Abstract
Background
Alcohol use disorder (AUD) is a chronic relapsing brain disorder. GABAA receptor (GABAAR) subunits are a target for the pharmacological effects of alcohol. Neurosteroids play an important role in the fine-tuning of GABAAR function in the brain. Recently, we have shown that AUD is associated with changes in DNA methylation mechanisms. However, the role of DNA methylation in the regulation of neurosteroid biosynthesis and GABAergic neurotransmission in AUD patients remains under-investigated.
Methods
In a cohort of postmortem brains from 20 male controls and AUD patients, we investigated the expression of GABAAR subunits and neurosteroid biosynthetic enzymes and their regulation by DNA methylation mechanisms. Neurosteroid levels were quantified by gas chromatography-mass spectrometry.
Results
The α 2 subunit expression was reduced due to increased DNA methylation at the gene promoter region in the cerebellum of AUD patients, a brain area particularly sensitive to the effects of alcohol. Alcohol-induced alteration in GABAAR subunits was also observed in the prefrontal cortex. Neurosteroid biosynthesis was also affected with reduced cerebellar expression of the 18kDa translocator protein and 3α-hydroxysteroid dehydrogenase mRNAs. Notably, increased DNA methylation levels were observed at the promoter region of 3α-hydroxysteroid dehydrogenase. These changes were associated with markedly reduced levels of allopregnanolone and pregnanolone in the cerebellum.
Conclusion
Given the key role of neurosteroids in modulating the strength of GABAAR-mediated inhibition, our data suggest that alcohol-induced impairments in GABAergic neurotransmission might be profoundly impacted by reduced neurosteroid biosynthesis most likely via DNA hypermethylation.
Keywords: cerebellum, GABAA receptor, neurosteroids, alcohol use disorder, DNA methylation
Significance Statement.
Alcohol use disorder (AUD) is a multifaceted chronic relapsing disorder affecting 6% of the US adult population. GABAergic neurotransmission mediates the behavioral tolerance and dependence to excessive alcohol drinking via brain-region specific alterations in GABAA receptor subunit expression. Endogenous neurosteroids, including allopregnanolone and pregnanolone, play a key role in fine-tuning GABAA receptor-mediated inhibition. Our study found reduced levels of neurosteroids in the postmortem cerebellum of AUD patients. These changes were associated with increased DNA methylation on key enzymes of the neurosteroidogenesis pathway (i.e., 3α-hydroxysteroid dehydrogenase) and related GABAergic genes. Our data suggest that alcohol-induced changes in GABAergic neurotransmission may be a result of reduced neurosteroid biosynthesis via DNA hypermethylation processes and implicates neurosteroid dysregulation as a primary mechanism for AUD neuropathophysiology. Thus, given the key role of allopregnanolone and pregnanolone in the fine-tuning of GABAAR-mediated inhibition, targeting neurosteroidogenesis may be a promising therapeutic strategy for the management of AUD.
Introduction
Alcohol use disorder (AUD) is a multifaceted chronic relapsing disorder often comorbid with mood disorders (Falk et al., 2008) and cognitive impairments (Keedwell et al., 2001). Altered GABAergic neurotransmission is thought to mediate the behavioral tolerance and dependence to protracted and excessive alcohol drinking and to contribute to the pathogenesis of AUD (Tabakoff and Hoffman, 2013). Synaptic changes in the strength of GABAergic neurotransmission are mediated by the structural and functional plasticity of both GABAA and GABAB receptors. The GABAA receptor (GABAAR) is a pentameric protein complex formed by subunits whose combination accounts for the diverse pharmacological properties of the receptor (Belelli and Lambert, 2005). On one hand, synaptic GABAAR, mainly containing α 1,2,3 along with β and γ subunit variants (Uusi-Oukari and Korpi, 2010; Fritschy and Panzanelli, 2014), mediate phasic currents (Brickley and Mody, 2012) and are sensitive to both benzodiazepines and barbiturates but are insensitive to the actions of 3α-hydroxy ring A-reduced pregnane steroids (GABAergic neurosteroids) (Costa and Guidotti, 1991; Hájos et al., 2000). On the other hand, peri- and extrasynaptic GABAAR containing α 4,5,6 with β and δ subunits instead of γ are responsible for a persistent tonic inhibition as a result of diffused extracellular GABA from nearby synapses (Belelli and Lambert, 2005; Uusi-Oukari and Korpi, 2010; Brickley and Mody, 2012). Importantly, the extrasynaptic GABAAR are highly sensitive (nM concentrations) to the positive allosteric modulation of neurosteroids, for example, allopregnanolone and its stereoisomer pregnanolone, while the synaptic receptors are less sensitive (µM) to these compounds (Belelli et al., 2009; Uusi-Oukari and Korpi, 2010; Brickley and Mody, 2012). The regulation of GABA steady-state levels is mediated by glutamate decarboxylases (i.e., GAD1 and GAD2) and high-affinity transporters primarily located in the presynaptic GABAergic neurons (i.e., GAT1) as well as in surrounding astrocytes (i.e., GAT2 and -3) (Schousboe et al., 2004). GABA may also be metabolized by GABA-transaminase (ABAT) (Madsen et al., 2008). Thus, GABA and neurosteroids interaction is crucial in maintaining physiological neurotransmission and brain function.
GABAAR adaptations have been largely implicated in alcohol dependence. In preclinical studies, long-term and continuous ethanol exposure has been found to downregulate α 1- and α 2-containing GABAAR mRNA expression in the cerebellum (Montpied et al., 1991). Of note, human genetic studies highlighted GABAAR α 2 single nucleotide polymorphisms in association with AUD (Edenberg et al., 2004). Furthermore, numerous preclinical and clinical studies have highlighted a role for GABAB receptors in mediating the effects of alcohol intake (Walker and Koob, 2007; Addolorato and Leggio, 2010; Maccioni and Colombo, 2019). GABAB receptor modulators are currently investigated to facilitate abstinence and reduce alcohol use (Farokhnia et al., 2018; Maccioni et al., 2019).
Mounting studies have shown evidence for a role for epigenetic mechanisms (e.g., DNA hypermethylation) in the pathophysiology of AUD and associated maladaptive behaviors (Warnault et al., 2013; Berkel and Pandey, 2017; Gatta et al., 2017, 2019). In a previous study in the cerebellum of AUD patients, we reported a marked decrease in the amounts of the GABAAR δ subunit, which was associated with increased DNA methylation of the corresponding gene promoter (Gatta et al., 2017). Additionally, we observed an impairment of the 1-carbon metabolism and a higher methylation index, suggesting the existence of an aberrant DNA methylation in the cerebellum of AUD patients (Gatta et al., 2017).
The cerebellum is a brain area particularly sensitive to the acute and chronic effects of ethanol (Dar, 2015; Valenzuela and Jotty, 2015). In addition to its well-known role in motor coordination (Ito, 2008), recent studies have demonstrated that cerebellar granule and Purkinje cells also integrate reward expectations (Wagner et al., 2017; Kostadinov et al., 2019). Alcohol affects motor coordination by enhancing tonic inhibition mediated by extrasynaptic receptor containing α 6 and δ subunits (Hanchar et al., 2005), which are almost exclusively present in cerebellar granule cells, where they generate a tonic inhibitory conductance controlling granule cells function (Nusser et al., 1999). Although the role of the δ subunit in mediating the pharmacological effects of alcohol has been previously studied, the effects of alcohol exposure on δ-containing GABAAR in the CNS remains controversial (Korpi et al., 2007). No changes in δ subunit expression have been observed in the brain of preclinical models of alcohol consumption (Mehta et al., 2007; Bohnsack et al., 2018). Additionally, δ-deficient mice showed reduced physiological responses to ethanol (Mihalek et al., 2001). However, extrasynaptic GABAAR are essential in mediating the physiological response to neurosteroids.
