Abstract
Purpose
Iron is essential to energy metabolism, cell proliferation and DNA synthesis, and sufficient iron availability may be required for tumor growth. The hormone hepcidin is a systemic regulator of iron concentration in plasma. Intra-tumor RNA expression of hepcidin has been linked to shorter metastasis-free survival in women with early breast cancer, but the prognostic implications of this inflammatory marker and iron-regulating plasma peptide in the blood are unknown.
Methods
Using an ELISA assay, hepcidin was measured in the banked blood of 518 women who were recruited from 1989 to 1996 for a prospective cohort study of diet and lifestyle factors in breast cancer. Blood samples were obtained 4–12 weeks post-operatively, prior to treatment with chemotherapy or tamoxifen.
Results
Hepcidin was not associated with time to distant breast cancer recurrence (primary outcome) nor time to death from any cause. However, a pre-planned interaction test of body mass index (BMI) was statistically significant (p < 0.01). Among obese women (BMI > 30 kg/m2), higher hepcidin was associated with a shorter time to distant breast cancer recurrence in both uni- and multivariable analyses (adjusted HR 1.84; 95% CI 1.04–3.25). For overall survival, a similar pattern was seen in the univariable model but the effect was diminished in a multivariable analysis. Plasma hepcidin was not associated with high-sensitivity C-reactive protein, but it was significantly associated (r ≥ 0.32) with iron indices, including total iron (p < 0.01), transferrin (p < 0.01) and soluble transferrin receptor (p < 0.01).
Conclusions
Hepcidin may be associated with poor breast cancer outcome in obese women, however, replication is required. The biologic basis for this prognostic association requires further research.
Keywords: Breast cancer, Hepcidin, Biomarker, Plasma iron, Obesity
Introduction
Hepcidin is an inflammatory marker and peptide hormone that regulates the concentration of plasma iron in the body by controlling the ferroportin transmembrane “valve”, which allows the outflow of intra-cellular iron into the plasma. When bound by hepcidin, ferroportin is occluded, ubiquitinated and undergoes endocytosis/proteolysis so that the flow of iron is ablated [1, 2]. Hence, hepcidin functions to inhibit flow of iron into the plasma, resulting in intra-cellular iron sequestration (Fig. 1) [2].
Fig. 1.
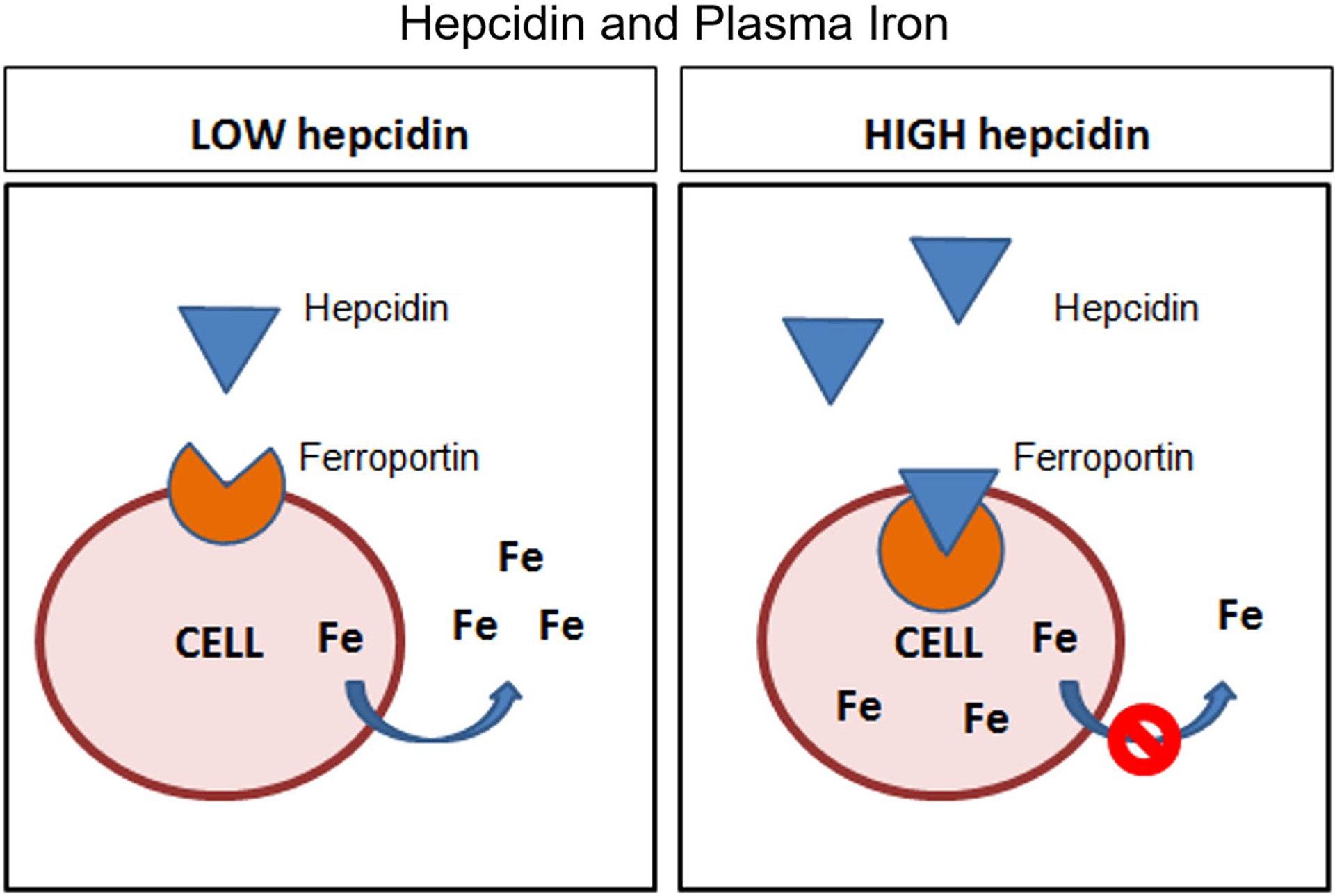
Influence of high versus low plasma hepcidin on plasma iron (Fe) levels
Hepcidin has been evolutionarily preserved across species because its iron-regulatory function helps maintain iron levels required for the function of many enzymes that are central to cell survival. Among iron-dependent enzymes is ribonucleotide reductase, which catalyzes the rate-limiting step for conversion of nucleic acids into 2′-deoxyribonucleotides that are required for DNA synthesis and repair [3]. The processes of cell growth, DNA replication and preparation for cell division are also tightly regulated by several iron-dependent molecules [4–12]. High hepcidin levels and consequent intra-cellular iron sequestration, may favor tumor cells and support their high rates of DNA synthesis and cell proliferation.
