Abstract
Rationale: Hypercapnia develops in one third of patients with advanced chronic obstructive pulmonary disease (COPD) and is associated with increased morbidity and mortality. Multiple factors in COPD are thought to contribute to the development of hypercapnia including increased carbon dioxide (CO2) production, increased dead space ventilation, and the complex interactions of deranged respiratory system mechanics, inspiratory muscle overload and the ventilatory control center in the brainstem. However, these factors have not previously been systematically analyzed in a large, well-characterized population of severe COPD patients.
Methods: This is a secondary analysis of the clinical, physiologic and imaging data from the National Emphysema Treatment Trial (NETT). All patients with complete baseline data for the key predictor variables were included. An inclusive list of 32 potential predictor variables were selected a priori based on consensus of the investigators and literature review. Stepwise variable selection yielded 10 statistically significant associations in multivariate regression.
Results: A total of 1419 patients with severe COPD were included in the analysis; mean age 66.4 years (standard deviation 6.3), 38% females, and 422 (29.7%) had baseline hypercapnia. Key variables associated with hypercapnia were low resting partial pressure of oxygen in blood, low minute ventilation (Ve), high volume of exhaled carbon dioxide, low forced expiratory volume in 1 second, high residual volume, lower % emphysema on chest computed tomography, use of oxygen, low ventilatory reserve (high Ve/maximal voluntary ventilation), and not being at high altitude. Low diffusing capacity for carbon monoxide showed a positive association with hypercapnia in univariate analysis but a negative correlation in multivariate analysis. Measures of dyspnea and quality of life did not associate with degree of hypercapnia in multivariable analysis.
Conclusions: Hypercapnia in a well-characterized cohort with severe COPD and emphysema is chiefly related to poor lung mechanics, high CO2 production, and a reduced ventilatory capability. Hypercapnia is less impacted by gas exchange abnormalities or the presence of emphysema.
Keywords: copd, hypercapnia, National Emphysema Treatment Trial, NETT
Introduction
This article contains supplemental material.
Chronic obstructive pulmonary disease (COPD) is a progressive inflammatory process in the airways, characterized by airway narrowing with expiratory flow limitation, air trapping/hyperinflation, and varying degree of destruction of alveoli (emphysema).1 Hypercapnia and chronic alveolar hypoventilation is common in severe COPD with a reported prevalence2,3 ranging from 23%-38%. Hypercapnia has prognostic implications with multiple studies demonstrating chronic hypercapnia is associated with increased hospitalization needs and higher mortality.4,5 In severe COPD, hypercapnia is accompanied by arterial hypoxemia.2,3,4 If severe enough, this leads to right ventricular overload, cor pulmonale, and the clinical “blue bloater” phenotype.4-6
The mechanisms underlying the development of hypercapnia in COPD are not well understood. Hypercapnia in patients with stable COPD has been associated with greater inspiratory muscle loads and less inspiratory muscle strength/endurance.7-20 Loads are largely imposed by increased airway resistance, hyperinflation and increased ventilation demands from increased muscle energy demands and wasted dead space ventilation.10-13 The lower muscle strength/endurance results from nutritional factors, neuromyopathic effects of systemic inflammation imposed by COPD, flattening of the diaphragm from hyperinflation, and chronic muscle overloading.13-29
The ventilatory control center in the brainstem receives inputs from central and peripheral partial pressure of oxygen (PO2) and partial pressure of carbon dioxide (PCO2) chemoreceptors as well as sophisticated load sensors in the lungs, chest wall and respiratory muscles.30-42 In the face of an imbalance between inspiratory muscle loads and inspiratory muscle pump capabilities (strength/endurance), a down regulation of respiratory drive and hypoventilation appears to occur to reduce muscle loads and energy needs and prevent overt muscle fatigue.34-43 However, the relative importance of specific pathophysiologic derangements in COPD (e.g., airway disease, emphysema, hypoxemia, vascular function, etc.) on the development of this imbalance and hypercapnia are not clear.7-10,39-43
The National Emphysema Treatment Trial (NETT) was a large National Institutes of Health-funded, multicenter trial evaluating the role of lung volume reduction surgery (LVRS) in patients with severe COPD, many with hypercapnia.44,45 Patients were well-characterized at baseline and we reasoned that this large and unique data set could be analyzed to better understand hypercapnia in COPD patients. We thus undertook a reanalysis of the NETT baseline data to identify physiologic and clinical parameters associated with hypercapnia in patients with severe COPD.
Methods
This was a secondary analysis of clinical, physiologic, and imaging data from the NETT. Inclusion criteria were severe airflow obstruction and hyperinflation on pulmonary function testing as well as radiographic evidence of bilateral emphysema with either heterogeneous or homogeneous distribution; and no smoking for 6 months prior to randomization. The criteria and study design have been detailed elsewhere.44,45 At baseline, patients completed questionnaires, lab analysis including arterial blood gas, full pulmonary function testing including body plethysmography according to American Thoracic Society standards,46 cardiopulmonary exercise testing and computed tomography (CT) scans of the chest.
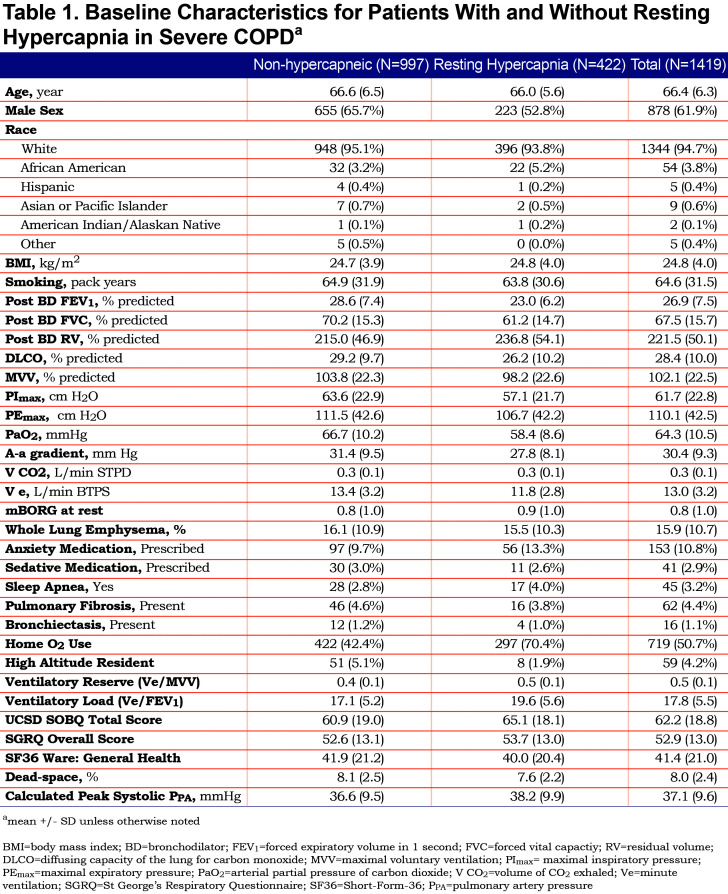
The primary outcome was resting arterial partial pressure of carbon dioxide (PaCO2) breathing ambient air (continuous variable). As a secondary sensitivity analysis, hypercapnia was also analyzed as a dichotomous outcome, defined as a PaCO2 ≥ 45 mmHg (6 kPa), a threshold often used to define significant hypercapnia. Thirty-two baseline demographic and clinical characteristics were identified as potential predictors of hypercapnia based on consensus of the investigators and review of the literature (Table 1). Pulmonary hypertension and other cardiac function data could not be analyzed due to high rate of missing data. A total of 1419 patients had data for the selected key predictor variables and were included. Dyspnea was measured using standard instruments, including the University of California San Diego Shortness of Breath Questionnaire (UCSD SOBQ), and the modified Borg (mBorg) dyspnea score. Quality of life was measured using St George’s Respiratory Questionnaire (SGRQ) and Short Form-36 (SF36).
Statistical Analysis
Associations between the factors and hypercapnia were analyzed using linear regression on the continuous outcome of PaCO2 and logistic regression in dichotomized analysis using previously validated general statistical methods.47 As dead-space and A-a gradient were derived from the outcome, they were excluded from the subsequent association analyses. Calculated peak systolic pulmonary artery pressure values were also removed from the data set because it had a large proportion of missing values. Eventually 1058 patients with complete records were included in the multivariable regression.
Variable selection was then performed to identify variables with strong association with degree of hypercapnia, and 3 models were obtained through a stepwise approach using Bayesian information criterion, Akaike information criteria, and least absolute shrinkage operator selector (LASSO) regression. Leave-one-out cross-validation was applied to compare the 3 models based on cross-validated prediction error. The model with variables selected by stepwise with BIC criterion was chosen as the final model as it yielded the lowest prediction error after cross validating 3 different models. This yielded 10 statistically significant variables associated with degree of hypercapnia. A p-value at < 0.05 was defined to be statistically significant. Dichotomous analysis using multivariable logistic regression yielded similar results to the linear regression modeling analysis. The analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina) and R (https://www.R-project.org/).
Ethical Considerations
All patients provided informed consent as part of the original trial. This study was performed in accordance with the Duke University Institutional Review Board and the NETT trial data stewards.
Results
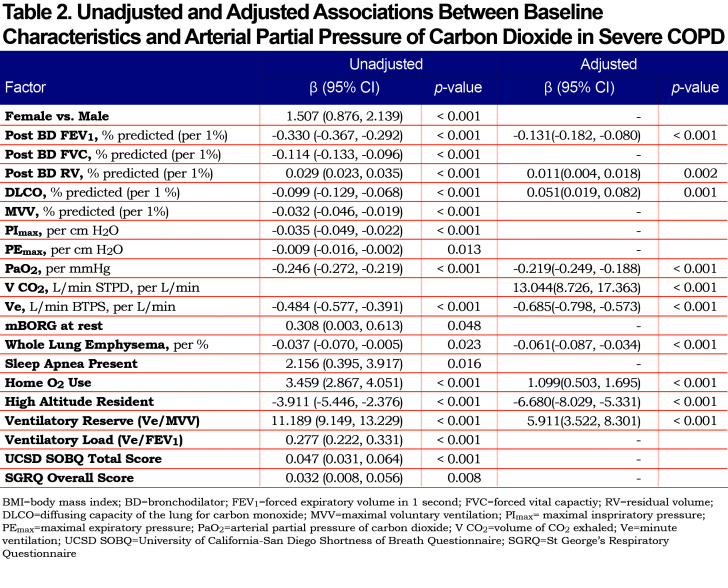
Of 1419 patients included in the analysis, 422 (29.7%) had baseline hypercapnia defined by PaCO2 ≥ 45 mm Hg. The baseline characteristics for patients with and without hypercapnia are shown in Table 1. Results of regression modeling are displayed in Table 2.
There were 20 predictors that were associated with hypercapnia in univariate analysis. Gender, forced vital capacity (FVC), maximal voluntary ventilation (MVV), maximal inspiratory pressure (PImax), mBorg at rest, sleep apnea, and ventilatory load (minute ventilation [Ve] to forced expiratory volume in 1 second [FEV1]) were associated with hypercapnia in univariable analysis, but not in multivariable analysis. In the final multivariable model, independent predictors of hypercapnia in severe COPD were low resting PaO2, low Ve, low post-bronchodilator FEV1, high post-bronchodilator residual volume (RV), lower percentage of emphysema observed on chest CT, use of oxygen, low ventilatory reserve (Ve/MVV), and not being at high altitude. After adjusting other predictors, the association of a high volume of CO2 exhaled (VCO2) with degree of hypercapnia became significant, and the univariate negative direction of the association between diffusing capacity for carbon monoxide (DLCO) % predicted and PaCO2 was reversed. Measures of dyspnea and quality of life did not associate with the degree of hypercapnia in multivariate analysis.
Discusssion
This analysis of detailed baseline clinical data in the NETT trial found that hypercapnia is associated with numerous mechanical and gas exchange factors. These findings support the notion that the mechanisms of chronic hypercapnia in COPD are multifactorial. Although the degree of airway obstruction and resulting resistive loads on the inspiratory muscles are important, there are a number of other important factors to consider.
Hypercapnia has been observed even in patients with mild airway obstruction,48 and chronic hypoventilation and PaCO2 retention does not occur in patients with bronchial asthma despite evidence of severe airflow obstruction.49 It is also known that the relationship between FEV1 and the ventilatory response to increasing inspired CO2 is highly variable.10 The present study suggests that factors besides FEV1 are involved in inspiratory muscle loading and likely include increased ventilation demands from dead space ventilation and increased oxygen demands, as well as CO2 production from excessive inspiratory muscle work.
Our results also suggest that reduced ventilatory capacity and low inspiratory muscle reserve likely play a role in the development of chronic hypercapnia. In severe COPD, inspiratory muscles are disadvantaged by hyperinflation, compromised oxygen delivery, structural changes from chronic overloading, and systemic effects from chronic inflammation.1,10-19
These load/capability imbalances likely influence the ventilatory control center in the brain stem. The ventilatory control center is a collection of neurons, feedback networks, and intrinsic pattern generators that must balance inputs from chemoreceptors sensing blood gases and acid base balance along with inputs from load and stretch receptors in the lungs, chest wall, and respiratory muscles.31-42 An attractive hypothesis is that the resulting neuronal output driving the ventilatory pattern is essentially attempting to provide adequate gas exchange while avoiding unnecessary lung stretch and not fatiguing the inspiratory muscles. Our results shed some insight into this concept. Most obvious is the observed overall reduction of minute ventilation and tolerance of hypercapnia and respiratory acidosis in these COPD patients with severe load/capability imbalances. Although hypercapneic COPD patients can voluntarily hyperventilate to lower the PaCO2, long term hypoventilation may be viewed as a “wise choice” to protect the inspiratory muscles at the expense of tolerable (non-life threatening) hypercapnia. More precise methods than those available in the present study to approximate the activity of the ventilatory control center will be needed to elucidate the contribution of downregulating the ventilatory control center to hypercapnia.
More complex is our observation that emphysema is negatively associated with hypercapnia. One explanation for this is the concept that load, stretch, and lung/thorax irritant receptor behavior in emphysema is different than in predominant airway disease. This might result in the ventilatory control center prioritizing ventilation over muscle protection and creating the “pink puffer” phenotype in predominantly emphysematous COPD. The negative associations of hypercapnia with emphysema are consistent with retrospective, postmortem reports that hypercapneic COPD patients have less obvious radiographic evidence of emphysema on chest CT and more normal DLCO on pulmonary function testing than those without hypercapnia.50 Given that this patient population was highly selected for severe emphysema rather than airway disease, our discussion above must be viewed with considerable caution.
The negative independent association of DLCO with hypercapnia in our patients may reflect this emphysematous effect. An interesting alternate explanation might be the fact that the alveolar hypoxia produced by hypercapnia could increase CO uptake, a phenomenon described by Roughton and Forester years ago.51 One final caveat is the observed association of an increased need for oxygen supplementation in hypercapneic patients. This is not surprising given the link between alveolar ventilation and both PCO2 and PO2. It may also suggest that impaired ventilation/matching and/or the Haldane effect may also contribute to hypercapnia in these patients.12,23,32
There are important limitations to this study. One is the cross sectional design which, although evaluating factors concurrently, limits causal inference. The potential mechanisms should be validated through randomized experimental trials. Evaluating the interplay between multiple factors is complex and may be affected by collinearity between variables. Collinearity was addressed in multiple ways including variable section based on subject matter knowledge, evaluation of multiple models with/without potential collinear factors, and techniques including Lasso method. Validation of the present findings in independent datasets is warranted.
Importantly, the present findings pertain to highly characterized patients with severe emphysema (as opposed to chronic bronchitis) with limits on hypercapnia (PCO2 > 60 mmHg), obesity (body mass index > 31), and hypoxemia (PO2 < 45 mmH2O). Unfortunately, because of limited data and exclusion of patients with moderate to severe pulmonary hypertension, we were also unble to characterize the effects of pulmonary hypertension on hypercapnia. Also, our participants were enrolled in a randomized controlled trial and may not be generalized to the broad population of patients with severe COPD or other phenotypes. Finally, our data set did not allow us to address the roles of extra-pulmonary factors, such as chest wall properties, hormones, systemic inflammatory cytokines, metabolic agents, nutritional status, cardiac function, psychological factors, and even genetic factors on the responses to load/capacity imbalances and the development of hypercapnia.9,52-54 These analyses will need to be the focus of future studies.
In summary, it is clear that the development of chronic hypercapnia in COPD is complex and multifactorial. However, our results suggest that an over-arching theme may be that a reduced ventilatory drive develops in the face of severe mechanical load/capabilities imbalances. Understanding hypercapnia and its effects is important as its presence is associated with worse hypoxemia, polycythemia and right ventricular hypertrophy with eventual right heart failure. These all translate into poorer outcomes including worse mortality. Indeed, there is growing interest in providing hypercapneic patients with chronic non-invasive positive pressure ventilation (NPPV) to conceptually unload respiratory muscles for prolonged periods of time (e.g., nocturnal use). While NPPV use in this fashion may reset the resting PaCO2 to lower levels, a risk of fostering hyperinflation and air trapping exists and long term outcome data are conflicting.54,55 Better understanding of the determinants and effects of hypercapnia will help us understand the role of therapies such as NPPV in the future.
Abbreviations
chronic obstructive pulmonary disease, COPD; carbon dioxide, CO2; National Emphysema Treatment Trial, NETT; minute ventilation,Ve; partial pressure of oxygen, PO2; partial pressure of carbon dioxide, PCO2; lung volume reduction surgery, LVRS; computed tomography, CT; arterial partial pressure of carbon dioxide, PaCO2; University of San Diego Shortness of Breath Questionnaire, UCSD SOBQ; modified Borg dyspnea score, mBorg; St George’s Respiratory Questionnaire, SGRQ; Short-Form-36, SF36; bronchodilator, BD; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; residual volume, RV; diffusing capacity for carbon monoxide, DLCO; maximal voluntary ventilation, MVV; maximal inspiratory pressure, PImax; maximal expiratory pressure, PEmax; volume of CO2 exhaled, V CO2; pulmonary artery pressure, PPA; non-invasive positive pressure ventilation, NPPV; least absolute shrinkage operator selector, LASSO
References
- 1.Barnes PJ. Pathophysiology of COPD. In: Crapo J, ed. Atlas of Chronic Obstructive Pulmonary Disease Springer Science;. 2008:19-32. [Google Scholar]
- 2.Burrows B,Earle RH. Course and prognosis of chronic obstructive lung disease. New Engl J Med. 1969;280:397-404. doi: https://doi.org/10.1056/NEJM196902202800801 [DOI] [PubMed] [Google Scholar]
- 3.Renzetti AD,McClement JH,Litt BO. The veterans administration cooperative study of pulmonary function III; mortality in relation to respiratory function in chronic obstructive pulmonary disease. Am J Med. 1966;41(1):115-129. doi: https://doi.org/10.1016/0002-9343(66)90009-X [DOI] [PubMed] [Google Scholar]
- 4.Nizet TA,van den Elshout FJJ,Heijdra YF,van de VenMarjo JT,Mulder PGH,Folgering HTM. Survival of chronic hypercapnic COPD patients is predicted by smoking habits, comorbidity, and hypoxemia. Chest. 2005;127(6):1904-1910. doi: https://doi.org/10.1378/chest.127.6.1904 [DOI] [PubMed] [Google Scholar]
- 5.Neff TA,Petty TL. Tolerance and survival in severe chronic hypercapnia. Arch Intern Med. 1972;29(4):591-596. doi: https://doi.org/10.1001/archinte.1972.00320040067008 [PubMed] [Google Scholar]
- 6.Johnson MA,Woodcock AA,Rehahn M,Geddes DM. Are "pink puffers" more breathless than "blue bloaters"?. Br Med J. 1983;286:179. doi: https://doi.org/10.1136/bmj.286.6360.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lourenco RV,Miranda JM. Drive and performance of the ventilatory apparatus in chronic obstructive lung disease. N Engl J Med. 1968;279:53-59. doi: https://doi.org/10.1056/NEJM196807112790201 [DOI] [PubMed] [Google Scholar]
- 8.Parot S,Saunier C,Gautier H,Milic-Emili J,Sadoul P. Breathing pattern and hypercapnoea in patients with obstructive pulmonary disease. Am Rev Respir Dis. 1980;121(6):985-991. [DOI] [PubMed] [Google Scholar]
- 9.Bégin P,Grassino A. Inspiratory muscle dysfunction and chronic hypercapnia in chronic pulmonary obstructive disease. Am Rev Respir Dis. 1991;143(5pt1):905-912. doi: https://doi.org/10.1164/ajrccm/143.5_Pt_1.905 [DOI] [PubMed] [Google Scholar]
- 10.Lane DJ,Howell JBL,Giblin B. Relation between airways obstuction and carbon dioxide tension in chronic obstructive pulmonary disease. Br Med J. 1968;3:707-709. doi: https://doi.org/10.1136/bmj.3.5620.707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardy RL,Roussos C. Endurance of hyperventilation in chronic airflow limitation. Chest. 1983;83(5):744-750. doi: https://doi.org/10.1378/chest.83.5.744 [DOI] [PubMed] [Google Scholar]
- 12.Gilbert R,Keighly GR Jr,Auechincloss JH. Mechanisms of chronic carbon dioxide retention in patients with obstructive pulmonary disease. Am J Med. 1965;38(2):217-225. doi: https://doi.org/10.1016/0002-9343(65)90175-0 [DOI] [PubMed] [Google Scholar]
- 13.Burrows B,Saskena FB,Diener CF. Carbon dioxide tension and ventilatory mechanics in chronic obstructive lung disease. Ann Intern Med. 1966;65(4):685-700. doi: https://doi.org/10.7326/0003-4819-65-4-685 [DOI] [PubMed] [Google Scholar]
- 14.Roussos C,Fixley M,Gross D,Macklem PT. Fatigue of the inspiratory muscles and their synergistic behaviour. J Appl Physiol. 1977;46(5):897-904. doi: https://doi.org/10.1152/jappl.1979.46.5.897 [DOI] [PubMed] [Google Scholar]
- 15.Bellemare F,Grassino A. Force reserve of the diaphragm in patients with chronic obstructive pulmonary disease. J Appl Physiol. 1983;55(1):8-15. doi: https://doi.org/10.1152/jappl.1983.55.1.8 [DOI] [PubMed] [Google Scholar]
- 16.Cohen CA,Zagelbaum G,Gross D,Roussos C,Macklem PT. Clinical manifestations of inspiratory muscle fatigue. Am J Med. 1982;73:308-316. doi: https://doi.org/10.1016/0002-9343(82)90711-2 [PubMed] [Google Scholar]
- 17.McKenzie DK,Allen GM,Butler JE,Gandevia SC. Task failure with lack of diaphragm fatigue during inspiratory resistive loading in human subjects. J Appl Physiol. 1997;82(6):2011-2019. doi: https://doi.org/10.1152/jappl.1997.82.6.2011 [DOI] [PubMed] [Google Scholar]
- 18.Zhu E,Petrof BJ,Gea J,Comtois N,Grassimo AE. Diaphragm muscle fiber injury after inspriatory resistive breathing. Am J Respir Crit Care Med. 1997;155(3):1110-1116. doi: https://doi.org/10.1164/ajrccm.155.3.9116995 [DOI] [PubMed] [Google Scholar]
- 19.Bellemare F,Grassino A. Effect pressure and timing of contraction on human diaphragm fatigue. J Appl Physiol. 1982;53(5):1190-1195. doi: https://doi.org/10.1152/jappl.1982.53.5.1190 [DOI] [PubMed] [Google Scholar]
- 20.Bellemare F,Grassino A. Force reserve of the diaphragm in patients with chronic obstructive pulmonary disease. J Appl Physiol. 1983;55(5):8-13. doi: https://doi.org/10.1152/jappl.1983.55.1.8 [DOI] [PubMed] [Google Scholar]
- 21.Topeil A,Laghi F,Tobin MJ. The voluntary drive to breathe is not decreased in hypercapnic patients with severe COPD. Eur Respir J. 2001;18(1):53-60. doi: https://doi.org/10.1183/09031936.01.00014101 [DOI] [PubMed] [Google Scholar]
- 22.Del Vecchio L,Polese G,Poggi R,Rossi A. "Intrinsic"positive end-expiratory pressure in stable patients with chronic obstructive pulmonary disease. Eur Respir J. 1990;3(1):74-80. [PubMed] [Google Scholar]
- 23.Haluszka J,Chartrand DA,Grassino AE,Milic-Emili J. Intrinsic PEEP and arterial PCO2 in stable patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1990;141(5):1194-1197. doi: https://doi.org/10.1164/ajrccm/141.5_Pt_1.1194 [DOI] [PubMed] [Google Scholar]
- 24.Burgel PR,Mannino D. Systemic inflammation in patients with chronic obstructive pulmonary disease: one size no longer fits all!. Am J Respir Crit Care Med. 2012;15:186(10):936-937. doi: https://doi.org/10.1164/rccm.201209-1634ED [DOI] [PubMed] [Google Scholar]
- 25.Meecham-Jones DF,Paul EA,Jones PW,Wedzicha JA. Nasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnic COPD. Am J Respir Crit Care Med. 1995;152(2):538-544. doi: https://doi.org/10.1164/ajrccm.152.2.7633704 [DOI] [PubMed] [Google Scholar]
- 26.Gigliotti F,Duranti R,Fabiani A,Schiavina M,Scano G. Suppression of ventilatory muscle activity in healthy subjects and COPD patients with negative pressure ventilation (NPV). Chest. 1991;99(5):1186-1192. doi: https://doi.org/10.1378/chest.99.5.1186 [DOI] [PubMed] [Google Scholar]
- 27.Goldstein RS,De Rosie JA,Avendano MA,et al. Influence of non-invasive postive pressure ventilation on inspiratory muscles. Chest. 1991;99(2):408-415. doi: https://doi.org/10.1378/chest.99.2.408 [DOI] [PubMed] [Google Scholar]
- 28.Sharp JT,Henry JP,Sweany SK,et al. The total work of breathing in normal and obese men. J Clin Invest. 1964;43(4):728-739. doi: https://doi.org/10.1172/JCI104957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Similowski T,Yan S,Gauthier AP,Macklem PT,Bellemare F. Contractile properties of the human diaphragm during chronic hyperinflation. N Engl J Med. 1991;325:917-325. doi: https://doi.org/10.1056/NEJM199109263251304 [DOI] [PubMed] [Google Scholar]
- 30.Hogg JC,McDonough J. Anatomic pathology of COPD. In: Crapo J, ed. Atlas of Chronic Obstructive Pulmonary Disease Springer Science;. 2008:73-90. [Google Scholar]
- 31.McIlroy M,Elridge F,Thomas JP,et al. The effect of added elastic and non-elastic resistances on the pattern of breathing in normal subjects. Clin Sci. 1956;15:337-344. [PubMed] [Google Scholar]
- 32.Mountain R,Zwillich C,Weil J. Hypoventilation in obstructive lung disease: The role of familial factors. N Engl J Med. 1978;298:521-525. doi: https://doi.org/10.1056/NEJM197803092981001 [DOI] [PubMed] [Google Scholar]
- 33.Arkinstall WW,Nirmel K,Kissouras V,Milic-Emili J. Genetic difference in the ventilatory response to inhaled CO2. J Appl Physiol. 1974;36(1):6-11. doi: https://doi.org/10.1152/jappl.1974.36.1.6 [DOI] [PubMed] [Google Scholar]
- 34.Richter DW,Ballanyi K,Schwarzacher S. Mechanisms of respiratory rhythm generation. Curr Opin Neurobiol. 1992;2(6):788-793. doi: https://doi.org/10.1016/0959-4388(92)90135-8 [DOI] [PubMed] [Google Scholar]
- 35.Ramirez JM,Zuperku EJ,Alheid GF,et al. Respiratory rhythm generation: converging concepts from in vitro in vivo approaches?. Respir Physiol Neurobiol. 2002;131(1-2):43-56. doi: https://doi.org/10.1016/S1569-9048(02)00036-8 [DOI] [PubMed] [Google Scholar]
- 36.Hlastala MP,Berger AJ. Central mechanisms of respiratory control. In: Hlastala MP, Berger AJ, eds. Physiology of Respiration, 2nd Ed. Oxford Univ Press: 1996:162-208. [Google Scholar]
- 37.Nattie E. CO2, brainstem chemoreceptors and breathing. Prog Neurobiol. 1999;59(4):299-331. doi: https://doi.org/10.1016/S0301-0082(99)00008-8 [DOI] [PubMed] [Google Scholar]
- 38.Nattie EE. Central chemosensitivity, sleep, and wakefulness. Respir Physiol. 2001;129(1-2):257-268. doi: https://doi.org/10.1016/S0034-5687(01)00295-X [DOI] [PubMed] [Google Scholar]
- 39.Bianchi AL,Denavit-Saubié M,Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75(1):1-45. doi: https://doi.org/10.1152/physrev.1995.75.1.1 [DOI] [PubMed] [Google Scholar]
- 40.Cateke RJ,Altose MD. Chemical and nonchemical influences on respriatory sensation and control of breathing. Fed Proc. 1983;42:742. [Google Scholar]
- 41.Lee LY,Milhorn HT. Central ventilatory response to O2 and CO2 at three levels of carotid chemoreceptor stimulation. Respir Physiol. 1975;25(3):319-333. doi: https://doi.org/10.1016/0034-5687(75)90007-9 [DOI] [PubMed] [Google Scholar]
- 42.Neubauer JA,Melton JE,Edelman NH. Modulation of respiration during hypoxia. J Appl Physiol. 1990;68(2):441-451. doi: https://doi.org/10.1152/jappl.1990.68.2.441 [DOI] [PubMed] [Google Scholar]
- 43.Shershow JC,King A,Robinson S. Carbon dioxide sensitivity and personality. Psychosom Med. 1973;22:19-33. doi: https://doi.org/10.1097/00006842-197303000-00008 [DOI] [PubMed] [Google Scholar]
- 44.NETT Research Group; A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059-2073. doi: https://doi.org/10.1056/NEJMoa030287 [DOI] [PubMed] [Google Scholar]
- 45.NETT Research Group; Rationale and design of the national emphysema treatment trial (NETT):A prospective randomized trial of lung volume reduction surgery. J Thorac Cardiovasc. 1999;118(3):518-528. doi: https://doi.org/10.1016/S0022-5223(99)70191-1 [DOI] [PubMed] [Google Scholar]
- 46.American Thoracic Society; Standards for the diagonsis and care of patients with chronic obstructive pulmonary disease. Am Rev Respir Crit Care Med. 1995;152(5pt2):S77-S121. [PubMed] [Google Scholar]
- 47.Harrell FE. Regression Modeling Strategies. 2nd ed. Springer Publications; 2015. [Google Scholar]
- 48.McNicol MW,Pride NB. Unexplained underventilation of the lungs. Thorax. 1965;20(1):53-65. doi: https://doi.org/10.1136/thx.20.1.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tai E,Read J. Blood-gas tensions in bronchial asthma. Lancet. 1967;1(7491):644-646. doi: https://doi.org/10.1016/S0140-6736(67)92541-X [DOI] [PubMed] [Google Scholar]
- 50.Burrows B,Fletcher CM,Heard BE,Jones NL,Wootliff JS. The emphysematous and bronchial types of chronic airways obstruction: a clinicopathological study of patients in London and Chicago. Lancet. 1966;287(7442):830-835. doi: https://doi.org/10.1016/S0140-6736(66)90181-4 [DOI] [PubMed] [Google Scholar]
- 51.Roughton FJ,Forster RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol. 1957;11(2):290-302. doi: https://doi.org/10.1152/jappl.1957.11.2.290 [DOI] [PubMed] [Google Scholar]
- 52.Lewis MI,Fournier M,Storer TW,Bhasin S,et al. Skeletal muscle adaptations to testosterone and resistance training in men with COPD. J Appl Physiol. 2007;103(4):1299-1310. doi: https://doi.org/10.1152/japplphysiol.00150.2007 [DOI] [PubMed] [Google Scholar]
- 53.Saunders NA,Heipern S,Rebuck AS. Relation between personality and ventilatory response to carbon dioxide in normal subjects: a role in asthma?. Br Med J. 1972;1:719-721. doi: https://doi.org/10.1136/bmj.1.5802.719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Struick FM,Lacasse Y,Goldstein R,Kerstjens HM,Wijkstra PJ. Nocturnal non-invasive positive pressure ventilation for stable COPD. Cochrane Database Syst Rev. 2013;(6):CD002878. doi: https://doi.org/10.1002/14651858.CD002878.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murphy PB,Rehal S,Arbane G,et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: a randomized clinical trial. JAMA. 2017;317(21):2177-2186. doi: https://doi.org/10.1001/jama.2017.4451 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article contains supplemental material.