Obstetricians will need to use the limited available data to guide their pregnant patients regarding benefits and risks of coronavirus disease 2019 (COVID-19) vaccination.
Abstract
Coronavirus disease 2019 (COVID-19) vaccines have begun to be distributed across the United States and to be offered initially to priority groups including health care personnel and persons living in long-term care facilities. Guidance regarding whether pregnant persons should receive a COVID-19 vaccine is needed. Because pregnant persons were excluded from the initial phase 3 clinical trials of COVID-19 vaccines, limited data are available on their efficacy and safety during pregnancy. After developmental and reproductive toxicology studies are completed, some companies are expected to conduct clinical trials in pregnant persons. Until then, pregnant persons and their obstetricians will need to use available data to weigh the benefits and risks of COVID-19 vaccines. Issues to be considered when counseling pregnant persons include data from animal studies and inadvertently exposed pregnancies during vaccine clinical trials when available, potential risks to pregnancy of vaccine reactogenicity, timing of vaccination during pregnancy, evidence for safety of other vaccines during pregnancy, risk of COVID-19 complications due to pregnancy and the pregnant person's underlying conditions, and risk of exposure to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and potential for risk mitigation. The Centers for Disease Control and Prevention, the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine have each issued guidance supportive of offering COVID-19 vaccine to pregnant persons. As additional information from clinical trials and from data collected on vaccinated pregnant persons becomes available, it will be critical for obstetricians to keep up to date with this information.
Less than a year after identification of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that causes coronavirus disease 2019 (COVID-19), safe and effective vaccines are beginning to be distributed across the United States, with the hope of bringing the COVID-19 pandemic to an end. Here we summarize what is currently known about COVID-19 vaccines and their use during pregnancy.
COVID-19 VACCINES AND THE VACCINE APPROVAL PROCESS IN THE UNITED STATES
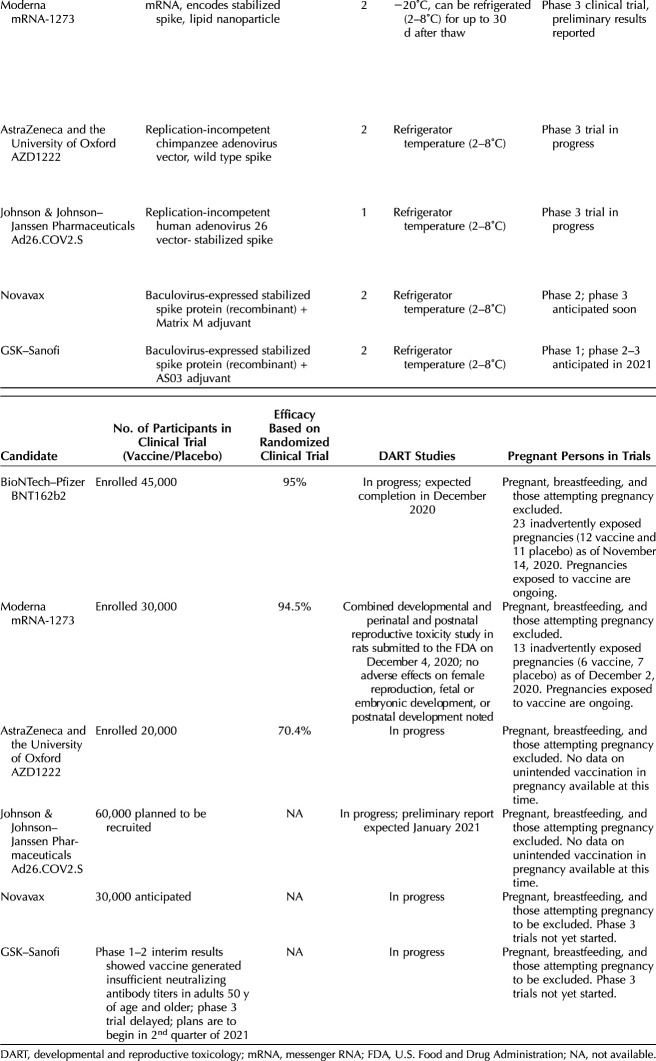
Six leading vaccine candidates have received some form of federal government support through Operation Warp Speed, the partnership between the U.S. government and the pharmaceutical industry (Table 1); of these, two are messenger RNA (mRNA)–based vaccine candidates, two are based on viral vectors, and two are recombinant proteins manufactured in a baculovirus (DNA virus that infects insect cells) system that are co-formulated with adjuvants (substances that are added to vaccines to boost the immune response). As of December 13, 2020, applications for Emergency Use Authorization (EUA) have been submitted to the U.S. Food and Drug Administration (FDA) for the two mRNA-based vaccine candidates; the other vaccines have not yet completed the clinical trial process. Emergency Use Authorization is an authority provided to the FDA to allow unapproved medical products to be used in a public health emergency to diagnose, treat, or prevent serious or life-threatening conditions when there are no adequate, approved, and available alternatives.1 Although the process of EUA is usually considered to be less rigorous than