The synthesis of neurosteroids starts in glial cells by the transport of cholesterol from the outer to the inner mitochondrial membrane by the 18kDa translocator protein (TSPO) (Costa and Guidotti, 1991; Rupprecht et al., 2009). Cholesterol is then converted by the mitochondrial cholesterol side-chain cleavage enzyme into pregnenolone, which is the precursor for all neurosteroids. Pregnenolone is subsequently taken into neurons where it is further metabolized into progesterone by the 3β-hydroxysteroid dehydrogenase (3β-HSD). Type-1 5α-reductase (5α-R1) and 3α-HSD then convert progesterone into allopregnanolone (Locci and Pinna, 2017). Neurosteroid synthesis has been shown in glutamatergic corticolimbic neurons as well as in cerebellar granule cells (Agís-Balboa et al., 2006). Neurosteroids act through specific binding sites located on the transmembrane domains of α 6 and δ subunits of extrasynaptic GABAARs where they potentiate GABAergic inhibition and produce pharmacological effects comparable with those of alcohol (Concas et al., 1998; Hosie et al., 2006; Follesa et al., 2000, 2006).
Acute alcohol intoxication increases serum allopregnanolone levels in human blood samples of adolescent individuals (Torres and Ortega, 2003, 2004) and in rat cerebral cortex (VanDoren et al., 2000). Conversely, chronic ethanol exposure in rodents reduces allopregnanolone levels in several brain regions, including cerebral cortex, hippocampus, medial prefrontal cortex, ventral tegmental area, amygdala, and striatum (Cagetti et al., 2004; Maldonado-Devincci et al., 2014). Interestingly, voluntary chronic ethanol intake decreases allopregnanolone in the amygdala and plasma of cynomolgus monkeys (Beattie et al., 2017). However, the alcohol-induced changes in GABAergic synaptic regulation as well as neurosteroidogenesis and associated DNA methylation mechanisms in human brain remain largely under-investigated. We therefore tested the hypothesis that alcohol drinking alters GABAergic neurotransmission in association with changes in allopregnanolone biosynthesis via epigenetic mechanisms in the cerebellum of individuals suffering from AUD.
Materials and Methods
Subjects
Frozen postmortem brain tissue was obtained from the New South Wales Brain Tissue Resource Centre (NSW BTRC, University of Sydney, Australia) as part of a cohort originally including 25 subjects per group (see Gatta et al., (2017) for details). Postmortem brain tissue was dissected as previously described (Sheedy et al., 2008). For each sample, cerebellar tissue was prepared according to standard NSW BTRC protocol. Briefly, the cerebellum was hemisected through the vermis then dissected into 3 segments along the parasagittal plane and frozen at −80°C. Fresh frozen cerebellar cortex was sampled at the level of the dentate nucleus. Use of postmortem brain tissue was approved by the University of Illinois at Chicago Institutional Review Board. Individuals were diagnosed according to the DSM-IV criteria for AUD. Because of lack of availability of female samples, we decided to focus our study in the male population and focused our analysis on 20 control and 20 AUD subjects for RNA analysis. Due to tissue availability, only 12 control and 15 AUD subjects were used for neurosteroid determination (supplementary Table 1). Focusing on males prevented confounding in allopregnanolone measurements due to menstrual cycle (Uzunova et al., 1998; Maguire et al., 2005). Subjects did not show any hepatic encephalopathy pathology. The AUD cohort of 20 subjects included 7 AUD subjects who had alcohol toxicology at the time of death (0.03–0.430 g/100 mL) and 13 AUD subjects that had unknown (n = 3) or undetected blood alcohol levels (n = 10). Four of the subjects with alcohol toxicology at the time of death were used for neurosteroid levels determination (supplementary Table 1).
Neurosteroid Measurements
Allopregnanolone and its isomer, pregnanolone, were extracted, derivatized, and quantified as previously described (Uzunov et al., 1996). After addition of deuterium-labeled internal standards to tissue samples, steroids were extracted, purified, and separated by high-pressure liquid chromatography. After derivatization, gas chromatography–mass spectrometry analysis in the standard electron impact mode was performed (Pinna et al., 2004; Locci and Pinna, 2019).
Reverse Transcriptase-Quantitative Polymerase Chain Reaction (qRT-PCR)
Total mRNA was extracted using miRNeasy kit following the manufacturer’s instructions (Qiagen, Valencia, CA). DNAse treatment was used to avoid any genomic DNA contamination. mRNA levels were measured in prefrontal cortex (BA10), hippocampus, striatum, and cerebellum of our control and AUD cohort by qRT-PCR following total RNA extraction as previously described. RNA Integrity Number (RIN) values were measured with the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). Samples with an RIN < 3 were excluded from the analyses. Primer sequences used for mRNA expression studies are listed in supplementary Table 2. Three reference genes (i.e., Beta-2-Microglobulin, Glyceraldehyde-3-Phosphate Dehydrogenase [GAPDH], and ß-actin) were chosen for normalization of mRNA levels (Gatta et al., 2017, 2019). Our study focused on α- and δ-containing GABAAR.
Methyl-DNA-Immunoprecipitation Assay
DNA methylation levels were assessed by methyl-DNA-immunoprecipitation (MeDIP) using the MagMeDIP kit (Diagenode, Denville, NJ) as previously described (Gavin et al., 2012; Gatta et al., 2017, 2019). Primers were designed in the promoter region of 3α-HSD (gene symbol: AKR1C2) and GABAA receptor subunit α 2 (GABRA2) genes (supplementary Table 2). A schematic representation of the genes’ structure is presented in supplementary Figure 1 for the main promoter (supplementary Figure 1A–B) and the promoter of upstream transcripts for the corresponding gene (supplementary Figure 1C– D). The efficiency of the MeDIP assay was validated by qRT-PCR using internal positive and negative DNA controls (methylated/hydroxymethylated and unmethylated DNA) as well as control primers for testis-specific H2B histone gene (which is methylated in all somatic cells but not in testis) and GAPDH promoter (which is poorly methylated) following the manufacturer’s instructions (see Gatta et al., 2017 and supplementary Figure 2) with a specificity of 97.75%. Data obtained were calculated as % recovery = 2^(input −3.32 −IP) × 100 as recommended by the manufacturer, then expressed as % of control to account for the fact that samples were run in 2 batches.
Western Blot
Cerebellar proteins were extracted as previously described (Gatta et al., 2017). Briefly, 30 µg of each sample was separated by electrophoresis on Novex 4%–12% Tris-Glycine gels (Invitrogen, Carlsbad, CA) then transferred to polyvinylidene difluoride membrane (Millipore, Billerica, MA). Membranes were incubated with the following primary antibodies: anti-GABRA2 protein (1:500, Alpha Diagnostic International, San Antonio, TX; #GAA21-A) and anti-GAPDH (1:8000 Millipore; #MAB374). Horseradish peroxidase (HRP)-conjugated secondary anti-rabbit or anti-mouse antibodies (1:10 000, GE Healthcare, Arlington Heights, IL) were used and membranes were developed with Immobilon Western Chemiluminescent HRP Substrate (Millipore). Densitometric analysis was performed with ImageJ software.
Statistical Analysis
Statistical differences were assessed with 2-tailed Student’s t tests, and comparisons were considered statistically significant at P < .05. Benjamini-Hochberg (1995) multiple comparisons were conducted to control for the false discovery rate as previously described (Gatta et al., 2017). ANCOVA was performed for adjusting co-variants on the results. When appropriate, data were analyzed by 1-way ANOVA followed by Tukey’s post-hoc comparison. Correlation analyses were performed using 2-tailed Pearson’s correlation analysis. All statistical tests were run using PASW v.18 software (SPSS).
