Abstract
Background
Endotoxin-induced neuroinflammation plays a crucial role in the pathogenesis and progression of various neurodegenerative diseases. A growing body of evidence supports that incretin-acting drugs possess various neuroprotective effects that can improve learning and memory impairments in Alzheimer’s disease models. Thus, the present study aimed to investigate whether alogliptin, a dipeptidyl peptidase-4 inhibitor, has neuroprotective effects against lipopolysaccharide (LPS)-induced neuroinflammation and cognitive impairment in mice as well as the potential mechanisms underlying these effects.
Methods
Mice were treated with alogliptin (20 mg/kg/d; p.o.) for 14 days, starting 1 day prior to intracerebroventricular LPS injection (8 μg/μL in 3 μL).
Results
Alogliptin treatment alleviated LPS-induced cognitive impairment as assessed by Morris water maze and novel object recognition tests. Moreover, alogliptin reversed LPS-induced increases in toll-like receptor 4 and myeloid differentiation primary response 88 protein expression, nuclear factor-κB p65 content, and microRNA‐155 gene expression. It also rescued LPS-induced decreases in suppressor of cytokine signaling gene expression, cyclic adenosine monophosphate (cAMP) content, and phosphorylated cAMP response element binding protein expression in the brain.
Conclusion
The present study sheds light on the potential neuroprotective effects of alogliptin against intracerebroventricular LPS-induced neuroinflammation and its associated memory impairment via inhibition of toll-like receptor 4/ myeloid differentiation primary response 88/ nuclear factor-κB signaling, modulation of microRNA-155/suppressor of cytokine signaling-1 expression, and enhancement of cAMP/phosphorylated cAMP response element binding protein signaling.
Keywords: Alogliptin, cognitive impairment, lipopolysaccharide, neuroinflammation
Significance Statement.
Endotoxin-induced neuroinflammation plays a crucial role in the pathogenesis and progression of various neurodegenerative diseases. A growing body of evidence supports that incretin-acting drugs possess various neuroprotective effects that can improve learning and memory impairments in AD models. Thus, this study aimed to investigate whether alogliptin, a DPP-4 inhibitor, has neuroprotective effects against LPS-induced neuroinflammation and cognitive impairment in mice as well as the potential mechanisms underlying these effects. Interestingly, alogliptin reversed ICV LPS-induced neuroinflammation and its associated amyloidogenesis, apoptosis, and memory impairment in mice. Our results demonstrate that alogliptin’s protective effects were associated with inhibition of TLR4/MYD88/NF-κB signaling, modulation of miRNA-155/SOCS-1 expression, and enhancement of cAMP/pCREB signaling in ICV LPS-treated mice.
Introduction
Neuroinflammation is a crucial driver of various neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease, and multiple sclerosis. Lipopolysaccharide (LPS), an endotoxin present in the outer membrane of gram-negative bacteria, is a potent activator of the innate immune system. LPS is commonly used to experimentally induce neuroinflammation (Zakaria et al., 2017; Khan et al., 2018). It mediates its action via binding to toll-like receptor 4 (TLR4), which is abundant on microglia in the central nervous system inducing nuclear factor-κB (NF-κB) phosphorylation and pro-inflammatory cytokines production through the myeloid differentiation primary response 88 (MyD88) adaptor (Zhou et al., 2019). Besides, LPS induces amyloidogenesis by increasing β-secretase (BACE1) and γ-secretase activation, thereby causing increased amyloid precursor protein (APP) cleavage and elevated β-amyloid (Aβ) peptide levels (Lee et al., 2008, 2013). Furthermore, studies have demonstrated increased microRNA‐155 (miRNA-155) expression following LPS administration (Cardoso et al., 2012; Paeschke et al., 2017; Sayed et al., 2018). MiRNA‐155 is known to downregulate the suppressor of cytokine signaling (SOCS-1), a negative regulator of cytokines signaling, resulting in upregulation of several inflammatory pathways (Contreras and Rao, 2012; Paeschke et al., 2017).
Type 2 diabetes mellitus is considered to be a major risk factor for developing AD. Both diseases share several pathological features, including defective insulin signaling and insulin resistance in addition to Aβ aggregation, enhanced glycogen synthase kinase-3β activity, dysregulated protein phosphorylation, and increased inflammatory response (Kosaraju et al., 2013b; Chen et al., 2019). Hence, antidiabetic drugs might be successful therapy in AD. Glucagon-like peptide-1 (GLP-1) is an endogenous peptide primarily secreted from L cells in the gastrointestinal tract in response to food ingestion (Rizzo et al., 2009). It exerts various antidiabetic effects, such as stimulating insulin secretion, inhibiting glucagon release, reducing gastric emptying, increasing satiety, and replenishing insulin stores, in addition to its cytoprotective and anti-inflammatory actions on β-cells (Rowlands et al., 2018). A growing body of evidence indicates that GLP-1 possesses various neuroprotective effects. For example, it ameliorated AD-like neurodegeneration in rodents by decreasing Aβ deposition and hyperphosphorylation of tau and neurofilament protein. These effects were associated with improvements in learning and memory impairments (Chen et al., 2019).
Dipeptidyl peptidase-4 (DPP-4) inhibitors are well-known antidiabetic drugs that treat type 2 diabetes mellitus by inhibiting the degradation of endogenous GLP-1, resulting in a glucose-dependent increase in insulin secretion and suppression of glucagon release (Gallwitz, 2019). In addition to GLP-1, DPP-4 has the capacity to degrade several other peptides, including glucose-dependent insulinotropic polypeptide, brain natriuretic peptide, substance P, neuropeptide Y, and stromal derived factor-1α, all of which have been associated with numerous neuroprotective effects (Cheng et al., 2020).
Apart from their glycemic effects, DPP-4 inhibitors have demonstrated neuroprotective actions where sitagliptin, vildagliptin, and saxagliptin were previously reported to prevent the accumulation of Aβ and abnormally phosphorylated tau, reduce inflammation, and reverse the behavioral deficits observed in streptozocin-induced AD rats (Kosaraju et al., 2013a, 2013b) and transgenic AD mice (D’Amico et al., 2010; Chen et al., 2019). Besides, alogliptin, a highly selective and potent DPP-4 inhibitor, was revealed to improve cognitive and depressive symptoms in obese ApoE−/− mice (Mori et al., 2017). In Zucker diabetic fatty rats, alogliptin normalized defective signaling responses to cyclic adenosine monophosphate (cAMP) response element binding protein (CREB), an important transcription factor that regulates various neuroprotective genes (Qin et al., 2016). These results suggest that alogliptin may reduce cognitive decline associated with neuroinflammation and amyloidogenesis and can be a potential treatment for AD. Thus, alogliptin was selected in our study to investigate for the first time, to our knowledge, its neuroprotective effects against LPS-induced neuroinflammation and cognitive impairment in mice through the possible modulation of TLR4/MYD88/NF-κB, miRNA-155/SOCS-1, and cAMP/phosphorylated CREB (pCREB) signaling pathways.
Materials and Methods
Animals
Adult male Swiss albino mice (18–22 g) were obtained from the animal facility of the National Research Center, Cairo, Egypt. Animals were allowed to acclimate for at least 1 week before the experiment started. The mice (4–5 per cage) were housed under temperature- and humidity-controlled conditions with a 12-h-light/-dark cycle and free access to food and water. The study complied with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 2011) and was approved by the Ethics Committee for Animal Experimentation of Faculty of Pharmacy, Cairo University (permit no. 2222). All efforts were made to minimize animal discomfort and suffering.
