Abstract
Background: The effect of high-flow nasal therapy (HFNT) in individuals with an exacerbation of chronic obstructive pulmonary disease (COPD) and hypercapnia is not well studied. We assessed patient tolerance and impact of air-gas therapy delivered by humidified HFNT (20-35 L/min) on gas exchange in hypercapnic COPD patients during hospitalization for COPD exacerbation. We hypothesized that HFNT use would be safe and well tolerated in individuals hospitalized for COPD exacerbation regardless of the degree of hypercapnia.
Methods: Patients hospitalized for a COPD exacerbation were included if they were hypercapnic (arterial partial pressure of carbon dioxide [PaCO2] > 45 mmHg), ≥ 10 pack-year history, and agreed to treatment with HFNT, along with daily arterial blood gas (ABG) samples and bedside spirometry. They were placed on a HFNT system following admission for at least 3 days with an air-gas blend to maintain a flow rate between 20-35 L/min and fraction of inspired oxygen (FiO2) titrated to keep oxygen saturation (SaO2) values > 90%. Patient tolerance of HFNT and evidence of clinical deterioration as defined by worsening hypoxia or hypercapnia was the primary endpoint.
Results: Ten consecutive patients participated in the study. The patients had frequent prior exacerbations, were hypercapnic, dyspneic, and gas trapped. Participants received an air-gas flow rate (median [interquartile range (IQR)] 25 (IQR 20-30) L/min and FiO2 of 30 (IQR 30-30) %. There was no increase in PaCO2 levels (p = 0.26) or dyspnea (Borg scale, p= 0.52) while using HFNT. No patient discontinued HFNT, had further decompensation, required non-invasive ventilation or intubation during the study period.
Conclusion: In a pilot study, patients experiencing a severe COPD exacerbation were able to tolerate continuous HFNT safely regardless of degree of hypercapnia.
Keywords: copd, chronic obstructive pulmonary disease, high flow oxygen
Introduction
High-flow nasal therapy (HFNT) in acute hypoxic respiratory failure reduces respiratory rate and improves oxygenation.1 Frat et al showed that HFNT was noninferior to conventional oxygen therapy (COT) or non-invasive ventilation (NIV) in preventing intubation in patients with non-hypercapnic acute hypoxic respiratory failure.2 Patients have also reported HFNT to be more comfortable and reduce dyspnea greater than NIV or COT.3-5
HFNT reduces dyspnea and improves respiratory effort in patients with chronic obstructive pulmonary disease (COPD) through several proposed mechanisms of action.6 Washout of nasopharyngeal dead space allows for increased fraction of alveolar ventilation which could improve hypercapnia and hypoxia.7-11 The warmed humidified delivery of HFNT keeps upper airways from becoming desiccated and allows for easier mobilization of secretions.12 HFNT provides a dose titratable increase in end expiratory pressure to improve work of breathing.13 Additionally, HFNT has been shown to increase end expiratory lung volumes and decrease respiratory rate.11,14,15
Despite HFNT's proven value in improving survival in patients with acute hypoxemic respiratory failure, HFNT has not been shown to reduce hospitalization or mortality in patients with COPD. Small studies for brief periods have shown improvement in gas exchange, reduced work of breathing and respiratory symptom relief with HFNT in patients with COPD. Comparing outcomes between studies is difficult because of variability in HFNT device settings, disease severity, and HFNT adherence. The clinical parameters that should be used to select patients to receive HFNT versus NIV during a severe COPD exacerbation that requires hospitalization are not established. Therefore, the timing and process for initiating HFNT in patients with COPD remains unclear, especially in patients with a severe exacerbation that requires hospitalization. A potential concern is that more severely hypercapnic patients hospitalized with a COPD exacerbation may need ventilatory support with NIV rather than HFNT.
The safety and tolerability, based on hours of use and adverse effects, of HFNT in patients with stable obstructive lung disease has been studied in the past. Rea et al studied those with stable COPD and bronchiectasis and found HFNT use can decrease days of exacerbation and improve lung function when compared to usual care.16 However, HFNT daily use only averaged 1.6 hours and baseline hypercapnia was not assessed. In those with stable COPD and baseline hypercapnia, HFNT has been compared to long-term oxygen therapy and NIV, with improvements in gas exchange.17,18 In those studies, adverse effects were mild and average daily use ranged from 5-7 hours.