full FDA approval through a Biologics License Application, the FDA issued guidance in October 2020 that set a higher standard for COVID-19 vaccines, given that they would be used on large populations of healthy people. In their guidance, the FDA recommended that, for issuance of an EUA, the evidence would need to be similar to that for full approval: benefits of the vaccine would need to outweigh its risks based on data from at least one well-designed phase 3 clinical trial (a trial in which participants are randomized to receive a new product or a placebo) that demonstrated the vaccine’s safety and efficacy in a clear and compelling manner.2
Table 1.
Leading Vaccine Candidates Under Consideration in the United States
Several levels of review are required before authorization and distribution of a COVID-19 vaccine. The first level of review is by the clinical trial’s Data and Safety Monitoring Board, a panel of independent scientists selected to review the evidence from the phase 3 clinical trial. Next, data are reviewed by career scientists at the FDA, followed by a review by the Vaccines and Related Biological Products Advisory Committee (VRBPAC), an independent advisory committee to the FDA, before issuance of the EUA. After the FDA’s issuance of an EUA, the Advisory Committee on Immunization Practices (ACIP), an independent advisory committee to the Centers for Disease Control and Prevention (CDC), reviews the EUA and provides recommendations for populations to be vaccinated. Because the demand for COVID-19 vaccines is believed to initially exceed the supply, the ACIP released interim recommendations on December 3, 2020, prioritizing health care personnel and persons living in long-term care facilities to be the first to be offered COVID-19 vaccines.3
The six vaccine candidates developed against COVID-19 use different technologies. Most previously approved vaccines work by introducing an antigen into the body to produce an immune response. The antigen can be an infectious agent that has been inactivated or a purified protein from the infectious agent. In contrast, the COVID-19 mRNA vaccines developed by Pfizer–BioNTech and Moderna work by carrying the genetic information necessary to manufacture the spike protein of SARS-CoV-2, the protein found on the surface of the virus. Once the vaccine is injected into muscle cells, they manufacture the spike protein, which is recognized by the immune system. The mRNA never enters the nucleus and is thus not integrated into DNA; within hours to days, the mRNA is degraded in the cell cytoplasm. The vaccines developed by AstraZeneca–Oxford and Janssen–Johnson and Johnson use a modified viral vector to deliver the spike protein of SARS-CoV-2 into the cells, which then triggers an immune response. The AstraZeneca–Oxford vaccine uses a chimpanzee adenovirus that has been modified so that it is unable to replicate, whereas the Janssen–Johnson and Johnson vaccine uses a human adenovirus 26 that has also been modified to be nonreplicating. The adenovirus 26 vector was previously used to make a successful vaccine against Ebola. The vaccines developed by Novavax and GSK–Sanofi are protein subunit vaccines in which a baculovirus is used to produce the recombinant protein in insect cells. Both of these vaccines will be mixed with adjuvants to boost the immune response. The protein subunit approach has been used to make other vaccines in common use (eg, types of influenza, hepatitis B, and human papillomavirus vaccines).
