Abstract
Purpose
Glaucoma is a neurodegenerative disease of the eye with an estimated prevalence of more than 111.8 million patients worldwide by 2040, with at least 6 to 8 million projected to become bilaterally blind. Clinically, the current method of slowing glaucomatous vision loss is to reduce intraocular pressure (IOP). In this manuscript, we describe the in vitro cytoprotective and in vivo long lasting IOP-lowering activity of the poly D, L-lactic-co-glycolic acid (PLGA) nanoparticle-encapsulated hybrid compound SA-2, possessing nitric oxide (NO) donating and superoxide radical scavenging functionalities.
Methods
Previously characterized primary human trabecular meshwork (hTM) cells were used for the study. hTM cells were treated with SA-2 (100 µM, 200 µM, and 1,000 µM), SA-2 PLGA-loaded nanosuspension (SA-2 NPs, 0.1%), or vehicle for 30 min. Cyclic guanosine monophosphate (cGMP) and super oxide dismutase (SOD) levels were analyzed using commercial kits. In another experiment, hTM cells were pretreated with tert-butyl hydrogen peroxide (TBHP, 300 µM) for 30 min followed by treatment with escalating doses of SA-2 for 24 h, and CellTiter 96 cell proliferation assay was performed. For the biodistribution study, the cornea, aqueous humor, vitreous humor, retina, choroid, and sclera were collected after 1 h of administration of a single eye drop (30 μl) of SA-2 NPs (1% w/v) formulated in PBS to rat (n = 6) eyes. Compound SA-2 was quantified using high performance liquid chromatography /mass spectrometry (HPLC/MS). For the IOP-lowering activity study, a single SA-2 NPs (1%) eye drop was instilled in normotensive rats eyes and in the IOP-elevated rat eyes (n = 3/group, in the Morrison model of glaucoma), or Ad5TGFβ2-induced ocular hypertensive (OHT) mouse eyes (n = 5/group). IOP was measured at various time points up to 72 h, and the experiment was repeated in triplicate. Mouse aqueous humor outflow facility was determined with multiple flow-rate infusion and episcleral venous pressure estimated with manometry.
Results
SA-2 upregulated cGMP levels (six- to ten-fold) with an half maximal effective concentration (EC50) of 20.3 µM in the hTM cells and simultaneously upregulated (40-fold) the SOD enzyme when compared with the vehicle-treated hTM cells. SA-2 also protected hTM cells from TBHP-induced decrease in cell survival with an EC50 of 0.38 µM. A single dose of slow-release SA-2 NPs (1% w/v) delivered as an eye drop significantly lowered IOP (by 30%) in normotensive and OHT rodent eyes after 3 h post-dose, with the effect lasting up to 72 h. A statistically significant increase in aqueous outflow facility and a decrease in episcleral venous pressure was observed in rodents at this dose at 54 h.
Conclusions
Hybrid compound SA-2 upregulated cGMP in hTM cells, increased outflow facility and decreased IOP in rodent models of OHT. Compound SA-2 possessing an antioxidant moiety provided additive cytoprotective activity to oxidatively stressed hTM cells by scavenging reactive oxygen species (ROS) and increasing SOD enzyme activity. Additionally, the PLGA nanosuspension formulation (SA-2 NPs) provided longer duration of IOP-lowering activity (up to 3 days) in comparison with the free non-encapsulated SA-2 drug. The data have implications for developing novel, non-prostaglandin therapeutics for IOP-lowering and cytoprotective effects with the possibility of an eye drop dosing regimen of once every 3 days for patients with glaucoma.
Introduction
It is estimated that by 2040, more than 111.8 million people worldwide will be affected with glaucoma, with the possibility of at least 6 to 8 million of them becoming bilaterally blind [1,2]. Primary open angle glaucoma (POAG), the most common form of glaucoma, is characterized by progressive loss of retinal ganglion cell (RGC) somas and their axons, as well as axonal degeneration of the optic nerve. In many patients with POAG, there is also a rise in intraocular pressure (IOP), which is thought to be a major contributing factor to the development of the disease. Thus far, the only clinically efficacious method for treating POAG is to reduce IOP, either medically or surgically. However, lowering IOP is only partially effective; in most cases, it slows but does not arrest the progression of neurodegeneration. Furthermore, lowering IOP does not adequately address the susceptibility to continued RGC degeneration [3]. Thus, there is an unmet need for new, effective, and safe pharmaceutical agents to treat glaucoma that lower IOP and have neuroprotective and cytoprotective effects.
Nitric oxide (NO) is a key target for glaucoma therapy as the polymorphisms of the endothelial nitric oxide synthase (eNOS) gene (Gene ID: 4846) is associated with POAG [4,5], and NO concentrations are decreased in aqueous humor of patients with glaucoma compared to healthy controls [6]. Signaling through the nitric oxide-cyclic guanosine monophosphate (NO-cGMP) pathway enhances aqueous outflow facility by relaxing the trabecular meshwork (TM) [7]. Recent studies emphasized that NO is directly implicated in the regulation of IOP. Latanoprostene bunod (LBN, Vyzulta) [8] is a NO-donating prostaglandin analog currently approved by the U.S. Food and Drug Administration (FDA) for POAG therapy which works by decreasing IOP [8]. NO-cGMP signaling also contributes to the reprogramming of neural precursors, derived from human embryonic stem cells, into functional neurons in animal models of Parkinson disease [9]. However, oxidative stress and mitochondrial dysfunction generate superoxide radicals, which may decrease NO bioavailability [10,11] by forming toxic peroxynitrite anions (ONOO•-). Recently, we reported that the compound SA-2 (Figure 1), incorporating a robust NO donor (sydnonimine) combined with a super oxide dismutase (SOD) mimetic functional group (nitrone) produces NO and scavenges reactive oxygen species (ROS) in human umbilical vein endothelial cells [12]. We also reported that SA-2 activates SOD in 661W photoreceptor neural cells in vitro [13]. We furthermore demonstrated that SA-2 attenuated RGC death in vivo in the retinas of mice subjected to the optic nerve crush (ONC) procedure treated with SA-2 in comparison with mice with ONC and treated with vehicle [14]. We believe that the target tissue for NO-mediated IOP-lowering effects is the TM, which in POAG undergoes pathological changes, leading to increased IOP and subsequent progression to optic neuropathy. Potentially, NO released from a NO donor such as SA-2 can enter the cells of the TM and the inner endothelial wall of Schlemm’s canal (SC), increasing the production of cGMP and leading to activation of protein kinase G and dephosphorylation of myosin light chain resulting in efflux of potassium ions through big potassium calcium-activated (BKCa) channels. Together, these changes could promote a decrease in TM cell contractility and volume, as well as rearrangement of the actin cytoskeleton and enhanced conventional outflow facility [15], leading to a decrease in IOP. Additionally, the ROS scavenging ability of SA-2 may be capable of protecting the human trabecular meshwork (hTM) cells from oxidative stress–induced cell death. In the present study, we tested whether the hybrid compound SA-2 was capable of elevating cGMP and SOD in hTM cells and was cytoprotective to hTM cells exposed to oxidative stress. We also determined whether nanoencapsulated slow release formulation of SA-2 (SA-2 NPs) can effectively lower IOP in normotensive and ocular hypertensive (OHT) rodent eyes and provide a longer duration of action in comparison with a free non-encapsulated SA-2 compound.
Figure 1.
