016/j.dadm.2016.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yousaf F, Seet E, Venkatraghavan L, et al. . Melatonin and postoperative delirium: a possible link? Can J Anaesth 2010;57:794–5. 10.1007/s12630-010-9340-2 [DOI] [PubMed] [Google Scholar]
- 46.Yoshitaka S, Egi M, Morimatsu H, et al. . Perioperative plasma melatonin concentration in postoperative critically ill patients: its association with delirium. J Crit Care 2013;28:236–42. 10.1016/j.jcrc.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 47.Ram E, Vishne TH, Weinstein T, et al. . General anesthesia for surgery influences melatonin and cortisol levels. World J Surg 2005;29:826–9. 10.1007/s00268-005-7724-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.