Herein we report the impact of continuous HFNT for 24 hours a day for 3 days in patients hospitalized with a COPD exacerbation, regardless of the extent of underlying hypercapnia, and its impact on patient tolerance and disease progression.
Materials and Methods
Patient Population
Consecutive patients were enrolled in the study if hospitalized with a primary diagnosis of COPD exacerbation. The study duration was 3 days. Additional criteria included age ≥ 40 years, ≥10 pack-year history of smoking, baseline hypercapnia defined as an arterial partial pressure of carbon dioxide (PaCO2) ≥ 45 mmHg, willingness to undergo daily arterial blood gas (ABG) draws and spirometry, and compliance with treatment with HFNT system. Diagnosis of COPD was based on patient self-report and review of physician past records. Patients were excluded if they were hemodynamically unstable, required urgent endotracheal intubation, if NIV was used, if there were self-directed care limitations in place, and if patients were unable to wear a HFNT device.
Data Collection
The study was approved by the local institutional review board. Baseline data were collected prospectively from each patient including age, gender, smoking history, and spirometry (according to American Thoracic Society/European Respiratory Society standard practice)19 and exacerbation history. Recent smoking was defined as smoking within 6 months of study initiation. Spirometry was performed as soon as possible after hospital admission and daily each morning for 3 days. Lung volumes were obtained from pulmonary function testing that was performed within 1 year prior to study initiation when available. COPD exacerbations that occurred in the year prior to study enrollment were recorded and classified as moderate if corticosteroids and/or antibiotics were prescribed, severe if the patient was hospitalized or visited the emergency department, and very severe if the patient required non-invasive or invasive ventilation. Vital signs, ABG, spirometry (pre- and post-bronchodilator), Borg questionnaire, modified Medical Research Council score (mMRC), and accessory respiratory muscle use was tracked daily.20,21 Comorbid conditions, pulmonary function testing and hospitalizations for COPD exacerbation in preceding 12 months were obtained from review of electronic medical records. Patients were seen daily by study coordinators and response to COPD treatment, need for escalation of therapy or transfer to the intensive care unit (ICU) was monitored. The study length was 3 days. Patients remained hospitalized after study termination if the primary medical team felt further care was warranted and length of hospital stay was recorded. The study was not an efficacy trial, but a pilot study and was powered based on the ability to detect safety issues or unforeseen problems. The sample size was estimated based on the method described by Viechtbauer et al.22 The safety issue identified was the need to intensify therapy because HFNT with oxygen did not achieve the targeted oxygen saturation of ≥ 90% resulting in the need to utilize more invasive treatments. Using a 95% confidence threshold and 30% probability that an event (need to intensify treatment) will occur, the sample size required is 8.3 participants, which was increased to 10 to accommodate dropouts.
Application of High-Flow Nasal Therapy
Patients were placed on HFNT with an AIRVO-2 heated humidifier and Optiflow+ cannula provided by Fisher and Paykel Healthcare (Auckland, New Zealand). The initial air-gas flow was 35 liters per minute (L/min) and titrated to patient comfort by a respiratory therapist. The FiO2 was titrated to keep oxygen saturation (SpO2) ≥ 90%. Humidified gas temperature was set between 34o-37oC. After initial titration, the patient would remain on HFNT for the duration of the study.
Data Analysis
After screening, all study-related procedures were completed during the initial 3 days of hospitalization and patients were followed until hospital discharge. Baseline ABG, spirometry and levels of dyspnea were compared over time. Numeric data are reported as mean and standard deviation (SD) or median and interquartile range (IQR) if significantly skewed. Continuous data are compared from baseline to day 3 using the Student’s t-test analysis with results displayed as p values.