Materials
LPS (Escherichia coli, serotype 0127: B8) was purchased from Sigma-Aldrich, St. Louis, Missouri. Alogliptin benzoate (Inhiglip) was obtained from Hikma Pharma (Giza, Egypt). LPS was dissolved in saline and alogliptin was freshly prepared in 1% (v/v) Tween 80 in saline.
Experimental Design
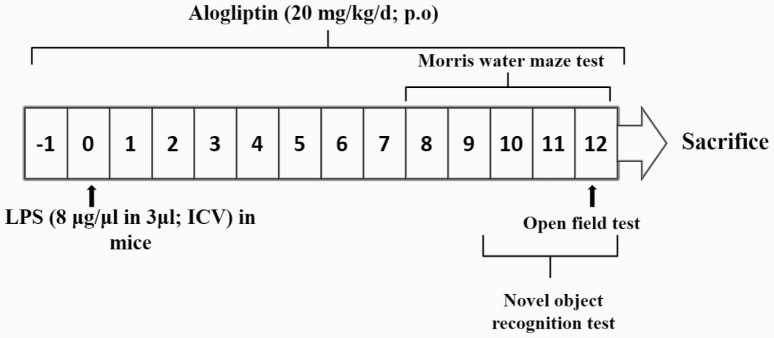
Sixty-four mice were randomly allocated into 4 groups (n = 16 per group). LPS (8 μg/μL in 3 μL) (Liu et al., 2018) or saline (3 μL) was administered by freehand intracerebroventricular (ICV) injection procedures under anesthesia as previously described by Sorial and EL Sayed (2017). Alogliptin (20 mg/kg/d) was orally administered for 14 days starting 1 day prior to LPS injection. Alogliptin dose was selected based on its significant anti-inflammatory effects in previous models of arterial injury (Akita et al., 2015), nondiabetic glomerular injury (Higashijima et al., 2015), and testicular toxicity (Kabel, 2018) in rodents. The control group received 1% (v/v) Tween 80 in saline (p.o.) and saline (ICV) injection. The alogliptin group received alogliptin (p.o.) followed by saline (ICV) injection. The LPS group was given 1% (v/v) Tween 80 in saline (p.o.) and LPS (ICV) injection to induce neuroinflammation. The LPS+alogliptin group received alogliptin (p.o.) followed by LPS (ICV) injection. Following treatments, mice were subjected to behavioral tests to assess cognitive functions and locomotor activity (Figure 1).
Figure 1.
Schematic representation of the experimental design.
Following the behavioral tests, animals were anesthetized using thiopental sodium (50 mg/kg i.p.) then killed by cervical dislocation. The whole brains were rapidly excised on ice and salt mixture and washed with ice-cold saline. The isolated brains from each group were divided for biochemical and western-blot analyses (n = 6) and real-time PCR analysis (n = 6), or kept in 10% formalin for immunohistochemical examination (n = 4).
Behavioral Assessments
Morris Water Maze Test
Spatial learning and memory were assessed using the Morris water maze (MWM) test (D’Hooge and De Deyn, 2001) as previously described by Sorial and EL Sayed (2017). Briefly, mice underwent 2 training trials per day (maximum trial time = 120 seconds) for 4 consecutive days. The escape latency was calculated as the average of the total time taken to find the platform during the 2 training sessions on each acquisition day. This metric was used as an index of acquisition learning. A probe test was performed on the fifth day. The platform was removed, and mice were allowed to explore the pool for 60 seconds. The time spent in the target quadrant was recorded as a measure of retrieval memory.
Novel Object Recognition Test
The novel object recognition (NOR) test is used to assess non-spatial memory in rodents (Cohen and Stackman Jr, 2015). The test was performed as described by Sayed and EL Sayed (2016). In brief, the test consisted of 3 phases conducted over 3 successive days: the habituation, familiarization, and test phases. In the habituation phase, each mouse was left to adapt to the surrounding wooden box (30 × 30 × 30) for 10 minutes. The second day was specified as the training phase in which familiarization with 2 wooden objects with the same size, shape, and color that were made from non-toxic materials was performed. The objects were placed 2 cm away from the walls in opposite corners inside the box. In the test phase, one of the identical objects used in the familiarization phase was replaced with a novel object. Each animal was allowed to explore the objects for 4 minutes while time was being recorded. The discrimination index was calculated as the difference between the time spent exploring the novel and the familiar objects divided by the total exploration time for both objects during the test phase (Sorial and EL Sayed, 2017).
Open Field Test
The open field test was used to assess exploratory locomotor activity to confirm that the behavioral test results were not attributable to changes in locomotor activity (Tatem et al., 2014). The test was conducted in a special square-shaped wooden box measuring 80 × 80 × 40 cm with red walls and a white floor. The floor was divided into 16 equal squares by black lines. Mice were individually placed in the central area of the open field and allowed to freely explore the area for 3 minutes. The latency time and frequency of ambulation, grooming, and rearing were recorded (Abdel Rasheed et al., 2018).
Biochemical Assays
Enzyme-Linked Immunosorbent Assay
NF-κB p65 (Cusabio, Hubei, China), tumor necrosis factor-α (TNF-α) (MyBioSource, San Diego, California, USA), Interleukin-6 (IL-6) (RayBiotech Inc., Peachtree Corners, Georgia, USA), Aβ (1–42; Cusabio, Hubei, China), and cAMP (R&D Systems Inc., Minneapolis, Minnesota, USA) content in the brain were estimated using commercially available enzyme-linked immunosorbent assay kits according to the manufacturers’ instructions. The results are expressed as pg/mg protein for all analytes except for cAMP, which is expressed as pmol/mg protein. The protein content was quantified using the method described by Lowry et al. (1951).
BACE1 Activity
BACE1 activity in the brain was spectrofluorometrically determined using a BACE1 Activity Assay Kit II (BioVision Inc., Milpitas, California, USA) according to the manufacturer’s instructions. The results are expressed as mU/mg protein.
Quantitative Real-Time PCR Analysis
Quantitative real-time PCR was used to assess miRNA-155 and SOCS-1 gene expression in brain. In brief, for miRNA-155 expression, miRNA was extracted from the brain samples using a mirVana PARIS kit (Ambion, Austin, Texas, USA). For SOCS-1, total RNA was extracted using SV total RNA isolation system (Promega, Madison, Wisconsin , USA). The complementary DNA was reverse transcribed from the miRNA-155 and SOCS-1 total RNA samples using a TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Waltham, Massachusetts, USA) and Reverse Transcription System (Promega, Madison, Wisconsin, USA), respectively. Quantitative real-time PCR of miRNA-155 and SOCS-1 was performed using the TaqMan MicroRNA Assay (Cat. no. 4427975, 002571, Applied Biosystems, Waltham, Massachusetts, USA) and SYBR Green JumpStart Taq ReadyMix (Cat. No. S4438, Sigma-Aldrich, St. Louis, Missouri), respectively, as described by the manufacturers. Primer sequences used for the SOCS-1 assay are listed in Table 1. The relative expression of the target genes was quantified using the 2−∆∆CT formula (Livak and Schmittgen, 2001) with snoRNA-202 (Cat. No. 4427975, 001232, Applied Biosystems, Waltham, Massachusetts, USA) and GAPDH as housekeeping genes for miRNA-155 and SOCS-1, respectively.
Table 1.
