Abstract
Introduction:
Although medroxyprogesterone acetate (MPA) is used commonly as a contraceptive in women and female nonhuman primates, its effects on social behavior remain unclear. This study examined whether MPA treatment and introduction of new adult males during the breeding season influence the social behaviors of group-housed adult female rhesus macaques.
Methods:
Subjects were 12 MPA-treated and 12 matched-case control females. Aggressive, affiliative and sexual behaviors were measured.
Results:
MPA-treated females showed less affiliative and sexual behavior compared to matched controls during the breeding season. MPA treatment was associated with decreased aggression emitted towards and received from females during the breeding season.
Conclusion:
MPA treatment is associated with differences in social behavior of female rhesus macaques during the breeding season, when normal hormonal cycles are attenuated by the treatment, but there is no indication that MPA-treated females bring an additional risk for more aggression during the male introduction and breeding season.
Keywords: aggression, animal management, breeding colony, contraception, sociosexual behavior
INTRODUCTION
Medroxyprogesterone acetate (MPA) is a synthetic progesterone that is commonly used as a contraceptive in females. MPA acts by suppressing the endogenous secretion of pituitary gonadotrophins (e.g. follicle stimulating hormone and luteinizing hormone) and ovarian steroid hormones, including estradiol and progesterone. Because of these inhibitory effects on the hypothalamic pituitary gonadal (HPG) axis, MPA prevents follicle maturation, ovulation and menstruation when given to women and female nonhuman primates.1,2 MPA is used in the management of captive nonhuman primates (NHPs) as a form of birth control to prevent conception, as well as a treatment for adverse reproductive clinical outcomes, such as endometriosis and abnormal uterine bleeding.3,4 The use of MPA to treat NHP females has the advantage of allowing these females, who otherwise may have been removed from their groups, to remain.3 While MPA has been recommended and used for decades to manage fertility and reproductive clinical conditions in socially housed, captive NHPs, much less is known about the impacts of MPA on social behaviors that are critical for the welfare, stability and management of NHP groups in captivity.5–7
MPA can impact female engagement in aggression and affiliative behaviors via the suppression of endogenous estradiol and progesterone levels that typically act to modulate these social behaviors.8,9 However, existing data across different NHP species have found equivocal effects of MPA treatment on these behaviors. For instance, MPA treatment increases aggression in captive, female stumptail (Macaca arctoides), Barbary (Macaca sylvanus), and pigtail (Macaca nemestrina) macaques, whereas it has no effects on aggressive behaviors in captive hamadryas baboons (Papio hamadryas).7,10–12 The effects of MPA on affiliative behaviors have also been equivocal, as MPA treatment of group-housed female hamadryas baboons and stumptail macaques does not impact grooming of males, but decreases male grooming in pair-housed stumptail macaques.7,13 Baboons have shown both reductions in affiliation and no differences in affiliation associated with MPA treatment.7,14 MPA treatment in females attenuates male approaches in stumptail macaques, as well as decreases proceptive (e.g. solicitations) and sexual behavior received from males.7,10,12,13,15 Taken together, existing data indicate that MPA may impact agonistic and affiliative behaviors in a manner that is dependent on the social context in which the animals are housed and on the NHP species being studied.
Rhesus macaques (Macaca mulatta) are commonly held and bred for biomedical research, but MPA effects on female rhesus aggression and affiliation remain unknown. This is a critical gap in knowledge, as rhesus macaques are considered to be the most despotic of the macaque species, showing frequent aggression in both wild and captive settings as a means of reinforcing dominance relationships between group members that function to maintain group stability.16,17 While it is natural for rhesus to use aggression during their social interactions, frequent and/or severe aggression can lead to serious injuries, negatively impacting animal welfare.17 Furthermore, social group instability can sometimes lead to matrilineal overthrows (in which related females fight and reverse rank with another group of related females) that can result in severe physical trauma and, in some cases, the death of members of the deposed matrilines.18,19 Rhesus aggression and wounding is more frequent and more intense in the breeding season with increases in competition for access to breeding partners.20,21 Coincident with breeding season is the introduction of new males to female social groups in some years, because females are more receptive to the presence of the unfamiliar males at that time.22,23 Therefore, understanding the impacts of MPA treatment on female rhesus social behavior, particularly during introductions of males during the breeding season, is important for maintaining the stability of matrilines in large captive breeding groups, and for mitigating rates of aggression and wounding, which are common concerns among those managing breeding colonies of rhesus macaques.24,25 The purpose of the current study was to evaluate the behavioral effects of MPA treatment on female rhesus macaques and to determine whether these effects are dependent on the breeding season when the HPG axis is not suppressed.26–28 We hypothesized that MPA-treated females would show greater aggression and less affiliation compared to matched control females, but only during the breeding season when MPA-treated females have suppressed ovarian hormones compared to control females. We also hypothesized that MPA-treated females would engage in and receive less sexual behavior than control females during the breeding season.
MATERIALS AND METHODS
Humane care guidelines
The Yerkes National Primate Research Center (YNPRC) facility and its programs are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Procedures involving animal subjects were approved by the Emory University Institutional Animal Care and Use Committee (IACUC) and were conducted in accordance with the United States Department of Agriculture (USDA) Animal Welfare Regulations, The Guide for the Care and Use of Laboratory Animals (2011), and institutional policies. Subjects had continuous access to fresh drinking water and unrestricted access to food. Routine enrichment provided to all monkeys included fresh produce, climbing and play structures, foraging devices with a variety of foods, and manipulanda.
Study location and subjects
The study was conducted between March 2017 and January 2020 at the YNPRC Field Station in Lawrenceville, Georgia. The rhesus macaque breeding colony at YNPRC is primarily housed in 242.8–1537.8 m2 outdoor, open air compounds with indoor, heated housing spaces, and are maintained in large breeding groups (18 to 170 animals), comprised of multiple, multigenerational matrilines of adult females, a smaller number of adult males, and offspring.