Results
Alcohol Impairs Neurosteroidogenesis in the Cerebellum of AUD Patients
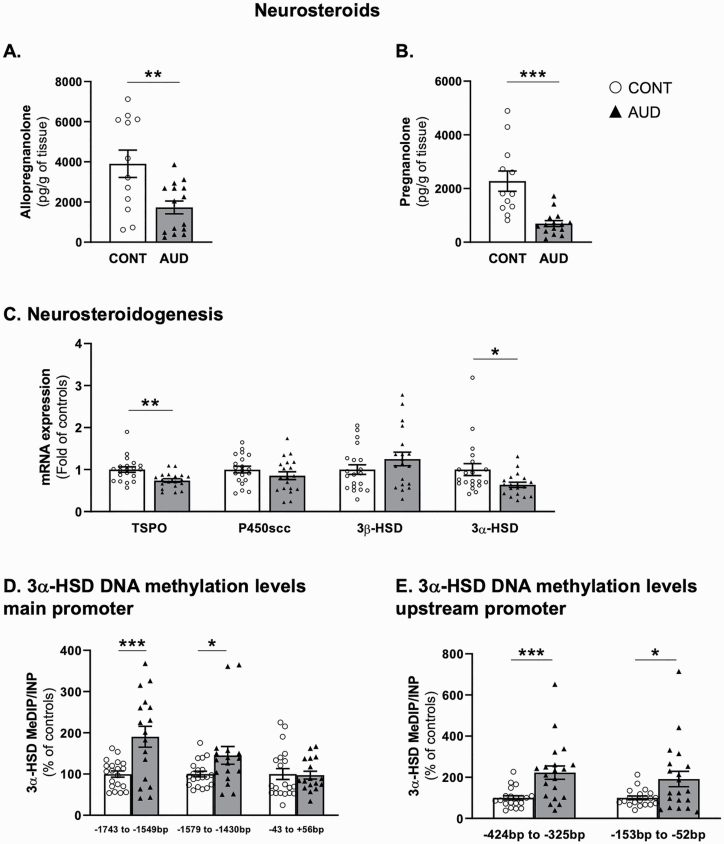
The mRNA expression of key components of the neurosteroid biosynthetic pathway and the levels of allopregnanolone and its isomer pregnanolone were altered in the cerebellum of AUD patients. We observed a decrease of approximately 60% in the amounts of allopregnanolone (Figure 1A) and pregnanolone (Figure 1B). These data remained significant after Benjamini-Hochberg’s correction (adjusted P = .005, .0004, respectively). Of note, the levels of allopregnanolone measured in our cohort negatively correlated with ethanol daily consumption and the number of standard drinks per week (supplementary Table 3). These correlations were not significant when only considering the AUD group. The AUD subjects with positive alcohol toxicology at the time of death did not differ from the other AUD subjects and showed significantly reduced levels of allopregnanolone (1-way ANOVA, F2,14 = 6.69, P = .009; Tukey’s post-hoc positive alcohol toxicology vs AUD P > .9999; alcohol toxicology vs control P = .03). In the cerebellum of the same subjects, we also observed reduced mRNA expression of TSPO and 3α-HSD (Figure 1C). These data remained significant after Benjamini-Hochberg multiple comparison (adjusted P values: TSPO P = .007, 3α-HSD P = .076). A significant inverse correlation was observed between the mRNA expression of TSPO and ethanol daily consumption levels (supplementary Table 3). mRNA expression of 3α-HSD correlated with the levels of allopregnanolone (r = 0.45, P = .02) and pregnanolone (r = 0.57, P = .002). These correlations were not significant when only considering the AUD group. No significant changes were found for P450scc and 3β-HSD mRNAs (Figure 1C).
Figure 1.
Cerebellar neurosteroidogenesis in individuals with alcohol use disorder (AUD). (A) Allopregnanolone (t1,25 = 3.1, P = .005) and (B) pregnanolone (t1,25 = 4.4, P = .0002) cerebellar levels (pg/g of tissue). mRNA expression of (C) 18 kDa translocator protein (TSPO, t1,37 = 3.3, P = .002), Mitochondrial Cholesterol Side-Chain Cleavage Enzyme (p450scc, t1,37 = 1.2, P = .2), 3β-hydroxysteroid dehydrogenase (3β-HSD, t1,37 = 1.3, P = .2), and 3α-HSD (t1,37 = 2.3, P = .03). Methylation levels measured by methylated DNA immunoprecipitation (MeDIP) of 3α-hydroxysteroid dehydrogenase (3α-HSD) (D) main promoter (from −1743 to −1594 bp: t1,35 = 3.6, P = .0008; −1579 to −1430 bp: t1,35 = 2.1, P = .04; −43 to +56 bp: t1,35 = 0.15, P = .88), (E) upstream promoter (from −424 to −325 bp: t1,38 = 3.6, P = .0009; from −153 to −52 bp: t1,38 = 2.4, P = .02). Methylation levels of 3α-HSD upstream promoter (−153 to −52 bp) negatively correlated with ethanol daily consumption (r = 0.31, P = .049); no significant correlation was observed with the number of standard drinks per week or the drinking years. These correlations were not significant when only considering the AUD group. Values are mean ± SEM of 12 control and 16 AUD samples for neurosteroid measurement, 18–20 samples per group for mRNA expression and 17–20 samples per group for DNA methylation levels determination depending on samples availability. *P < .05, **P < .01, ***P < .001, Student’s t test vs controls.
DNA Methylation and Neurosteroid Biosynthesis
We next investigated whether reductions in mRNA expression of 3α-HSD in the cerebellum of AUD subjects were related to changes in DNA methylation of gene promoter. We observed a hypermethylation of the 3α-HSD gene promoter (AKR1C2, Figure 1D) at −1743 to −1594 bp and −1579 to −1430 bp, while no differences in methylation levels were observed at −43 bp to +56 bp. Increased methylation levels were also detected for a promoter of upstream transcripts site at −424 to −325 bp and −153 to −52 bp (Figure 1E). These data remained significant after Benjamini-Hochberg multiple comparison (main promoter, −1743 to −1594 bp: adjusted P = .0004, −1579 to −1430 bp: adjusted P = 0,01; upstream promoter sites, from −424 to −325 bp: adjusted P = .0004; from −153 to −52 bp: adjusted P = .002). A detailed gene structure for AKR1C2 is shown in supplementary Figure 1A and C. These results suggest that DNA hypermethylation of 3α-HSD in the cerebellum of AUD is responsible for deficits in 3α-HSD expression and might be associated with altered neurosteroid levels in AUD.
Expression of GABAA/B Receptor Subunits in the Brain of AUD Patients
The expression of GABAAR subunits is brain region specific. To study whether chronic alcohol abuse induces changes in GABAAR mRNA subtypes expression, we presented our data as a fold change of control. In a previous work, we found reduced mRNA expression of GABAAR δ subunit in the cerebellum of AUD subjects (Table 1; Gatta et al., 2017). Here, we extended this study to other GABAAR subunits not only in the cerebellum but also in other brain regions. We observed a statistically significant reduction in the α 2 subunit mRNA expression in prefrontal cortex (PFC) and cerebellum (Table 1) of AUD subjects. However, no significant change was observed in the hippocampus or striatum (Table 1). Importantly, the GABAAR δ subunit mRNA expression was significantly decreased in PFC, with reduced but not significant levels in the hippocampus and no significant changes detected in striatum (Table 1). GABAAR α 5 subunit mRNA expression tended to increase but failed to reach statistical significance in the cerebellum and striatum (Table 1, P = .16, P = .23, respectively). After Benjamini-Hochberg multiple comparison, the changes observed in PFC (α 2,6 and δ subunits adjusted P = .035) and cerebellum (α 2 and δ subunits adjusted P = .042) remained significant. The β 2,3 and γ 2 subunits of the GABAA receptor failed to change in a significant manner in all brain areas studied, except γ 2 was significantly reduced in the cerebellum (supplementary Table 4) of AUD subjects. Taken together, our data showed that both the synaptic (α 2) and the extrasynaptic (δ) GABAergic transmission are affected in AUD. Cerebellar mRNA levels of the α 2 subunit correlated with postmortem interval (PMI) (α 2: r = −0.39, P = .01) and ethanol daily consumption (supplementary Table 5). Adjusting for PMI using ANCOVA maintained a significant group effect (group effect, F1,38 = 5.3, P = .027).