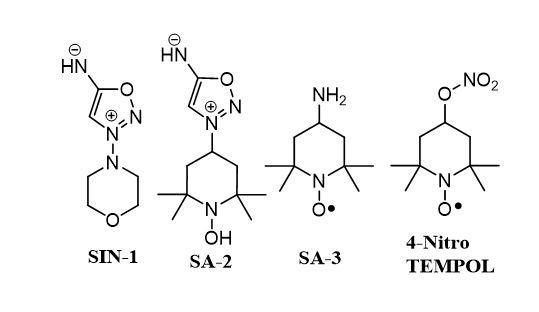
Chemical structures of the compounds. 4-Nitro TEMPOL, Linsidomine (SIN-1), SA-2, and 4-amino TEMPOL (SA-3).
Methods
cGMP assay
Previously characterized human primary TM cells (hTM-80 and hTM-34) that were obtained from human donor eyes (age 80 and 34 years old) with no history of ocular disease or surgery were used in these studies [16-18]. TM cells were obtained from whole human donor eyes purchased from the Lions Eye Institute (Tampa, FL) within 24 to 30 h of death or from corneal scleral rims that were stored in corneal storage medium (Optisol; Chiron Ophthalmics, Irvine, CA) at 4 °C. Human tissue use was approved by UNTHSC Institutional Review Board. All human tissue was handled in accordance to the tenets of the Declaration of Helsinki and the ARVO statement on human subjects. Passages 3 to 5 of the hTM cells were grown in six-well culture dishes at 37 °C in 5% CO2 in low-glucose Dulbecco's modified Eagle medium (DMEM) Cat. No. 11885084, Gibco, Frederick, MD) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S). Two days before the experiments, the cell medium was changed to serum-free media and subsequently treated with SA-2 (100 µM, 200 µM, and 1,000 µM), SA-2 NPs (0.1% equivalent to 250 µM of SA-2), or vehicle Dulbecco’s Phosphate Buffered Saline (Cat. No. 59331C, Millipore-Sigma) for 30 min. The cell lysates were used to assess cGMP using the enzyme immunoassay kit from Cayman Chemicals (Ann Arbor, MI) following the manufacturer’s instructions. The half maximal effective concentration (EC50) was calculated using GraphPad Prism 7.0 (San Diego, CA).
Superoxide dismutase activity assay
Human primary TM cells were maintained in serum-free DMEM for 2 days and treated overnight with vehicle or 100 µM of SIN-1 (linsidomine), a known NO donor, or SA-3 (4-amino TEMPOL, Millipore Sigma, St. Louis, MO; Figure 1), a known SOD mimetic/antioxidant and ROS scavenger, or SA-2 (100 µM). Cell lysates were analyzed using a commercially available kit from Cayman Chemicals following the manufacturer’s instruction.
Determination of ROS activity
Human primary TM cells were seeded in a dark, clear bottom 96-well microplate with 25,000 cells per well and were allowed to adhere overnight. Cells were co-treated with tert-butyl hydrogen peroxide (TBHP, Millipore Sigma) for 30 min and compounds (SIN-1, SA-2, or SA-3). After 1 h of treatment, cytosolic ROS levels were measured using 6-carboxy-2',7'-dichlorodihydrofluorescein diacetate (DCF, Millipore Sigma) following the manufacturer’s protocol on a ultraviolet (UV)–vis spectrometer (Infinite M200 plate reader, Tecan, Durham, NC) at a wavelength of 485/530 nm (excitation/emission). The fluorescence intensities of DCF were proportionated to the cytosolic ROS.
Cell proliferation and cytoprotection assay
For the cell viability experiment, hTM cells were treated with 300 µM of TBHP (the dose was standardized previously in a dose–response study), in the absence or presence of SA-2 (1.0, 2.0, 4.0, 8.0, 16, 32, 64, 125, 250, 500, and 1,000 µM) for 24 h. CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI) was performed to estimate the number of viable cells. Similarly to previous assays, the cells were deprived of serum for 2 days before the assay, and all treatments were performed in serum-free media.
Animals
Animal studies were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) resolution for the Use of Animals in Ophthalmic and Vision Research and approved by the University of North Texas Health Science Center (UNTHSC) Institutional Animal Care and Use Committee (IACUC-2019–0036, IACUC-2018-0035, and IACUC-2017–0024). Retired breeder male Brown Norway rats (8 to 11 months old, n = 3/group) and adult (10-week-old) Sprague Dawley female rats n = 3/group) were obtained from Charles River (Wilmington, MA) and maintained in constant dim light conditions (90 lux) with food and water provided ad libitum. C57BL/6J mice (female, 10–12 weeks) were also used in the study (n = 5 animals/group were purchased from the Jackson Laboratory, Bar Harbor, ME). The mice were maintained on a 12 h:12 h light-dark cycle (lights on at 6:00 AM) with food and water available ad libitum.
Formulation and storage
Either free drug SA-2 or SA-2 NPs (weight/volume, w/v) was reconstituted in sterile PBS 7.4 (1X) and vortexed to obtain a colorless solution or a milky nanosuspension respectively. All formulations were freshly prepared before dosing, and leftover was discarded.
Ocular biodistribution after administration of SA-2NP eye drop
The ocular concentration of compound SA-2 was measured in the corneal, retinal, choroidal, and scleral tissues, aqueous humor (AH), and vitreous humor (VH) 1 h post dosing (30 µl topical ocular eye drop of 1% w/v SA-2 NPs, equivalent to 2.5 mM of free SA-2) in the rat eyes (n = 12). A modified protocol for mouse eyes [19] was used and the high performance liquid chromatography /mass spectrometry (HPLC/MS) method to detect SA-2 was developed in-house.
The Morrison glaucoma model in rats
The Morrison model of IOP elevation was used to induce elevated IOP in male retired breeder Brown Norway rats (8–11 months old) as we previously described [20-23]. The rats were anaesthetized with an injection of a cocktail of ketamine (100 mg/kg body wt), xylazine (5 mg/kg body wt), and acepromazine (10 mg/kg body wt). IOP was induced in one eye with a single injection 50 µl of 1.8 M saline through episcleral veins using a glass needle (TIP01TW1F, WPI) at a rate of 309 µl/min for 10–20 s), while the contralateral eye served as control.
The Ad5.CMV.hTGFß2C226/228S transduction OHT mouse model
The ocular hypertensive mouse model was generated as follows: C57BL/6J mice were anesthetized with isoflurane, 2.5%; O2(g) 0.8 l/min which was administered through a mask sized for use with mice. Following attainment of a surgical plane of anesthesia, each eye to be injected was given one to two drops of proparacaine HCl 0.5% (Alcaine, Alcon, Fort Worth, TX) as topical anesthetic. The globe was then proptosed, and a 0.5 inch 33-gauge needle (Hamilton Company, Reno, NV) with a 12° bevel, connected to a 10 μl glass microsyringe filled with a 2 μl bolus of Ad5.CMV.hTGFβ2C226/228S (2.5 × 107 pfu/μl, Gene Transfer Core Facility, University of Iowa, Iowa City, IA), was inserted through the equatorial sclera and into the vitreous, taking care not to touch the posterior lens capsule, lens, or retina. The bolus was then injected into the vitreous over a period of 15 to 30 s. Following the injection, the needle was left in place for an additional 30 s to allow time for mixing, after which time the needle was rapidly withdrawn. The animal was then returned to its cage and allowed to recover and develop IOP elevation.