COVID-19 VACCINES AND PREGNANCY
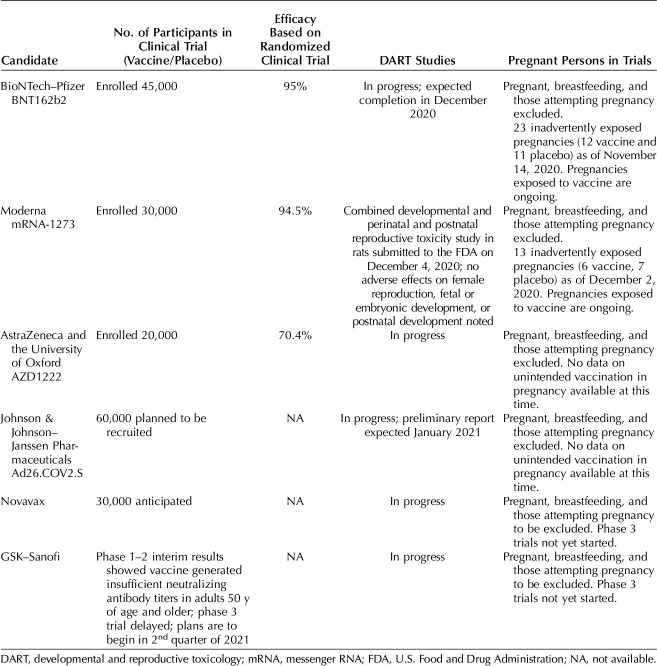
As is the case thus far with COVID-19 vaccines, pregnant persons have traditionally been excluded from clinical trials of new medications and vaccines because of concerns for effects on the fetus. However, in recent years, federal agencies have recognized the challenges to this approach. In 2013, the National Institutes of Health’s National Institute of Allergy and Infectious Diseases established an expert panel that developed guidelines for protocol design and safety assessment for clinical trials conducted in pregnant persons.4 In 2018, the FDA published draft guidance that provided a framework for consideration of inclusion of pregnant persons in clinical trials.5 Also in 2018, the Task Force on Research Specific to Pregnant Women and Lactating Women, a federal group established to advise the Secretary of Health and Human Services, recognized that, “inclusion of pregnant women and lactating women in clinical trials is essential, unless there are compelling scientific reasons for their exclusion.”6 Despite the recognition of the need for inclusion of pregnant persons in clinical trials, the speed at which the COVID-19 vaccines were developed and trials conducted precluded inclusion of pregnant persons. In guidance released in June of 2020, the FDA recommended that pharmaceutical companies conduct developmental and reproductive toxicology studies before enrolling pregnant people or persons who are not actively avoiding pregnancy in their trials.7 Developmental and reproductive toxicology studies, which typically use animals to assess the potential effects of a new medication or vaccine on the full spectrum of reproduction, have not yet been completed for any of the COVID-19 vaccines, except for the Moderna vaccine, which submitted results to the FDA on December 4, 20208; thus, pregnant persons have yet to be enrolled. A preliminary report of developmental and reproductive toxicology studies on the Pfizer–BioNTech vaccine is expected to be sent to the FDA in the near future. According to the VRBPAC briefing documents on the Moderna vaccine,8 a combined developmental and perinatal and postnatal study was conducted in rats and showed no adverse effects on female reproduction, fetal or embryonic development, or postnatal development. It is anticipated that clinical trials of COVID-19 vaccine safety and immunogenicity for pregnant persons could begin in January 2021. Because the vaccine is already shown to be efficacious in adults and adolescents older than 16 years of age, the trials may use correlates of immunity (eg, neutralizing antibodies) rather than COVID-19 acquisition as an outcome.
Some companies that developed COVID-19 vaccines may plan to include pregnant persons in clinical trials in the future, but all current studies not only excluded pregnant participants but requested confirmation of a contraception plan for weeks to months after injection. Thus, very little information is currently available on safety and efficacy in pregnancy. As of November 14, Pfizer reported that there were 23 pregnant persons inadvertently enrolled in their clinical trial, including 12 in the vaccine group; these pregnancies are ongoing. Moderna reported 13 pregnancies in their clinical trial, including six in the vaccine group and seven in the placebo group. The pregnancies exposed to vaccine are ongoing. The CDC's ACIP released interim guidance recommending that health care personnel be included in the initial phase to receive the vaccine because of the high risk of exposure. Given that approximately 75% of the health care workforce are women, the CDC estimates that approximately 300,000 health care personnel could be pregnant or recently postpartum at the time of vaccine implementation.