Results
Patient Population
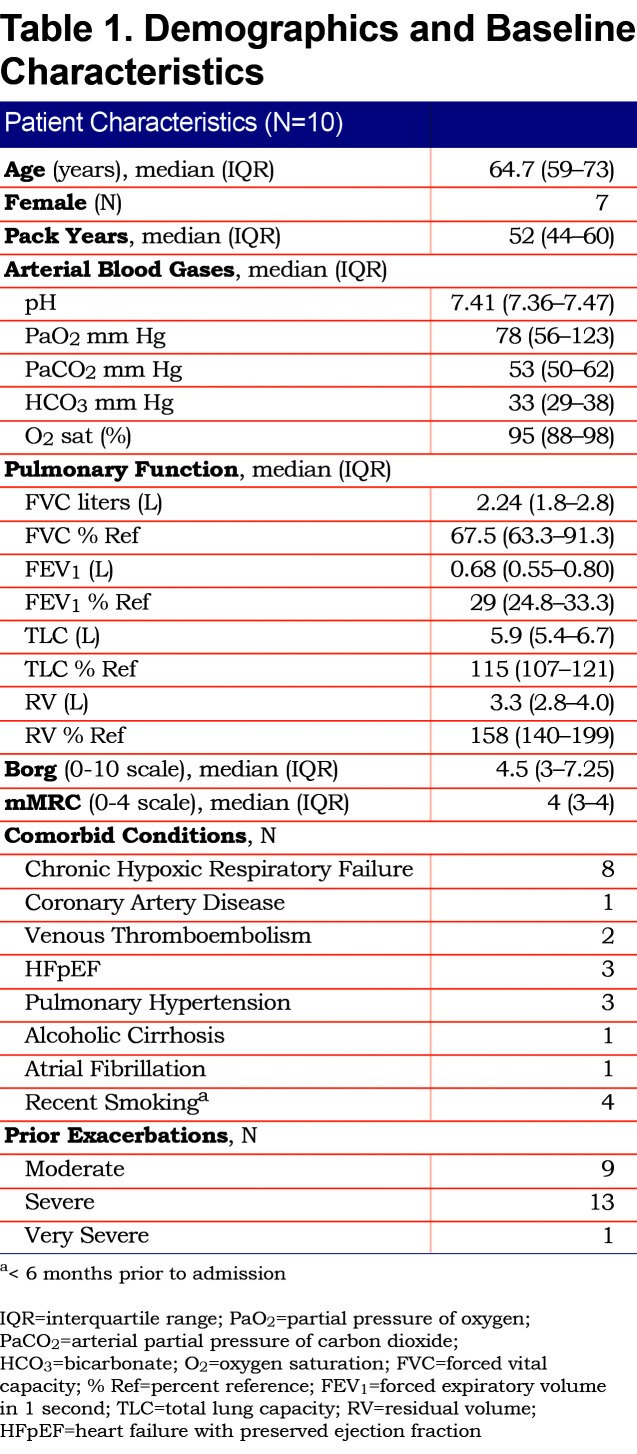
Ten patients were enrolled in the study. Demographic and baseline characteristics including comorbid conditions are listed in Table 1. The patients had a median of 2 exacerbations in the 12 months prior to study enrollment. All patients were markedly hypercapnic, severely dyspneic, had Global Initiative for Chronic Obstructive Lung Disease23 stage 3 or 4 airflow limitation and severe gas trapping. Eight had chronic hypoxic respiratory failure and were prescribed supplementary oxygen therapy at home.