Indeed, several studies favor the hypothesis that hepcidin may have pro-growth properties in breast and other cancers. Pinnix et al. evaluated the mRNA and protein expression of prohepcidin (a precursor to hepcidin) in a panel of healthy and malignant breast cells. They found that prohepcidin levels were higher in malignant cell lines than in normal breast cells [13]. In a cross-sectional study of 131 patients, plasma hepcidin levels were significantly higher among 84 patients (64%) with breast cancer compared to those 47 (36%) of whom had benign breast disease [14]. Some data also suggest that hepcidin may be implicated in the development of cancer. For example, in a mouse lung cancer model, knock out of Hamp1 (the gene encoding hepcidin) resulted in a marked reduction in the development of cancer [15]. Only 6% of Hamp1 knockout mice developed lung tumors (n = 1/18), whereas 47% of wildtype mice (n = 8/17) developed a lung malignancy [15]. The overall survival was significantly greater among Hamp1 knockout mice than their wildtype counterparts (p = 0.0058) [15].
The RNA expression of both hepcidin and ferroportin have been assessed in four publicly available databases [16–19]. In a combined cohort of unselected breast cancer patients (n = 504), there was a non-significant association between high hepcidin expression in tumor tissue and poor distant metastasis-free survival (p = 0.06) [13]. Among those patients with high ferroportin expression (n = 219), high hepcidin was associated with a shorter distant metastasis-free survival (p = 0.001), whereas among those 285 patients with low ferroportin expression outcomes were uniformly poor regardless of hepcidin levels [13]. This finding suggests that hepcidin’s effect on iron sequestration is less pronounced when the concentration of its target protein, ferroportin, is low [13]. Although these results are intriguing, definite conclusions regarding the role of hepcidin as a prognostic biomarker cannot be made based on the aforementioned study. The study population was heterogenous, with some patients having no active cancer, and others having early breast cancer or even locally advanced disease. Further, plasma levels of hepcidin were not assessed.
In this study, we explored the prognostic associations between post-surgical plasma hepcidin levels (obtained prior to initiation of systemic therapy) and clinical outcomes of interest in a prospective cohort of women with early breast cancer. We hypothesized that low hepcidin levels were associated with adverse clinical outcomes, including time to distant breast cancer recurrence. The correlation between plasma hepcidin and metabolic markers, iron indices, as well as a marker of inflammation, high-sensitivity C-reactive protein (hs-CRP), was also investigated.
Methods
We studied a prospectively recruited cohort of 535 women with newly diagnosed early breast cancer and median follow-up of 12.1 years. Participants were identified from surgical lists at one of three University of Toronto hospitals (Mount Sinai Hospital, Women’s College Hospital, and St. Michael’s Hospital) and recruited post-operatively between 1989 and 1996 for a prospective cohort study regarding diet and lifestyle factors in breast cancer [20]. Patients did not have diabetes nor any established chronic disease. Only pre-menopausal women were included before June 1992 but the study was expanded to increase generalizability of results; both pre-/peri- and post-menopausal patients were included thereafter [20]. Tumor size, grade, estrogen receptor (ER) and progesterone receptor (PR) expression were abstracted from pathology reports. Human epidermal growth factor receptor-2 (HER2z All patients were followed prospectively for local and distant recurrences, new primary cancers, and death. The REMARK criteria was used to report these findings [21].
Blood was obtained after a 12-h overnight fast, between 4 and 12 weeks postoperatively (median 7 weeks) and prior to any treatment with adjuvant systemic therapy. Blood was collected in accordance with previously published methods and stored at minus 70 °C [20]. Hepcidin levels were measured in 25 μL of plasma using a commercially available and previously validated ELISA assay by Intrinsic Life Sciences in San Diego, California [22]; storage at minus 70 °C was maintained for shipping. All patients with a successful hepcidin assay result were included in the analysis. Blood specimens were identified with a study number only so that the lab was blinded to all patient and tumor characteristics, as well as patient outcomes. Since prior literature indicates that hepcidin levels fluctuate with iron ingestion [23], it is notable that blood was collected after an overnight fast (for 12 h or longer) in this study.
The study has received Research Ethics Board for approval as part of a goal to study obesity and nutritional factors in early breast cancer. It has also received administrative approval by the University of Toronto Research Ethics Board.
Statistical methods
The primary outcome, time to distant breast cancer recurrence, was defined as the time from definitive breast cancer surgery to the first of clinical, radiologic or pathologic documentation of recurrence outside of the breast and ipsilateral axilla. Patients were censored at the earliest of a non-breast cancer diagnosis or death. Those patients with contralateral breast cancer or in situ disease were not censored nor labelled as having recurrent disease, as per the original design of the study. The secondary outcome, time to death due to any cause, was defined as the time from definitive breast cancer surgery to the time of death due to any cause. In total, 134 women died and 136 had distant recurrences with a median follow-up of 12.1 years (range 0.2 to 17 years). Although the majority (n = 113) of patients who expired died from breast cancer, 21 died from other causes (13 due to other cancers, 7 without prior distant breast cancer recurrence and 1 with unknown breast recurrence status prior to death).
The pre-specified primary analysis was a multivariable Cox regression model with hepcidin as a continuous variable. A multivariable Cox proportional hazards model adjusted for the following variables a priori: (i) age (continuous variable), (ii) T stage (T2, T3 vs. T1), (iii) tumor grade (3 vs. 2 or 1), (iv) N stage (node positive vs. negative), (v) hormone receptor expression (defined as both ER and PR negative vs. either or both positive with a concentration ≥ 10 fmol/L). Hazard ratios (HRs) for hepcidin as a continuous variable were calculated using the 75th percentile (P75) vs the 25th percentile (P25) of the hepcidin distribution as convenient comparison points to aid in the interpretation of the HRs.
Missing values for these co-variates were systematically labelled and accounted for in all analyses using dummy variables. Tests for proportionality of the hazard were performed and were not significant. In secondary analyses, two pre-specified interactions were tested, namely between plasma hepcidin concentration and (i) menopausal status (pre- versus peri/post-menopausal) and (ii) obesity (BMI > 30 kg/m2 versus ≤ 30 kg/m2) because obese pre-menopausal women have been reported to have higher hepcidin levels than their non-obese counterparts [24] and obesity has been associated with poor breast cancer outcomes. Tests of interaction were employed to determine whether prognostic associations of plasma hepcidin differed by these pre-specified variables.
Finally, associations between hepcidin and the inflammatory marker hs-CRP, metabolic factors and iron indices listed below were explored using serial Pearson correlation coefficients. The normality of all inflammatory, metabolic and iron markers was assessed both visually and using the Shapiro–Wilk and Kolmogorov–Smirnov tests. Non-normal variables were log transformed to allow consistent use of Pearson’s coefficients in this exploratory component of the study.
Results
The hepcidin assay was successful in all 518 patients for whom study blood was available. In this cohort of 518 women with early breast cancer, the average age was 50.3 ± 9.7 years (Table 1). A minority of patients were obese (16% having a body mass index > 30 kg/m2) reflecting the era in which they were recruited (1989–1996) and 297 (57%) were either peri- or post-menopausal. Among the included women, 30% (n = 156) had node-positive disease. 35% (n = 181) had grade 3 tumors and 71% (n = 370) had tumors that were ER and/or PR positive. HER2 status was unknown. A majority (n = 401; 77%) of women underwent breast-conserving surgery, 73% (n = 380) received adjuvant radiotherapy and 39% (n = 203) were treated with adjuvant chemotherapy. At the time that this study was conducted, the efficacy of endocrine therapy in pre-menopausal women was not proven and, hence, a minority (n = 200; 39%) of patients received tamoxifen.
Table 1.
Baseline patient characteristics
| Variable | n (%) |
|---|---|
| Age | |
| < 50 | 303 (58.5) |
| ≥ 50 | 215 (41.5) |
| Menopausal status | |
| Pre-menopausal | 221 (42.7) |
| Peri/post-menopausal | 297 (57.3) |
| Height (cm) | |
| Mean ± SD | 162.0 ± 6.9 |
| Median (IQR) | 162.0 (157, 166.3) |
| Weight (kg) | |
| Mean ± SD | 66.8 ± 13.5 |
| Median (IQR) | 64.1 (57.8, 73.0) |
| Body mass index (kg/m2) | |
| ≤ 25 | 283 (54.6) |
| 25–30 | 150 (29.0) |
| > 30 | 85 (16.4) |
| Hormone receptor expression | |
| Either ER or PR positive/equivocal | 370 (71.4) |
| Both ER and PR negative | 75 (14.5) |
| Missing | 73 (14.1) |
| Tumour stage | |
| T1 | 290 (56.0) |
| T2 | 167 (32.2) |
| T3 | 25 (4.8) |
| TX | 36 (7.0) |
| Nodal status | |
| Negative | 362 (69.9) |
| Positive | 156 (30.1) |
| Tumour grade | |
| 1 | 77 (14.9) |
| 2 | 216 (41.7) |
| 3 | 181 (34.9) |
| Missing | 44 (8.5) |
| Surgery type | |
| Mastectomy | 117 (22.6) |
| Lumpectomy | 401 (77.4) |
| Adjuvant chemotherapy | |
| No | 315 (60.8) |
| Yes | 203 (39.2) |
| Adjuvant hormonal therapy | |
| No | 318 (61.4) |
| Yes | 200 (38.6) |
| Adjuvant radiation | |
| No | 138 (26.6) |
| Yes | 380 (73.4) |
Plasma hepcidin levels in patients ranged from 4.7 ng/mL to 190.7 ng/mL; the median was 16.3 ng/mL, the 25th percentile (P25) was 11.9 ng/mL and the 75th percentile (P75) was 28.3 ng/mL (Fig. 2). Visual inspection and statistically significant Shapiro–Wilk and Kolmogorov–Smirnov tests (p < 0.01) confirmed a non-normal distribution of plasma hepcidin. Hence, the plasma hepcidin values were transformed to minimize the effect of outliers on the outcomes of interest. The log transformation did not adequately normalize the data, and so an alternative − 1/sqrt (x) transformation was employed.
Fig. 2.
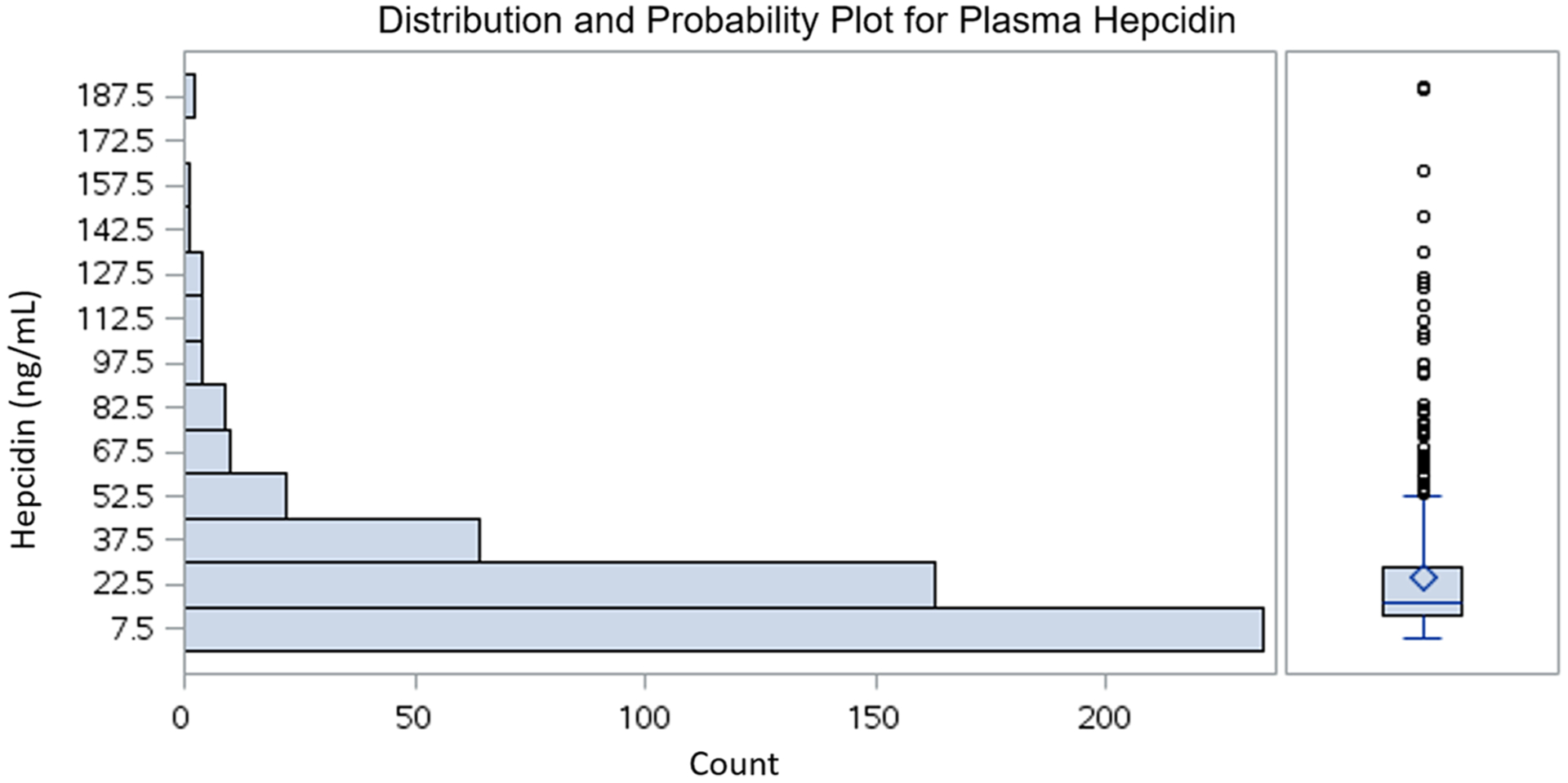
Distribution of plasma hepcidin
The distribution of plasma hepcidin by patient characteristics, tumor features and therapies employed is provided in Table 2. Hepcidin levels were lower among women age < 50 as compared to ≥ 50 (median 13.6 vs 23.8 ng/mL, p < 0.01) and they were lower among those who were pre-menopausal than among peri/post-menopausal women (median 13.5 vs 24.6 ng/mL, p < 0.01). In addition, hepcidin levels were progressively lower with decreasing BMI (Supplementary Fig. S1); the median hepcidin level among women with a BMI > 30 kg/m2 was 21.8 ng/mL (IQR 12.9–39.7), whereas the medians among women with a BMI 25–30 kg/m2 and BMI less than 25 kg/m2 were 18.1 ng/mL (IQR 13.6–28.3) and 14.5 ng/mL (IQR 11.4–23.2), respectively. There was no difference in hepcidin levels according to nodal status, T-stage or tumor grade.
Table 2.
Hepcidin levels according to patient and tumor characteristics
| Variable | Hepcidin (ng/mL) | p-value | |
|---|---|---|---|
| Median | IQR | ||
| All patients | 16.3 | (11.9, 28.3) | – |
| Age (years) | |||
| < 50 | 13.6 | (10.6, 18.3) | < 0.01 |
| ≥ 50 | 23.8 | (15.8, 39.7) | |
| Body mass index (kg/m2) | |||
| ≤ 25 | 14.5 | (11.4, 23.2) | < 0.01 |
| 25–30 | 18.1 | (13.6, 28.3) | |
| > 30 | 21.8 | (12.9, 39.7) | |
| Menopausal status | |||
| Pre | 13.5 | (10.5, 17.0) | < 0.01 |
| Peri/post | 24.6 | (16.7, 39.9) | |
| Tumor grade | |||
| 3 | 16.8 | (11.8, 30.6) | 0.87 |
| 2 or 1 | 16.7 | (12.1, 28.0) | |
| T-stage | |||
| X, 2, 3 | 15.6 | (11.6, 30.3) | 0.96 |
| 1 | 16.7 | (12.1, 25.9) | |
| Nodal status | |||
| Positive | 16.9 | (11.8, 31.3) | 0.47 |
| Negative | 16.0 | (12.0, 25.9) | |
| Hormone receptor status | |||
| ER and/or PR positive | 15.3 | (11.3, 24.7) | 0.01 |
| ER and PR negative | 19.9 | (13.0, 33.7) | |
| Adjuvant chemotherapy | |||
| Yes | 14.7 | (11.3, 25.4) | 0.02 |
| No | 17.5 | (12.2, 30.5) | |
| Adjuvant radiation | |||
| Yes | 17.6 | (12.9, 30.4) | < 0.01 |
| No | 14.0 | (10.7, 21.5) | |
| Adjuvant hormone therapy | |||
| Yes | 20.5 | (13.5, 36.5) | < 0.01 |
| No | 14.6 | (11.1, 22.9) | |
| Primary surgery | |||
| Mastectomy | 12.8 | (10.4, 18.2) | < 0.01 |
| Lumpectomy | 17.7 | (12.9, 30.7) | |
Associations between plasma hepcidin and clinical outcomes
When assessed as a continuous variable, plasma hepcidin was not associated with time to distant breast cancer recurrence (primary outcome) in univariable [HR 1.02 for hepcidin P75 versus P25 (95% CI 0.79–1.32), p = 0.88] or multivariable (HR 1.13 (95% CI 0.87–1.47), p = 0.35] analyses. Similarly, plasma hepcidin was not associated with time to death due to any cause (secondary outcome) in either a uni-variable [HR 1.23 (95% CI 0.95–1.59), p = 0.12] or multi-variable [HR 1.19 (95% CI 0.91–1.56), p = 0.21] Cox proportional hazards model (Table 3).
Table 3.
Association between plasma hepcidin and (i) time to distant recurrence, as well as (ii) time to death due to any cause, in univariable and multivariable models
| Outcome | Time to distant recurrence | Time to death due to any cause | ||
|---|---|---|---|---|
| Hazard ratio (95% confidence interval) | p-value | Hazard ratio (95% confidence interval) | p-value | |
| Hepcidin (continuous) 75th vs. 25th percentile | 1.02 (0.79–1.32) | 0.88 | 1.23 (0.95–1.59) | 0.12 |
| Multivariable | ||||
| Hepcidin (continuous) 75th vs. 25th percentile | 1.13 (0.87–1.47) | 0.35 | 1.19 (0.91–1.56) | 0.21 |
| Age (continuous/year) | 0.99 (0.97–1.01) | 0.27 | 1.01 (0.99–1.03) | 0.24 |
| T-stage (TX, 2, 3 versus 1) | 1.21 (0.82–1.79) | 0.33 | 1.37 (0.92–2.04) | 0.12 |
| Tumor grade (3 versus 2 or 1) | 1.43 (0.73–2.83) | 0.30 | 1.43 (0.96–2.15) | 0.08 |
| Node status (positive versus negative) | 2.90 (2.01–4.17) | < 0.01 | 2.55 (1.77–3.69) | 0.01 |
| Hormone receptor status (ER or PR positive versus negative) | 1.16 (0.69–1.96) | 0.57 | 0.94 (0.57–1.53) | 0.79 |
The association of hepcidin with the outcomes of interest was different in women who were obese (BMI > 30 kg/ m2) versus those who were not obese (BMI ≤ 30 kg/m2) as shown by a test of interaction (interaction p-value 0.01, Table 4). Univariably, higher plasma hepcidin levels were significantly associated with a shorter time to distant breast cancer recurrence (primary outcome) among obese women [HR 1.81 (95% CI 1.06–3.10)] but not among women with a BMI ≤ 30 kg/m2 [HR 0.80 (95% CI 0.59–1.09). Likewise, after adjusting for the pre-specified confounding variables, higher plasma hepcidin was independently associated with a shorter time to distant breast cancer recurrence among obese women [HR 1.84 (95% CI 1.04–3.25)] but not among their non-obese counterparts [HR 0.92 (95% CI 0.67–1.28)], with an interaction p-value of 0.04. For overall survival, a similar pattern was seen in the univariable model but the effect was diminished in a multivariable analysis (Table 4). HRs for both the population overall and the obese versus non-obese groups of women are illustrated in Supplementary Fig. S2.
Table 4.
Prognostic association between hepcidin and (i) time to distant recurrence, as well as (ii) time to death due to any cause with an interaction term for BMI *Interaction p-value
| Model | Outcome | Time to distant recurrence | Time to death due to any cause | ||
|---|---|---|---|---|---|
| Hazard ratio (95% confidence interval) | p-value | Hazard ratio (95% confidence interval) | p-value | ||
| Hepcidin (continuous) (75th vs. 25th percentile) | |||||
| Uni-variable | BMI > 30 | 1.81 (1.06–3.10) | < 0.01* | 1.91 (1.13–3.22) | 0.03* |
| BMI ≤ 30 | 0.80 (0.59–1.09) | 0.98 (0.72–1.33) | |||
| Hepcidin (continuous) (75th vs. 25th percentile) | |||||
| Multi-variable | BMI > 30 | 1.84 (1.04–3.25) | 0.02* | 1.58 (0.91–2.72) | 0.11* |
| BMI ≤ 30 | 0.92 (0.67–1.28) | 0.97 (0.69–1.37) | |||
Interaction p-value
Exploratory associations
Plasma hepcidin was not associated with the pre-specified inflammatory marker hs-CRP. There were only weak [25] associations with metabolic factors (all r < 0.32). However, plasma hepcidin was significantly associated (r ≥ 0.32) with iron indices, including total iron, transferrin and soluble transferrin receptor (sTfR) [25]. Substantially significant (r ≥ 0.45) and highly significant (r ≥ 0.60) associations were not observed [25].
Discussion
In this study, we sought to explore the prognostic associations of plasma hepcidin, an inflammatory marker and iron-regulatory peptide, in early breast cancer. We found that plasma hepcidin, when measured as a continuous variable, was not prognostic for time to distant breast cancer recurrence (primary outcome) or time to death due to any cause (secondary outcome). However, in a pre-specified test of interaction, we found that the association between hepcidin and the outcomes of interest was different in women who were obese (BMI > 30 kg/m2) versus those who were not obese (BMI ≤ 30 kg/m2).
Higher plasma hepcidin levels were significantly associated with a shorter time to distant breast cancer recurrence among obese women in both uni- and multi-variable models (p < 0.01). Higher hepcidin levels were also associated with shorter time to death due to any cause among obese women in a univariable analysis [HR 1.91 (95% CI 1.13–3.22), p = 0.03], but this effect was lost in the multivariable model. Proposed explanations for elevated hepcidin levels in obese women include the activation of the JAK/STAT3 pathway, which regulates hepcidin; increased hepcidin levels may result in ineffective dietary iron absorption and a reduction of intra-cellular iron stores [26, 27]. Although this mechanism may explain higher levels of hepcidin in obese women in our study, the reasons for a differential association of hepcidin with breast cancer outcomes in an obese (vs non-obese) population remain unclear. One possibility is that adverse outcomes among obese women are due to obesity-related inflammation, with hepcidin acting as its biomarker. Although links between hepcidin and inflammatory markers such as IL-6 have been noted previously [28–30], no associations were seen in this study or other studies [14].
Another possibility is that a relative state of iron deficiency in obese women limits the potential of hepcidin-induced ferroptosis, a non-apoptotic form of cell death in response to excessive levels of intracellular iron and high levels of reactive oxygen species [31]. Obesity may also mediate the differential prognostic associations of hepcidin with breast cancer outcomes via as yet unidentified mechanisms or simply by chance.
Moderate correlations between plasma hepcidin and iron-related variables, but not hs-CRP were identified in our study. Plasma hepcidin was associated with iron markers in the blood, including total iron (r = 0.35), transferrin (r = − 0.35) and sTfR (r = − 0.39). There was an inverse correlation between plasma hepcidin and both transferrin and sTfR, which are expected to increase in iron deficiency when plasma iron levels are low. However, the direct correlation between plasma hepcidin and total iron was unexpected because a primary increase in hepcidin should cause greater intracellular iron sequestration and consequently lower iron levels in the plasma. These correlations raise the possibility that hepcidin was physiologically responding to changes in iron parameters induced by dietary factors or blood loss that acted as the primary drivers of cancer outcomes in obese women. That being said, plasma iron levels in this cohort of women were in the low range of normal, with a mean of 9.4 μmol/L (standard deviation 4.7 μmol/L) and range of 1 to 36 μmol/L; the reference range in healthy women is 7 to 28 μmol/L [32]. This narrow range of iron levels in the studied cohort of women may limit our ability to detect the expected correlations with plasma hepcidin. Given the stability of iron levels over time [33], low levels in our study are unlikely due to a decrease in iron concentration after long-term storage, but rather due to a high proportion of young women who are more prone to iron deficiency.
One of the major limitations of this study is the assessment of plasma hepcidin at a single time point. Blood had been obtained between 4 and 12 weeks post-operatively, prior to treatment with chemotherapy or hormonal therapy. While the method and timing of blood collection was consistent for all patients in this prospective cohort, the hepcidin levels among women during their entire course of follow-up (median 12.1 years) is unknown. The generalizability of our results is another limitation of this study. Patients had been recruited between 1989 and 1996, when a breast cancer screening program was not in place. Hence, women presented with clinically palpable and generally more advanced disease. The patterns of death and recurrence also differed during the era of the current study, largely due to changes in the pattern of medical co-morbidities and older treatment regimens. That being said, recruitment of patients during this time period allowed for long-term follow-up (median 12.1 years, range 0.2 to 17 years) and a relatively high event rate.
Although higher plasma hepcidin levels in obese women may be associated with a shorter time to distant breast cancer recurrence and death due to chance alone (false positive finding), it is also possible that prognostic associations of hepcidin may be due to an underlying state of inflammation or intracellular iron sequestration. The former is less likely given that hepcidin was not associated with inflammatory markers in this study. However, given that iron it is essential to energy metabolism, cell proliferation and DNA synthesis, it is possible that hepcidin may be causally linked with breast cancer outcomes via regulation of plasma iron in the body. Due to the nature of this hypothesis-generating study and lack of available breast cancer tissue for analysis, the reasons for the observed prognostic associations cannot be established. However, further investigation regarding the role of iron-regulatory processes in the development and progression of breast and other cancers is warranted. A larger cohort study to validate the prognostic significance of plasma hepcidin among obese women with early breast cancer may also be of interest.
Supplementary Material
Funding
This study was funded by the Hold’ Em for Life Foundation.
Footnotes
Data availability The datasets generated during and/or analysed during the current study are not publicly available due to privacy or ethical restrictions, but are available from the corresponding author on reason-able request.
Conflict of interest KJJ has served as a Consultant/Speaker for and/ or attended Advisory Boards for Amgen, Apobiologix, Eli Lilly, Esai, Genomic Health, Inc., Novartis, Pfizer, Purdue Pharma and Roche; she has also received research funding from Astra Zeneca and Eli Lilly. TG and EN are Scientific Founders and Shareholders of Intrinsic LifeSciences and Silarus Pharma, and Consultants for Protagonist, Ionis and Regeneron. TG is a Consultant for Akebia. EN is a Consultant for Vifor.
Ethical approval The study has received Research Ethics Board for approval as part of a goal to study obesity and nutritional factors in early breast cancer. It has also received administrative approval by the University of Toronto Research Ethics Board.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s1054-020-05903-z) contains supplementary material, which is available to authorized users.
References
- 1.Drakesmith H, Nemeth E, Ganz T (2015) Ironing out ferroportin. Cell Metab 22:777–787. 10.1016/j.cmet.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganz T (2015) Hepcidin and the global burden of iron deficiency. Clin Chem 61:577–578. 10.1373/clinchem.2014.229179 [DOI] [PubMed] [Google Scholar]
- 3.Kolberg M, Strand KR, Graff P, Andersson KK (2004) Structure, function, and mechanism of ribonucleotide reductases. Biochim Biophys Acta Proteins Proteomics 1699:1–34. 10.1016/S1570-9639(04)00054-8 [DOI] [PubMed] [Google Scholar]
- 4.Yu Y, Kovacevic Z, Richardson DR (2007) Tuning cell cycle regulation with an iron key. Cell Cycle 6:1982–1994. 10.4161/cc.6.16.4603 [DOI] [PubMed] [Google Scholar]
- 5.Wang G, Miskimins R, Miskimins WK (2000) Mimosine arrests cells in G1 by enhancing the levels of p27(Kip1). Exp Cell Res 254:64–71. 10.1006/excr.1999.4743 [DOI] [PubMed] [Google Scholar]
- 6.Fukuchi K, Tomoyasu S, Watanabe H et al. (1995) Iron deprivation results in an increase in p53 expression. Biol Chem Hoppe Seyler 376:627–630 [DOI] [PubMed] [Google Scholar]
- 7.Kulp KS, Green SL, Vulliet PR (1996) Iron deprivation inhibits cyclin-dependent kinase activity and decreases cyclin D/CDK4 protein levels in asynchronous MDA-MB-453 human breast cancer cells. Exp Cell Res 229:60–68. 10.1006/excr.1996.0343 [DOI] [PubMed] [Google Scholar]
- 8.Lucas JJ, Szepesi A, Domenico J et al. (1995) Effects of iron-depletion on cell cycle progression in normal human T lymphocytes: selective inhibition of the appearance of the cyclin A-associated component of the p33(cdk2) kinase. Blood 86:2268–2280 [PubMed] [Google Scholar]
- 9.Vidal A, Koff A (2000) Cell-cycle inhibitors: three families united by a common cause. Gene 247:1–15. 10.1016/S0378-1119(00)00092-5 [DOI] [PubMed] [Google Scholar]