The vast majority of vaccines are allowed during pregnancy when the benefit of vaccine is deemed to outweigh the potential risk.9 Unfortunately, other than the small number of inadvertent exposures during pregnancy reported in recent COVID-19 vaccine clinical trials, no previous experience with pregnant persons is available for mRNA vaccines, the type of vaccines first to be available in the United States. However, there is no reason to expect that mRNA vaccines will work differently in pregnant persons than in other adults. As is often the case with vaccines, data on the Pfizer–BioNTech mRNA vaccines show reactogenicity in a proportion of patients, with fever, fatigue, headache, chills, and muscle and joint pain. Fever (38°C or higher) occurred in 3.7% of participants after dose 1 and 15.8% after dose 2 of the Pfizer–BioNTech mRNA vaccines in persons aged 18–55 years in the clinical trials.10 Neonates born to pregnant persons with fevers in their first trimester of pregnancy have been shown to have an increased risk for certain types of birth defects, although the absolute risk is small.11 The risks associated with fever appear to be lowered with antipyretic medication. Judicious use of antipyretic medications after vaccination is recommended, given the possible concerns that have been raised about acetaminophen use during pregnancy12 as well as concerns regarding whether antipyretic medications could decrease vaccine efficacy.13 Recommendations from the ACIP are to use antipyretic medications for treatment rather than for prophylaxis. Based on what is known about how mRNA vaccines act locally (at the site of injection) and are rapidly degraded and removed by the lymphatic system, the likelihood of the vaccine reaching and crossing the placenta is believed to be low.
The VRBPAC meeting to discuss the Pfizer–BioNTech vaccine was held on December 10, 2020, and the meeting to discuss the Moderna vaccine is scheduled for December 17, 2020. At the meeting on December 10, the VRBPAC voted to support an EUA for the Pfizer–BioNTech vaccine, and the next day, the FDA announced an EUA for this vaccine. In a news briefing held on December 12, 2020, Dr. Peter Marks, Director of the FDA’s Center for Biologics Evaluation and Research, noted that, given that pregnant persons were not enrolled in vaccine clinical trials, there were insufficient data to make a recommendation; however, he stated, “it will be something that providers will need to consider on an individual basis for patients.”14 On December 12, 2020, the CDC’s ACIP voted to recommend the Pfizer–BioNTech COVID-19 vaccine for persons 16 years of age and older in the U.S. population under the FDA’s EUA, a recommendation that was subsequently accepted by the CDC Director. The ACIP noted that persons who are part of a group recommended to receive the COVID-19 vaccine (eg, health care personnel) who are pregnant may choose to be vaccinated and that a discussion with their obstetrician might assist them in making an informed decision,15 consistent with a recent statement from the Society for Maternal-Fetal Medicine (SMFM)16 and a Practice Advisory from the American College of Obstetricians and Gynecologists (ACOG).17
COUNSELING PREGNANT PERSONS ABOUT COVID-19 VACCINES
In weighing the risks and benefits of COVID-19 vaccination, several issues should be considered (Box 1). The obstetrician should review what is known from developmental and reproductive toxicology studies on animals regarding the vaccine (when available) and the limited data on outcomes of pregnant persons inadvertently exposed to vaccine during the clinical trials. Another issue to be discussed is the risks of COVID-19 illness to a pregnant person and the fetus. Current data suggest that pregnant persons are more likely to be admitted to an intensive care unit, to require invasive ventilation, to receive extracorporeal membrane oxygenation, and to die than nonpregnant women of reproductive age.18 The effects on the fetus are not completely understood. Intrauterine transmission can occur but appears to be rare19; however, data suggest that neonates born to persons with SARS-CoV-2-infection may be more likely to be born preterm.20 The risk of complications to the pregnant person based on factors other than pregnancy also need to be considered: does the person have an underlying condition (eg, diabetes, obesity, or heart disease) that could cause increased risk for severe COVID-19 complications.21 Another consideration is whether the risk of acquiring infection can be mitigated. Pregnant persons who can minimize their exposure to persons with SARS-CoV-2 infection (eg, those who can work from home) are at less risk. Whether there is a risk of exposure to children who might acquire infection in schools or other settings also needs to be considered. Timing during pregnancy is another consideration. Given the vaccine reactogenicity, including fever, vaccination in the first trimester could increase the risk of neural tube and other congenital defects.11 In addition, exposures earlier in pregnancy are more likely to cause adverse outcomes. However, the risks of vaccine reactogenicity on the pregnant person and the fetus need to be weighed against the risks of COVID-19 itself. Data collection on persons who received a COVID-19 vaccine during pregnancy will be needed to provide information to guide future vaccine recommendations regarding pregnancy. Pfizer Inc. has committed to conducting postauthorization observational studies on vaccine use in several populations, including pregnant persons.22
Box 1.