Sequences of the primers used for quantitative real time-PCR analysis
| SOCS-1 | Forward 5’-TGGGCACCTTCTTGGTGCGC-3′ Reverse 5’-GGCAGTCGAAGGTCTCGCGG-3′ |
| GAPDH | Forward 5’- ACCACAGTCCATGCCATCAC-3′ Reverse 5’-TCCACCACCCTGTTGCTGTA-3′ |
Western-Blot Analysis
The brains were homogenized in lysis buffer and protein levels were quantified using a Bicinchoninic Acid Protein Assay Kit (Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA). Protein expression was assessed as previously described (Ahmed et al., 2014) using anti-TLR4, anti-MyD88, anti-Bax, anti-Bcl-2, and anti-p-CREB (Ser133) antibodies (Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA). The amount of protein was quantified by densitometric analysis of the autoradiograms using a scanning laser densitometer (Biomed Instrument Inc., Fullerton, California, USA). Results are expressed as arbitrary units normalized to β-actin protein expression.
Immunohistochemical Staining
Brain samples were fixed in 10% neutral buffered formalin for 48 hours. Samples were then processed in serial grades of ethanol, cleared in xylene, then infiltrated and embedded in paraffin. For glial fibrillary acidic protein (GFAP) immunohistochemical staining, deparaffinized 4-micron tissue sections were treated with 3% hydrogen peroxide for 20 minutes, washed with phosphate-buffered saline (PBS), and incubated with anti-GFAP according to the manufacturer’s instructions using mouse monoclonal antibody (1:100) (Cat. no. MS-280-P1, Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA) overnight at 4°C. After washing with PBS, sections were incubated with a secondary antibody HRP Envision Kit (Dako, Carpinteria, California, USA) for 30 minutes, washed with PBS, then incubated with diaminobenzidine for 10 minutes. Finally, the sections were washed with PBS, counterstained with hematoxylin, dehydrated and cleared in xylene, then coverslipped for microscopic analysis. For microscopic analysis, 6 random, non-overlapping fields from the hippocampus of each tissue section were analyzed for determination of the mean area percentage of GFAP expression in the hippocampus. All micrographs and data were obtained by using a full HD microscopic camera operated by a Leica application module for tissue sections analysis (Leica Microsystems, Wetzlar, Germany).
Statistical Analysis
Data were assessed for normality and homogeneity of variance using Kolmogorov-Smirnov and Bartlett’s tests, respectively. Data that met the assumptions for parametric analysis were analyzed using 1-way ANOVA followed by Tukey’s multiple comparisons test. The mean escape latency during the MWM acquisition phase was analyzed using repeated-measures 2-way ANOVA followed by Tukey’s multiple comparisons test. The significance level was fixed at P < .05 for all statistical tests. Data are expressed as mean ± SEM. Statistical analysis was performed using GraphPad Prism software, version 6 (GraphPad Software Inc., San Diego, California, USA).
Results
Overall, no significant differences were observed between the control and alogliptin groups.
Effects of LPS and Alogliptin on Spatial Learning and Memory Deficits
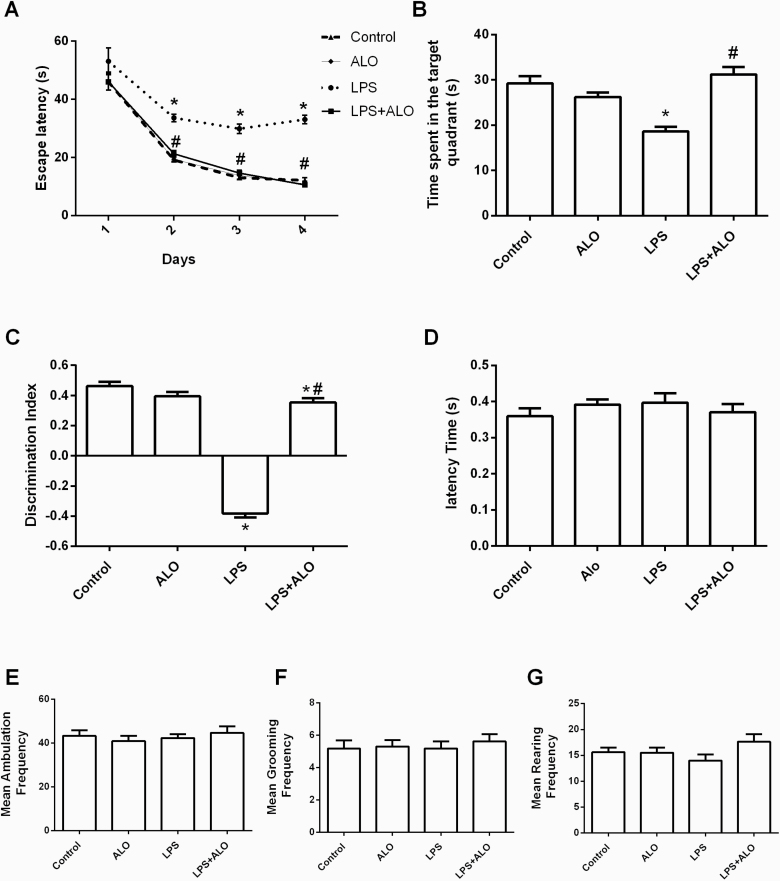
In the MWM, LPS treatment significantly increased the escape latency on training days 2–4 compared with the control mice (F [3, 60] = 41.44), suggesting that LPS administration impaired spatial learning abilities in mice. Notably, alogliptin treatment significantly reduced the escape latency on training days 2–4 in LPS-treated mice, restoring them to control levels (Figure 2A).
Figure 2.
Effects of lipopolysaccharide (LPS) and alogliptin on the escape latency (A) and time spent in the target quadrant (B) in the Morris water maze (MWM) test; the novel object recognition (NOR) discrimination index (C); and the latency time (D), mean ambulation frequency (E), mean grooming frequency (F), and mean rearing frequency (G) in the open field test. Data are expressed as mean ± SEM (n = 16). Statistical analysis was done using 1-way ANOVA followed by Tukey’s post-hoc test, except for the escape latency in MWM test, which was analyzed using repeated measures 2-way ANOVA followed by Tukey’s post-hoc test. *P < .05 vs control, #P < .05 vs LPS group. ALO; alogliptin.
In the probe trial, LPS treatment reduced the time spent in the target quadrant by 36.3% compared with the control mice (F [3, 60] = 16.8). However, alogliptin treatment significantly increased the time spent in the target quadrant in LPS-treated mice, restoring it to the control value. These results indicate that alogliptin treatment beneficially affected deteriorated spatial learning and memory in LPS-challenged mice (Figure 2B).
Effects of LPS and Alogliptin on Recognition Memory Impairment
The NOR test revealed that LPS treatment significantly impaired non-spatial memory quantified by the discrimination index (F [3, 60] = 196). Alogliptin administration significantly rescued the LPS-induced memory impairment (Figure 2C).
Effects of LPS and Alogliptin on Spontaneous Locomotor Activity
The open field test was conducted to evaluate whether LPS-induced cognitive impairments could be attributed to altered locomotor activity. The treatments did not significantly alter the latency time or the frequency of ambulation, grooming, and rearing. Thus, LPS-induced cognitive impairments were not associated with decreased locomotor ability (Figure 2D–G).
Effects of LPS and Alogliptin on the TLR4/MyD88/NF-κB Signaling Pathway
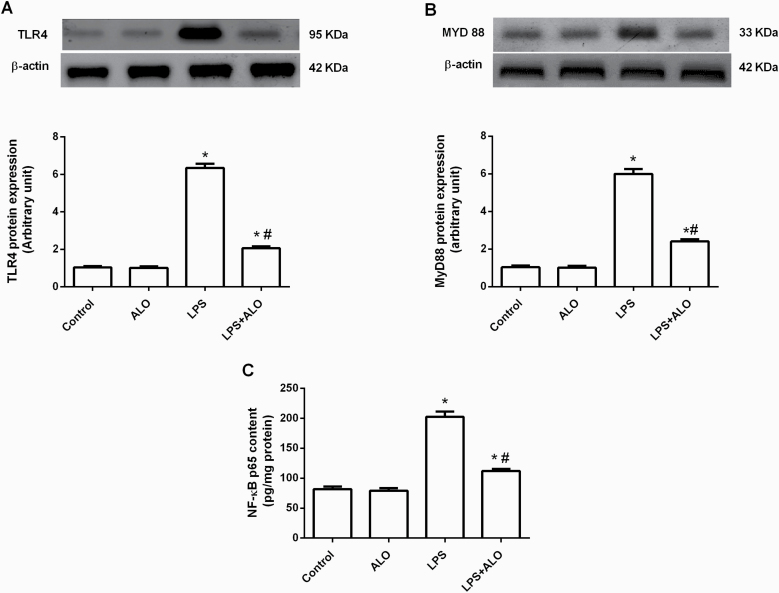
ICV LPS injection significantly increased protein expression of TLR4 and its adaptor protein MyD88 by approximately sixfold in the brain (F [3, 20] = 380 and 240, respectively). This was successfully rescued by alogliptin treatment, which significantly decreased TLR4 and MyD88 protein expression compared with the LPS-treated group (Figure 3A–B). The TLR4/MyD88 signaling pathway is a key inflammatory pathway that results in NF-κB activation and subsequent pro-inflammatory cytokine expression (Naqvi et al., 2016). As hypothesized, LPS treatment significantly increased the NF-κB p65 content in the brain by 146.94% compared with control mice (F [3, 20] = 106). This LPS-induced increase in NF-κB p65 was significantly reduced by alogliptin treatment (Figure 3C).
Figure 3.
Effects of lipopolysaccharide (LPS) and alogliptin on protein expression of toll-like receptor 4 (TLR4) (A) and its adaptor protein myeloid differentiation primary response88 (MyD88) (B), as well as nuclear factor-κB (NF-κB) p65 content (C) in the brain. Data are expressed as mean ± SEM (n = 6). Statistical analysis was done using 1-way ANOVA followed by Tukey’s post-hoc test. *P < .05 vs control, #P < .05 vs LPS group. ALO; alogliptin.
Effects of LPS and Alogliptin on miRNA-155 and SOCS-1 Gene Expression
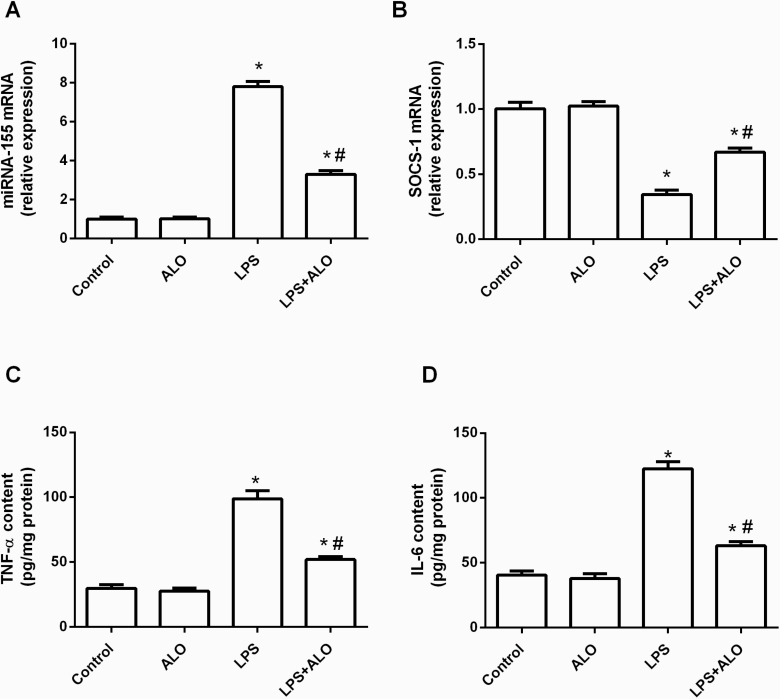
ICV LPS injection significantly elevated miRNA-155 gene expression along with a consequent reduction of the expression of its target gene, SOCS-1, reaching 772.28% and 34% of their respective control values (F [3, 20] = 344 and 70.1, respectively). Alogliptin treatment significantly rescued the LPS-induced increase in miRNA-155 gene expression and decrease in SOCS-1 gene expression (Figure 4A–B).
Figure 4.
Effects of lipopolysaccharide (LPS) and alogliptin on gene expression of microRNA-155 (miRNA-155) (A) and its target, suppressor of cytokine signaling (SOCS-1) (B), as well as tumor necrosis factor-α (TNF-α) (C) and interleukin-6 (IL-6) (D) contents in the brain. Data are expressed as mean ± SEM (n = 6). Statistical analysis was done using 1-way ANOVA followed by Tukey’s post-hoc test. *P < .05 vs control, #P < .05 vs LPS group. ALO; alogliptin.
Effects of LPS and Alogliptin on Pro-inflammatory Cytokines
The neuroinflammatory state triggered by LPS administration was further evidenced by a spike in pro-inflammatory cytokines in the brain. TNF-α and IL-6 levels were triple their respective control values (F [3, 20] = 81.5 and 95.3, respectively). Conversely, alogliptin displayed remarkable anti-inflammatory effects and significantly reduced the LPS-induced TNF-α and IL-6 levels (Figure 4C–D).
Effects of LPS and Alogliptin on Amyloidogenesis and Apoptosis
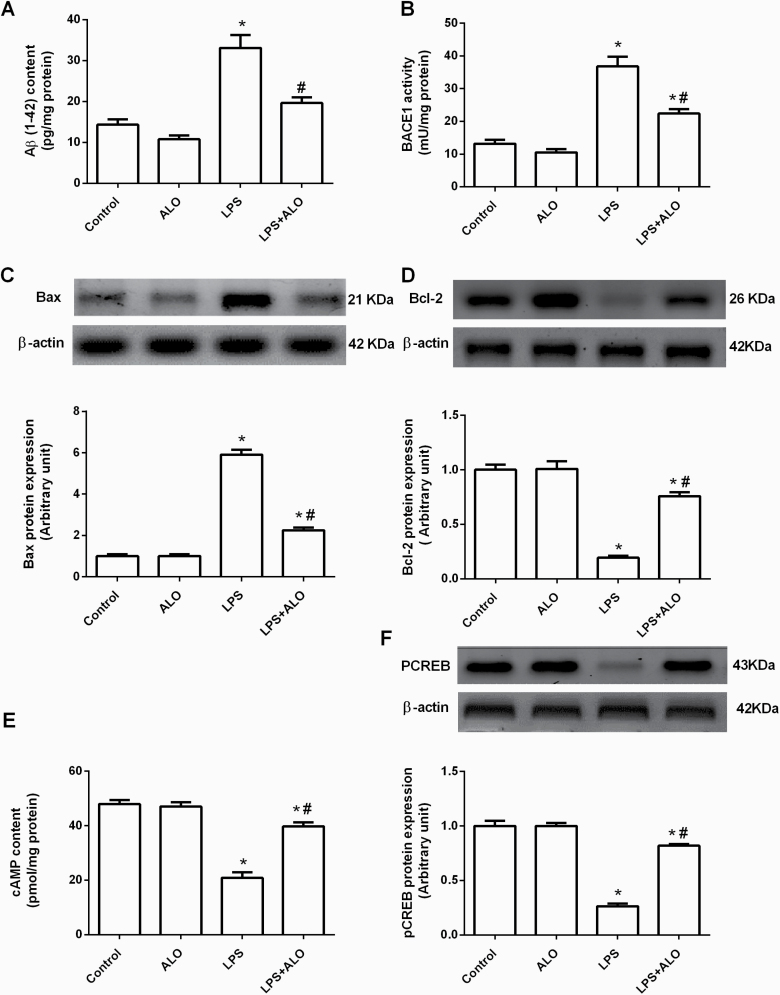
Marked amyloidogenesis was indicated by elevated Aβ (1–42) content in the brains of LPS-challenged mice (F [3, 20] = 26.8). Similarly, LPS increased the activity of BACE1, the key enzyme responsible for Aβ (1–42) production, to 278.79% of the control level (F [3, 20] = 42.9). Alogliptin treatment restored LPS-induced Aβ (1–42) to its control level and significantly reduced BACE1 activity compared with LPS-injected mice (Figure 5A–B).
Figure 5.
Effects of lipopolysaccharide (LPS) and alogliptin on β-amyloid (Aβ) (1-42) content (A), β-secretase (BACE1) activity (B), Bax protein expression (C), Bcl-2 protein expression (D), cyclic adenosine monophosphate (cAMP) content (E), and phosphorylated cAMP response element binding protein (pCREB) protein expression (F) in the brain. Data are expressed as mean ± SEM (n = 6). Statistical analysis was done using 1-way ANOVA followed by Tukey’s post-hoc test. *P < .05 vs control, #P < .05 vs LPS group. ALO; alogliptin.
Furthermore, ICV LPS injection triggered apoptotic cell death in the brain, which was reflected by increased pro-apoptotic Bax protein expression and a concomitant decrease in anti-apoptotic Bcl-2 protein expression, reaching 585.15% and 19% of their respective control levels (F [3, 20] = 249 and 68.7, respectively). Alogliptin administration protected against LPS-induced apoptotic cell death by significantly decreasing Bax protein expression and increasing Bcl-2 protein expression (Figure 5C–D).
Effects of LPS and Alogliptin on the cAMP/pCREB Signaling Pathway
cAMP content and pCREB protein expression were assessed in the brain to determine their role in alogliptin’s beneficial effects against LPS-induced damage. LPS induced a dramatic decrease in cAMP brain content accompanied by downregulation of pCREB protein expression to 43.63% and 26% of their respective control values (F [3, 20] = 54.7 and 128, respectively). Alogliptin reversed these LPS-induced deficits by significantly increasing cAMP content and pCREB protein expression compared with the LPS group (Figure 5E–F).
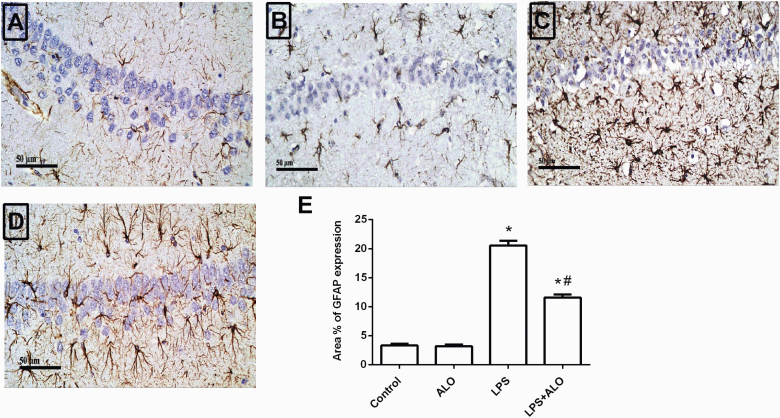
Effects of LPS and Alogliptin on Astrocyte Activation
Glial activation is widely implicated in LPS-induced neuroinflammation. On activation, glial cells are an important source of inflammatory cytokines (Khan et al., 2018). Immunohistochemical staining was used to assess GFAP expression as a marker of astrocyte activation in the hippocampus. Marked astrogliosis was observed in the LPS-treated group, with the percent of area with GFAP expression reaching 620.48% of the control group (F [3, 12] = 243). Notably, alogliptin treatment significantly suppressed LPS-induced GFAP protein expression (Figure 6).
Figure 6.
Effects of lipopolysaccharide (LPS) and alogliptin on glial fibrillary acidic protein (GFAP) immunohistochemical analysis in the hippocampus. Representative photomicrographs (scale bars = 50 μm) for the control (A), alogliptin (ALO) (B), LPS (C) and LPS+ALO groups (D). The quantitative analysis of the mean area percentage of GFAP expression (E). Data are expressed as mean ± SEM (n = 4). Statistical analysis was done using 1-way ANOVA followed by Tukey’s post-hoc test. *P < .05 vs control, #P < .05 vs LPS group.
Discussion
The present data reveal that alogliptin possessed significant neuroprotective effects against ICV LPS-induced neuroinflammation in mice. The results show that alogliptin treatment ameliorated LPS-induced memory impairment via its anti-inflammatory, anti-amyloidogenic, and anti-apoptotic effects. This study also provides insights into the potential molecular pathways underlying alogliptin’s neuroprotective actions.
It is well established that ICV injection of LPS produces cognitive impairment in rodents (Miwa et al., 2011; Lee et al., 2018; Liu et al., 2018; Zhang et al., 2018). In the current study, its administration resulted in marked deterioration of memory and learning functions in mice in the MWM and NOR tests. These LPS-induced cognitive dysfunctions were successfully attenuated by alogliptin treatment. These results are in line with Mori et al. (2017) and Rahman et al. (2020), who showed that alogliptin treatment improved cognitive functions in obese ApoE−/− mice and Aβ (1–42) fibrils injected rats, respectively.
In microglia, LPS activates the NF-κB signaling cascade via the TLR4/MyD88 pathway (Dai et al., 2015; Zhou et al., 2019). NF-κB is an important nuclear transcription factor in the inflammatory cascade that stimulates inflammation-related genes expression (Liu et al., 2017). Accordingly, the LPS-treated brains in the present study showed an increased TLR4 and MyD88 protein expression resulting in elevation of NF-κB p65 and consequent increase in TNF-α and IL-6 levels. This activated TLR4/MyD88/NF-κB pathway in ICV LPS-treated mice is consistent with the findings of Zhou et al. (2019). Notably, alogliptin treatment effectively reversed these deleterious inflammatory effects, signifying that alogliptin possesses protective effects against LPS-induced neuroinflammation that are mediated via the TLR4/MyD88/NF-κB pathway. Other DPP-4 inhibitors have also shown inhibitory effects on the TLR4/NF-κB signaling cascade both in vitro and in vivo, highlighting their anti-inflammatory potential (Makdissi et al., 2012; Lee et al., 2016; Sherif and Al-Shaalan, 2018; El-kashef and Serrya, 2019).
MiRNA-155 is a key pro-inflammatory regulator in the central nervous system that promotes inflammatory responses by negatively targeting various anti-inflammatory proteins, including SOCS-1 (Slota and Booth, 2019). MiRNA-155 expression is induced in response to LPS and other TLR ligands, such as TNF-α (Guedes et al., 2014). Consistently in the current study, ICV LPS injection increased miRNA-155 gene expression, decreased expression of its target gene, SOCS-1, and increased the content of inflammatory cytokines in the brain. This is in agreement with Sayed et al. (2018) following systemic LPS-induced neuroinflammation in mice.