Table 1.
mRNA expression of GABAA (GABAAR) and GABAB (GABABR) receptor subunits in the PFC (BA10), HPC, STR, and CB of CONT and AUD subjects
| GABAAR | GABABR | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| α1 | α2 | α4 | α5 | α6 | δ | 1 | 2 | ||
| PFC | CONT | 1.00 ± 0.04 | 1.00 ± 0.07 | 1.00 ± 0.09 | 1.00 ± 0.11 | 1.00 ± 0.14 | 1.00 ± 0.12 | 1.00 ± 0.05 | 1.00 ± 0.06 |
| AUD | 0.95 ± 0.04 | 0.77 ± 0.06* | 0.98 ± 0.08 | 0.95 ± 0.08 | 0.64 ± 0.06* | 0.71 ± 0.05* | 0.84 ± 0.04* | 0.80 ± 0.03** | |
| HPC | CONT | 1.00 ± 0.08 | 1.00 ± 0.13 | 1.00 ± 0.11 | 1.00 ± 0.12 | ND | 1.00 ± 0.24 | 1.00 ± 0.07 | 1.00 ± 0.12 |
| AUD | 0.86 ± 0.09 | 0.84 ± 0.07 | 1.00 ± 0.07 | 0.89 ± 0.07 | 0.67 ± 0.10 | 0.87 ± 0.05 | 1.00 ± 0.06 | ||
| STR | CONT | 1.00 ± 0.16 | 1.00 ± 0.15 | 1.00 ± 0.10 | 1.00 ± 0.16 | 1.00 ± 0.13 | 1.00 ± 0.21 | 1.00 ± 0.06 | 1.00 ± 0.15 |
| AUD | 0.82 ± 0.07 | 0.81 ± 0.08 | 1.07 ± 0.12 | 0.57 ± 0.32 | 1.07 ± 0.12 | 1.12 ± 0.21 | 0.89 ± 0.06 | 0.81 ± 0.11 | |
| CB | CONT | 1.00 ± 0.11a | 1.00 ± 0.11/1.00 ± 0.15 | 1.00 ± 0.13 | 1.00 ± 0.15 | 1.00 ± 0.09† | 1.00 ± 0.08† | 1.00 ± 0.05 0.85 ± 0.05* |
1.00 ± 0.05 0.89 ± 0.04 |
| AUD | 0.92 ± 0.07 | 0.66 ± 0.08*/0.65 ± 0.08* | 1.11 ± 0.15 | 1.40 ± 0.24 | 0.99 ± 0.13 | 0.75 ± 0.07* |
Abbreviations: AUD, alcohol use disorder; CB, cerebellum; CONT, control; HPC, hippocampus; PFC, prefrontal cortex; STR, striatum.
a These data were part of our previously published study and have been revised to include only the males of the same cohort (Gatta et al., 2017). In cerebellum, similar results were obtained with 2 different set of primers targeting GABAAR α 2 subunit (see supplementary Table 2). Values are mean ± SEM of 19–20 samples per group *P < .05, **P < .01. Student’s t test vs control. α 6 mRNA levels were not detectable in the HPC in our experimental conditions (ND).
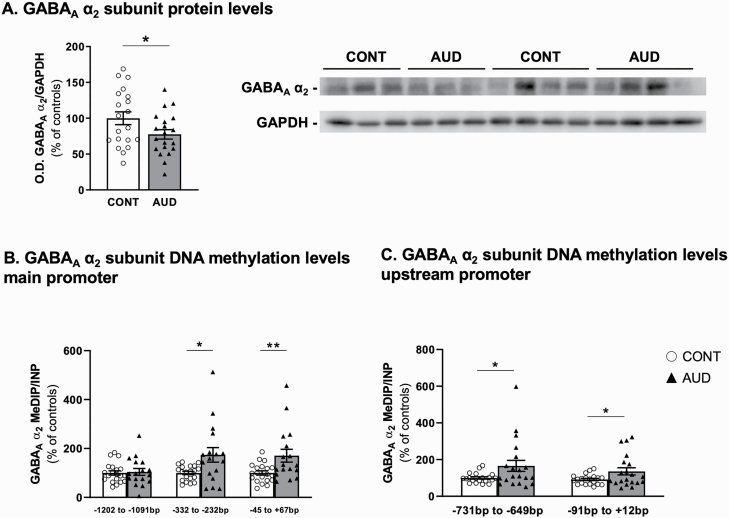
We also observed reduced GABAAR α 2 protein levels in the cerebellum of male AUD subjects (Figure 2A). Interestingly, the GABAA α 2 subunit protein levels negatively correlated with the number of drinking years (r = −0.36, P = .02). In addition, the mRNA expression corresponding to the GABAB subunits 1 and 2 was reduced by approximately 20% in PFC of male AUD subjects; no changes in the levels of these mRNAs were detected in hippocampus or striatum (Table 1). Only GABAB subunit 1 was reduced in the cerebellum (Table 1).
Figure 2.
GABAA receptor α 2 subunit protein levels and promoter methylation in cerebellum of individuals with alcohol use disorder (AUD). Protein levels of GABAA receptor subunit α 2 measured as ratio of α 2 /GAPDH optical density (O.D.) expressed as percent of controls (t1,38 = 2.03, P = .049). Representative western blots are shown on the right side. Methylation levels measured by methylated DNA immunoprecipitation (MeDIP) of GABAA receptor subunit α 2 (GABRA2) (B) main promoter region (from −1202 to −1091 bp: t1,35 = 0.27, P = .79; −332 to −232 bp: t1,35 = 2.61, P = .01 −45 bp to +67 bp: t1,35 = 2.73, P = .0098), (C) upstream promoter (from −731 to −649 bp: t1,38 = 2.15, P = .04; from −91 to +12 bp: t1,38 = 2.15, P = .04). GABAAR α 2 upstream promoter methylation levels at both measured locations inversely correlated with protein expression (−731 to −649 bp, r = −0.4, P = .01; −91 to +12 bp, r = −0.39, P = .01). No significant correlation was observed with α 2 mRNA expression (r = −0.08, P = .59; r = −0.08, P = .61, respectively). Values are mean ± SEM of 19–20 samples per group for protein levels. A total 17–20 samples per group were used for DNA methylation levels determination depending on samples availability. *P < .05, **P < .01, Student’s t test vs controls.
Chronic Alcohol Consumption Impacts Promoter Methylation of Key GABAergic-Related Genes
The reduced expression of GABAAR α 2 subunit was associated with increased DNA methylation levels in the promoter region of GABAAR α 2 gene (GABRA2, from −332 to −232 bp, −45 bp to +67 bp; Figure 2B). No differences in methylation levels were observed upstream of the CpG island from −1202 to −1091 bp (Figure 2B). Methylation data for an upstream promoter region are provided in Figure 2C and show higher methylation in the AUD group from −731 to −649 bp and from −91 to +12 bp. These data remained significant after Benjamini-Hochberg multiple comparison (main promoter sites, 332 to −232 bp and −45 bp to +67 bp: adjusted P = .007; upstream promoter, −731 to −649 bp and −91 to +12 bp: adjusted P = .01). Detailed gene structure for GABRA2 is presented in supplementary Figure 1B and D.
Alcohol Transcriptional Effects on Presynaptic GABAergic-Related Genes
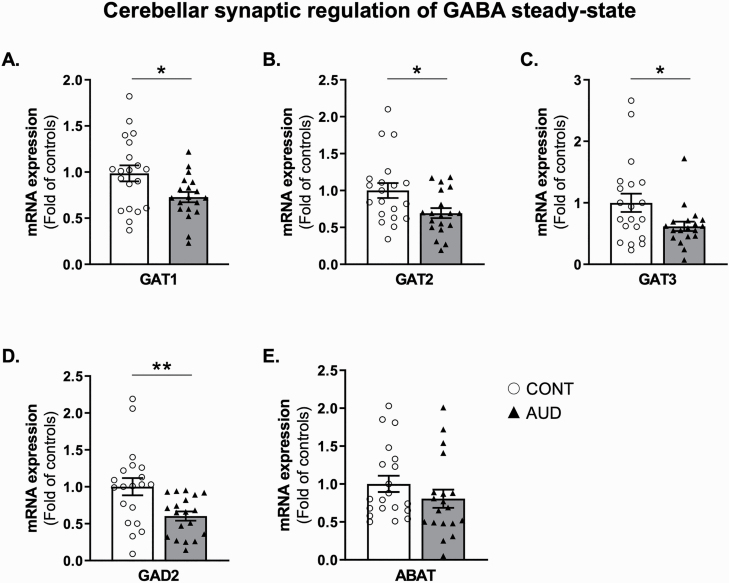
We also studied whether chronic alcohol alters the expression of neuronal and glial GABA transporter mRNAs. We observed reduced expression of the neuronal GAT1 mRNA (Figure 3A) and glial GAT2 and GAT3 mRNAs (Figure 3B–C, respectively) in the cerebellum of AUD subjects. mRNA expression of GAT2 correlated with pH (r = 0.35, P = .03). Hence, using ANCOVA, we tested the changes in GAT2 after adjusting for the effect of pH. GAT2 mRNA expression remained significantly different between control and AUD groups (group effect, F1,38 = 7.7, P = .009). We also measured a decrease in GAD2 mRNA in AUD subjects (Figure 3D). However, no changes were noted for the GABA transaminase ABAT mRNA expression (Figure 3E). The Benjamini-Hochberg correction maintained the significance of our results (GAT1 and GAT2 adjusted P = .007, GAT3 adjusted P = .008, GAD2 adjusted P = .007, ABAT adjusted P = .052).
Figure 3.
Characterization of the cerebellar GABAergic synapse in individuals with alcohol use disorder (AUD). mRNA expression of (A) high affinity GABA transporters 1 (GAT1, t1,37 = 2.5, P = .02), (B) GAT2 (t1,37 = 2.5, P = .02), (C) GAT3 (t1,37 = 2.2, P = .03), (D) glutamate decarboxylase 2 (GAD2, t1,37 = 2.9, P = .005), and (E) GABA-transaminase (ABAT, t1,37 = 1.2, P = .2). Values are mean ± SEM of 19–20 samples per group. *P < .05, **P < .01, Student’s t test vs controls.
Discussion
GABAergic synapses are a recognized target for the behavioral and molecular actions of alcohol. Chronic alcohol abuse induces tolerance and dependence to its anxiolytic and anticonvulsant properties via GABAAR-mediated processes (Olsen, 2018). Allopregnanolone and pregnanolone play a pivotal neurophysiological role by potently and allosterically modulating extrasynaptic GABAAR signaling (Belelli et al., 2009; Uusi-Oukari and Korpi, 2010; Brickley and Mody, 2012). In the current study, we observed reduced levels of allopregnanolone and pregnanolone measured by gas chromatography–mass spectrometry in the cerebellum of AUD patients compared with controls. The drastic decrease (approximately 60%) in the levels of these neurosteroids observed in the cerebellum of AUD subjects is consistent with the significantly reduced expression of the neurosteroidogenic enzymes (5-αR1 that we previously observed [Gatta et al., 2017] and 3α-HSD showed here) that are rate-limiting steps for allopregnanolone biosynthesis. In our study, chronic alcohol use was also associated with the reduced expression of α 2 (synaptic) and δ (extrasynaptic) containing GABAAR in PFC and cerebellum (Gatta et al., 2017) but not in striatum and hippocampus of AUD male subjects. Hence, long-term alcohol drinking history differentially alters the expression levels of selected GABAAR subunits in a brain region–specific manner in humans. The inhibitory control of cerebellar output on deep cerebellar nuclei is exclusively GABAergic. Thus, we focused on this brain region to study whether chronic alcohol abuse affects GABAergic neurotransmission and neurosteroidogenesis via epigenetic mechanisms.
Preclinical studies have shown that chronic ethanol exposure and withdrawal alter brain biosynthesis of allopregnanolone. Similar to our findings in human postmortem brain, reduced allopregnanolone has been observed in corticolimbic brain areas in rats (Cagetti et al., 2004) and mice (Maldonado-Devincci et al., 2014) chronically exposed to ethanol. Only a few studies have examined the effects of chronic alcohol on neurosteroid levels in the human postmortem brain. Hasirci and colleagues (2017), using an immunohistochemical approach, reported increased allopregnanolone-like content in ventral tegmental area (VTA) neurons of AUD subjects. If one excludes demographic and methodological divergences, the difference in allopregnanolone levels observed between VTA and cerebellum of AUD subjects suggests the existence of a cell and brain region–specific regulation of neurosteroid biosynthesis following alcohol exposure. The reduced neurosteroid levels we observed in the cerebellum of AUD subjects could contribute to a biochemical adaptation to the GABAmimetic effects of alcohol. Accordingly, our data indicate that chronic alcohol exposure reduces the expression of neurosteroidogenic enzymes in the cerebellum of AUD subjects. Since acute alcohol stimulates neurosteroidogenesis (Korpi et al., 2001; Khisti et al., 2002), the dampened response we observed after long-term chronic alcohol consumption may be related to an adaptive mechanism leading to alcohol dependence. This may provide a rationale for the use of synthetic neurosteroids (e.g., allopregnanolone) in the treatment of AUD (Morrow et al., 2020). Our previous work showed similar effects in the hippocampus of rats exposed to chronic intermittent ethanol administration (Cagetti et al., 2004). Upon investigating the epigenetic regulation of key enzymes of the neurosteroidogenesis, we observed increased DNA methylation levels at the promoter region of the gene encoding 3-αHSD in the cerebellum of AUD subjects. These epigenetic changes are likely the result of an increased methylation index associated with the higher rates of DNA methylation we previously detected in the cerebellum of AUD subjects, where decreased levels of DNA methylation erasers (i.e., TETs) have been observed (Gatta et al., 2017).
The cerebellum is the host of a complex neuronal circuitry. While the Purkinje cells are considered the sole inhibitory output of the cerebellum, the inputs arriving to these cells are mediated by multiple interneurons. Purkinje cell dendrites synapse with glutamatergic projections coming from the granule cells, that is, the parallel fibers. The effectiveness of these synapses is modulated by glutamatergic mossy fibers projecting to the granule cells. The inhibitory activity of the Purkinje cells is also tightly regulated by local GABAergic interneurons projecting on the cell body (basket cells) or on the dendrites (stellate cells). Stellate and basket cells also receive inputs from the parallel fibers that send inputs to the Golgi cells, which in turn release GABA on the granule cell (Ito, 2008). The activation of the inhibitory synaptic transmission, which is triggered by Golgi cells releasing GABA that binds to extrasynaptic δ-containing GABAAR located on granule cells, is particularly evident in the cerebellum (Hanchar et al., 2005). Of note, the cerebellar granule cells are considered the most abundant class of neurons in the human brain (Purves et al., 2001). These cells are known to play a fundamental role in the regulation of neurosteroid biosynthesis and release (Follesa et al., 2000; Agís-Balboa et al., 2006), which may explain why cerebellar GABAergic transmission is particularly sensitive to the effects of alcohol (Figure 4). At the same time, this suggests that neurosteroid dysregulation may be a leading mechanism underlying AUD neuropathophysiology.
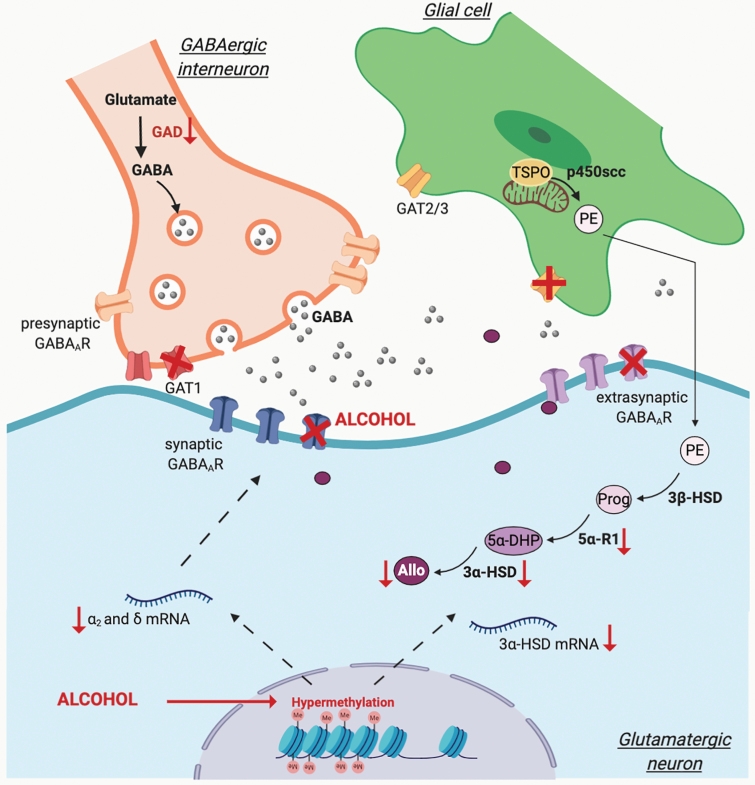
Figure 4.
Schematic representation of the effect of alcohol on GABAergic synapse and neurosteroid synthesis via DNA methylation mechanisms. Γ-Aminobutyric acid (GABA) is synthesized by glutamate decarboxylases (GAD). Upon release from GABAergic interneurons, GABA acts on synaptic (phasic) and extrasynaptic (tonic) GABAA receptors (GABAAR). Synaptic GABA levels are maintained by high-affinity transporters located in the presynaptic GABAergic interneurons (GAT1) as well as in surrounding astrocytes (GAT2/3). Allopregnanolone (Allo), released by glutamatergic neurons, plays a key role in facilitating GABAAR-mediated inhibition. The synthesis of Allo results from the transport of cholesterol to the outer membrane of the mitochondria by the 18kDa translocator protein (TSPO), where it will be converted by the mitochondrial cholesterol side-chain cleavage enzyme (p450scc) into pregnenolone (PE). In glutamatergic neurons, PE will then be metabolized by the 3β-hydroxysteroid dehydrogenase (3β-HSD) into progesterone (Prog), which by the action of type-1 5α-reductase (5α-R1) will be converted into 5α-dihydroprogesterone (5α-DHP). 3α-HSD will then convert 5α-DHP into Allo. The function of GABAergic synapses is altered by chronic alcohol consumption, which increases DNA methylation (Me, hypermethylation) with a consequent downregulation of the rate-limiting steps of Allo biosynthesis (as indicated by the red arrows) and alteration of GABAAR subunit composition. Chronic alcohol exposure also affects GAT expression in the cerebellum (indicated by red crosses). Created with BioRender.com
Brain region–specific alterations of GABAAR subunits expression have been observed in postmortem human brain of individuals with AUD (Jin et al., 2012). Here, we report alcohol-induced alterations in both the synaptic and the extra-synaptic GABAAR composition. Specifically, we observed reductions in α 2 and δ subunits mRNA expression in the PFC and cerebellum of AUD subjects compared with controls. In agreement with our findings, chronic alcohol administration in rodents reduces α 2 subunit expression in cortex (Montpied et al., 1991) and cerebellum (Marutha Ravindran et al., 2007). Although the association between GABAAR polymorphisms and the vulnerability for AUD has been somewhat controversial (Cui et al., 2012), genetic alteration of the synaptic α 2 receptor subunit have been associated with AUD (Edenberg et al., 2004). Thus, we focused our studies on the epigenetic mechanisms underlying the reduction of α 2 receptor subunit and found that this change was associated with a significant promoter hypermethylation, consistent with our previous study showing alteration of the methylation index in the cerebellum of AUD subjects (Gatta et al., 2017). Whether these epigenetic modifications elicited by alcohol are specific to granule cells or differ in distinct neuronal populations remains to be determined. In association with changes in neurosteroidogenic enzymes and GABAAR subunit composition, we also observed a decrease in the neuronal (GAT1) and glial (GAT2/3) GABA transporters. We believe that this may represent a compensatory epigenetic mechanism that maintains sufficient steady-state GABA levels at synapses during chronic alcohol intoxication. Interestingly, GAT3 expression has been shown to play a key role in alcohol preference, and reduced GAT3 expression was also observed in the central amygdala of AUD subjects compared with controls (Augier et al., 2018). Furthermore, we observed reduced GABAB receptor mRNA expression in the PFC of AUD subjects. The role for GABAB receptors in mediating the effects of alcohol intake has been provided by the evidence that baclofen (a GABAB receptor agonist) administration decreased withdrawal symptoms in Wistar rats (Colombo et al., 2004) and AUD patients (Addolorato and Leggio, 2010). However, the association of single nucleotide polymorphisms in GABBR1 and GABBR2 genes has raised some controversy (Sander et al., 1999; Köhnke et al., 2006; Terranova et al., 2014; Caputo et al., 2017). In contrast to our observations in the PFC, Flatscher-Bader et al., 2005 showed increased mRNA expression of GABBR1 using cDNA microarrays. However, this study was conducted in a smaller cohort of control and AUD subjects, and the investigators did not further validation of these findings. Conversely, reduced transcript levels for GABBR2 were observed in the hippocampus of alcohol-dependent rats (Ribeiro et al., 2012). A growing body of evidence suggests that the conformational alteration of the GABAB subunits is necessary for effective activation of these receptors (Villas Boas et al., 2012; Shaye et al., 2020). Further investigation is needed to determine whether the alterations we observed could be responsible for an alcohol-induced disbalance between the 2 GABAB subunits.
Conclusion
Chronic alcohol exposure alters synaptic and extrasynaptic GABAAR function through changes in subunit expression, resulting in altered signaling and neurosteroid sensitivity and thus contributing to alcohol tolerance and dependence (Kumar et al., 2009). Collectively, our data suggest that allopregnanolone plays a significant role in the alcohol-induced impairments of GABAergic neurotransmission. Whether changes in GABAAR subunit expression result from a direct effect of alcohol or whether this is secondary to alcohol impacting neurosteroid biosynthesis remains to be further investigated. Several studies have demonstrated that alcohol addiction is associated with a loss of GABAergic inhibition, suggesting the existence of an imbalance between excitatory and inhibitory signaling and the loss of control over neuronal firing. Thus, given the key role of allopregnanolone and pregnanolone in the fine-tuning of GABAAR-mediated inhibition, targeting neurosteroidogenesis may be a promising therapeutic strategy for the management of AUD. Furthermore, preclinical studies have shown that treatment with the DNA methyltransferases inhibitor, 5-azacytidine, reduces ethanol intake and preference (Warnault et al., 2013; Sakharkar et al., 2019), suggesting that DNA methylation is a putative target for correcting GABAergic neurotransmission and neurosteroid biosynthesis in AUD.
Supplementary Material
Acknowledgments
The authors thank the NSW BTRC at the University of Sydney (Australia), supported by NIH-NIAAA R28AA012725, for providing postmortem brain tissues used in this study.
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) grant P50AA022538 (Center for Alcohol Research in Epigenetics) to S.C.P. and A.G., as well as a senior VA research career scientist award to S.C.P., and by a Department of Defense Grant W81XWH-15-1-0521 to G.P.
Statement of Interest
G.P. has 2 pending patent applications; 1 on PEA and PPAR-α agonists (US 2018/0369171) and 1 on allopregnanolone’s analogs in the treatment of neuropsychiatric disorders (WO 2018/237282). All other authors have no conflicts to declare.