Considerations for Counseling Pregnant Persons Regarding Coronavirus Disease 2019 (COVID-19) Vaccination
Data from animal studies (once developmental and reproductive toxicology studies become available)
Lack of data on pregnancies during vaccine clinical trials
Risks of vaccine reactogenicity, including fever; treatment with antipyretic medications (eg, acetaminophen) might reduce this risk
Timing of planned vaccination during pregnancy
Extensive evidence for safety of other vaccines during pregnancy
Risk of COVID-19 complications due to pregnancy (increased risk to pregnant person of severe disease and death)
Risk of COVID-19 complications due to underlying conditions (eg, diabetes, obesity, heart disease)
Risk of COVID-19 to fetus or newborn (intrauterine transmission is rare, but preterm birth appears to be increased)
Risk of exposure to SARS-CoV-2 and potential for mitigation with working from home, wearing masks, and physical distancing
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
In summary, pregnant persons and their obstetricians will need to use the limited available data to weigh the benefits and risks of COVID-19 vaccine during pregnancy, taking into account the patient’s specific risk of SARS-CoV-2 exposure. The CDC,15 ACOG,17 and SMFM16 have each issued guidance supporting the offer of COVID-19 vaccines to pregnant persons. As additional information from clinical trials and from data collected on vaccinated pregnant persons outside clinical trials becomes available, it will be critical for obstetricians to keep up to date with the latest information from federal agencies (eg, CDC and FDA) and professional organizations (eg, SMFM and ACOG), which will be updated regularly.
On December 18, 2020, an EUA was issued by the FDA for the Moderna COVID-19 vaccine.23 The CDC's ACIP met on December 19, 2020, and approved a recommendation for the Moderna COVID-19 vaccine for persons 18 years of age and older.
Footnotes
Financial Disclosure Sonja A. Rasmussen reports serving on the Teva Pregnancy Registry Advisory Committee and the Solriamfetol Pregnancy Registry Advisory Committee. In addition, she serves as a litigation consultant on behalf of Hoffmann-La Roche for a product liability claim regarding an alleged birth defect. These are not relevant to this article or COVID-19. Colleen F. Kelley is supported by research grants to her institution from Gilead Sciences, ViiV, Moderna, and Novavax. The other authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal's requirements for authorship.
Published online ahead-of-print December 23, 2020.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/C184.
Figure.
No available caption
REFERENCES
- 1.U.S. Food and Drug Administration. Emergency use authorization. Accessed December 13, 2020. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization [Google Scholar]
- 2.U.S. Food and Drug Administration. Emergency use authorization for vaccines to prevent COVID-19: guidance for industry—October 2020. Accessed December 13, 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/emergency-use-authorization-vaccines-prevent-covid-19 [Google Scholar]
- 3.Dooling K, McClung N, Chamberland M, Marin M, Wallace M, Bell BP, et al. The Advisory Committee On Immunization Practices' interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1857–9. doi: 10.15585/mmwr.mm6949e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munoz FM, Sheffield JS, Beigi RH, Read JS, Swamy GK, Jevaji I, et al. Research on vaccines during pregnancy: protocol design and assessment of safety. Vaccine 2013;31:4274–9. doi: 10.1016/j.vaccine.2013.07.042 [DOI] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration. Pregnant women: scientific and ethical considerations for inclusion in clinical trials guidance for industry—draft guidance. Accessed December 13, 2020. https://www.fda.gov/media/112195/download [Google Scholar]
- 6.Task Force on Research Specific to Pregnant Women and Lactating Women. Report to Secretary, Health and Human Services and Congress. Accessed December 13, 2020. https://www.nichd.nih.gov/sites/default/files/2018-09/PRGLAC_Report.pdf [Google Scholar]
- 7.U.S. Food and Drug Administration. Development and licensure of vaccines to prevent COVID-19—guidance for industry. Accessed December 13, 2020. https://www.fda.gov/media/139638/download [Google Scholar]
- 8.U.S. