Abstract
Background
Buprenorphine treatment is not equally effective in all patients with opioid use disorder (OUD). Two retrospective studies showed that, among African Americans (AAs), rs678849, a polymorphism in the delta-opioid receptor gene, moderated the therapeutic effect of sublingual buprenorphine.
Methods
We examined rs678849 as a moderator of the response to an extended-release subcutaneous buprenorphine formulation (BUP-XR) in a 24-week OUD treatment study of 127 AAs and 327 European Americans (EAs). Participants were randomly assigned to receive: (1) BUP-XR as 2 monthly injections of 300 mg followed by either 300 mg monthly or 100 mg monthly for 4 months, or (2) monthly volume-matched placebo injections. Generalized estimating equations logistic regression analyses tested, per population group, the main and interaction effects of treatment (BUP-XR vs placebo) and genotype group (rs678849*CC vs CT/TT) on weekly urine drug screens (UDS).
Results
Among AAs, the placebo group had higher rates of opioid-positive UDS than the BUP-XR group (log odds ratio = 1.67, 95% CI = 0.36, 2.98), but no genotype by treatment effect (P = .80). Among EAs, the placebo group also showed higher rates of opioid-positive UDS than the BUP-XR group (log odds ratio = 1.97, 95% CI = 1.14, 2.79) but a significant genotype by treatment interaction (χ 2(1) = 4.33, P = .04).
Conclusion
We found a moderating effect of rs678849 on the response to buprenorphine treatment of OUD in EAs, but not AAs. These findings require replication in well-powered, prospective studies of both AA and EA OUD patients treated with BUP-XR and stratified on rs678849 genotype.
Keywords: Buprenorphine, delta-opioid receptor, rs678849, opioid use disorder, pharmacogenetic
Significance Statement.
To advance precision medicine treatment of opioid use disorder, we tested whether variation in the gene that encodes the delta-opioid receptor is associated with the response to buprenorphine treatment. Using data from a large clinical trial of extended-release buprenorphine, we found that one genotype was associated with a greater likelihood of having an opioid-positive urine drug screen, but only in European-ancestry individuals. This contrasts with the findings from 2 prior studies, both of which showed a similar effect, but only in African-ancestry individuals. Thus, additional studies of both population groups are needed to evaluate this potential predictor of a robust response to buprenorphine treatment of opioid use disorder.
Introduction
Opioid use and opioid-related overdose are epidemic in the United States, affecting all major segments of the population (Hedegaard et al., 2017; Rudd et al., 2016). Opioid analgesic prescriptions in the country quadrupled over the 2 decades beginning in 1999 (Paulozzi et al., 2011). Despite subsequent decreases in prescribing, in 2018, 3.7% of US adults reported misuse of a prescription pain reliever in the past year (Substance Abuse and Mental Health Services Administration, 2019). Increased demand for prescription opioids and their decreasing availability due to administrative and legal efforts to combat the epidemic have contributed to a transition from prescribed opioids to illicit ones, including heroin and illicitly manufactured fentanyl, a high-potency opioid (Compton et al., 2016). In 2018, nearly 2 million US adults (0.8% of the population aged 18 and older) were reported to have an opioid use disorder (OUD) involving prescription opioids, and another half million adults (0.2%) had an OUD involving heroin (Substance Abuse and Mental Health Services Administration, 2019).
In 2002, buprenorphine, a mu-opioid receptor partial agonist and kappa-opioid receptor antagonist, was approved in the United States for treating OUD. Buprenorphine treatment aims to alleviate the signs and symptoms of opioid withdrawal, reduce craving, and block the subjective effects of abused opioids. Because buprenorphine is not equally effective in all patients with OUD, identifying those who are most likely to respond well to the medication prior to its initiation is an important clinical challenge. Differentiating patients unlikely to respond well to buprenorphine treatment could potentially prevent a delay in their being treated with methadone or long-acting naltrexone, 2 other medications approved for treating OUD. In 2 retrospective analyses of sublingual buprenorphine (BUP-SL), an intronic single nucleotide polymorphism (SNP), rs678849 in OPRD1, which encodes the delta-opioid receptor, moderated the response to buprenorphine treatment of OUD in African Americans (AAs) (Crist et al., 2013, 2019). In the first study, a 24-week comparison of buprenorphine vs methadone in 77 AAs, of the 41 buprenorphine-treated patients, those with the rs678849*CT or TT genotype (n = 17 with either CT or TT) had significantly fewer opioid-positive urine drug screens (UDS) (30.7% ± 32.3) than those with the CC genotype (n = 24; 60.7% ± 37.2; P < .004). The study showed no moderating effect of the SNP in the 566 European-American (EA) participants (Crist et al., 2013). In an independent sample of 55 AA buprenorphine-treated patients (Crist et al., 2019), the risk ratio for opioid-positive UDS associated with the CC genotype was 1.69, thereby replicating the initial finding that Crist et al. (2013) obtained in AAs, but not EAs. Combining data across the 2 studies, buprenorphine-treated AA patients with the CC genotype had opioid-positive UDS 56.3% of the time, compared with 30.7% for patients in the combined CT and TT genotype groups (P < .0001).