Subjects were 24 adult female rhesus macaques (M age=15.6 yrs; Age range= 8–22 yrs) belonging to five large social groups. Each social group had 14 to 39 breeding age females and their offspring, and one to seven adult males. Study subjects were uniquely dyemarked and tattooed for identification by observers. At the YNPRC, breeder male introductions occur every few years to prevent inbreeding between young females and their fathers as the females mature.29 Male introductions are conducted during the breeding season when females are more receptive to the presence of the unfamiliar males.22 Each of our study groups were scheduled to undergo a male introduction during the breeding season we observed.
New adult male cohorts were introduced to each of the female social groups during the breeding seasons included in the current study (September – January). Since breeding season and the introduction of new males occurred during the same time period, we will refer to this factor as the “male breeding season introduction period.” Data collected during the non-breeding seasons included resident males that were fully integrated into the group.
Subject selection and treatment with MPA
Twelve of the 24 subjects received MPA treatment as their birth control strategy about every three months, and had been receiving it for at least five months prior to study onset. Treatment with MPA was determined by veterinarians at the YNPRC based on best clinical practice. The reasons for the 12 subjects undergoing treatment with MPA in this study included: insufficient lactation to support infant (2), reproductive complications (1 cervical abnormality, 1 vaginal prolapse, 4 retained products of conception), and underlying chronic inflammatory conditions (4 inflammatory bowel disease). Due to a simultaneous study involving the same subjects, observers were not blind to MPA treatments, but they were not aware that we were going to assess MPA, so bias during the data collection is unlikely. The procedure for MPA treatment was administered by members of YNPRC Colony Management Staff by removing each individual subject from her social group and placing her into a cage within the indoor housing unit. Each subject was given 200 mg of MPA by intramuscular injection if she weighed less than 10 kg, or 300 mg if she weighed more than 10 kg. Subjects were then released back into their social groups. The entire procedure lasted about 20 minutes.
The MPA-treated subjects’ weights, ages, and relative ranks were used to select 12 females to serve as matched-case controls for statistical comparison. Archival weight data were assessed to calculate a mean weight for each subject during the period of time observational data were collected. These weights were categorized using a histogram to identify natural breaks in the data: 6.37–9.39 kg; 9.4–12.39 kg; 12.4–15 kg. Ages of the treatment subjects were also categorized: 8–13 yrs, 14–19 yrs, 20–23 yrs. Social rank category was defined as relative rank within an individual’s social group based on a status signaling and dominance interactions matrix assessment.30,31 ‘High’ ranking subjects were ranked within the top one-third of their social group, ‘Low’ ranking within the lower third, and the remainder were considered ‘Middle’ ranking. The control subjects were considered a match for treatment subjects if they fell into the same category for at least two of the three variables; of the 14 matched pairs, five pairs disagreed on one category.
Behavioral data collection and summary
Behavioral data were collected over the four-year period of study. Aggressive, affiliative, and sexual behaviors were collected on each subject simultaneously (see Table 1 for the ethogram). Four observers were involved in the collection of these data over the course of the study. Inter-rater reliability was tested before any data were collected. During initial training, all observers achieved a reliability score of 100% agreement in animal identification and 95% agreement on identifying behaviors. Reliabilities were calculated for each individual behavior during data collection sessions, using Krippendorff’s alpha and ranged between 0.85–1.00 across all observers.32
TABLE 1.
Descriptive ethogram of the observed behaviors
| Behavior | Definition |
|---|---|
| Aggression | |
| Open mouth stare | Unwavering gaze with widening of the eyes and mouth open |
| Head bob | Abrupt lowering of the head while staring at another individual |
| Brow flash | Staring with exaggerated raising and release of the eyebrows |
| Ear flaps | Staring while quickly pulling the ears forward and then releasing them |
| Lunge | Jump quickly at another individual |
| Slap | Hitting or striking another individual, contact is brief |
| Push | Shoving another individual, with hands, away from their present location. Contact is brief. |
| Chase | Running threateningly after the recipient |
| Wrestle/Grapple | Prolonged physical contact with another individual where one animal attempts to restrain or restrict the other’s movement |
| Pin | Holding an animal to the ground for at least 3 seconds |
| Bite | Physical contact involving the teeth |
| Affiliation | |
| Groom | Picking, scraping, spreading, mouth picking, or licking of a monkey’s hair or skin for at least 5 seconds |
| Huddle | Motionless contact between two individuals for at least 5 seconds; does not count if tails are the only body part touching |
| Proximity | Remaining within arm’s length of another individual without touching for at least 5 seconds |
| Sexual | |
| Mount | A male monkey grabs the hind legs of another monkey with his own feet and places his hands on the lower back of the recipient, thus hoisting himself off the ground; must include deliberate thrusting |
| Solicitation | Can be displayed as head bobs, hand slaps, jaw thrusts, lipsmacking, crouching, enlisting, presenting, or otherwise flirting |
| Hip Touch | A male is seen to touch the hips of the subject in as if to position themselves for a mount. This may or may not be done in response to a present and may or may not be followed by a mount |
Aggression and affiliation data were collected in both the non-breeding season (March – October) (M=154.5 hours per individual, Min= 96.5 hours) and the breeding season (September– January) (M= 40.6 hours per individual, Min=6 hours). Sexual behaviors were only collected during the breeding season (M=40.6 hours per individual, Min=6) as many of the included behaviors are only expressed during this time of year in rhesus macaques.
Aggressive behaviors were recorded by one observer using all occurrences, event-based sampling for adults in the group. Aggressive behaviors ranged from minor (e.g. vocal threats, brow flashes) to severe attacks (e.g. bites, pinning) and were included in the analysis only if our subjects were involved in the initial stage of the conflict with a single other individual (dyadic conflicts). Affiliation data (grooming and huddling/proximity) were collected by a second observer using a scan-sampling method with a 20-minute intersample interval. Records of huddling and proximity (See Table 1) were combined for this analysis. Sexual behavior data were collected by a third observer via all occurrences, event-based sampling.
Hourly rates were generated for each individual for the categories of aggressive behavior and sexual behavior by adding hourly rates of the individual behaviors subsumed within each category, and dividing by hours observed. These were calculated separately for the breeding and non-breeding seasons. Since affiliative (grooming and huddling/proximity) data were collected with a point sampling technique, the percentage of the total data points possible in which each subject could have been recorded expressing each affiliative behavior was generated. These were calculated separately for the breeding and non-breeding seasons.
Statistical analyses
Data were summarized as mean ± standard error (SEM) and a p≤0.05 was considered significant. Analysis of variance (ANOVAs) were used to assess for differences in affiliative and aggressive behaviors due to treatment (control vs. MPA), season (breeding vs. non-breeding), and their interaction. ANOVAs were also used to assess for differences in sexual behaviors due to treatment (control vs. MPA) during the breeding season.
RESULTS
Effects of MPA treatment and season on aggression
The rate of aggression directed towards other females was not influenced by MPA treatment (F(1,22)=0.62, p=0.44), but was influenced by season (F(1,22)=17.2, p<0.001);lower rates of aggression were initiated towards others in the breeding compared to the non-breeding season. There was a significant interaction of MPA treatment and season (F(1,22)=16.7, p<0.001). MPA-treated females showed significantly less aggression towards other females compared to control females during the breeding season (p=0.014; Figure 1A). The rate of female aggression toward males was only influenced by a main effect of season (F(1,22)=35.7, p<0.001), as aggression towards males was significantly higher in the breeding season compared to the non-breeding season (Figure 1B). There were no effects of MPA treatment (F(1,22)=0.89, p=0.36), nor a season by treatment interaction on aggression towards males (F(1,22)=1.07, p<0.31).
FIGURE 1:
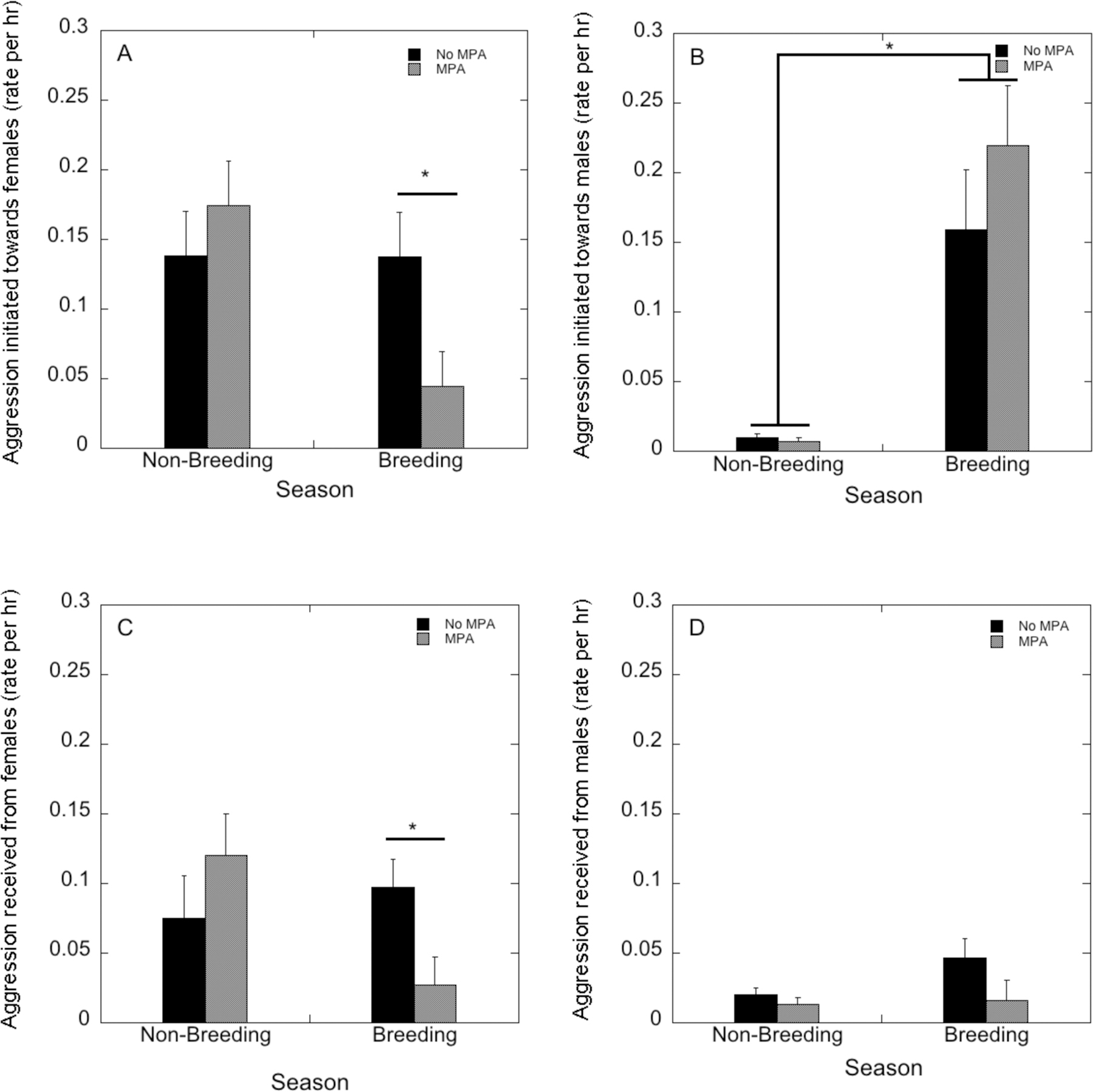
Rates of aggressive behaviors initiated towards female (A) and male (B) groupmates, and rates of aggressive behaviors received from female (C) and male (D) groupmates by medroxyprogesterone acetate (MPA)-treated and control females in the non-breeding and male breeding season introduction periods.
The rate of aggression received from other females was influenced by season (F(1,22)=4.22, p=0.052) with less received in the breeding season, but not by MPA treatment (F(1,22)=0.16, p=0.69), and there was a significant season-by-MPA treatment interaction (F(1,22)=11.3, p=0.003). In the breeding season, MPA-treated females received significantly less aggression from other females compared to controls (p=0.021; Figure 1C). The rates of aggression received from males were not influenced by season (F(1,22)=1.74, p=0.20), MPA treatment (F(1,22)=3.43, p=0.08), or their interaction (F(1,22)=1.04, p=0.32; Figure 1D).
Effects of MPA treatment and season on affiliative behavior
Rates of grooming other females was not influenced by MPA treatment (F(1,22)=0.41, p=0.53), but was influenced by season (F(1,22)=7.35, p=0.01), as rates were significantly lower in the breeding season than during the non-breeding season (Figure 2A). There was no significant interaction of MPA treatment and season (F(1,22)=3.59, p=0.07). Rates of grooming males were influenced by season (F(1,22)=7.56, p=0.012), with more grooming of males in the breeding season than during the non-breeding season. There was also an effect of treatment on grooming of males (F(1,22)=8.88, p=0.007), as the control females groomed males more than MPA-treated females did. Finally, there was a significant interaction between treatment and season (F(1,22)=12.14, p=0.002), such that control females directed more grooming to males during the breeding season than MPA-treated females (p=0.003; Figure 2B).
FIGURE 2:
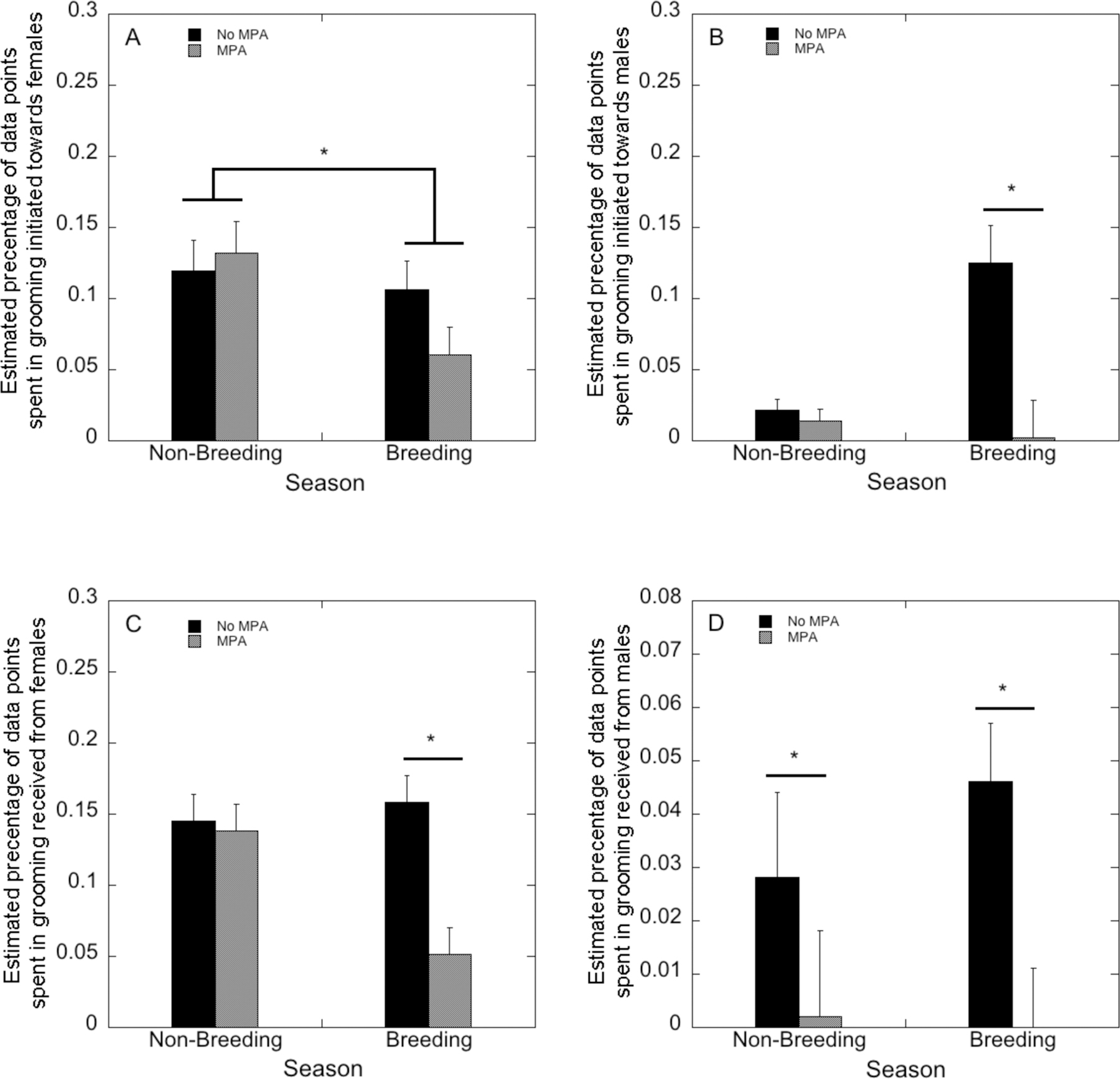
Estimated percentage of data points spent grooming female (A) and male (B) groupmates, and estimated percentage of data points spent receiving grooms from female (C) and male (D) groupmates by MPA-treated and control females in the non-breeding and male breeding season introduction periods.
Estimated rates of grooming received from other females was influenced by season (F(1,22)=8.34, p=0.008), with less grooming received during the breeding season, and MPA treatment (F(1,22)=5.63, p=0.027) with less grooming received by MPA-treated females. There was also a significant season-by-MPA interaction (F(1,22)=15.44, p=0.001). In the breeding season, MPA-treated females received significantly less grooming from other females than did the control females (Figure 2C). The rates of grooming received from males were not influenced by season (F(1,22)=.61, p=0.44), however there was an effect of MPA treatment (F(1,22)=4.76, p=0.04). MPA-treated females received significantly less grooming from males across both the non-breeding and breeding seasons (Figure 2D). There was no significant interaction of the season and treatment on grooming received from males (F(1,22)=.91, p=0.35).
Estimated rates of huddling/proximity were influenced by MPA treatment (F(1,22)=5.89, p=0.024), as the MPA-treated females displayed less huddling/proximity than controls. A seasonal effect was also measured (F(1,22)=8.70, p=0.007) with elevated levels in the breeding season. However, there was a significant interaction of MPA treatment and season (F(1,22)=6.59, p=0.018; Figure 3) such that huddle/proximity behaviors were observed more in control females than in MPA-treated females during the breeding season.
FIGURE 3:
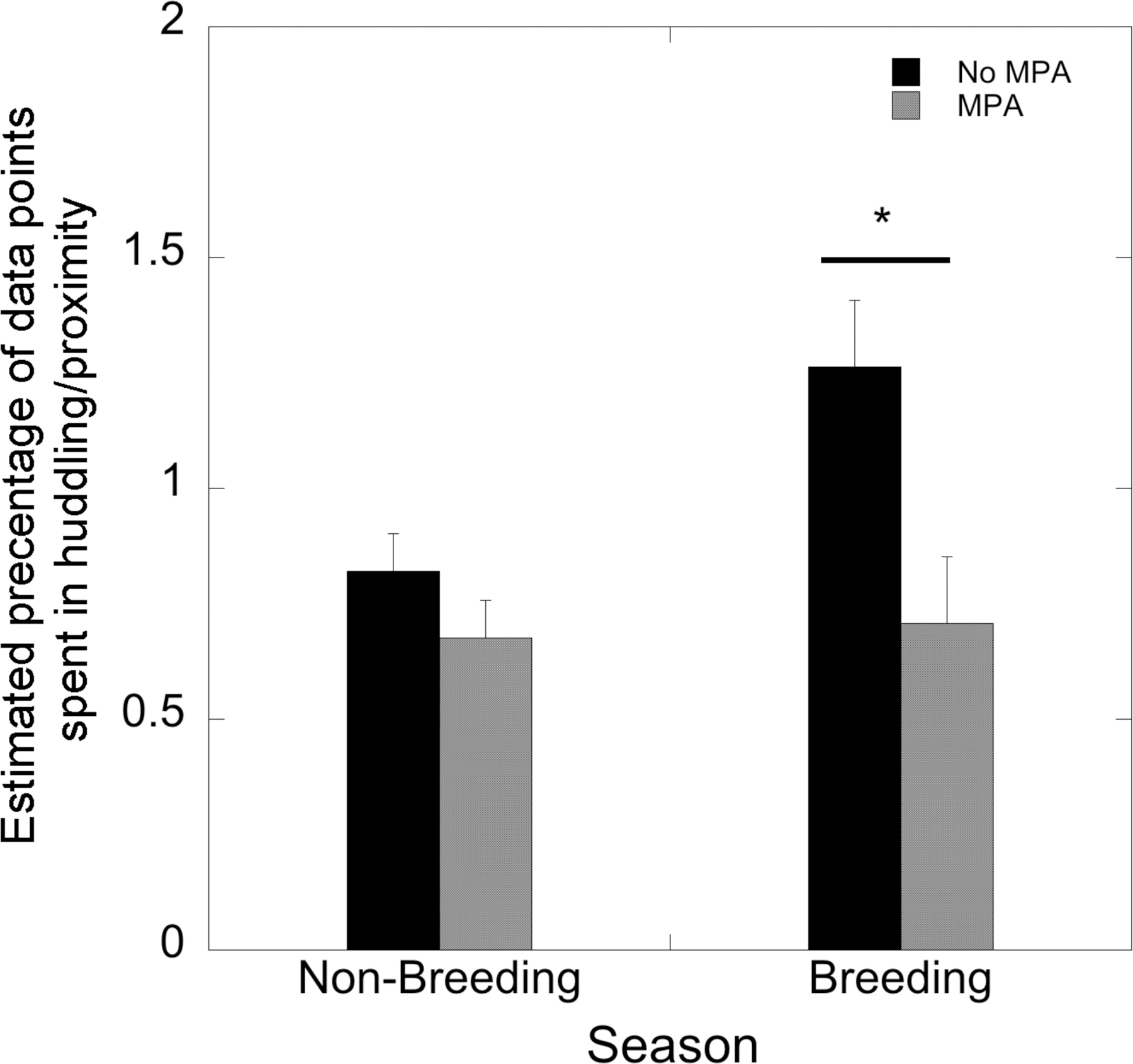
Estimated percentage of data points spent in proximity/huddling for MPA-treated and control females in the non-breeding and male breeding season introduction periods.
Effects of MPA treatment on sexual behaviors during the breeding season
Males mounted (F1,22=12.95, p=0.002) and hip-touched (F1,22=9.77, p=0.005) control females significantly more than they did MPA-treated females (Figure 4A). Additionally, MPA-treated females solicited males (F1,22=12.23, p=0.002; Figure 4B) and presented their rumps at a significantly lower rates (F1,22=12.701, p=0.001; Figure 4B) than control females. However, there was no significant difference in the rate at which MPA-treated females received solicitations from males compared to control females (F1,22=2.18, p=0.154; Figure 4B).
FIGURE 4:

Rates of sexual behaviors received (A) and emitted (B) by MPA-treated and control females in the male breeding season introduction periods.
DISCUSSION
In the current matched case-control study, we assessed whether MPA treatment is associated with differences in the social behavior of group housed, female rhesus macaque in a manner that is dependent upon male breeding season introduction. Our results showed that MPA treatment was associated with decreased aggression emitted towards and received from female groupmates during the breeding season, but was not associated with differences in rates of aggression towards and received from male groupmates. Our results also showed that MPA treatment was associated with decreased engagement in affiliation, including grooming, proximity and huddling, and sexual behaviors during the breeding season. Overall, these results suggest that MPA treatment in rhesus females does not adversely influence aggressive behaviors in a manner that may compromise social group stability and animal welfare in capture rhesus monkeys.
Contrary to our prediction that MPA treatment would be associated with heightened levels of aggression, we found that MPA treatment had main effect on aggression emitted in female rhesus macaque subjects. The MPA-treated females did not direct or receive more aggression in interactions with males or females as compared to the control females in the current study. Our findings regarding aggressive behavior contradict those in stumptail and pigtail macaques but are generally consistent with findings in hamadryas baboons in which there were no changes in female/female aggression.7,10,12 More specifically, MPA-treated stumptail females increase their agonistic behavior directed toward subadults, juveniles and infants who were ranked below them, but decrease their threats towards at higher ranking group members.10 However, MPA-treated pigtail macaque females show a large increase in the rate of aggression in which they were involved.12 While the differences in MPA effects on aggression between species may be due to species differences, other contextual factors, including composition of social groups and timing of studies during the breeding season or new male introduction may also important factor to considering when assessing the effects of MPA on behavior. This notion is supported by our finding that the effects of MPA on aggression were dependent on the breeding season, a time when endogenous levels of estradiol and progesterone that typically act to modulate impact aggression are suppressed by MPA administration.8,9 Indeed, MPA-treated females showed significantly less aggression toward other females and received less aggression from females compared to control females during the breeding season, but not during the non-breeding season. These findings suggest that MPA treatment in rhesus females for clinical and contraceptive purposes will not affect female involvement in group aggression during the introduction of unfamiliar adult males during the breeding season. In fact, the decrease in aggressive interactions upon MPA treatment during would likely contribute to greater group stability and success of new male introduction.
Similarly, our results showed the ability for MPA to influence affiliative behavior was dependent on the breeding season when levels of estradiol and progesterone act to facilitate affiliative behavior,8,9 as MPA-treated rhesus females spent significantly less time in proximity to other monkeys during the breeding season when males were introduced. MPA-treated females also groomed males less than the non-treated controls in the breeding season, although their grooming of other females was similar to that of the controls. Furthermore, MPA-treated females received less grooming by males and by females than the controls, and directed less grooming toward males during the male breeding season introduction period than during the non-breeding season. Thus, MPA impacted affiliation in rhesus females more so than that reported for female stumptail macaques or for hamadryas baboons. In the case of the baboons this difference may also be related to the difference in social organization that favor the importance of adult female relationships with the one adult male in their groups.7,10 Overall, MPA-treated female rhesus macaques were less socially engaged with female and male group members in comparison to control females, who showed an increases in huddle/proximity behaviors during the breeding season, consistent with past research.14
The ability of MPA to attenuate engagement in affiliation behaviors in the current study extended to sexual behavior during the breeding season, as MPA-treated females in the current study solicited and presented to males at lower rates than untreated females. This MPA associated decrease in proceptive behavior has been previously described in female pigtail macaques and linked to inhibition of the HPG axis and reductions in circulating estradiol concentrations associated with MPA treatment.2,12 However, studies in stumptail macaques have previously shown no effect of MPA on female proceptive and affiliative behavior towards males, and have suggested that MPA-treatment reduces female sexual attractiveness to males.13 Indeed, MPA’s ability to suppress typical alterations in genital swellings that signal reproductive status cues to conspecifics and are dependent upon the HPG axis coming online during the breeding season.14,36,37 The current data show that MPA-treatment impacts male behavior towards females similar what has been described in hamadryas baboons, stumptail macaques and pigtail macaques, 7,10,12 as male macaques groomed, hip-touched, and mounted the MPA-treated females at lower rates than control females. However, male sexual solicitation was not associated with MPA-treatment, possibly because these male solicitations typically occur at a distance and out of olfactory range of the females, as has been described in ring-tailed lemur (Lemur catta) males that spend time closer to non-MPA-treated females’ scents.35
Overall, our findings showing that MPA-treated females showed lower rates of aggressive affiliative and sexual behavior compared to matched controls have significant implications for the use of MPA in captive, socially housed rhesus monkeys, who are the most despotic of captive macaque species, are seasonal breeders, and undergo frequent introductions of novel males to female groups during the breeding season when females are most receptive to males.8 While we did find expected increases in aggression towards males during the breeding season male introduction period for all females, MPA-treated was associated with less aggression emitted towards other females during the breeding season and did not impact the amount of aggression received from males or females.20,21 These findings indicate that MPA treatment in captive macaques groups does not contribute to group instability, which has previously been shown to be impacted by increased female aggression,41,42 and may actually improve group stability, as MPA treatment is associated with less affiliation with males. Furthermore, our findings that MPA treatment of rhesus females did not have major effects on aggressive behavior informs the use of MPA to retain socially-central females in groups who would otherwise be removed for clinical reasons and whose removal could cause extreme social upheaval.43
In conclusion, the current findings indicate that behavioral changes associated with MPA treatment of female rhesus macaques do not significantly influence social behaviors that are central to group stability, providing more support for the use MPA on in the management of rhesus breeding.7 Limitations of the current study include its between-subjects design, the use of scan based observations for affiliative behavior, the lack of observer blinding to MPA-treatment status, and the confounding of breeding season with the introduction of new males. Future within-subjects studies of MPA administration in captive, female rhesus monkeys are necessary to determine the causal impacts of MPA on these behaviors across the breeding season and in the presence of familiar versus new males. Future studies are also necessary to better understand how MPA treatment in females influences genital swellings that may change sociosexual behavior,38–40 as well how MPA influences overall group dynamics in captive macaque groups and other nonhuman primate species.33
ACKNOWLEGEMENTS
We appreciate all Colony Management, Veterinary, and Animal Care staff members of the YNPRC for their hard work throughout this project. In particular we would like to thank our behavioral observers Shannon Moss, and Caroline Long. Thank you to YNPRC veterinarian Devon Owens for her input and clarity on MPA use within our study groups. This project and the costs associated were funded by the National Institutes of Health Office of Research Infrastructure Programs, Office of the Director, grant R24OD020349 awarded to the YNPRC. Additionally, we acknowledge support from the NIH Office of Research Infrastructure Programs, Office of the Director, grant P51OD011132 to the YNPRC.
Footnotes
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal have been adhered to. The authors received approval by the Institutional Animal Care and Use Committee. The US National Research Council’s guidelines for the Care and Use of Laboratory Animals were followed.
REFERENCES
- 1.Ortiz A, Hiroi M, Stanczyk FZ, Goebelsmann U, & Mishell DR (1977). Serum medroxyprogesterone acetate (MPA) concentrations and ovarian function following intramuscular injection of depo-MPA. J Clin Endocrinol Metab 44(1), 32–39. doi: 10.1210/jcem-44-1-32 [DOI] [PubMed] [Google Scholar]
- 2.Lan PT, Aedo AR, Landgren BM, Johannisson E, Diczfalusy E. Return of ovulation following a single injection of depo-medroxyprogesterone acetate: A pharmacokinetic and pharmacodynamic study. Contraception. 1984;29(1):1–18. doi: 10.1016/0010-7824(84)90054-4 [DOI] [PubMed] [Google Scholar]
- 3.Wallace P, Asa C, Agnew M, Cheyne S. A review of population control methods in captive-housed primates. Anim Welfare. 2016;25(1):7–20. doi: 10.7120/09627286.25.1.007 [DOI] [Google Scholar]
- 4.Cruzen CL, Baum ST, Colman RJ. Glucoregulatory function in adult rhesus macaques (Macaca mulatta) undergoing treatment with medroxyprogesterone acetate for endometriosis. J Am Assoc Lab Anim Sci. 2011;50(6):921–925. [PMC free article] [PubMed] [Google Scholar]
- 5.Dragoman MV, Gaffield ME. The safety of subcutaneously administered depot medroxyprogesterone acetate (104 mg/0.65 mL): A systematic review. Contraception 2016;94(3):202–215. doi: 10.1016/j.contraception.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 6.Mora G, Johansson EDB. Plasma levels of medroxyprogesterone acetate (MPA), estradiol and progesterone in the rhesus monkey after intramuscular administration of depo-provera. Contraception 1976;14(3):343–350. doi: 10.1016/0010-7824(76)90101-3 [DOI] [PubMed] [Google Scholar]
- 7.Guy AJ, Schuerch FS, Heffernan S, Thomson PC, O’Brien JK, McGreevy PD. The effect of medroxyprogesterone acetate on behavioural responses of captive female hamadryas baboons (Papio hamadryas). Anim Reprod Sci 2008;108(3–4):412–424. doi: 10.1016/j.anireprosci.2007.09.008 [DOI] [PubMed] [Google Scholar]
- 8.Wallen K, Tannenbaum PL. Hormonal Modulation of Sexual Behavior and Affiliation in Rhesus Monkeys. Ann NY Acad Sci 1997;807(1 Integrative N):185–202. doi: 10.1111/j.1749-6632.1997.tb51920.x [DOI] [PubMed] [Google Scholar]
- 9.Michael RP, Zumpe D. A review of hormonal factors influencing the sexual and aggressive behavior of macaques. Am J Primatol 1993;30(3):213–241. doi: 10.1002/ajp.1350300306 [DOI] [PubMed] [Google Scholar]
- 10.Linn GS, Steklis HD. The effects of depo-medroxyprogesterone acetate (DMPA) on copulation-related and agonistic behaviors in an island colony of stumptail macaques (Macaca arctoides). Physiol Behav 1990;47(3):403–408. doi: 10.1016/0031-9384(90)90100-i [DOI] [PubMed] [Google Scholar]
- 11.Maijer AM, Semple S. Investigating potential Effects of the contraceptive Implanon on the behavior of free-ranging adult female barbary macaques. J Appl Anim Welf Sci 2015;19(1):16–23. doi: 10.1080/10888705.2015.1083432 [DOI] [PubMed] [Google Scholar]
- 12.Pazol K, Wilson ME, Wallen K. Medroxyprogesterone acetate antagonizes the effects of estrogen treatment on social and sexual behavior in female macaques. J Clin Endocrinol Metab 2004;89(6):2998–3006. doi: 10.1210/jc.2003-032086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steklis HD, Linn GS, Howard SM, Kling AS, Tiger L. Effects of medroxyprogesterone acetate on socio-sexual behavior of stumptail macaques. Physiol Behav 1982;28(3):535–544. doi: 10.1016/0031-9384(82)90152-4 [DOI] [PubMed] [Google Scholar]
- 14.Portugal MM, Asa CS. Effects of chronic melengestrol acetate contraceptive treatment on perineal tumescence, body weight, and sociosexual behavior of hamadryas baboons (Papio hamadryas). Zoo Biol. 1995;14(3):251–259. doi: 10.1002/zoo.1430140306 [DOI] [Google Scholar]
- 15.Michael RP, Saayman GS, Zumpe D. The suppression of mounting behaviour and ejaculation in male rhesus monkeys (Macaca mulatta) by administration of progesterone to their female partners. J Endocrinol 1968;41(3):421–431. doi: 10.1677/joe.0.0410421 [DOI] [PubMed] [Google Scholar]
- 16.Bernstein IS, Gordon TP. The function of aggression in primate societies. Am Sci 1974;62(3):304–311. [PubMed] [Google Scholar]
- 17.Bernstein IS, Gordon TP, Rose RM. Aggression and social controls in rhesus monkey (Macaca mulatta) groups revealed in group formation studies. Folia Primatol 1974;21(2):81–107. doi: 10.1159/000155607 [DOI] [PubMed] [Google Scholar]
- 18.Oates-O’Brien RS, Farver TB, Anderson-Vicino KC, McCowan B, Lerche NW. Predictors of matrilineal overthrows in large captive breeding groups of rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci. 2010;49(2):196–201. [PMC free article] [PubMed] [Google Scholar]
- 19.Dettmer AM, Woodward RA, Suomi SJ. Reproductive consequences of a matrilineal overthrow in rhesus monkeys. Am J Primatol 2014;77(3):346–352. doi: 10.1002/ajp.22350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stavisky RC, Ramsey JK, Meeker T, Stovall M, Crane MM. Trauma rates and patterns in specific pathogen free (SPF) rhesus macaque (Macaca mulatta) groups. Am J Primatol 2018;80(3). doi: 10.1002/ajp.22742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson AP, Boelkins R. Evidence for seasonal variation in aggressive behaviour by Macaca mulatta. Anim Behav 1970;18:719–724. doi: 10.1016/0003-3472(70)90017-5 [DOI] [PubMed] [Google Scholar]
- 22.Bernstein IS, Rose RM, Gordon TP. Behavioural and hormonal responses of male rhesus monkeys introduced to females in the breeding and non-breeding seasons. Anim Behav 1977;25:609–614. doi: 10.1016/0003-3472(77)90111-7 [DOI] [PubMed] [Google Scholar]
- 23.Lindburg DG. Rhesus monkeys: mating season mobility of adult males. Science 1969;166(3909):1176–1178. doi: 10.1126/science.166.3909.1176 [DOI] [PubMed] [Google Scholar]
- 24.Judge PG, de Waal FB, Paul KS, Gordon TP. Removal of a trauma-inflicting alpha matriline from a group of rhesus macaques to control severe wounding. Lab Anim Sci 1994;44(4):344–350. [PubMed] [Google Scholar]
- 25.McCowan B, Anderson K, Heagarty A, Cameron A. Utility of social network analysis for primate behavioral management and well-being. Appl Anim Behav Sci 2008;109(2–4):396–405. doi: 10.1016/j.applanim.2007.02.009 [DOI] [Google Scholar]
- 26.Drickamer LC. A ten-year summary of reproductive data for free-ranging Macaca mulatta. Folia Primatol 1974;21(1):61–80. doi: 10.1159/000155596 [DOI] [PubMed] [Google Scholar]
- 27.Ghosh D, Sengupta J. Patterns of ovulation, conception and pre-implantation embryo development during the breeding season in rhesus monkeys kept under semi-natural conditions. Acta Endocrinol (Copenh). 1992;127(2):168–173. doi: 10.1530/acta.0.1270168 [DOI] [PubMed] [Google Scholar]
- 28.Dunk RDP, Petto AJ, Mayer GC, Campbell BC. Seasonality of conceptions in captive rhesus macaques (Macaca mulatta). Int J Primatol 2015;36(4):855–870. doi: 10.1007/s10764-015-9858-9 [DOI] [Google Scholar]
- 29.Bailey KL, Young LA, Long CE, Remillard CM, Moss S, Meeker TL, Bloomsmith MA. Introducing the introduction enclosure: a novel approach to integrating multi-male cohorts into rhesus macaque (Macaca mulatta) female groups. J Am Assoc Lab Anim Sci In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michopoulos V, Reding KM, Wilson ME, Toufexis D. Social subordination impairs hypothalamic–pituitary–adrenal function in female rhesus monkeys. Horm Behav 2012;62(4):389–399. doi: 10.1016/j.yhbeh.2012.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernstein IS. Dominance, aggression and reproduction in primate societies. J Theor Biol 1976;60(2):459–472. doi: 10.1016/0022-5193(76)90072-2 [DOI] [PubMed] [Google Scholar]
- 32.Krippendorff K Computing Krippendorff’s alpha-reliability. University of Pennsylvania ScholarlyCommons; https://repository.upenn.edu/cgi/viewcontent.cgi?article=1043&context=asc_papers. Published January 25, 2011. Accessed July 9, 2020. [Google Scholar]
- 33.Huchard E, Cowlishaw G. Female–female aggression around mating: an extra cost of sociality in a multimale primate society. Behavl Ecol 2011;22(5):1003–1011. doi: 10.1093/beheco/arr083 [DOI] [Google Scholar]
- 34.Baum MJ, Everitt BJ, Herbert J, Keverne EB, Greef WJD. Reduction of sexual interaction in rhesus monkeys by a vaginal action of progesterone. Nature 1976;263(5578):606–608. doi: 10.1038/263606a0 [DOI] [PubMed] [Google Scholar]
- 35.Crawford JC, Boulet M, Drea CM. Smelling wrong: hormonal contraception in lemurs alters critical female odour cues. P Roy Soc B-Biol Sci 2010;278(1702):122–130. doi: 10.1098/rspb.2010.1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bourry O, Peignot P, Rouquet P. Contraception in the chimpanzee: 12-year experience at the CIRMF Primate Centre, Gabon. J Med Primatol 2005;34(1):25–34. doi: 10.1111/j.1600-0684.2004.00088.x [DOI] [PubMed] [Google Scholar]
- 37.Butler K, Ritter J, Ellis S, et al. Analysis of putative mucosal SHIV susceptibility factors during repeated DMPA treatments in pigtail macaques. J Med Primatol 2015;44(5):286–295. doi: 10.1111/jmp.12188 [DOI] [PubMed] [Google Scholar]
- 38.Wallis J Chimpanzee genital swelling and its role in the pattern of sociosexual behavior. American Journal of Primatology. 1992;28(2):101–113. doi: 10.1002/ajp.1350280203 [DOI] [PubMed] [Google Scholar]
- 39.Bettinger T, Cougar D, Lee DR, Lasley BL, Wallis J. Ovarian hormone concentrations and genital swelling patterns in female chimpanzees with Norplant implants. Zoo Biol 1997;16(3):209–223. doi: 10.1002/(sici)1098-2361(1997)16:33.0.co;2-e [DOI] [Google Scholar]
- 40.AZA Ape TAG. Chimpanzee (Pan troglodytes) care manual. Silver Spring: Association of Zoos and Aquariums; 2010. https://assets.speakcdn.com/assets/2332/chimpanzeecaremanual2010r.pdf [Google Scholar]
- 41.McCowan B, Beisner B, Hannibal D. Social management of laboratory rhesus macaques housed in large groups using a network approach: a review. Behav Process 2018;156:77–82. doi: 10.1016/j.beproc.2017.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCowan B, Beisner BA, Capitanio JP, et al. Network stability is a balancing act of personality, power, and conflict dynamics in rhesus macaque societies. PLoS ONE 2011;6(8). doi: 10.1371/journal.pone.0022350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beisner BA, Jackson ME, Cameron AN, McCowan B. Detecting instability in animal social networks: genetic fragmentation is associated with social instability in rhesus macaques. PLoS ONE 2011;6(1). doi: 10.1371/journal.pone.0016365 [DOI] [PMC free article] [PubMed] [Google Scholar]