Modulation of the miRNA-155/SOCS-1 pathway has been implicated in the anti-inflammatory effects of different agents against LPS-induced inflammation (Chen et al., 2013; Ma et al., 2017; Pourgholi et al., 2017; Zheng et al., 2018). SOCS-1 negatively regulates TLR-4 signaling, thereby inhibiting NF-κB activation (Kinjyo et al., 2002; Nakagawa et al., 2002; Chen et al., 2013). Expectedly, LPS-induced elevations in GFAP expression and pro-inflammatory cytokines were prevented by alogliptin treatment through decreasing NF-κB and miRNA-155 expression, and increasing SOCS-1 expression in the brain. This reduction in pro-inflammatory cytokines was also reported using alogliptin in atherosclerotic plaques in diabetic apoE-deficient mice (Ta et al., 2011) and in plasma and adipose tissues of stressed mice (Yisireyili et al., 2016).
LPS-induced neuroinflammation causes Aβ accumulation, leading to learning and memory impairment in animal models. ICV LPS is an established model that provokes inflammatory cytokines, stimulating APP mRNA upregulation, and inducing amyloidogenesis through activation of β- and γ-secretase activity (Lee et al., 2009; Gong et al., 2011). Our study clearly demonstrated these effects, with the LPS-treated mice displaying marked increases in Aβ (1–42) contents in the brain. This was associated with enhanced BACE1 activity, a limiting enzyme that produces Aβ and is known to be induced by NF-κB and inflammatory cytokines (Choi et al., 2017), as observed herein. Interestingly, alogliptin treatment succeeded to prevent LPS-induced increase in Aβ (1–42) content, which could be related to the reduced BACE1 activity and inflammatory cytokines contents. In the same context, D’Amico et al. (2010) found that sitagliptin decreased Aβ and APP levels in the brain of AD mice, attributing this protective effect to the concomitant increase in GLP-1 levels.
Consistent with previous studies (Zhao et al., 2017; Goel et al., 2018), LPS-induced neuroinflammation in the current study was associated with increased apoptotic cell death as indicated by elevated pro-apoptotic Bax protein expression and decreased anti-apoptotic Bcl-2 protein expression. Alogliptin treatment shifted this balance in favor of the anti-apoptotic axis by reducing Bax protein expression while increasing Bcl-2 protein expression. In the same context, saxagliptin, another DPP-4 inhibitor, was also reported to increase striatal Bcl-2 levels in a rat model of Parkinson’s disease, thereby inhibiting neuronal apoptotic loss (Nassar et al., 2015).
CREB is an important nuclear transcription factor that, on phosphorylation through cAMP-dependent protein kinase A, induces expression of various neuroprotective genes, including Bcl-2 and brain-derived neurotrophic factor (Lonze and Ginty, 2002; Kitagawa, 2007; Velmurugan et al., 2012). In addition, emerging evidence suggests that CREB can promote anti-inflammatory immune responses by inhibiting NF-κB activation and inducing anti-inflammatory cytokine expression (Wen et al., 2010; Jung et al., 2017; Li et al., 2018). Importantly, CREB plays a vital role in learning and memory functions (Lonze and Ginty, 2002). Drugs that promote CREB-mediated neuroprotection can improve cognition, whereas disrupting CREB expression can result in neuronal death and neurodegeneration (Dragunow, 2004). In the current study, LPS administration substantially decreased cAMP content and pCREB protein expression, similar to the findings of Guo et al. (2014) and Zou et al. (2017). On the other hand, alogliptin treatment effectively increased cAMP content and pCREB protein expression. Qin et al. (2016) also found that alogliptin normalized defective CREB signaling in the hippocampus of diabetic rats, resulting in increased expression of its neuroprotective target proteins, including Bcl-2. Hence, our findings suggest that reversing LPS-induced reductions in cAMP and pCREB might underlie alogliptin’s protective effects against LPS-induced learning and memory impairment.
Conclusion
In conclusion, the present study sheds light on the potential neuroprotective effects of alogliptin against ICV LPS-induced neuroinflammation and its associated amyloidogenesis, apoptosis, and memory impairment in mice. Our results demonstrate that alogliptin’s protective effects were mediated via inhibition of TLR4/MYD88/NF-κB signaling, modulation of miRNA-155/SOCS-1 expression, and enhancement of cAMP/pCREB signaling.
Author Contributions
A.E.E., N.A.S., N.S.E., and L.A.A. developed the idea and designed the experimental approach. N.A.S. performed the experiments. N.A.S., A.E.E., and L.A.A. contributed to data analysis and manuscript writing. A.E.E., N.A.S., N.S.E., and L.A.A. revised and approved the final manuscript.
Acknowledgments
The authors are thankful to Dr Mohamed A. Khattab, Assistant Professor of Cytology and Histology, Faculty of Veterinary Medicine, Cairo University, Cairo, Egypt, for his effort in performing and analyzing the immunohistochemical examinations.
This research received no specific grant from any funding agency in the public or commercial or not-for-profit sectors.
Statement of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Abdel Rasheed NO, El Sayed NS, El-Khatib AS (2018) Targeting central β2 receptors ameliorates streptozotocin-induced neuroinflammation via inhibition of glycogen synthase kinase3 pathway in mice. Prog Neuropsychopharmacol Biol Psychiatry 86:65–75. [DOI] [PubMed] [Google Scholar]
- Ahmed LA, Shehata NI, Abdelkader NF, Khattab MM (2014) Tempol, a superoxide dismutase mimetic agent, ameliorates cisplatin-induced nephrotoxicity through alleviation of mitochondrial dysfunction in mice. PLoS One 9:e108889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akita K, Isoda K, Shimada K, Daida H (2015) Dipeptidyl-peptidase-4 inhibitor, alogliptin, attenuates arterial inflammation and neointimal formation after injury in low-density lipoprotein (LDL) receptor-deficient mice. J Am Heart Assoc 4:e001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso AL, Guedes JR, Pereira de Almeida L, Pedroso de Lima MC (2012) miR-155 modulates microglia-mediated immune response by down-regulating SOCS-1 and promoting cytokine and nitric oxide production. Immunology 135:73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhou M, Sun J, Guo A, Fernando RL, Chen Y, Peng P, Zhao G, Deng Y (2019) DPP-4 inhibitor improves learning and memory deficits and AD-like neurodegeneration by modulating the GLP-1 signaling. Neuropharmacology 157:107668. [DOI] [PubMed] [Google Scholar]
- Chen Y, Liu W, Sun T, Huang Y, Wang Y, Deb DK, Yoon D, Kong J, Thadhani R, Li YC (2013) 1,25-dihydroxyvitamin D promotes negative feedback regulation of toll-like receptor signaling via targeting MicroRNA-155-SOCS1 in macrophages. J Immunol 190:3687–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Cheng J, Cordato D, Gao J (2020) Can dipeptidyl peptidase-4 inhibitors treat cognitive disorders? Pharmacol Ther 212:107559. [DOI] [PubMed] [Google Scholar]
- Choi JY, Jang JS, Son DJ, Im HS, Kim JY, Park JE, Choi WR, Han SB, Hong JT (2017) Antarctic krill oil diet protects against lipopolysaccharide-induced oxidative stress, neuroinflammation and cognitive impairment. Int J Mol Sci 18:2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SJ, Stackman RW Jr (2015) Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res 285:105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras J, Rao DS (2012) MicroRNAs in inflammation and immune responses. Leukemia 26:404–413. [DOI] [PubMed] [Google Scholar]
- D’Amico M, Di Filippo C, Marfella R, Abbatecola AM, Ferraraccio F, Rossi F, Paolisso G (2010) Long-term inhibition of dipeptidyl peptidase-4 in Alzheimer’s prone mice. Exp Gerontol 45:202–207. [DOI] [PubMed] [Google Scholar]
- D’Hooge R, De Deyn PP (2001) Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev 36:60–90. [DOI] [PubMed] [Google Scholar]
- Dai XJ, Li N, Yu L, Chen ZY, Hua R, Qin X, Zhang YM (2015) Activation of BV2 microglia by lipopolysaccharide triggers an inflammatory reaction in PC12 cell apoptosis through a toll-like receptor 4-dependent pathway. Cell Stress Chaperones 20:321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragunow M (2004) CREB and neurodegeneration. Front Biosci 9:100–103. [DOI] [PubMed] [Google Scholar]
- El-Kashef DH, Serrya MS (2019) Sitagliptin ameliorates thioacetamide-induced acute liver injury via modulating TLR4/NF-KB signaling pathway in mice. Life Sci 228:266–273. [DOI] [PubMed] [Google Scholar]
- Gallwitz B (2019) Clinical Use of DPP-4 Inhibitors. Front Endocrinol (Lausanne) 10:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel R, Bhat SA, Hanif K, Nath C, Shukla R (2018) Angiotensin II receptor blockers attenuate lipopolysaccharide-induced memory impairment by modulation of NF-κB-mediated BDNF/CREB expression and apoptosis in spontaneously hypertensive rats. Mol Neurobiol 55:1725–1739. [DOI] [PubMed] [Google Scholar]
- Gong QH, Wang Q, Pan LL, Liu XH, Xin H, Zhu YZ (2011) S-propargyl-cysteine, a novel hydrogen sulfide-modulated agent, attenuates lipopolysaccharide-induced spatial learning and memory impairment: involvement of TNF signaling and NF-κB pathway in rats. Brain Behav Immun 25:110–119. [DOI] [PubMed] [Google Scholar]
- Guedes JR, Custódia CM, Silva RJ, de Almeida LP, Pedroso de Lima MC, Cardoso AL (2014) Early miR-155 upregulation contributes to neuroinflammation in Alzheimer’s disease triple transgenic mouse model. Hum Mol Genet 23:6286–6301. [DOI] [PubMed] [Google Scholar]
- Guo J, Lin P, Zhao X, Zhang J, Wei X, Wang Q, Wang C (2014) Etazolate abrogates the lipopolysaccharide (LPS)-induced downregulation of the cAMP/pCREB/BDNF signaling, neuroinflammatory response and depressive-like behavior in mice. Neuroscience 263:1–14. [DOI] [PubMed] [Google Scholar]
- Higashijima Y, Tanaka T, Yamaguchi J, Tanaka S, Nangaku M (2015) Anti-inflammatory role of DPP-4 inhibitors in a nondiabetic model of glomerular injury. Am J Physiol Renal Physiol 308:F878–F887. [DOI] [PubMed] [Google Scholar]
- Jung JS, Choi MJ, Lee YY, Moon BI, Park JS, Kim HS (2017) Suppression of lipopolysaccharide-induced neuroinflammation by morin via MAPK, PI3K/Akt, and PKA/HO-1 signaling pathway modulation. J Agric Food Chem 65:373–382. [DOI] [PubMed] [Google Scholar]
- Kabel AM (2018) Zinc/alogliptin combination attenuates testicular toxicity induced by doxorubicin in rats: role of oxidative stress, apoptosis and TGF-β1/NF-κB signaling. Biomed Pharmacother 97:439–449. [DOI] [PubMed] [Google Scholar]
- Khan A, Ali T, Rehman SU, Khan MS, Alam SI, Ikram M, Muhammad T, Saeed K, Badshah H, Kim MO (2018) Neuroprotective effect of quercetin against the detrimental effects of LPS in the adult mouse brain. Front Pharmacol 9:1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinjyo I, Hanada T, Inagaki-Ohara K, Mori H, Aki D, Ohishi M, Yoshida H, Kubo M, Yoshimura A (2002) SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity 17:583–591. [DOI] [PubMed] [Google Scholar]
- Kitagawa K (2007) CREB and cAMP response element-mediated gene expression in the ischemic brain. Febs J 274:3210–3217. [DOI] [PubMed] [Google Scholar]
- Kosaraju J, Gali CC, Khatwal RB, Dubala A, Chinni S, Holsinger RM, Madhunapantula VS, Muthureddy Nataraj SK, Basavan D (2013a) Saxagliptin: a dipeptidyl peptidase-4 inhibitor ameliorates streptozotocin induced Alzheimer’s disease. Neuropharmacology 72:291–300. [DOI] [PubMed] [Google Scholar]
- Kosaraju J, Murthy V, Khatwal RB, Dubala A, Chinni S, Muthureddy Nataraj SK, Basavan D (2013b) Vildagliptin: an anti-diabetes agent ameliorates cognitive deficits and pathology observed in streptozotocin-induced Alzheimer’s disease. J Pharm Pharmacol 65:1773–1784. [DOI] [PubMed] [Google Scholar]
- Lee B, Shim I, Lee H (2018) Gypenosides attenuate lipopolysaccharide-induced neuroinflammation and memory impairment in rats. Evid Based Complement Alternat Med 2018:4183670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DS, Lee ES, Alam MM, Jang JH, Lee HS, Oh H, Kim YC, Manzoor Z, Koh YS, Kang DG, Lee DH (2016) Soluble DPP-4 up-regulates toll-like receptors and augments inflammatory reactions, which are ameliorated by vildagliptin or mannose-6-phosphate. Metabolism 65:89–101. [DOI] [PubMed] [Google Scholar]
- Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, Hong JT (2008) Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation 5:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Choi DY, Yun YP, Han SB, Oh KW, Hong JT (2013) Epigallocatechin-3-gallate prevents systemic inflammation-induced memory deficiency and amyloidogenesis via its anti-neuroinflammatory properties. J Nutr Biochem 24:298–310. [DOI] [PubMed] [Google Scholar]
- Lee YK, Yuk DY, Lee JW, Lee SY, Ha TY, Oh KW, Yun YP, Hong JT (2009) (-)-Epigallocatechin-3-gallate prevents lipopolysaccharide-induced elevation of beta-amyloid generation and memory deficiency. Brain Res 1250:164–174. [DOI] [PubMed] [Google Scholar]
- Li C, Chen T, Zhou H, Feng Y, Hoi MPM, Ma D, Zhao C, Zheng Y, Lee SMY (2018) BHDPC Is a novel neuroprotectant that provides anti-neuroinflammatory and neuroprotective effects by inactivating NF-κB and activating PKA/CREB. Front Pharmacol 9:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Zhang L, Joo D, Sun SC (2017) NF-κB signaling in inflammation. Signal Transduct Target Ther 2:17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang Y, Zheng X, Fang T, Yang X, Luo X, Guo A, Newell KA, Huang XF, Yu Y (2018) Galantamine improves cognition, hippocampal inflammation, and synaptic plasticity impairments induced by lipopolysaccharide in mice. J Neuroinflammation 15:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD (2002) Function and regulation of CREB family transcription factors in the nervous system. Neuron 35:605–623. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275. [PubMed] [Google Scholar]
- Ma C, Wang Y, Shen A, Cai W (2017) Resveratrol upregulates SOCS1 production by lipopolysaccharide-stimulated RAW264.7 macrophages by inhibiting miR-155. Int J Mol Med 39:231–237. [DOI] [PubMed] [Google Scholar]
- Makdissi A, Ghanim H, Vora M, Green K, Abuaysheh S, Chaudhuri A, Dhindsa S, Dandona P (2012) Sitagliptin exerts an antiinflammatory action. J Clin Endocrinol Metab 97:3333–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa M, Tsuboi M, Noguchi Y, Enokishima A, Nabeshima T, Hiramatsu M (2011) Effects of betaine on lipopolysaccharide-induced memory impairment in mice and the involvement of GABA transporter 2. J Neuroinflammation 8:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, He W, Kawasaki Y, Kato N, Kasamaki Y, Kanda T (2017) Alogliptin, DPP4 inhibitor, improved cognitive and depressive function in obese apoe-/-mice with modification of BDNF in hippocampus. Int J Pharmacol 13:1079–1085. [Google Scholar]
- Nakagawa R, Naka T, Tsutsui H, Fujimoto M, Kimura A, Abe T, Seki E, Sato S, Takeuchi O, Takeda K, Akira S, Yamanishi K, Kawase I, Nakanishi K, Kishimoto T (2002) SOCS-1 participates in negative regulation of LPS responses. Immunity 17:677–687. [DOI] [PubMed] [Google Scholar]
- Naqvi AR, Zhong S, Dang H, Fordham JB, Nares S, Khan A (2016) Expression profiling of LPS responsive miRNA in primary human macrophages. J Microb Biochem Technol 8:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar NN, Al-Shorbagy MY, Arab HH, Abdallah DM (2015) Saxagliptin: a novel antiparkinsonian approach. Neuropharmacology 89:308–317. [DOI] [PubMed] [Google Scholar]
- Paeschke N, Von Haefen C, Endesfelder S, Sifringer M, Spies CD (2017) Dexmedetomidine prevents lipopolysaccharide-induced microRNA expression in the adult rat brain. Int J Mol Sci 18:1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourgholi F, Hajivalili M, Razavi R, Esmaeili S, Baradaran B, Movasaghpour AA, Sadreddini S, Goodarzynejad H, Mirshafiey A, Yousefi M (2017) The role of M2000 as an anti-inflammatory agent in toll-like receptor 2/microRNA-155 pathway. Avicenna J Med Biotechnol 9:8–12. [PMC free article] [PubMed] [Google Scholar]
- Qin L, Chong T, Rodriguez R, Pugazhenthi S (2016) Glucagon-like peptide-1-mediated modulation of inflammatory pathways in the diabetic brain: relevance to Alzheimer’s disease. Curr Alzheimer Res 13:1346–1355. [DOI] [PubMed] [Google Scholar]
- Rahman SO, Kaundal M, Salman M, Shrivastava A, Parvez S, Panda BP, Akhter M, Akhtar M, Najmi AK (2020) Alogliptin reversed hippocampal insulin resistance in an amyloid-beta fibrils induced animal model of Alzheimer’s disease. Eur J Pharmacol 889:173522. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Rizvi AA, Spinas GA, Rini GB, Berneis K (2009) Glucose lowering and anti-atherogenic effects of incretin-based therapies: GLP-1 analogues and DPP-4-inhibitors. Expert Opin Investig Drugs 18:1495–1503. [DOI] [PubMed] [Google Scholar]
- Rowlands J, Heng J, Newsholme P, Carlessi R (2018) Pleiotropic effects of GLP-1 and analogs on cell signaling, metabolism, and function. Front Endocrinol (Lausanne) 9:672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed AS, El Sayed NS (2016) Co-administration of 3-acetyl-11-keto-beta-boswellic acid potentiates the protective effect of celecoxib in lipopolysaccharide-induced cognitive impairment in mice: possible implication of anti-inflammatory and antiglutamatergic Pathways. J Mol Neurosci 59:58–67. [DOI] [PubMed] [Google Scholar]
- Sayed AS, Gomaa IEO, Bader M, El Sayed NSED (2018) Role of 3-acetyl-11-keto-beta-boswellic acid in counteracting LPS-induced neuroinflammation via modulation of miRNA-155. Mol Neurobiol 55:5798–5808. [DOI] [PubMed] [Google Scholar]
- Sherif IO, Al-Shaalan NH (2018) Vildagliptin attenuates hepatic ischemia/reperfusion injury via the TLR4/NF-κB signaling pathway. Oxid Med Cell Longev 2018:3509091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slota JA, Booth SA (2019) MicroRNAs in neuroinflammation: implications in disease pathogenesis, biomarker discovery and therapeutic applications. Non-coding RNA 5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorial ME, El Sayed NSED (2017) Protective effect of valproic acid in streptozotocin-induced sporadic Alzheimer’s disease mouse model: possible involvement of the cholinergic system. Naunyn Schmiedebergs Arch Pharmacol 390:581–593. [DOI] [PubMed] [Google Scholar]
- Ta NN, Schuyler CA, Li Y, Lopes-Virella MF, Huang Y (2011) DPP-4 (CD26) inhibitor alogliptin inhibits atherosclerosis in diabetic apolipoprotein E-deficient mice. J Cardiovasc Pharmacol 58:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatem KS, Quinn JL, Phadke A, Yu Q, Gordish-Dressman H, Nagaraju K (2014) Behavioral and locomotor measurements using an open field activity monitoring system for skeletal muscle diseases. J Vis Exp 91: e51785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velmurugan K, Balamurugan AN, Loganathan G, Ahmad A, Hering BJ, Pugazhenthi S (2012) Antiapoptotic actions of exendin-4 against hypoxia and cytokines are augmented by CREB. Endocrinology 153:1116–1128. [DOI] [PubMed] [Google Scholar]
- Wen AY, Sakamoto KM, Miller LS (2010) The role of the transcription factor CREB in immune function. J Immunol 185:6413–6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yisireyili M, Takeshita K, Hayashi M, Wu H, Uchida Y, Yamamoto K, Kikuchi R, Hao CN, Nakayama T, Cheng XW, Matsushita T, Nakamura S, Murohara T (2016) Dipeptidyl peptidase- IV inhibitor alogliptin improves stress-induced insulin resistance and prothrombotic state in a murine model. Psychoneuroendocrinology 73:186–195. [DOI] [PubMed] [Google Scholar]
- Zakaria R, Wan Yaacob WM, Othman Z, Long I, Ahmad AH, Al-Rahbi B (2017) Lipopolysaccharide-induced memory impairment in rats: a model of Alzheimer’s disease. Physiol Res 66:553–565. [DOI] [PubMed] [Google Scholar]
- Zhang F, Zhang JG, Yang W, Xu P, Xiao YL, Zhang HT (2018) 6-Gingerol attenuates LPS-induced neuroinflammation and cognitive impairment partially via suppressing astrocyte overactivation. Biomed Pharmacother 107:1523–1529. [DOI] [PubMed] [Google Scholar]
- Zhao WX, Zhang JH, Cao JB, Wang W, Wang DX, Zhang XY, Yu J, Zhang YY, Zhang YZ, Mi WD (2017) Acetaminophen attenuates lipopolysaccharide-induced cognitive impairment through antioxidant activity. J Neuroinflammation 14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Huang H, Liu J, Li M, Liu M, Luo T (2018) Propofol attenuates inflammatory response in LPS-activated microglia by regulating the miR-155/SOCS1 pathway. Inflammation 41:11–19. [DOI] [PubMed] [Google Scholar]
- Zhou J, Deng Y, Li F, Yin C, Shi J, Gong Q (2019) Icariside II attenuates lipopolysaccharide-induced neuroinflammation through inhibiting TLR4/MyD88/NF-κB pathway in rats. Biomed Pharmacother 111:315–324. [DOI] [PubMed] [Google Scholar]
- Zou ZQ, Chen JJ, Feng HF, Cheng YF, Wang HT, Zhou ZZ, Guo HB, Zheng W, Xu JP (2017) Novel phosphodiesterase 4 inhibitor FCPR03 alleviates lipopolysaccharide-induced neuroinflammation by regulation of the cAMP/PKA/CREB signaling pathway and NF-κB inhibition. J Pharmacol Exp Ther 362:67–77. [DOI] [PubMed] [Google Scholar]