To extend these findings by reducing the potentially confounding effects of nonadherence to BUP-SL (Tkacz et al., 2012), we examined the moderating effect of rs678849 on the response to treatment using an extended-release, monthly subcutaneous buprenorphine formulation (BUP-XR; Haight et al., 2019) in AA and EA participants with OUD. The current study also included a placebo group to control for nonpharmacological effects, whereas the prior published studies did not.
Methods
Study Design
The study was a double-blind, placebo-controlled trial conducted at 36 US treatment centers, as described in Haight et al. (2019) and in the study protocol included in the FDA Briefing Document (FDA Briefing Document RBP-6000, 2017). Eligible participants were those 18–65 years of age who met DSM-5 (American Psychiatric Association 2013) criteria for moderate or severe OUD for the 3 months prior to providing written informed consent and were seeking treatment. Potential participants were excluded if they received medications for the treatment of OUD within 90 days of enrollment, required chronic opioid treatment for a current diagnosis other than OUD, or had a current substance use disorder involving substances other than opioids, cocaine, cannabis, alcohol, or tobacco. Participants with moderate or severe alcohol use disorder were excluded, as were those with moderate or severe cocaine or cannabis use disorders if their screening UDS was positive for cocaine or cannabinoids, respectively.
A centralized institutional review board reviewed and approved the protocol in accordance with principles and requirements of the International Council for Harmonisation Good Clinical Practice guidelines. Written informed consent was obtained from participants before any study-related procedure was initiated.
Procedures
Following induction onto BUP-SL for 3 days, participants completed a 4- to 11-day period of dose adjustment with 8–24 mg daily of BUP-SL. After receiving at least 7 days of BUP-SL, participants with no significant opioid craving or withdrawal were randomly assigned (4:4:1:1) to receive BUP-XR 300 mg/300 mg (6 monthly injections of 300 mg), BUP-XR 300 mg/100 mg (2 monthly injections of 300 mg plus 4 monthly injections of 100 mg), or volume-matched placebos.
After randomization, participants attended weekly visits for 24 weeks at which a UDS, self-reported illicit drug use via the timeline follow-back method, measures of opioid withdrawal and craving, and safety assessments were obtained. The UDS tested for stimulants, barbiturates, benzodiazepines, cannabinoids, cocaine metabolite, methadone, opioids, oxycodone, and phencyclidine. Confirmatory testing for opioids used gas chromatography combined with mass spectrometry for codeine, hydrocodone, hydromorphone, methadone, morphine, oxycodone, and oxymorphone.
Analyses
We compared BUP-XR with placebo separately by population group given the large difference in rs678849 allele frequencies between AAs and EAs. Of the 504 randomized participants, there were 127 AAs and 327 EAs with genotype data. The outcome variable was a binary indicator of the presence/absence of an opioid-positive UDS. The time frame for the group comparisons was visits 1 through 24, inclusive.
We used generalized estimating equations logistic regression models with an independence working correlation and robust standard errors (Garrett et al., 2011). The main explanatory variables for the analyses were binary indicators of treatment group and of genotype. For the treatment group variable, we combined the BUP-XR groups (who all received 300 mg monthly for the first 2 months and then either 300 mg or 100 mg monthly for 4 months) to form a combined active BUP-XR treatment group (tx = 1), which we compared with a placebo group that combined the 2 volume-matched groups (tx = 0). Thus, the ratio of BUP-XR to placebo treatment was 4:1. For genotype, we combined the TT and CT groups (cc = 0) and compared them with the CC group (cc = 1).
The models also included time trends, modeling weekly visit as a continuous variable, and interaction terms comprised of treatment group, genotype group, and time. Because the 3-way interaction was not significant in either of the populations, we removed it from the model before testing the 2-way interactions. Similarly, because the genotype by time interaction was not significant in either population, it too was removed. The results below are for models with main effects for treatment, genotype and time, with treatment by genotype and treatment by time interactions. For each of the 2 population groups, we used least squares means estimates from the models to estimate opioid-positive UDS rates within treatment by genotype groups and of treatment effects within genotype groups.
We used Cox proportional hazards regression models to compare rates of dropout for treatment and genotype groups within each population group. In secondary analyses, we assessed the effects of missing data on the models by repeating the analyses equating a missed visit to an opioid-positive UDS. We also performed pattern-mixture analyses using a binary indicator for treatment completer as our pattern variable.
Results
Participants
The demographic and clinical characteristics of the sample by population and genotype groups are shown in Tables 1 and 2. The AA sample was predominantly (78.7%) male, with a mean (SD) age of 47.5 years (10.5) and a mean (SD) body mass index of 25.8 kg/m2 (4.1). About one-third (36.0%) of the AA participants used opioids intravenously at baseline. The genotype by treatment groups were well balanced at baseline, with only 1 comparison (of 36 made at baseline) attaining significance at uncorrected levels. The EA sample was also largely (62.7%) male, with a mean (SD) age of 37.0 years (10.0) and a mean body mass index of 25.6 kg/m2 (4.4). Nearly one-half (47.4%) of EA participants used opioids intravenously at baseline. Comparisons by treatment group, genotype group, and their interaction showed no significant subgroup differences.
Table 1.
Baseline Demographic and Clinical Characteristics of African-American Participants (N = 127)
| BUP-XR (n = 108) | Placebo (n = 19) | ||||||
|---|---|---|---|---|---|---|---|
| Genotype | Genotype | Significance level | |||||
| CC | CT/TT | CC | CT/TT | P value (treatment) | P value (genotype) | P value (interaction) | |
| N (%) | 70 (64.8) | 38 (35.2) | 13 (68.4) | 6 (31.6) | |||
| Mean (SD) age, y | 47.5 (9.6) | 47.5 (11.7) | 46.6 (11.3) | 49.0 (13.9) | .74 | .64 | .67 |
| Sex, % male | 54 (77.1%) | 32 (84.2%) | 9 (69.2%) | 5 (83.3%) | .96 | .52 | .80 |
| Mean (SD) BMI, kg/m2 | 25.4 (3.9) | 26.5 (4.5) | 25.5 (4.5) | 27.3 (2.5) | .65 | .37 | .74 |
| IV opioid usea | 24 (34.3%) | 15 (39.5%) | 5 (38.5%) | 2 (33.3%) | .78 | .83 | .69 |
| Mean (SD) COWS score | 2.7 (2.9) | 2.4 (3.0) | 2.8 (2.8) | 2.2 (1.5) | .87 | .68 | .88 |
| Mean (SD) SOWS score | 4.5 (6.6) | 4.2 (7.2) | 7.8 (8.7) | 2.7 (3.0) | .62 | .13 | .20 |
Abbreviations: BMI, body mass index; COWS, Clinical Opiate Withdrawal Scale (Wesson and Ling, 2003); SOWS, Subjective Opiate Withdrawal Scale (Handelsman et al., 1987).
a% whose most common route of opioid use at baseline was i.v.
Table 2.
Baseline Demographic and Clinical Characteristics of European-American Participants (N = 327)
| BUP-XR (n = 253) | Placebo (n = 74) | ||||||
|---|---|---|---|---|---|---|---|
| Genotype | Genotype | Significance level | |||||
| CC | CT/TT | CC | CT/TT | P value (treatment) | P value (genotype) | P value (interaction) | |
| N (%) | 64 (25.3) | 189 (74.7) | 20 (27.0) | 54 (73.0) | |||
| Mean (SD) age, y | 37.1 (10.4) | 36.8 (9.9) | 37.85 (10.4) | 37.09 (10.0) | .86 | .77 | .86 |
| Sex, % male | 39 (60.9%) | 120 (63.5%) | 15 (75.0%) | 31 (57.4%) | .42 | .17 | .17 |
| Mean (SD) BMI | 26.3 (4.6) | 25.57 (4.3) | 24.05 (4.1) | 25.51 (4.4) | .93 | .20 | .10 |
| IV opioid usea | 32 (50%) | 83 (43.9%) | 14 (70.0%) | 26 (48.2%) | .60 | .10 | .28 |
| Mean (SD) COWS score | 2.1 (2.0) | 1.9 (2.2) | 3.3 (2.8) | 1.9 (2.4) | .95 | .02 | .08 |
| Mean (SD) SOWS score | 4.1 (4.6) | 3.5 (4.9) | 5.5 (5.0) | 3.7 (5.4) | .78 | .17 | .42 |
Abbreviations: BMI, body mass index; COWS, Clinical Opiate Withdrawal Scale (Wesson and Ling, 2003); SOWS, Subjective Opiate Withdrawal Scale (Handelsman et al., 1987).
a% whose most common route of opioid use at baseline was i.v.
Among AAs, the rs678849 genotype frequencies were CC: N = 83 (65.4%), CT: N = 37 (29.1%), and TT: N = 7 (5.5%), consistent with Hardy-Weinberg equilibrium expectations [χ 2(1) = 1.08, P = .58]. Among EAs, the rs678849 genotype frequencies were CC: N = 84 (25.7%), CT: N = 164 (50.2%), and TT: N = 79 (24.2%), also consistent with Hardy-Weinberg equilibrium expectations [χ 2(1) = 0.0035, P = .99].
Treatment Outcomes and Moderating Effects
African Americans (n = 127)
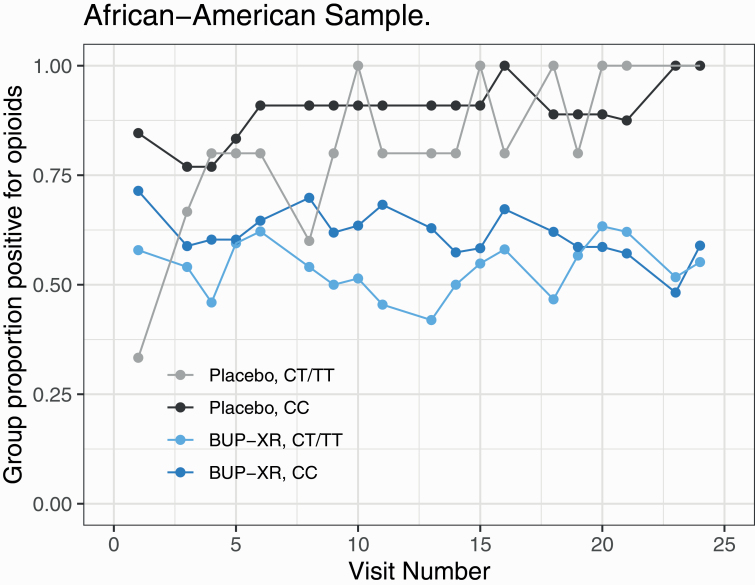
—The interaction effects were not significant for genotype by treatment by time (P = .43) and genotype by time (P = .16). There was a significant treatment by time interaction [χ 2(1) = 7.37, P = .007], with the log odds ratio (LOR) of an opioid-positive UDS for placebo relative to BUP-XR increasing by 0.11 (95% CI = 0.02, 0.21) weekly (see Figure 1).
Figure 1.
Proportion of opioid-positive urine drug screens by treatment and genotype groups in African Americans.
The genotype by treatment interaction in AAs was not significant [χ 2(1) = 0.07, P = .80]. As shown in Table 3, among participants with the CC genotype, the estimated log odds of an opioid-positive UDS in the placebo group was 2.30 (SE = 0.92) compared with 0.48 (SE = 0.20) in the BUP-XR group, with a LOR for placebo relative to BUP-XR of 1.83 (95% CI = –0.02, 3.67, P = .05). Among CT/TT participants, the estimated log odds of an opioid-positive UDS in the placebo group was 1.67 (SE = 0.79) compared with 0.15 (SE = 0.27) in the BUP-XR group, with an LOR for placebo relative to BUP-XR of 1.52 (95% CI = –0.12, 3.16, P = .07). Because there were few AA participants with the TT genotype (N = 7), we did not calculate the LOR for that subgroup. Thus, among AAs, the placebo group showed higher rates of opioid-positive UDS than the BUP-XR group, yielding a significant main effect of treatment [χ 2(1) = 6.27, P = .01, LOR = 1.67, 95% CI = 0.36, 2.98]. Although rates of opioid-positive UDS in the CT/TT genotype were lower than those in the CC genotype for both the placebo and BUP-XR groups, the main effect of genotype was not significant [χ 2(1) = 0.59, P = .44, LOR = –0.48, 95% CI = –1.63, 0.67].
Table 3.
Genotype by Treatment Group Effects on the Likelihood of an Opioid-Positive UDS
| African Americans (n = 127) | European Americans (n = 327) | ||||
|---|---|---|---|---|---|
| [Interaction χ 2(1) = 0.07, P = .80] | [Interaction χ 2(1) = 17.35, P < .0001] | ||||
| Genotype | Medication | Log odds of + UDS (SE) | LOR (95%CI) | Log odds of + UDS (SE) | LOR (95%CI) |
| CC | Placebo | 2.30 (0.93) | 1.83 (-0.02, 3.67) | 1.04 (0.57) | 1.08 (-0.11, 2.27) |
| CC | BUP-XR | 0.48 (0.20) | -0.04 (0.21) | ||
| CT/TT | Placebo | 1.67 (0.79) | 1.52 (-0.12, 3.16) | 2.25 (0.42) | 2.86 (2.00, 3.71) |
| CT/TT | BUP-XR | 0.15 (0.27) | -0.61 (0.12) | ||
Abbreviations: BUP-XR, buprenorphine formulation; 95%CI = 95% confidence interval; LOR, log odds ratio; UDS, urine drug screen.
Dropout
There were no significant effects of genotype by treatment (χ 2(1) = 1.09, P = .30), genotype [χ 2(1) = 0.01, P = .92, hazard ratio (HR) of CC relative to CT/TT = 1.04], or treatment [χ 2(1) = 1.96, P = .16, HR of placebo relative to BUP-XR = 1.83] on time to dropout.
Missing Data Analyses
Supplementary Table 1 shows the estimated log odds of opioid use for each treatment by genotype combination for AAs under the 3 different modeling approaches, with positive values indicating rates of opioid-positive UDS higher than 50%. For each genotype, positive LOR values reflect higher rates of opioid-positive UDS in the placebo group than the BUP-XR group. For the CC genotype group, the estimated LORs under ignorability, missing = opioid use, and pattern-mixture models were 1.83, 1.91, and 2.53, respectively, and for the CT/TT genotype group they were 1.52, 1.57, and 0.00, respectively, so all 3 approaches had larger estimated LORs for the CC than for the CT/TT genotypes. The differences between the CC and CT/TT LORs were similar for the ignorable and missing = opioid use models; the larger difference in the pattern mixture model appears to be a small-sample effect. The interaction P values show that none of these estimated differences between the effects in the CC and CT/TT groups are statistically significant.
European Americans (n = 327)
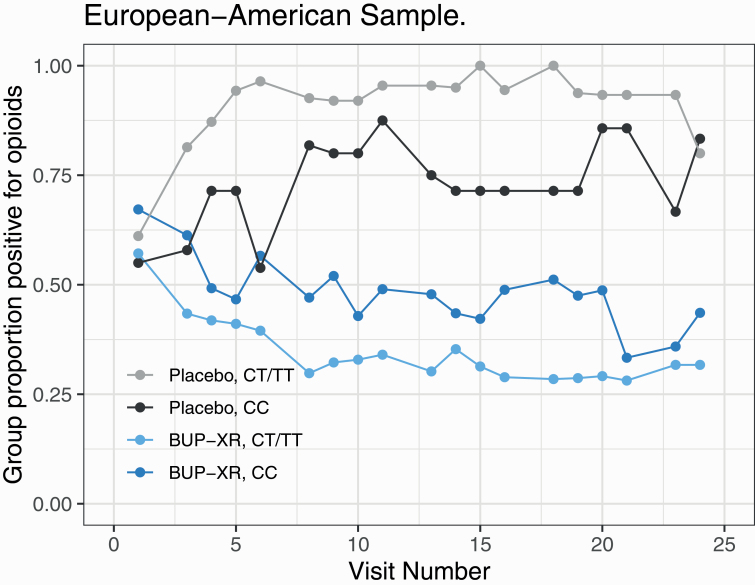
—The interaction effects for genotype by treatment by time (P = .44) and genotype by time (P = .98) were nonsignificant. There was a significant treatment by time interaction [χ 2(1) = 17.35, P < .0001], with the LOR for opioid-positive UDS in the placebo group relative to the BUP-XR group increasing by 0.11 (95% CI = 0.03, 0.19) weekly (see Figure 2).
Figure 2.
Proportion of opioid-positive urine drug screens by treatment and genotype groups in European Americans.
The genotype by treatment interaction was also significant [χ 2(1) = 4.33, P = .040]. As can be seen in Table 3, among the CC genotype group, the estimated log odds (SE) of an opioid-positive UDS in the placebo arm was 1.04 (0.57) compared with –0.04 (0.21) in the BUP-XR group, with a LOR of 1.08 (95% CI = –0.11, 2.27, P = .07). For the CT/TT group, the estimated log odds of an opioid-positive UDS in the placebo group was 2.25 (0.42), while for the BUP-XR group it was –0.61 (0.12), with a LOR of 2.86 (95% CI = 2.00, 3.71, P < .0001). Thus, among EAs, within each genotype group, placebo-treated participants had higher rates of opioid-positive UDS than the BUP-XR group, with a larger treatment effect in the CT/TT group, yielding a significant main effect for treatment [χ 2(1) = 21.92, P < .0001, LOR = 1.97, 95% CI = 1.14, 2.79). Among EAs, the main effect of genotype was not significant [χ 2(1) = 1.03, P = .31, LOR = 0.32, 95% CI = –0.30, 0.94], despite slightly higher rates of positive UDS among the CT/TT group than the CC group.
Dropout
Neither the genotype by treatment interaction [χ 2(1) = 0.37, P = .54] nor the main effect of genotype [χ 2(1) = 0.05, P = .82, HR of CC relative to CT/TT = 1.04] had significant effects on time to dropout. There was, however, a significant effect of treatment [χ 2(1) = 35.91, P < .0001], with an HR for placebo relative to BUP-XR of 2.80, reflecting greater risk of dropout in the placebo arm than the BUP-XR arm.
Missing Data Analyses
Supplementary Table 2 shows the estimated log odds of opioid use for each treatment by genotype combination among EAs, with corresponding LORs, under the 3 different modeling approaches. For the CC genotype, the estimated LORs for the ignorable, missing = opioid use, and pattern-mixture model were 1.08, 1.62, and 1.89, respectively, with corresponding LORs of 2.86, 3.28, and 3.72, respectively, for the CT/TT genotype. The differences between the CC and CT/TT LORs were similar across the 3 models. Under all 3 models, we see higher estimated LORs in the CT/TT genotype than in the CC genotype. The CC vs CT/TT genotype group differences in the LORs are significant for the ignorable (P < .0001) and missing = opioid use (P = .04) models and not for the pattern-mixture model. Also, for each genotype, positive LOR values reflect higher rates of opioid-positive UDS in the placebo group than the BUP-XR group.
Discussion
In this study, as expected given the report by Haight et al. (2019), BUP-XR–treated participants in both population groups had fewer opioid-positive UDS than those treated with placebo irrespective of rs678849 genotype. Analysis of the moderating effects of rs678849 showed that, in AAs, BUP-XR had a modestly larger beneficial effect in the CC genotype group, as evidenced by an LOR for placebo relative to BUP-XR in the CC genotype group that was 0.31 greater than that in the CT/TT genotype group (indicating an OR that was 1.36 times greater). In contrast, among EAs, the LOR for placebo relative to BUP-XR in the CC group was 1.78 smaller than in the CT/TT group, indicating a substantially smaller treatment effect in the CC genotype group than the CT/TT genotype group (the OR was 0.17 of that in the CT/TT group). We lacked the statistical power to test the ratio of ORs, but the difference in effects related to genotype group justified further evaluation.
In AAs, the odds in the CC genotype group did not differ significantly from those in the CT/TT group (P = .80), that is, there was no effect of genotype group on the response to placebo compared with BUP-XR. In EAs, the LORs differed significantly (P<.0001) by genotype group (–1.78 vs 0.17 for CC vs CT/TT), reflecting genetic moderation of the treatment effect. The missing data analyses showed genotype by medication effects with the same direction and magnitude as those in the primary analyses. Thus, although we did not replicate the findings of Crist et al. (2013, 2019) of a moderating effect of rs678849 on the response to buprenorphine in AAs, we found such an effect in EAs, which Crist et al. (2013) did not.
A number of factors may explain our lack of replication of the findings of Crist et al. (2013, 2019). Whereas we examined the pharmacogenetic effects of rs678849 in a randomized, placebo-controlled, blinded trial of a long-acting BUP-XR, the Crist et al. studies (2013, 2019) assessed only a sublingual formulation and did not include a placebo condition. The use of a long-acting formulation could reduce potential confounding from missed doses of the medication. The presence of a placebo group, in addition to providing evidence of the efficacy of BUP-XR, also reduces potential confounding, such as that attributable to pleiotropic genetic effects and population stratification (Ross et al., 2012). Further, the AA subsample in the BUP-XR clinical trial (Haight et al., 2019) is substantially smaller than the EA subsample, limiting the statistical power for replication. Additionally, there could be differences in the clinical effects of BUP-SL and BUP-XR. Although the active medication is the same in both formulations, daily treatments like BUP-SL and methadone frequently suffer from poor adherence (Roux et al., 2014), resulting in a greater risk of illicit opioid use (Blum et al., 2018). Extended-release injectable formulations such as BUP-XR reduce the frequency of administration from daily to monthly and ensure drug delivery once administered. The formulations also differ pharmacokinetically, resulting in different buprenorphine plasma concentrations and consistency of exposure during the day. While BUP-SL may not sustain buprenorphine plasma concentrations at therapeutic levels with daily dosing (Greenwald et al., 2007), monthly injections of the BUP-XR formulation provide sustained buprenorphine plasma concentrations over the entire dosing interval at levels sufficient to block drug-liking of abused opioids while controlling withdrawal symptoms and craving (Haight, et al., 2019). Accounting for differences in pharmacokinetics through pharmacokinetic/pharmacodynamic modelling could be considered for future analyses.