References
- Addolorato G, Leggio L (2010) Safety and efficacy of baclofen in the treatment of alcohol-dependent patients. Curr Pharm Des 16:2113–2117. [DOI] [PubMed] [Google Scholar]
- Agís-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A (2006) Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci U S A 103:14602–14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augier E, Barbier E, Dulman RS, Licheri V, Augier G, Domi E, Barchiesi R, Farris S, Nätt D, Mayfield RD, Adermark L, Heilig M (2018) A molecular mechanism for choosing alcohol over an alternative reward. Science 360:1321–1326. [DOI] [PubMed] [Google Scholar]
- Beattie MC, Maldonado-Devincci AM, Porcu P, O’Buckley TK, Daunais JB, Grant KA, Morrow AL (2017) Voluntary ethanol consumption reduces GABAergic neuroactive steroid (3α,5α)3-hydroxypregnan-20-one (3α,5α-THP) in the amygdala of the cynomolgus monkey. Addict Biol 22:318–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW (2009) Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci 29:12757–12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ (2005) Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci 6:565–575. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 57:289–300. [Google Scholar]
- Berkel TD, Pandey SC (2017) Emerging role of epigenetic mechanisms in alcohol addiction. Alcohol Clin Exp Res 41:666–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack JP, Hughes BA, O’Buckley TK, Edokpolor K, Besheer J, Morrow AL (2018) Histone deacetylases mediate GABAA receptor expression, physiology, and behavioral maladaptations in rat models of alcohol dependence. Neuropsychopharmacology 43:1518–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Mody I (2012) Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron 73:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagetti E, Pinna G, Guidotti A, Baicy K, Olsen RW (2004) Chronic intermittent ethanol (CIE) administration in rats decreases levels of neurosteroids in hippocampus, accompanied by altered behavioral responses to neurosteroids and memory function. Neuropharmacology 46:570–579. [DOI] [PubMed] [Google Scholar]
- Caputo F, Ciminelli BM, Jodice C, Blasi P, Vignoli T, Cibin M, Zoli G, Malaspina P (2017) Alcohol use disorder and GABAB receptor gene polymorphisms in an Italian sample: haplotype frequencies, linkage disequilibrium and association studies. Ann Hum Biol 44:384–388. [DOI] [PubMed] [Google Scholar]
- Colombo G, Addolorato G, Agabio R, Carai MA, Pibiri F, Serra S, Vacca G, Gessa GL (2004) Role of GABA(B) receptor in alcohol dependence: reducing effect of baclofen on alcohol intake and alcohol motivational properties in rats and amelioration of alcohol withdrawal syndrome and alcohol craving in human alcoholics. Neurotox Res 6:403–414. [DOI] [PubMed] [Google Scholar]
- Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G (1998) Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci U S A 95:13284–13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E, Guidotti A (1991) Diazepam binding inhibitor (DBI): a peptide with multiple biological actions. Life Sci 49:325–344. [DOI] [PubMed] [Google Scholar]
- Cui WY, Seneviratne C, Gu J, Li MD (2012) Genetics of GABAergic signaling in nicotine and alcohol dependence. Hum Genet 131:843–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar MS (2015) Ethanol-induced cerebellar ataxia: cellular and molecular mechanisms. Cerebellum Lond Engl 14:447–465. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, et al. (2004) Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet 74:705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hilton ME (2008) Age of onset and temporal sequencing of lifetime DSM-IV alcohol use disorders relative to comorbid mood and anxiety disorders. Drug Alcohol Depend 94:234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farokhnia M, Deschaine SL, Sadighi A, Farinelli LA, Lee MR, Akhlaghi F, Leggio L (2018) A deeper insight into how GABA-B receptor agonism via baclofen may affect alcohol seeking and consumption: lessons learned from a human laboratory investigation. Mol Psychiatry. doi: 10.1038/s41380-018-0287-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatscher-Bader T, van der Brug M, Hwang JW, Gochee PA, Matsumoto I, Niwa S, Wilce PA (2005) Alcohol-responsive genes in the frontal cortex and nucleus accumbens of human alcoholics. J Neurochem 93:359–370. [DOI] [PubMed] [Google Scholar]
- Follesa P, Serra M, Cagetti E, Pisu MG, Porta S, Floris S, Massa F, Sanna E, Biggio G (2000) Allopregnanolone synthesis in cerebellar granule cells: roles in regulation of GABA(A) receptor expression and function during progesterone treatment and withdrawal. Mol Pharmacol 57:1262–1270. [PubMed] [Google Scholar]
- Follesa P, Biggio F, Talani G, Murru L, Serra M, Sanna E, Biggio G (2006) Neurosteroids, GABAA receptors, and ethanol dependence. Psychopharmacology (Berl) 186:267–280. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Panzanelli P (2014) GABAA receptors and plasticity of inhibitory neurotransmission in the central nervous system. Eur J Neurosci 39:1845–1865. [DOI] [PubMed] [Google Scholar]
- Gatta E, Auta J, Gavin DP, Bhaumik DK, Grayson DR, Pandey SC, Guidotti A (2017) Emerging role of one-carbon metabolism and DNA methylation enrichment on δ-containing GABAA receptor expression in the cerebellum of subjects with alcohol use disorders (AUD). Int J Neuropsychopharmacol 20:1013–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatta E, Grayson DR, Auta J, Saudagar V, Dong E, Chen Y, Krishnan HR, Drnevich J, Pandey SC, Guidotti A (2019) Genome-wide methylation in alcohol use disorder subjects: implications for an epigenetic regulation of the cortico-limbic glucocorticoid receptors (NR3C1). Mol Psychiatry 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin DP, Sharma RP, Chase KA, Matrisciano F, Dong E, Guidotti A (2012) Growth arrest and DNA-damage-inducible, beta (GADD45b)-mediated DNA demethylation in major psychosis. Neuropsychopharmacology 37:531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N, Nusser Z, Rancz EA, Freund TF, Mody I (2000) Cell type- and synapse-specific variability in synaptic GABAA receptor occupancy. Eur J Neurosci 12:810–818. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M (2005) Alcohol-induced motor impairment caused by increased extrasynaptic GABA(A) receptor activity. Nat Neurosci 8:339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasirci AS, Maldonado-Devincci AM, Beattie MC, O’Buckley TK, Morrow AL (2017) Cellular GABAergic neuroactive steroid (3α,5α)-3-hydroxy-pregnan-20-one (3α,5α-THP) immunostaining levels are increased in the ventral tegmental area of human alcohol use disorder patients: a postmortem study. Alcohol Clin Exp Res 41:299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HMA, Smart TG (2006) Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature 444:486–489. [DOI] [PubMed] [Google Scholar]
- Ito M (2008) Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci 9:304–313. [DOI] [PubMed] [Google Scholar]
- Jin Z, Bazov I, Kononenko O, Korpi ER, Bakalkin G, Birnir B (2012) Selective changes of GABAA channel subunit mRNAs in the hippocampus and orbitofrontal cortex but not in prefrontal cortex of human alcoholics. Front Cell Neurosci 5 https://www.frontiersin.org/articles/10.3389/fncel.2011.00030/full Accessed October 30, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedwell PA, Kumari V, Poon L, Marshall EJ, Checkley SA (2001) Information processing deficits in withdrawing alcoholics. Addict Biol 6:239–245. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Penland SN, VanDoren MJ, Grobin AC, Morrow AL (2002) GABAergic neurosteroid modulation of ethanol actions. World J Biol Psychiatry 3:87–95. [DOI] [PubMed] [Google Scholar]
- Köhnke M, Schick S, Lutz U, Köhnke A, Vonthein R, Kolb W, Batra A (2006) The polymorphism GABABR1 T1974C[rs29230] of the GABAB receptor gene is not associated with the diagnosis of alcoholism or alcohol withdrawal seizures. Addict Biol 11:152–156. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Debus F, Linden A-M, Malécot C, Leppä E, Vekovischeva O, Rabe H, Böhme I, Aller MI, Wisden W, Lüddens H (2007) Does ethanol act preferentially via selected brain GABAA receptor subtypes? the current evidence is ambiguous. Alcohol 41:163–176. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Mäkelä R, Romeo E, Guidotti A, Uusi-Oukari M, Furnari C, di Michele F, Sarviharju M, Xu M, Rosenberg PH (2001) Increased behavioral neurosteroid sensitivity in a rat line selectively bred for high alcohol sensitivity. Eur J Pharmacol 421:31–38. [DOI] [PubMed] [Google Scholar]