Administration of eye drops and intraocular pressure measurements in rat eyes
Nine Sprague Dawley normotensive rats (n = 3 per group) were used to evaluate the IOP-lowering properties of SA-2 and SA-2 NPs under a masked protocol. Topical eye drops (30 µl/drop) containing vehicle (Saline), Travoprost (0.004%)-positive control, SA-2 (75 mM), or SA-2 NPs (1%, 2.5 mM of SA-2) were administered in three rats, and IOP was measured using the TonoLab rebound tonometer (TonoLab; Tiolat Oy; Helsinki, Finland) as described previously [22] at various time points: 0, 3, 6, 24, 48, and 72 h post-dosing. The entire dosing schedule was repeated three times in each rat following 7-day drug wash-out. IOP plots were generated from IOP values obtained from the treated eye and the contralateral control eye.
Administration of eye drops and intraocular pressure measurements in mouse eyes
Five days following intravitreal injection of Ad5.CMV.hTGFβ2C226/228S, each animal was given a single drop (topical-ocular) of SA-2-NP (1%) suspension (2 µl) in the IOP-elevated eye only, while the contralateral eye was left untreated as a control. IOP was measured and recorded in conscious animals at various time points up to 54 h with TonoLab rebound tonometry. All IOP measurements were conducted between 10:00 AM and 12:00 PM. Six consecutive IOP measurements were taken in a single sitting and averaged to obtain an IOP value using the TonoLab rebound tonometer (TonoLab; Tiolat Oy). Following the final IOP measurements, aqueous outflow facility was measured.
Outflow facility assessment: C measurement and Pe estimation
For measurement of aqueous humor outflow facility (C) and estimation of episcleral venous pressure (Pe), animals were anesthetized with an intraperitoneal injection of an anesthetic cocktail composed of ketamine (72.7 mg/kg, Fort Dodge Animal Health, Fort Dodge, IA), xylazine (1.8 mg/kg, Vetus; Butler Animal Health Supply, Westbury, NY), and acepromazine (1.8 mg/kg, Butler Animal Health Supply). Maintenance doses of anesthetic cocktail (one half to one fourth the induction dose) were given subsequently as required. Each animal was then placed on a heated pad (37 °C) for maintenance of body temperature and left for 30 min to develop a suitable plane of surgical anesthesia, as judged by the absence of the blink reflex and the hind limb flexor withdrawal response to a toe pinch. After development of a surgical plane of anesthesia, the IOP of each eye was measured with a rebound tonometer (TonoLab; Colonial Medical Supply, Franconia, NH), as described previously [24]. For local anesthesia, one to two drops of proparacaine HCl 0.5% (Alcaine; Alcon) was applied topically to each eye. The eyes were then cannulated intracamerally with a 30-gauge steel needle inserted through the peripheral cornea approximately 1 to 2 mm from the limbus and pushed toward the region of the opposing chamber angle, taking care not to strike the corneal endothelium, anterior surface of the iris, anterior lens capsule, or chamber angle itself. The needle was connected via PE60 tubing filled with PBS to a flow-through pressure transducer (BLPR; World Precision Instruments, Sarasota, FL) for the continuous determination of pressure within the system. The opposing terminal of the pressure transducer was connected via further tubing to a three-way valve, the other two ports of which were connected to a PBS-filled 50 μl glass microsyringe (Hamilton Company) loaded into a microdialysis infusion pump (SP101I Syringe Pump; World Precision Instruments (WPI), Sarasota, FL) and a PBS-filled open-ended, variable-height, raised reservoir manometer. When the three-way valve was adjusted to switch the manometer into the circuit, adjusting the height of the reservoir relative to the eye allowed for artificial manipulation of intracameral pressure.
The following steps were then implemented in succession: (1) estimation of Pe and (2) measurement of C. For estimation of Pe, using the manometers, intracameral pressure was adjusted to precannulation but post-anesthesia values as estimated with tonometry. The eyes were then given approximately 5 min to equilibrate, after which time intracameral pressure was dropped at the rate of 1 mmHg/min, while the anterior segment of the eye was visualized through a dissection microscope at 30X magnification, under powerful illumination. Following each stepwise drop in intracameral pressure, the episcleral veins and Schlemm’s canal were observed carefully, until after sufficient drops in pressure, the intracameral pressure was approximately equal to the episcleral venous pressure, as evidenced by visible injection with blood of the episcleral veins and Schlemm’s canal. When this occurred, intracameral pressure was read from the pressure transducer and was accepted as a reasonable estimate for Pe. For measurement of C, intracameral pressure was then reset to equal precannulation IOP using the manometer once again. The three-way valve was then adjusted to switch the manometer out of, and the infusion pump in the circuit. The pump was then switched on and set to a flow rate of 0.1 μl/min. The pump remained running until the pressure (continuously recorded via the BLPR2 transducers) in the system stabilized (typically within 15–20 min). Pressure was then recorded for an additional 10 min, and then the flow rate was increased sequentially in 0.1 μl/min increments, until it reached 0.5 μl/min. Stabilized pressure values at each flow rate were recorded over a 10-min period. For each eye, aqueous humor outflow facility was calculated as the reciprocal of the slope of the respective pressure-flow rate curves [25,26].
Results
In the present study, we found that compound SA-2 increased cGMP levels in primary hTM cells (Figure 2A), increased SOD enzyme levels (Figure 3A), and protected the hTM cells from TBHP-induced oxidative stress (Figure 4). SA-2 formulated as a poly D, L-lactic-co-glycolic acid (PLGA)-encapsulated nanosuspension with a slow drug release profile lowered IOP in normotensive and ocular hypertensive rodent eyes following topical ocular dosing (Figure 5 and 6).
Figure 2.
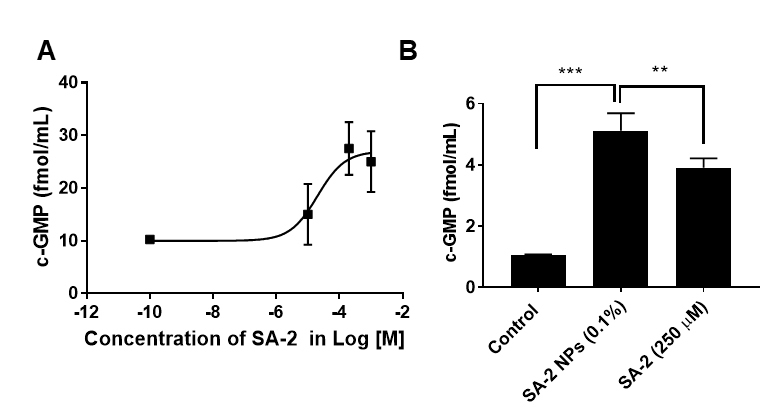
Cyclic guanosine monophosphate (cGMP) levels in primary human trabecular meshwork (hTM) cells. A:SA-2 (100, 200, 1,000 µM) increased the cyclic guanosine monophosphate (cGMP) level in primary human trabecular meshwork (hTM) cells. hTM cells (hTM-80, hTM-34) were treated with SA-2 (100 µM, 200 µM, and 1,000 µM) or vehicle for 30 min. cGMP was assayed using the cell lysate using the enzyme immunoassay kit from Cayman Chemicals (Ann Arbor, MI) following the manufacturer’s instructions. The EC50 was calculated to be 20.3 µM using GraphPad Prism 7.0. B: SA-2 NPs (0.1% equivalent to 250 µM of SA-2) increased cGMP in hTM cells. *p<0.05, ***p<0.001, unpaired t test. Experiments were performed in triplicate with n = 4 technical replicates. The results showed that SA-2 and SA-2 NPs were able to significantly increase cGMP levels in two different types of primary hTM cells. Values are expressed in mean ± standard error of the mean (SEM).
Figure 3.
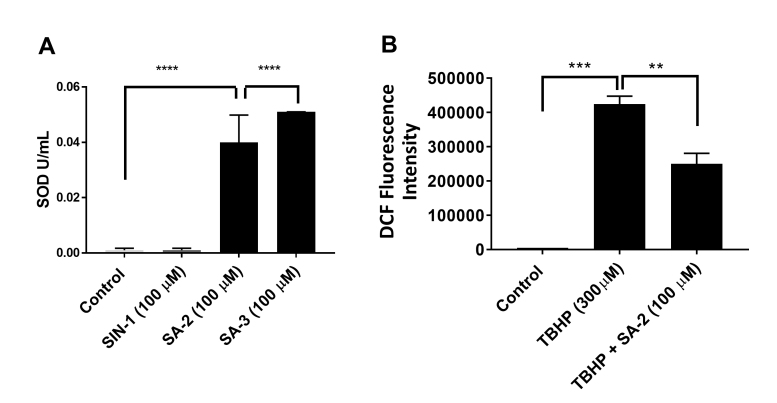
Superoxide dismutase (SOD) enzyme level in human trabecular meshwork (hTM)-80 cells. A: SA-2 increased the SOD enzyme level in hTM-80 cells. Compound SIN-1 (linsidomine, a NO donor) was used as negative control and did not increase the SOD enzyme in hTM-80 cells (as expected) after 24 h. B: SA-2 decreased the reactive oxygen species (ROS) level in hTM cells observed after 1 h. *p<0.01, **p = 0.0072, ***p = 0.0005, ****p<0.0001, one-way analysis of variance (ANOVA), Dunnett’s multiple comparisons test. Experiments were performed in triplicate with n = 4 technical replicates. Values are expressed in mean ± standard error of the mean (SEM).
Figure 4.
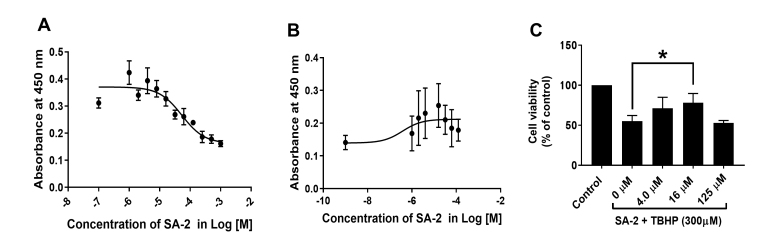
Viability of human trabecular meshwork (hTM) cells. A:SA-2 dose-dependently increased the number of viable hTM cells and exhibited no toxicity up to 125 µM. The half maximal effective concentration (EC50) was calculated to be 53.5 µM. B: SA-2 dose-dependently increased the number of viable hTM cells in the presence of tert-butyl hydroperoxide (TBHP, 300 µM). The EC50 was calculated to be 0.38 µM. C: SA-2 dose-dependently protected hTM cells against TBHP (300 µM) and induced cell proliferation up to the 16 µM dose. hTM cell viability is represented as % of control. Experiments were performed in triplicate with n = 4 technical replicates. Values are expressed in mean ± standard error of the mean (SEM). *p<0.01, one-way analysis of variance (ANOVA).
Figure 5.
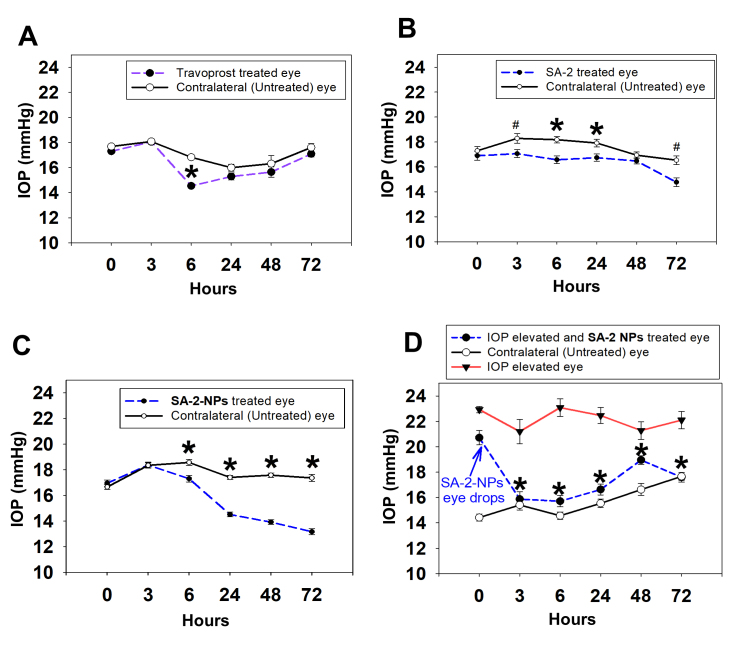
Intraocular pressure profiles in rats treated with SA-2, Travoprost and SA2 NPs eye drops. Effect of single dose topically administered SA-2 (75 mM) and SA-2 NPs (1%, 2.5 mM of SA-2) eye drop formulated in PBS in normotensive (A–C, n = 3, Sprague Dawley) and the Morrison intraocular pressure (IOP) elevation model (D, n = 3,, Brown Norway) rats at 3, 6, 24, 48, and 72 h post dose. Travoprost (0.004%) was used as positive control. *p<0.001 and #p<0.05 (rank-sum test), n = 3 rats per group. Contralateral eyes were used as controls. Values are expressed in mean ± standard error of the mean (SEM).
Figure 6.
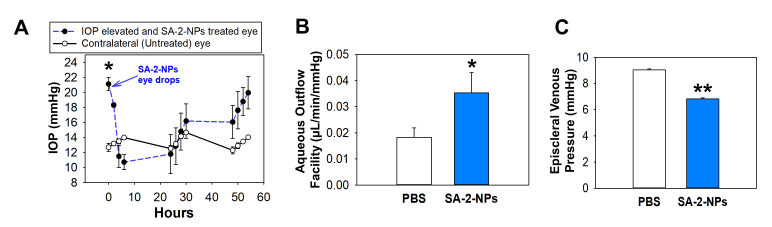
Assessment of aqueous humor dynamics in the mice treated with SA-2 NPs eye drops. A: The effect of SA-2 NPs (1%) topical eye drop administration in adenovirus 5 (Ad5) viral vector expressing mutated human TGFβc226s/c228s (Ad5.TGFβ-2) induced ocular hypertensive (OHT) mice eyes (n = 5) at various time points post dose. The decrease in intraocular pressure (IOP) was statistically significant up to 30 h. *p<0.001, one-way analysis of variance (ANOVA), followed by Dunnett’s post-hoc paired comparison. B: SA-2 NPs increased aqueous humor outflow facility in OHT mice, *p<0.002, paired t test. C: SA-2 NPs caused a decrease in episcleral venous pressure (Pe), **p<0.001, paired t test. Values are expressed in mean ± standard error of the mean (SEM).
cGMP release from hTM cells
Previously, using the Griess assay, we reported that [13] compound SA-2 releases NO similarly to the standard NO donor compound SIN-1 (Figure 1) at concentrations of 100 µM and above. In the present study, we demonstrated that SA-2 increased the cGMP level in hTM cells in a dose-dependent manner with an EC50 of 20.3 µM and plateaued at the 1 mM concentration (Figure 2A). We also tested SA-2 NPs (0.1%, equivalent to 250 µM of free SA-2 compound) in hTM cells for their ability to upregulate cGMP and as shown in Figure 2B. We found that the cGMP release mediated by 0.1% SA-2 NPs treatment was statistically significantly higher than that from non-encapsulated SA-2 (250 µM), suggesting that a sufficient amount of drug release is achievable after the nanosuspension formulation.
Increase in SOD enzyme and decrease in ROS after SA-2 treatment of hTM cells
SA-2 at 100 µM concentration significantly upregulated SOD in primary hTM-80 cells (Figure 3A) and the effect was comparable to that of a standard SOD mimetic SA-3 compound (Figure 1), where as expected, only NO donor SIN-1 did not increase the SOD level. We also noticed that when the hTM cells were treated with 300 µM of TBHP for 30 min, there was a statistically significant increase in ROS (as detected with DCF fluorescence intensity assay), and treatment with SA-2 (100 µM) significantly decreased the ROS level within 1 h by more than 40% (Figure 3B).
Increase in cell viability after SA-2 treatment of hTM cells
A dose–response study was conducted to determine the cytotoxicity of SA-2 in hTM cells after 24 h of treatment at different concentrations (EC50 of 53.5 µM, Figure 4A). TM cell viability was statistically significantly decreased when the cells were treated with 300 µM of TBHP (Figure 4C), and treatment with compound SA-2 statistically significantly and dose-dependently protected against loss of cell viability; the EC50 was calculated as 0.38 µM (Figure 4B). This provides about a 40-fold safety margin for SA-2 over the efficacy dose (0.38 µM vs. 53.5 µM) in hTM cells.
Bioavailability of SA-2 from SA-2 NPs eye drop in rat eyes
We conducted a biodistribution study of SA-2 NPs dosed as a nanosuspension eye drop in rat eyes to determine the concentration of SA-2 in the tissues of the posterior regions of the eye. Using the HPLC/MS method standardized in our laboratory and an established protocol for processing tissues and ocular fluids [19], we were able to quantify the amount of compound SA-2 in various ocular tissues, including the cornea, AH, VH, retina, choroid, and sclera (Table 1, n = 12 eyes) 1 h after dosing with a 30 µl topical ocular eye drop of 1% w/v SA-2 NPs, equivalent to 2.5 mM of free SA-2 in rat eyes. For this study, we selected a 1% dose (a ten-fold higher concentration than that used in vitro, 0.1% w/v SA-2NPs, Figure 2B), with the understanding that following the eye drop administration, there would be retention of only 5–10% volume of the instilled drug, while the remaining 90–95% would be lost via nasolacrimal drainage or systemic absorption. The results demonstrated the release of SA-2 from SA-2 NPs within 1 h after dosing and bioavailability to anterior and posterior segments, with the highest concentration of SA-2 being recoverable from VH (9.4 pg/mg) and the retina (9.7 pg/mg).
SA-2 and SA-2 NPs decreases IOP in rodent eyes
At the beginning of the experiment (before administration of SA-2), the IOP baseline was measured using a TonoLab rebound tonometer as published previously [22,24]. First, we assessed the ability of free SA-2 compound (75 mM) and 1% SA-2 NPs suspension (containing 2.5 mM of SA-2) to lower IOP in normotensive Sprague Dawley rats in comparison with positive control Travoprost (0.004%). We selected the 1% SA-2 dose for that study based on our recent report that 2% saline solution of SA-2 has an acceptable ocular safety profile and provided IOP independent neuroprotection in mouse eyes [14]. Following the administration of a single eye drop of the compounds, the IOP was monitored at various time points up to 72 h. Travoprost significantly lowered IOP (by 14%) in treated normotensive rat eyes only at the 6 h time point (Figure 5A), consistent with the literature [27]. Free compound SA-2 (Figure 5B) and SA-2NPs (Figure 5C) statistically significantly lowered IOP in normotensive-treated rat eyes compared to contralateral control eyes at 6, 24, 48, and 72 h. Vehicle (normal saline) eyes showed no differences in IOP with contralateral eyes (data not shown).
Next, we tested SA-2 NPs in two OHT rodent glaucoma models. In the Morrison rat model of OHT, which recapitulates the hallmark features of glaucoma, including the increase in IOP, RGC death, and optic nerve degeneration, IOP was statistically significantly lowered (by >30%) initially at 3–6 h post-dose, with prolonged duration of action (48–72 h) as shown in Figure 5D. The second OHT model in the mouse is achieved with an intravitreal injection of Ad5.CMV.hTGFβ2C226/228S viral particles. This model recapitulates the extracellular matrix (ECM) dysregulation in the TM and ROS-mediated injury that results in increased IOP [28]. A decrease in IOP (by 50%) occurred in this model rapidly within 3–6 h of SA-2 NPs administration and was maintained up to 30 h. Complete loss of IOP-lowering activity was observed by the 50 h time point in this model (Figure 6A).
Effect of SA-2 NPs treatment on aqueous humor dynamics and episcleral venous pressure
Additionally, we assessed various parameters of aqueous humor dynamics in the Ad5.CMV.hTGFβ2C226/228S-injected mice using our reported technique of constant flow infusion [25,26]. We found a statistically significant increase in outflow facility (Figure 6B), and a decrease in episcleral venous pressure (Figure 6C) in the OHT-SA-2 NPs-treated eyes when compared with the OHT and vehicle-treated (PBS) eyes. These data support our hypothesis that SA-2 may relax contractile elements within TM cells, thus increasing outflow facility of the aqueous humor and leading to a decrease in IOP. We measured outflow facility and episcleral venous pressure only in animals following SA-2 NPs treatment in one eye and PBS treatment in the contralateral eye. A theoretical possibility exists that systemic absorption of SA-2-NPs could have had some effect on the contralateral eye. However, previously we measured bilateral aqueous humor dynamics in various strains (A/J, BALB/cJ, C57BL/6J, C3H/HeJ) of untreated naïve mice. The PBS-treated (control) eyes in the present study in C57BL/6J animals showed similar (and not statistically significantly different) outflow facility and episcleral venous pressure values to age-matched C57BL/6J naïve animals which we reported [25].
Discussion
Current therapies for glaucoma include the use of prostaglandin analog-based IOP-lowering agents. However, about 10% of patients with glaucoma do not respond adequately to these therapies. Brimonidine, an α2 agonist, lowers IOP and has been shown to have neuroprotective properties [29], but it has the potential to cause many side effects, such as allergic reactions and corneal disorders [30,31]. IOP-lowering and neuroprotective activity of Rho kinase inhibitors was recently demonstrated in preclinical experimental models of glaucoma [32]; however, some adverse effects have been found to occur with this treatment. Along with IOP, an age-related decline in antioxidant enzymes in ocular tissues contributes to the death of RGCs and TM cells, which is not addressed by any of the commercially available glaucoma treatments. The NO system could potentially be targeted to enhance aqueous outflow facility by relaxing TM cells to lower IOP; however, an increase in superoxide levels can deplete the NO level by forming toxic peroxynitrite. Thus, the presence of a radical scavenger is beneficial to maintain the therapeutic level of NO. In support of this hypothesis, we previously reported [33] that the SOD mimetic-NO donating hybrid compound 4-nitro-TEMPOL (Figure 1) has superior IOP-lowering activity in a laser-induced glaucoma model in monkeys (by 20% at 6 h at the dose of 150 µg/eye drop) compared to only a NO donor such as glyceryl trinitrate (GTN, 13% at 6 h at the dose of 500 µg). We and others suggested that the presence of an N-oxide radical as in 4-nitro TEMPOL may be responsible for scavenging the surrounding ROS thus resulting in improved NO bioavailability and better IOP-lowering activity [34]. However, the 20% IOP-lowering effect observed with 4-nitro TEMPOL was not a therapeutically significant IOP-lowering effect. Similarly, the NO-donating prostaglandin analog Vyzulta (LBN) was recently approved by the FDA for POAG therapy due to Vyzulta’s ability to decrease IOP [8]. However, Vyzulta has not yet been demonstrated to be neuroprotective. The difference in the IOP-lowering ability of LBN (−9.0 mm/Hg) versus latanoprost (−7.7 mm/Hg) indicates that the potency of the nitrate ester in LBN may be due to its NO-donating ability, allowing an additional IOP decrease within a range of 1–2 mm/Hg. In principle, the nitrate ester as present in LBN or in 4-nitro TEMPOL requires thiol-mediated enzymatic bioactivation to release NO. In contrast, the compound SA-2 is a spontaneous pH-dependent NO donor and releases NO at biologic pH without requiring any enzymatic activation. Along with the NO-donating activity, SA-2 is a SOD mimetic (containing the N-oxide radical) antioxidant. SOD is required to dismute the superoxide radicals and is found to decline age-dependently in the TM [35]. There is a correlation between the decrease in the antioxidant activities in the TM and the progression of POAG [36]. Cultured TM cells exposed to hydrogen peroxide (H2O2), display a reduction in cell adhesion to the growth matrix, followed by cell death. Repeated oxidative insult also leads to compromised tissue integrity and enhanced expression of inflammatory markers [37,38]. When compared with healthy age-matched controls, it has been found that circulating plasma levels of the reduced glutathione (rGSH) antioxidant were consistently lower in patients with POAG [39]. Additional reports indicate a significantly higher level of oxidative DNA damage to nuclear and mitochondrial DNA in TM tissue in glaucoma versus healthy human eyes [40-42]. Additionally, a comparison between human TM specimens from age-matched normal and glaucomatous eyes showed significantly fewer TM cells in the glaucomatous tissue [43]. Iomdina et al. demonstrated that topically administered 10-(6’-plastoquinonyl) decyltriphenylphosphonium (SkQ1), an antioxidant that has direct ROS scavenging activity, was able to decrease IOP and promote neuroprotection of RGCs in rabbits [44]. We recently reported that compound SA-2 increased SOD levels in the mouse retina and minimized the loss of RGCs from ischemic reperfusion–induced cell death, as well as optic nerve crush injury–mediated loss of RGCs demonstrating its strong IOP-independent neuroprotective properties in vivo [14]. This process was partially reversed when a SOD inhibitor was used alone and in combination with SA-2 demonstrating the neuroprotective role of SA-2 is due to the presence of the SOD mimetic functional group. In the present study, in normal hTM cells, compound SA-2 inhibited TBHP-induced cell death, possibly via upregulating SOD and scavenging the ROS generated from TBHP. We acknowledge that glaucomatous hTM cells may have a different response to SA-2 treatment and be more vulnerable to cell death than normal hTM cells when exposed to the cytotoxic insult. We carefully considered various limitations of the use of glaucomatous hTM cells, including the difficulties in maintaining a viable cell culture for more than several passages [45], an increase in endoplasmic reticulum (ER) stress markers [46], possible genetic mutations [47], and previous exposure to glaucomatous drugs. To overcome those variations, we decided to use normal hTM cells in combination with a well-established oxidative stress, glaucoma-like insult induced with stable hydrogen peroxide, TBHP [48]. Along with the cytoprotective activity, the observed IOP-lowering effect in two animal models of OHT is possibly via the release sufficient amounts of NO, the increase in cGMP, and relaxation of TM cells, resulting in an increase in aqueous humor outflow.
Poor patient compliance with non-adherence to eye drop–dosing regimens compromises treatment outcomes in glaucoma. Estimates of non-compliance for patients with glaucoma range from 23% to 60% [49] resulting in progressive vision loss and eventual blindness. To mitigate these patient compliance issues, many sustained release modalities are being researched and are entering the clinic to provide options to patients to instill eye drops either once a day or following longer intervals [50]. Ibrahim et al. [51] reported a sustained release brimonidine nanogel formulation using PLGA as the drug delivery vehicle, where they demonstrated that the IOP-lowering effect of brimonidine can last up to 12 h anticipating to translate into once a day dosing regimen for patients with glaucoma and potentially help in minimizing patient compliance issues in the future. In the present study, with our unique PLGA-encapsulated nanosuspension formulation of compound SA-2 (SA-2 NPs) [12], the IOP-lowering effect could be achieved for up to 30 and 72 h in mouse and rat eyes, respectively. We prepared PLGA-encapsulated nanoparticles according to our previously reported protocol [12] that provided a sustained SA-2 release profile at 37 °C. The release composed of a burst release (16% release of SA-2 within 2 days), followed by nearly 35% of a slow release over 30 days [12]. A biodistribution study in rat eyes demonstrated that within 1 h of installation of a single nanosuspension eye drop, there is a measurable quantity of SA-2 (picomolar) in anterior and posterior segments of the eye (Table 1). This result indicates that the SA-2 NPs formulation delivered topically reaches the aqueous humor and the TM region. Based on this preliminary biodistribution study, we assume that SA-2-NPs may create a depot in vitreous humor, from where SA-2 NPs is released to the anterior chamber as well as to the retina which can explain the longer duration of IOP-lowering activity. Additionally, we predict that the SA-2 NPs eye drop will provide therapeutic concentration of SA-2 in the retina comparable to that we reported previously via intravitreal dosing of SA-2 [14]. However, further studies with different doses of SA-2 NPs and time points are needed to determine the full pharmacokinetic parameters and drug release profile in larger animal eyes.
Table 1. Bioavailability of SA-2 in the rat ocular tissue and fluids 1 h post dosing of 30 µl topical ocular eye drop of 1% w/v SA-2 NPs (n=12).
| SA-2 NPs dose | Cornea | AH | VH | Retina | Choroid + Sclera |
|---|---|---|---|---|---|
| 1% (w/v, 2.5 mM SA-2) | 2.6 pg/mg | 1.5 pg/mg | 9.4 pg/mg | 9.7 pg/mg | 3.37 pg/mg |
The present results demonstrated that in human primary TM cells, the hybrid NO donor-SOD mimetic compound SA-2 increased cGMP and SOD levels and protected hTM cells from TBHP-induced oxidative stress-mediated cell death. To provide sustained release and longer duration of action (>3 days) in ocular tissues, a PLGA-encapsulated nanosuspension of SA-2 was prepared with a slow drug release profile over days. The nanoencapsulated SA-2 NPs (1%) instilled as eye drops in rat eyes were bioavailable in the retina, vitreous humor, aqueous humor, as well as corneal and choroidal tissue at the picogram per milligram level after 1 h of post dosing. A single dose of eye drop containing 1% SA-2 NPs reduced IOP in the Morrison OHT rat model and the mouse Ad5.CMV.hTGFβ2C226/228S OHT mouse model. The IOP-lowering effect lasted up to 30 h in mice and 72 h in rats indicating the slow SA-2 release provided the desired therapeutic effect. These studies provide the basic foundation for developing a novel, efficacious, and long-acting antiglaucoma topical eye medication that may provide neuroprotective and IOP-lowering activity.
Acknowledgments
This work is funded by the grant awarded to SA from Bright Focus Foundation (G2018056) and to both SA and DS from National Institute of Health (R01EY029823), USA. We thank Prof. Thomas Yorio for several helpful discussions and manuscript review.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Pascale A, Drago F, Govoni S. Protecting the retinal neurons from glaucoma: lowering ocular pressure is not enough. Pharmacol Res. 2012;66:19–32. doi: 10.1016/j.phrs.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Jeoung JW, Kim DM, Oh S, Lee JS, Park SS, Kim JY. The Relation Between Endothelial Nitric Oxide Synthase Polymorphisms and Normal Tension Glaucoma. J Glaucoma. 2017;26:1030–5. doi: 10.1097/IJG.0000000000000751. [DOI] [PubMed] [Google Scholar]
- 5.Kang JH, Wiggs JL, Rosner BA, Hankinson SE, Abdrabou W, Fan BJ, Haines J, Pasquale LR. Endothelial Nitric Oxide Synthase Gene Variants and Primary Open-Angle Glaucoma: Interactions with Sex and Postmenopausal Hormone Use. Invest Ophthalmol Vis Sci. 2010;51:971–9. doi: 10.1167/iovs.09-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doganay S, Evereklioglu C, Turkoz Y, Er H. Decreased nitric oxide production in primary open-angle glaucoma. Eur J Ophthalmol. 2002;12:44–8. doi: 10.1177/112067210201200109. [DOI] [PubMed] [Google Scholar]
- 7.Gabelt BAT, Kaufman PL, Rasmussen CA. Effect of nitric oxide compounds on monkey ciliary muscle in vitro. Exp Eye Res. 2011;93:321–7. doi: 10.1016/j.exer.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Addis VM, Miller-Ellis E. Latanoprostene bunod ophthalmic solution 0.024% in the treatment of open-angle glaucoma: design, development, and place in therapy. Clin Ophthalmol. 2018;12:2649–57. doi: 10.2147/OPTH.S156038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madhusoodanan KS, Murad F. NO-cGMP signaling and regenerative medicine involving stem cells. Neurochem Res. 2007;32:681–94. doi: 10.1007/s11064-006-9167-y. [DOI] [PubMed] [Google Scholar]
- 10.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci. 2011;120:357–75. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witting PK, Rayner BS, Wu BJ, Ellis NA, Stocker R. Hydrogen peroxide promotes endothelial dysfunction by stimulating multiple sources of superoxide anion radical production and decreasing nitric oxide bioavailability. Cell Physiol Biochem. 2007;20:255–68. doi: 10.1159/000107512. [DOI] [PubMed] [Google Scholar]
- 12.Le DQ, Kuriakose AE, Nguyen DX, Nguyen KT, Acharya S. Hybrid Nitric Oxide Donor and its Carrier for the Treatment of Peripheral Arterial Diseases. Sci Rep. 2017;7:8692. doi: 10.1038/s41598-017-08441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acharya S, Rogers P, Krishnamoorthy RR, Stankowska DL, Dias HV, Yorio T. Design and synthesis of novel hybrid sydnonimine and prodrug useful for glaucomatous optic neuropathy. Bioorg Med Chem Lett. 2016;26:1490–4. doi: 10.1016/j.bmcl.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 14.Stankowska DL, Dibas A, Li L, Zhang W, Krishnamoorthy VR, Chavala SH, Nguyen TP, Yorio T, Ellis DZ, Acharya S. Hybrid Compound SA-2 is Neuroprotective in Animal Models of Retinal Ganglion Cell Death. Invest Ophthalmol Vis Sci. 2019;60:3064–73. doi: 10.1167/iovs.18-25999. [DOI] [PubMed] [Google Scholar]
- 15.Ellis DZ, Sharif NA, Dismuke WM. Endogenous regulation of human Schlemm’s canal cell volume by nitric oxide signaling. Invest Ophthalmol Vis Sci. 2010;51:5817–24. doi: 10.1167/iovs.09-5072. [DOI] [PubMed] [Google Scholar]
- 16.Ellis DZ, Dismuke WM, Chokshi BM. Characterization of Soluble Guanylate Cyclase in NO-Induced Increases in Aqueous Humor Outflow Facility and in the Trabecular Meshwork. Invest Ophthalmol Vis Sci. 2009;50:1808–13. doi: 10.1167/iovs.08-2750. [DOI] [PubMed] [Google Scholar]
- 17.Stamer WD, Roberts BC, Howell DN, Epstein DL. Isolation, culture, and characterization of endothelial cells from Schlemm’s canal. Invest Ophthalmol Vis Sci. 1998;39:1804–12. [PubMed] [Google Scholar]
- 18.Dismuke WM, Mbadugha CC, Ellis DZ. NO-induced regulation of human trabecular meshwork cell volume and aqueous humor outflow facility involve the BKCa ion channel. Am J Physiol Cell Physiol. 2008;294:C1378–86. doi: 10.1152/ajpcell.00363.2007. [DOI] [PubMed] [Google Scholar]
- 19.Tse DY, Kim SJ, Chung I, He F, Wensel TG, Wu SM. The ocular toxicity and pharmacokinetics of simvastatin following intravitreal injection in mice. Int J Ophthalmol. 2017;10:1361–9. doi: 10.18240/ijo.2017.09.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minton AZ, Phatak NR, Stankowska DL, He S, Ma H-Y, Mueller BH, Jiang M, Luedtke R, Yang S, Brownlee C, Krishnamoorthy RR, Endothelin B. Receptors Contribute to Retinal Ganglion Cell Loss in a Rat Model of Glaucoma. PLoS One. 2012;7:e43199. doi: 10.1371/journal.pone.0043199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phatak NR, Stankowska DL, Krishnamoorthy RR. Bcl-2, Bcl-xL, and p-AKT are involved in neuroprotective effects of transcription factor Brn3b in an ocular hypertension rat model of glaucoma. Mol Vis. 2016;22:1048–61. [PMC free article] [PubMed] [Google Scholar]
- 22.Stankowska DL, Minton AZ, Rutledge MA, Mueller BH, 2nd, Phatak NR, He S, Ma HY, Forster MJ, Yorio T, Krishnamoorthy RR. Neuroprotective effects of transcription factor Brn3b in an ocular hypertension rat model of glaucoma. Invest Ophthalmol Vis Sci. 2015;56:893–907. doi: 10.1167/iovs.14-15008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stankowska DL, Nam M-H, Nahomi RB, Chaphalkar RM, Nandi SK, Fudala R, Krishnamoorthy RR, Nagaraj RH. Systemically administered peptain-1 inhibits retinal ganglion cell death in animal models: implications for neuroprotection in glaucoma. Cell Death Dis. 2019;5:112. doi: 10.1038/s41420-019-0194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W-H, Millar JC, Pang I-H, Wax MB, Clark AF. Noninvasive Measurement of Rodent Intraocular Pressure with a Rebound Tonometer. Invest Ophthalmol Vis Sci. 2005;46:4617–21. doi: 10.1167/iovs.05-0781. [DOI] [PubMed] [Google Scholar]
- 25.Millar JC, Phan TN, Pang IH, Clark AF. Strain and Age Effects on Aqueous Humor Dynamics in the Mouse. Invest Ophthalmol Vis Sci. 2015;56:5764–76. doi: 10.1167/iovs.15-16720. [DOI] [PubMed] [Google Scholar]
- 26.Millar JC, Clark AF, Pang IH. Assessment of aqueous humor dynamics in the mouse by a novel method of constant-flow infusion. Invest Ophthalmol Vis Sci. 2011;52:685–94. doi: 10.1167/iovs.10-6069. [DOI] [PubMed] [Google Scholar]
- 27.Ota T, Murata H, Sugimoto E-i, Aihara M, Araie M. Prostaglandin Analogues and Mouse Intraocular Pressure: Effects of Tafluprost, Latanoprost, Travoprost, and Unoprostone, Considering 24-Hour Variation. Invest Ophthalmol Vis Sci. 2005;46:2006–11. doi: 10.1167/iovs.04-1527. [DOI] [PubMed] [Google Scholar]
- 28.Shepard AR, Millar JC, Pang IH, Jacobson N, Wang WH, Clark AF. Adenoviral gene transfer of active human transforming growth factor-β2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Invest Ophthalmol Vis Sci. 2010;51:2067–76. doi: 10.1167/iovs.09-4567. [DOI] [PubMed] [Google Scholar]
- 29.Galanopoulos A, Goldberg I. Clinical efficacy and neuroprotective effects of brimonidine in the management of glaucoma and ocular hypertension. Clin Ophthalmol. 2009;3:117–22. [PMC free article] [PubMed] [Google Scholar]
- 30.Blondeau P, Rousseau JA. Allergic reactions to brimonidine in patients treated for glaucoma. Can J Ophthalmol. 2002;37:21–6. doi: 10.1016/s0008-4182(02)80094-1. [DOI] [PubMed] [Google Scholar]
- 31.Maruyama Y, Ikeda Y, Yokoi N, Mori K, Kato H, Ueno M, Kinoshita S, Sotozono C. Severe Corneal Disorders Developed After Brimonidine Tartrate Ophthalmic Solution Use. Cornea. 2017;36:1567–9. doi: 10.1097/ICO.0000000000001370. [DOI] [PubMed] [Google Scholar]
- 32.Tanna AP, Johnson M. Rho Kinase Inhibitors as a Novel Treatment for Glaucoma and Ocular Hypertension. Ophthalmology. 2018;125:1741–56. doi: 10.1016/j.ophtha.2018.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hellberg MR, Klimko PG, May JA, Mohapatra S. Ophthalmic composition with nitric oxide donor compound and method of forming and using same. 2020: US 20100197702A1. [Google Scholar]
- 34.Anggard EE, Abdullah Ibrahim H-Y. Piperidine and pyrrolidine derivatives comprising a nitric oxide donor for treating stress. 2020:US 20026455542. [Google Scholar]
- 35.De La Paz MA, Epstein DL. Effect of age on superoxide dismutase activity of human trabecular meshwork. Invest Ophthalmol Vis Sci. 1996;37:1849–53. [PubMed] [Google Scholar]
- 36.Hohberger B, Welge‐Lüßen UC, Yu A. Trabecular Meshwork and Intraocular Pressure Dynamics: Oxidative Stress‐Induced Changes. In: Ichhpujani P, editor. Glaucoma - Intraocular Pressure and Aqueous Dynamics. Rijeka: InTech; 2016. p. Ch. 02. [Google Scholar]
- 37.Li G, Luna C, Liton PB, Navarro I, Epstein DL, Gonzalez P. Sustained stress response after oxidative stress in trabecular meshwork cells. Mol Vis. 2007;13:2282–8. [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou L, Li Y, Yue BY. Oxidative stress affects cytoskeletal structure and cell-matrix interactions in cells from an ocular tissue: the trabecular meshwork. J Cell Physiol. 1999;180:182–9. doi: 10.1002/(SICI)1097-4652(199908)180:2<182::AID-JCP6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 39.Gherghel D, Griffiths HR, Hilton EJ, Cunliffe IA, Hosking SL. Systemic reduction in glutathione levels occurs in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2005;46:877–83. doi: 10.1167/iovs.04-0777. [DOI] [PubMed] [Google Scholar]
- 40.Izzotti A, Sacca SC, Longobardi M, Cartiglia C. Mitochondrial damage in the trabecular meshwork of patients with glaucoma. Arch Ophthalmol. 2010;128:724–30. doi: 10.1001/archophthalmol.2010.87. [DOI] [PubMed] [Google Scholar]
- 41.Sacca SC, Pascotto A, Camicione P, Capris P, Izzotti A. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005;123:458–63. doi: 10.1001/archopht.123.4.458. [DOI] [PubMed] [Google Scholar]
- 42.Izzotti A, Saccà SC, Cartiglia C, De Flora S. Oxidative deoxyribonucleic acid damage in the eyes of glaucoma patients. Am J Med. 2003;114:638–46. doi: 10.1016/s0002-9343(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 43.Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984;91:564–79. doi: 10.1016/s0161-6420(84)34248-8. [DOI] [PubMed] [Google Scholar]
- 44.Iomdina EN, Khoroshilova-Maslova IP, Robustova OV, Averina OA, Kovaleva NA, Aliev G, Reddy VP, Zamyatnin AA, Jr, Skulachev MV, Senin II, Skulachev VP. Mitochondria-targeted antioxidant SkQ1 reverses glaucomatous lesions in rabbits. Front Biosci (Landmark Ed) 2015;20:892–901. doi: 10.2741/4343. [DOI] [PubMed] [Google Scholar]
- 45.Keller KE, Bhattacharya SK, Borrás T, Brunner TM, Chansangpetch S, Clark AF, Dismuke WM, Du Y, Elliott MH, Ethier CR, Faralli JA, Freddo TF, Fuchshofer R, Giovingo M, Gong H, Gonzalez P, Huang A, Johnstone MA, Kaufman PL, Kelley MJ, Knepper PA, Kopczynski CC, Kuchtey JG, Kuchtey RW, Kuehn MH, Lieberman RL, Lin SC, Liton P, Liu Y, Lütjen-Drecoll E, Mao W, Masis-Solano M, McDonnell F, McDowell CM, Overby DR, Pattabiraman PP, Raghunathan VK, Rao PV, Rhee DJ, Chowdhury UR, Russell P, Samples JR, Schwartz D, Stubbs EB, Tamm ER, Tan JC, Toris CB, Torrejon KY, Vranka JA, Wirtz MK, Yorio T, Zhang J, Zode GS, Fautsch MP, Peters DM, Acott TS, Stamer WD. Consensus recommendations for trabecular meshwork cell isolation, characterization and culture. Exp Eye Res. 2018;171:164–73. doi: 10.1016/j.exer.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peters JC, Bhattacharya S, Clark AF, Zode GS. Increased Endoplasmic Reticulum Stress in Human Glaucomatous Trabecular Meshwork Cells and Tissues. Invest Ophthalmol Vis Sci. 2015;56:3860–8. doi: 10.1167/iovs.14-16220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiggs JL. Genetic etiologies of glaucoma. Arch Ophthalmol. 2007;125:30–7. doi: 10.1001/archopht.125.1.30. [DOI] [PubMed] [Google Scholar]
- 48.Yu AL, Fuchshofer R, Kampik A, Welge-Lüssen U. Effects of oxidative stress in trabecular meshwork cells are reduced by prostaglandin analogues. Invest Ophthalmol Vis Sci. 2008;49:4872–80. doi: 10.1167/iovs.07-0984. [DOI] [PubMed] [Google Scholar]
- 49.Richardson C, Brunton L, Olleveant N, Henson DB, Pilling M, Mottershead J, Fenerty CH, Spencer AF, Waterman H. A study to assess the feasibility of undertaking a randomized controlled trial of adherence with eye drops in glaucoma patients. Patient Prefer Adherence. 2013;7:1025–39. doi: 10.2147/PPA.S47785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gooch N, Molokhia SA, Condie R, Burr RM, Archer B, Ambati BK, Wirostko B. Ocular drug delivery for glaucoma management. Pharmaceutics. 2012;4:197–211. doi: 10.3390/pharmaceutics4010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ibrahim MM, Abd-Elgawad AE, Soliman OA, Jablonski MM. Novel topical ophthalmic formulations for management of glaucoma. Pharm Res. 2013;30:2818–31. doi: 10.1007/s11095-013-1109-1. [DOI] [PubMed] [Google Scholar]