- 10.Nurtjahja-Tjendraputra E, Fu D, Phang JM, Richardson DR (2007) Iron chelation regulates cyclin D1 expression via the proteasome: a link to iron deficiency-mediated growth suppression. Blood 109:4045–4054. 10.1182/blood-2006-10-047753 [DOI] [PubMed] [Google Scholar]
- 11.Gao J, Richardson DR (2001) The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiprolifera-tive agents, IV: the mechanisms involved in inhibiting cell-cycle progression. Blood 98:842–850. 10.1182/blood.V98.3.842 [DOI] [PubMed] [Google Scholar]
- 12.Defamie N, Chepied A, Mesnil M (2014) Connexins, gap junctions and tissue invasion. FEBS Lett 588:1331–1338. 10.1016/j.febslet.2014.01.012 [DOI] [PubMed] [Google Scholar]
- 13.Pinnix ZK, Miller LD, Wang W et al. (2010) Ferroportin and iron regulation in breast cancer progression and prognosis. Sci Transl Med 2:43ra56 10.1126/scisignal.3001127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciniselli CM, De Bortoli M, Taverna E et al. (2015) Plasma hepcidin in early-stage breast cancer patients: no relationship with interleukin-6, erythropoietin and erythroferrone. Expert Rev Proteomics 12:695–701. 10.1586/14789450.2015.1099436 [DOI] [PubMed] [Google Scholar]
- 15.Guo W, Zhang S, Chen Y et al. (2015) An important role of the hepcidin-ferroportin signaling in affecting tumor growth and metastasis. Acta Biochim Biophys Sin (Shanghai) 47:703–715. 10.1093/abbs/gmv063 [DOI] [PubMed] [Google Scholar]
- 16.Sørlie T, Sørlie T, Tibshirani R et al. (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 100:8418–8423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van De Vijver MJ, He YD, Van’t Veer LJ, et al. (2002) A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347:1999–2009. 10.1056/NEJMoa021967 [DOI] [PubMed] [Google Scholar]
- 18.Miller LD, Smeds J, George J et al. (2005) An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci USA 102:13550–13555. 10.1073/pnas.0506230102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall P, Ploner A, Bjöhle J et al. (2006) Hormone-replacement therapy influences gene expression profiles and is associated with breast-cancer prognosis: a cohort study. BMC Med: 10.1089/dis.2006.9.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodwin PJ, Ennis M, Pritchard KI et al. (2002) Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol 20:42–51. 10.1200/JCO.20.1.42 [DOI] [PubMed] [Google Scholar]
- 21.McShane LM, Altman DG, Sauerbrei W et al. (2006) REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 100:229–235. 10.1007/s10549-006-9242-8 [DOI] [PubMed] [Google Scholar]
- 22.Ganz T, Olbina G, Girelli D et al. (2008) Immunoassay for human serum hepcidin. Blood 112:4292–4297. 10.1182/blood-2008-02-139915 [DOI] [PubMed] [Google Scholar]
- 23.Troutt JS, Rudling M, Persson L et al. (2012) Circulating Human hepcidin-25 concentrations display a diurnal rhythm, increase with prolonged fasting, and are reduced by growth hormone administration. Clin Chem 58:1225–1232. 10.1373/clinchem.2012.186866 [DOI] [PubMed] [Google Scholar]
- 24.Tussing-Humphreys LM, Nemeth E, Fantuzzi G et al. (2010) Elevated systemic hepcidin and iron depletion in obese premenopausal females. Obesity 18:1449–1456. 10.1038/oby.2009.319 [DOI] [PubMed] [Google Scholar]
- 25.Burnand B, Kernan WN, Feinstein AR (1990) Indexes and boundaries for “quantitative significance” in statistical decisions. J Clin Epidemiol 43:1273–1284. 10.1016/0895-4356(90)90093-5 [DOI] [PubMed] [Google Scholar]
- 26.Lainé F, Jouannolle AM, Morcet J et al. (2005) Phenotypic expression in detected C282Y homozygous women depends on body mass index. J Hepatol 12:319–322. 10.1016/j.jhep.2005.05.027 [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann MB, Zeder C, Muthayya S et al. (2008) Adiposity in women and children from transition countries predicts decreased iron absorption, iron deficiency and a reduced response to iron fortification. Int J Obes 32:1098–1104. 10.1038/ijo.2008.43 [DOI] [PubMed] [Google Scholar]
- 28.Nemeth E, Rivera S, Gabayan V et al. (2004) IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Investig 113:1271–1276. 10.1172/JCI200420945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicolas G, Chauvet C, Viatte L et al. (2002) The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Investig 110:1037–1044. 10.1172/JCI0215686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nemeth E, Valore EV, Territo M et al. (2003) Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 101:2461–2463. 10.1182/blood-2002-10-3235 [DOI] [PubMed] [Google Scholar]
- 31.Abdel-Qadir H, Austin PC, Lee DS et al. (2017) A population-based study of cardiovascular mortality following early-stage breast cancer. JAMA Cardiol 2:88–93. 10.1001/jamacardio.2016.3841 [DOI] [PubMed] [Google Scholar]
- 32.Gomella LG, Haist SA (2007) Chapter 4. Laboratory diagnosis: chemistry, immunology, serology In: Clinician’s pocket reference: The Scut Monkey, 11e [Google Scholar]
- 33.Gislefoss RE, Grimsrud TK, Mørkrid L (2009) Stability of selected serum proteins after long-term storage in the Janus Serum Bank. Clin Chem Lab Med 47:596–603. 10.1515/CCLM.2009.121 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