Impact of High-Flow Nasal Therapy on Gas Exchange, Dyspnea and Lung Function
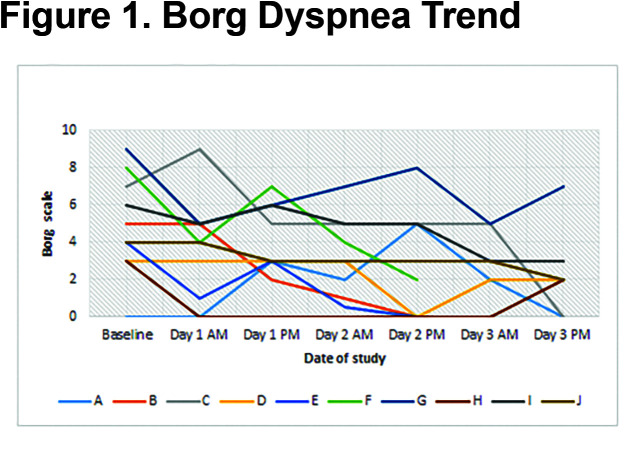
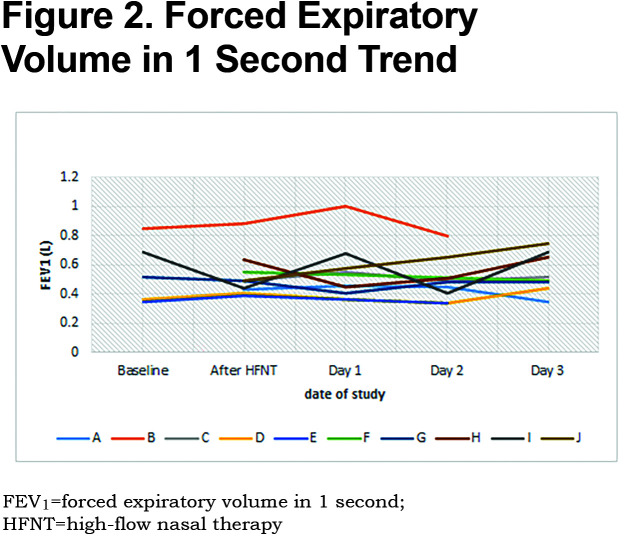
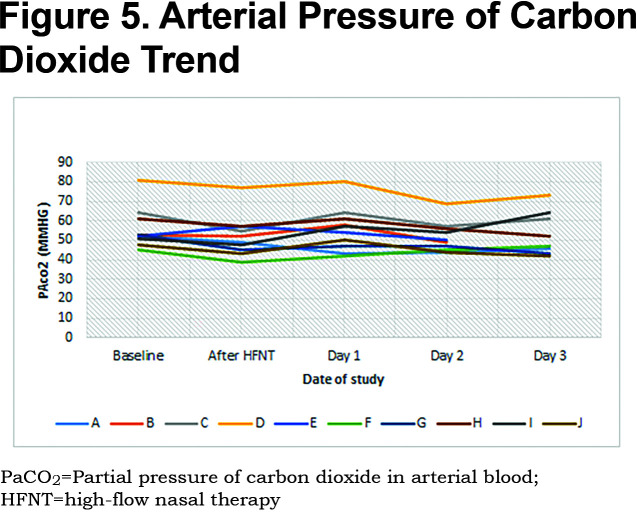
Patients received a median humidified air-gas flow of 25 (IQR 20-30) L/min and FiO2 of 30 (IQR 30-30) %. There were no statistically significant differences between baseline and Day 3 of the study with regards to pH (p = 0.54) and arterial partial pressure of oxygen (PaO2) (p = 0.42) (Figures 1, 2, 3, 4, 5). There were no statistically significant differences between the baseline spirometry values of forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC), (p = 0.71 and p = 0.82, respectively). The PaCO2 and the patient reported dyspnea did not increase during study period. Of note, in 3 patients with markedly elevated PaCO2 levels at baseline (ranging from 61 to 81 mmHg), the PaCO2 levels decreased.
Seven of the patients had accessory respiratory muscle use at study initiation. Five of those patients had no accessory muscle use by the end of study Day 1. One patient had no accessory muscle use by end of study Day 2 and another by study Day 3 three. The length of hospital stay (LOS) for the patients was a mean of 7.2 days (standard deviation ± 2.49). All patients continued to receive HFNT for the duration of their hospitalizations.
Tolerability of High-Flow Nasal Therapy
Nine of the patients were able to tolerate a temperature range of 34o-37oC. One patient desired the temperature to be reduced to 32oC but continued using the HFNT apparatus. Initial titration of air-gas blend flow rate was between 35 and 20 L/min and FiO2 ranged from 50%-28%. No individuals prematurely stopped the study due to intolerance of the HFNT system, required intubation or placement on non-invasive mechanical ventilation.
Discussion
The main findings of the study demonstrated that HFNT was safe and well tolerated in patients hospitalized with a COPD exacerbation and hypercapnic respiratory failure. This study adds to the literature by its prospective nature and inclusion of decompensated hypercapnic COPD patients, with significantly impaired pulmonary mechanics, severe baseline dyspnea, and frequent exacerbations.
Studies have shown that HFNT can be used in acute hypercapnic failure and will result in a significant decrease24,25 in PaCO2. However, these patients were not followed prospectively, nor did they all have COPD. Short term studies performed in emergency departments have shown the effectiveness of HFNT in acute dyspnea and hypoxic respiratory failure.4,25,26 These studies did not prospectively follow patients and patients had heterogeneous lung pathology. A single center study by Braunlich et al did examine HFNT in individuals with COPD exacerbation and found improvement in hypercapnia. However, that study was retrospective, and some participants had been placed on NIV during the admission prior to enrollment in study.27 Additionally, a single center study by Pilcher et al used HFNT versus COT to assess improvement in transcutaneous carbon dioxide tension in patients admitted with COPD exacerbation.28 However, patients were only placed on HFNT for 30 minutes, so assessment of tolerability and impact on treatment failure would be difficult to ascertain.
The mean PaCO2 for patients was 56 mmHg and ranged from a low of 45 mmHg to a high of 81 mmHg. While there was a broad range of hypercapnia among the patients, they were able to equally tolerate the therapy. Regardless of degree of hypercapnia, there were no trends to worsening of subjective dyspnea, gas exchange, accessory respiratory muscle use, need for escalation of COPD therapy, and need for invasive/non-invasive ventilation. While PaCO2 did not significantly change in this study, other studies have shown significant reduction in participants with higher baseline PaCO2 (>55 mmHg).11,25
The patients’ use of HFNT was monitored by hospital nurses and respiratory therapists as well as research staff members. HFNT was not stopped after study initiation by any of the patients. HFNT was used on a continuous basis in a non-ICU setting during the entire study period (approximately 72 hours). Other studies have applied HFNT on a continuous basis only in an ICU setting.24 Finally, the LOS for the patients in the study was similar to prior studies reporting LOS for COPD exacerbation in patients with severe airflow obstruction.29
Regarding the limitations of this study, it was a small, single center pilot study. The sample size was chosen to estimate the chance of a significant adverse effect (i.e., transfer to ICU or failure of COPD therapy). However, this was not powered with the purpose of finding clinical significance. Additionally, there was no matched cohort to compare HFNT to continuous oxygen therapy.
Studies involving the clinical effects of HFNT on sleep architecture, impact on rate of COPD exacerbation and hospitalizations, long term effects on gas exchange, exercise tolerance, and mortality are needed. Additionally, optimal patient selection criteria with regard to degree of baseline hypoxic and hypercapnic respiratory failure, extent of underlying airflow obstruction, and subjective dyspnea require further investigation.
In conclusion, patients hospitalized with COPD exacerbation, regardless of degree of hypercapnic respiratory failure, can safely tolerate continuous HFNT and not suffer from decompensation.
Abbreviations
high-flow nasal therapy, HFNT; chronic obstructive pulmonary disease, COPD; arterial partial pressure of carbon dioxide, PaCO2; arterial blood gas, ABG; fraction of inspired oxygen, FiO2; oxygen saturation, SaO2; conventional oxygen therapy, COT; non-invasive ventilation, NIV; interquartile range, IQR; modified Medical Research Council, mMRC; intensive care unit, ICU; percentage of reference,% ref; total lung capacity, TLC; residual volume, RV; heart failure with preserved ejection fraction, HFpEI; arterial partial pressure of oxygen, PaO2; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; length of hospital stay, LOS; standard deviation, SD
Funding Statement
Nasal high-flow oxygen system (AIRVO-2 heated humidifier with Optiflow+ nasal cannula) provided by Fisher and Paykel Healthcare
References
- 1.Benjamin S,Jonathan M,Thomas M,Hugo L,Didier D,Jean-Damien R. Impact of high-flow nasal cannula oxygen therapy on intensive care unit patients with acute respiratory failure: a prospective observational study. J Crit Care. 2012;27(3):324.e9-.e13. doi: https://doi.org/10.1016/j.jcrc.2011.07.075 [DOI] [PubMed] [Google Scholar]
- 2.Jean-Pierre F,Stéphanie R,Arnaud WT. High-flow nasal cannula oxygen in respiratory failure. New Eng J Med. 2015;373(14):1373-1375. doi: https://doi.org/10.1056/NEJMc1508390 [Google Scholar]
- 3.Norbert S,Björn B,Gunnar B,Michael H,Jürgen H,Reimer R. Nasal high-flow oxygen therapy in patients with hypoxic respiratory failure: effect on functional and subjective respiratory parameters compared to conventional oxygen therapy and non-invasive ventilation (NIV). BMC Anesthesiol. 2014;14(1):66. doi: https://doi.org/10.1186/1471-2253-14-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rittayamai N,Tscheikuna J,Praphruetkit N,Kijpinyochai S. Use of high-flow nasal cannula for acute dyspnea and hypoxemia in the emergency department. Respir Care. 2015;60(10):1377-1382. doi: https://doi.org/10.4187/respcare.03837 [DOI] [PubMed] [Google Scholar]
- 5.Kazuma N,Takeshi M,Daichi F,et al. Efficacy of high-flow nasal cannula therapy in acute hypoxemic respiratory failure: decreased use of mechanical ventilation. Respir Care. 2015;60(10):1390-1396. doi: https://doi.org/10.4187/respcare.04026 [DOI] [PubMed] [Google Scholar]
- 6.Gerard C. Sorting out the mechanisms of benefit of high-flow nasal cannula in stable COPD. Chronic Obstr Pulm Dis. 2017;4(4):259-261. doi: https://doi.org/10.15326/jcopdf.4.4.2017.0176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wissam C,Tom N,Gwendolyn V,John G,Gerard JC. The effects of high-flow vs low-flow oxygen on exercise in advanced obstructive airways disease. Chest. 2004;126(4):1108-1115. doi: https://doi.org/10.1378/chest.126.4.1108 [DOI] [PubMed] [Google Scholar]
- 8.Kevin D,Thomas LM,Marla RW,Thomas HS. Research in high-flow therapy: mechanisms of action. Respir Med. 2009;103(10):1400-1405. doi: https://doi.org/10.1016/j.rmed.2009.04.007 [DOI] [PubMed] [Google Scholar]
- 9.Meg F,Thomas LM,Maria Elena R,et al. High‐flow nasal cannula: impact on oxygenation and ventilation in an acute lung injury model. Pediatr Pulmonol. 2011;46(1):67-74. doi: https://doi.org/10.1002/ppul.21326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giulia S,Mona A,Francesco B,Nicholas SH. Heated humidified high-flow nasal oxygen in adults. Chest. 2015;148(1):253-261. doi: https://doi.org/10.1378/chest.14-2871 [DOI] [PubMed] [Google Scholar]
- 11.Jens B,Friederike M,Hubert W. Effectiveness of nasal high-flow in hypercapnic COPD patients is flow and leakage dependent. BMC Pulm Med. 2018;18(1):14. doi: https://doi.org/10.1186/s12890-018-0576-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emma K,Nigel R,Stuart R,Rodger P. Mucociliary function deteriorates in the clinical range of inspired air temperature and humidity. Intensive Care Med. 2004;30(7):1491-1494. doi: https://doi.org/10.1007/s00134-004-2235-3 [DOI] [PubMed] [Google Scholar]
- 13.Rachael LP,Michelle LE,Shay PM. The effects of flow on airway pressure during nasal high-flow oxygen therapy. Respir Care. 2011;56(8):1151-1155. doi: https://doi.org/10.4187/respcare.01106 [DOI] [PubMed] [Google Scholar]
- 14.Jordi R,Purificación P,Jordi C,Oriol R,Joan Ramon M,Jordi R. Effect of high-flow nasal cannula and body position on end-expiratory lung volume: a cohort study using electrical impedance tomography. Respir Care. 2013;58(4):589-596. doi: https://doi.org/10.4187/respcare.02086 [DOI] [PubMed] [Google Scholar]
- 15.Miyuki O,Nobuya T,Kazuyuki N,et al. Evaluation by various methods of the physiological mechanism of a high-flow nasal cannula (HFNC) in healthy volunteers. BMJ Open Respir Res. 2017;4(1):e000200. doi: https://doi.org/10.1136/bmjresp-2017-000200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rea H,McAuley S,Jayaram L,et al. The clinical utility of long-term humidification therapy in chronic airway disease. Respir Med. 2010;104(4):525-533. doi: https://doi.org/10.1016/j.rmed.2009.12.016 [DOI] [PubMed] [Google Scholar]
- 17.Bräunlich J,Dellweg D,Bastian A,et al. Nasal high-flow versus noninvasive ventilation in patients with chronic hypercapnic COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:1411-1421. doi: https://doi.org/10.2147/COPD.S206111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kazuma N,Takashi K,Takeo H,et al. Domiciliary high-flow nasal cannula oxygen therapy for patients with stable hypercapnic chronic obstructive pulmonary disease. A multicenter randomized crossover trial. Ann Am Thorac Soc. 2018;15(4):432-439. doi: https://doi.org/10.1513/AnnalsATS.201706-425OC [DOI] [PubMed] [Google Scholar]
- 19. Standardization of spirometry 2019 update. Am J Respir Crit Care Med. 2019;200(8):e70-e88. doi: https://doi.org/10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller MR,Hankinson J,Brusasco V,et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319-338. doi: https://doi.org/10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 21.Donald AM,Carolyn KW. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580-586. doi: https://doi.org/10.1378/chest.93.3.580 [DOI] [PubMed] [Google Scholar]
- 22.Wolfgang V,Luc S,Daniel K,et al. A simple formula for the calculation of sample size in pilot studies. J Clin Epidemiol. 2015;68(11):1375-1379. doi: https://doi.org/10.1016/j.jclinepi.2015.04.014 [DOI] [PubMed] [Google Scholar]
- 23.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management and prevention of COPD. GOLD website. Published 2020 Accessed May 2020 https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf [Google Scholar]
- 24.Eun Sun K,Hongyeul L,Se Joong K,et al. Effectiveness of high-flow nasal cannula oxygen therapy for acute respiratory failure with hypercapnia. J Thorac Dis. 2018;10(2):882-888. doi: https://doi.org/10.21037/jtd.2018.01.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin Hee J,Dong Hoon K,Seong Chun K,et al. Changes in arterial blood gases after use of high-flow nasal cannula therapy in the ED. Am J Emerg Med. 2015;33(10):1344-1349. doi: https://doi.org/10.1016/j.ajem.2015.07.060 [DOI] [PubMed] [Google Scholar]
- 26.Ruiz VR,Mayer GF,Roux NG,Peralta HA. Use of high-flow nasal cannula in the emergency department. Eur Respr J. 2016;48:PA357. doi: https://doi.org/10.1183/13993003.congress-2016.PA357 [Google Scholar]
- 27.Jens B,Hubert W. Nasal high-flow in acute hypercapnic exacerbation of COPD. Int J Chronic Obstr Pulmon Dis. 2018;13:3895-3897. doi: https://doi.org/10.2147/COPD.S185001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janine P,Leonie E,Michael R,et al. Physiological effects of titrated oxygen via nasal high‐flow cannulae in COPD exacerbations: a randomized controlled cross‐over trial. Respirology. 2017;22(6):1149-1155. doi: https://doi.org/10.1111/resp.13050 [DOI] [PubMed] [Google Scholar]
- 29.Mamta R,Jose Luis L-C,Ady C-A,Sylvia H,Francisco P-R,Roberts CM. Understanding variation in length of hospital stay for COPD exacerbation: European COPD audit. ERJ Open Res. 2016;2(1):00034-2015. doi: https://doi.org/10.1183/23120541.00034-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]