- Kostadinov D, Beau M, Blanco-Pozo M, Häusser M (2019) Predictive and reactive reward signals conveyed by climbing fiber inputs to cerebellar Purkinje cells. Nat Neurosci 22:950–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL (2009) The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 205:529–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locci A, Pinna G (2017) Neurosteroid biosynthesis down-regulation and changes in GABAA receptor subunit composition: a biomarker axis in stress-induced cognitive and emotional impairment. Br J Pharmacol 174:3226–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locci A, Pinna G (2019) Stimulation of peroxisome proliferator-activated receptor-α by N-palmitoylethanolamine engages allopregnanolone biosynthesis to modulate emotional behavior. Biol Psychiatry 85:1036–1045. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Colombo G (2019) Potential of GABAB receptor positive allosteric modulators in the treatment of alcohol use disorder. CNS Drugs 33:107–123. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Fara F, Lorrai I, Acciaro C, Mugnaini C, Corelli F, Colombo G (2019) Suppressing effect of CMPPE, a new positive allosteric modulator of the GABAB receptor, on alcohol self-administration and reinstatement of alcohol seeking in rats. Alcohol 75:79–87. [DOI] [PubMed] [Google Scholar]
- Madsen KK, Larsson OM, Schousboe A (2008) Regulation of excitation by GABA neurotransmission: focus on metabolism and transport. In: Inhibitory regulation of excitatory neurotransmission. Results and problems in cell differentiation. (Darlison MG, ed), pp201–221. Berlin, Heidelberg: Springer; 10.1007/400_2007_036. Accessed November 5, 2019. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I (2005) Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci 8:797–804. [DOI] [PubMed] [Google Scholar]
- Maldonado-Devincci AM, Cook JB, O’Buckley TK, Morrow DH, McKinley RE, Lopez MF, Becker HC, Morrow AL (2014) Chronic intermittent ethanol exposure and withdrawal alters (3α,5α)-3-hydroxy-pregnan-20-one immunostaining in cortical and limbic brain regions of C57BL/6J mice. Alcohol Clin Exp Res 38:2561–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marutha Ravindran CR, Mehta AK, Ticku MK (2007) Effect of chronic administration of ethanol on the regulation of the δ-subunit of GABAA receptors in the rat brain. Brain Res 1174:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AK, Marutha Ravindran CR, Ticku MK (2007) Low concentrations of ethanol do not affect radioligand binding to the delta-subunit-containing GABAA receptors in the rat brain. Brain Res 1165:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalek RM, Bowers BJ, Wehner JM, Kralic JE, VanDoren MJ, Morrow AL, Homanics GE (2001) GABAA-receptor δ subunit knockout mice have multiple defects in behavioral responses to ethanol. Alcohol Clin Exp Res 25:1708–1718. [PubMed] [Google Scholar]
- Montpied P, Morrow AL, Karanian JW, Ginns EI, Martin BM, Paul SM (1991) Prolonged ethanol inhalation decreases gamma-aminobutyric acidA receptor alpha subunit mRNAs in the rat cerebral cortex. Mol Pharmacol 39:157–163. [PubMed] [Google Scholar]
- Morrow AL, Boero G, Porcu P (2020) A rationale for allopregnanolone treatment of alcohol use disorders: basic and clinical studies. Alcohol Clin Exp Res 44:320–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Ahmad Z, Tretter V, Fuchs K, Wisden W, Sieghart W, Somogyi P (1999) Alterations in the expression of GABAA receptor subunits in cerebellar granule cells after the disruption of the alpha6 subunit gene. Eur J Neurosci 11:1685–1697. [DOI] [PubMed] [Google Scholar]
- Olsen RW (2018) GABAA receptor: positive and negative allosteric modulators. Neuropharmacology 136:10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A (2004) Fluoxetine and norfluoxetine stereospecifically facilitate pentobarbital sedation by increasing neurosteroids. Proc Natl Acad Sci U S A 101:6222–6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia A-S, McNamara JO, Williams SM (2001) Circuits within the cerebellum. 2nd ed. Neurosci. https://www.ncbi.nlm.nih.gov/books/NBK10865/. Accessed February 12, 2020. [Google Scholar]
- Ribeiro AF, Correia D, Torres AA, Boas GRV, Rueda AVL, Camarini R, Chiavegatto S, Boerngen-Lacerda R, Brunialti-Godard AL (2012) A transcriptional study in mice with different ethanol-drinking profiles: possible involvement of the GABAB receptor. Pharmacol Biochem Behav 102:224–232. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, et al. (2009) Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science 325:490–493. [DOI] [PubMed] [Google Scholar]
- Sakharkar AJ, Kyzar EJ, Gavin DP, Zhang H, Chen Y, Krishnan HR, Grayson DR, Pandey SC (2019) Altered amygdala DNA methylation mechanisms after adolescent alcohol exposure contribute to adult anxiety and alcohol drinking. Neuropharmacology 157:107679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander T, Peters C, Kämmer G, Samochowiec J, Zirra M, Mischke D, Ziegler A, Kaupmann K, Bettler B, Epplen JT, Riess O (1999) Association analysis of exonic variants of the gene encoding the GABAB receptor and idiopathic generalized epilepsy. Am J Med Genet 88:305–310. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Sarup A, Larsson OM, White HS (2004) GABA transporters as drug targets for modulation of GABAergic activity. Biochem Pharmacol 68:1557–1563. [DOI] [PubMed] [Google Scholar]
- Shaye H, Ishchenko A, Lam JH, Han GW, Xue L, Rondard P, Pin JP, Katritch V, Gati C, Cherezov V (2020) Structural basis of the activation of a metabotropic GABA receptor. Nature 584:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheedy D, Garrick T, Dedova I, Hunt C, Miller R, Sundqvist N, Harper C (2008) An Australian brain bank: a critical investment with a high return! Cell Tissue Bank 9:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL (2013) The neurobiology of alcohol consumption and alcoholism: an integrative history. Pharmacol Biochem Behav 113:20–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova C, Tucci M, Di Pietra L, Ferrara SD (2014) GABA receptors genes polymorphisms and alcohol dependence: no evidence of an association in an italian male population. Clin Psychopharmacol Neurosci 12:142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres JM, Ortega E (2003) Alcohol intoxication increases allopregnanolone levels in female adolescent humans. Neuropsychopharmacology 28:1207–1209. [DOI] [PubMed] [Google Scholar]
- Torres JM, Ortega E (2004) Alcohol intoxication increases allopregnanolone levels in male adolescent humans. Psychopharmacology (Berl) 172:352–355. [DOI] [PubMed] [Google Scholar]
- Uusi-Oukari M, Korpi ER (2010) Regulation of GABA(A) receptor subunit expression by pharmacological agents. Pharmacol Rev 62:97–135. [DOI] [PubMed] [Google Scholar]
- Uzunov DP, Cooper TB, Costa E, Guidotti A (1996) Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proc Natl Acad Sci U S A 93:12599–12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A (1998) Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci U S A 95:3239–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela CF, Jotty K (2015) Mini-review: effects of ethanol on GABAA receptor-mediated neurotransmission in the cerebellar cortex–recent advances. Cerebellum 14:438–446. [DOI] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL (2000) Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci 20:1982–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villas Boas GR, Zamboni CG, Peretti MC, Correia D, Rueda AV, Camarini R, Brunialti-Godard AL, Boerngen-Lacerda R (2012) GABA(B) receptor agonist only reduces ethanol drinking in light-drinking mice. Pharmacol Biochem Behav 102:233–240. [DOI] [PubMed] [Google Scholar]
- Wagner MJ, Kim TH, Savall J, Schnitzer MJ, Luo L (2017) Cerebellar granule cells encode the expectation of reward. Nature 544:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF (2007) The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res 31:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnault V, Darcq E, Levine A, Barak S, Ron D (2013) Chromatin remodeling–a novel strategy to control excessive alcohol drinking. Transl Psychiatry 3:e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.