The mechanism by which rs678849 could regulate the response to BUP-XR is not clear. Intronic variants may tag 1 or more other functional variants, although this seems unlikely to explain the rs678849 effect given the haplotype structure of OPRD1 in the EA population (Crist et al., 2013). Intronic variants like rs678849 can also affect alternative splicing or alter gene expression by disrupting cis regulatory elements (Cooper 2010). Such an effect has been observed for rs3778150, an intronic variant in OPRM1 that is both an expression quantitative trait locus for the gene and associated with OUD (Hancock et al., 2015). The delta-opioid receptor is also believed to form heterodimers with mu- and kappa-opioid receptors (Valentino and Volkow, 2018). The signaling of these heterodimers may differ from that of the component receptors (Valentino and Volkow, 2018), providing another potential mechanism by which variation in the delta-opioid receptor could directly affect buprenorphine’s efficacy. Finally, although buprenorphine is thought to act at mu- and kappa-opioid receptors, there is evidence that it also has a strong affinity for the delta-opioid receptor (Negus et al., 2002). Further, norbuprenorphine, a major metabolite of buprenorphine, may be a delta-opioid receptor agonist (Huang et al., 2001).
This study has limitations that should be considered. In addition to the modest size of the study groups, particularly when differentiated by race, the study was not designed to evaluate pharmacogenetic effects. To prevent potential confounding of the genetic and treatment effects requires that the randomization be stratified based on genotype so that all participants would have undergone genotyping during screening. Second, although treatment response may be partially genetically determined (Motsinger-Reif et al., 2013), it is a complex trait and is influenced by multiple genetic variants of small effect (Manolio et al., 2009), which are likely to differ by population group.
Despite the lack of a moderating effect in AAs, rs678849 moderated the efficacy of BUP-XR in EAs, with the CC genotype group showing a poorer treatment response than the CT/TT group. Although this finding requires independent replication, EAs are the largest population group affected by OUD and opioid overdose (Rudd et al., 2016), so the findings have potentially important implications for the treatment of OUD. Future studies of SNPs such as rs678849 or others that could be identified in genome-wide association studies will require large, treatment samples comprising different population groups. A promising alternative to the use of SNPs is the use of polygenic risk scores, which capture the genetic contributions of a potentially large number of variants, to identify a priori individuals with OUD who are most likely to respond to buprenorphine treatment.
Supplementary Material
Acknowledgments
None.
Funding
This work was supported by the National Institute on Drug Abuse of the U.S. National Institutes of Health (P30 DA046345) and the Mental Illness Research, Education and Clinical Center of the Veterans Integrated Service Network 4, U.S. Department of Veterans Affairs. The clinical study on which the analyses are based was supported and sponsored by Indivior, Inc. and is registered with ClinicalTrials.gov, number NCT02357901.
Statement of Interest
H.R.K. is an advisory board member for Dicerna Pharmaceuticals and a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, which was sponsored in the past three years by AbbVie, Alkermes, Amygdala Neurosciences, Arbor Pharmaceuticals, Ethypharm, Indivior, Lilly, Lundbeck, Otsuka, and Pfizer. H.R.K. is named as an inventor on PCT patent application #15/878,640 entitled: “Genotype-guided dosing of opioid agonists,” filed January 24, 2018. A.L.M. and C.L. are employees of Indivior, Inc. A.A. was an employee of Indivior, Inc. at the time the work was performed.
References
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders: diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Association. [Google Scholar]
- Blum K, Han D, Modestino EJ, Saunders S, Roy AK 3rd, Jacobs W, Inaba DS, Baron D, Oscar-Berman M, Hauser M, Badgaiyan RD, Smith DE, Femino J, Gold MS (2018) A systematic, intensive statistical investigation of data from the Comprehensive Analysis of Reported Drugs (CARD) for compliance and illicit opioid abstinence in substance addiction treatment with buprenorphine/naloxone. Subst Use Misuse 53:220–229. [DOI] [PubMed] [Google Scholar]
- Compton WM, Jones CM, Baldwin GT (2016) Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med 374:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DN (2010) Functional intronic polymorphisms: buried treasure awaiting discovery within our genes. Hum Genomics 4:284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist RC, Clarke TK, Ang A, Ambrose-Lanci LM, Lohoff FW, Saxon AJ, Ling W, Hillhouse MP, Bruce RD, Woody G, Berrettini WH (2013) An intronic variant in OPRD1 predicts treatment outcome for opioid dependence in African-Americans. Neuropsychopharmacology 38:2003–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist RC, Phillips KA, Furnari MA, Moran LM, Doyle GA, McNicholas LF, Cornish JW, Kampman KM, Preston KL, Berrettini WH (2019) Replication of the pharmacogenetic effect of rs678849 on buprenorphine efficacy in African-Americans with opioid use disorder. Pharmacogenomics J 19:260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA Briefing Document RBP-6000 (extended-release buprenorphine) Joint Meeting of Psychopharmacologic Drugs Advisory Committee and Drug Safety and Risk Management Advisory Committee October 31, 2017. https://www.fda.gov/media/108382/download. Accessed March 22, 2020.
- Fitzmaurice GM, Laird NM, Ware JH (2011) Applied longitudinal analysis. 2nd ed. New York: Wiley Series in Probability and Statistics. [Google Scholar]
- Greenwald M, Johanson CE, Bueller J, Chang Y, Moody DE, Kilbourn M, Koeppe R, Zubieta JK (2007) Buprenorphine duration of action: mu-opioid receptor availability and pharmacokinetic and behavioral indices. Biol Psychiatry 61:101–110. [DOI] [PubMed] [Google Scholar]
- Haight BR, Learned SM, Laffont CM, Fudala PJ, Zhao Y, Garofalo AS, Greenwald MK, Nadipelli VR, Ling W, Heidbreder C; RB-US-13-0001 Study Investigators (2019) Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 393:778–790. [DOI] [PubMed] [Google Scholar]
- Hancock DB, et al. (2015) Cis-expression quantitative trait loci mapping reveals replicable associations with heroin addiction in OPRM1. Biol Psychiatry 78:474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD (1987) Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse 13:293–308. [DOI] [PubMed] [Google Scholar]
- Hedegaard H, Warner M, Miniño AM (2017) Drug overdose deaths in the United States, 1999–2016. NCHS Data Brief 294: 1– 8. [PubMed] [Google Scholar]
- Huang P, Kehner GB, Cowan A, Liu-Chen LY (2001) Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist. J Pharmacol Exp Ther 297:688–695. [PubMed] [Google Scholar]
- Manolio TA, et al. (2009) Finding the missing heritability of complex diseases. Nature 461:747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motsinger-Reif AA, Jorgenson E, Relling MV, Kroetz DL, Weinshilboum R, Cox NJ, Roden DM (2013) Genome-wide association studies in pharmacogenomics: successes and lessons. Pharmacogenet Genomics 23:383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Bidlack JM, Mello NK, Furness MS, Rice KC, Brandt MR (2002) Delta opioid antagonist effects of buprenorphine in rhesus monkeys. Behav Pharmacol 13:557–570. [DOI] [PubMed] [Google Scholar]
- Paulozzi LJ, Jones C, Mack K, Rudd R (2011) Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. Morb Mortal Wkly Rep 60:1487–1492. [PubMed] [Google Scholar]
- Ross S, Anand SS, Joseph P, Paré G (2012) Promises and challenges of pharmacogenetics: an overview of study design, methodological and statistical issues. JRSM Cardiovasc Dis 1:cvd.2012.012001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux P, Lions C, Michel L, Cohen J, Mora M, Marcellin F, Spire B, Morel A, Carrieri PM, Karila L; ANRS Methaville Study Group (2014) Predictors of non-adherence to methadone maintenance treatment in opioid-dependent individuals: implications for clinicians. Curr Pharm Des 20:4097–4105. [DOI] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F, Scholl L (2016) Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep 65:1445–1452. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2019) Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19-5068, NSDUH Series H-54). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; https://www.samhsa.gov/data/report/2018-nsduh-detailed-tables. Accessed April 25, 2020. [Google Scholar]
- Tkacz J, Severt J, Cacciola J, Ruetsch C (2012) Compliance with buprenorphine medication-assisted treatment and relapse to opioid use. Am J Addict 21:55–62. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Volkow ND (2018) Untangling the complexity of opioid receptor function. Neuropsychopharmacology 43:2514–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DR, Ling W (2003) The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs 35:253–259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.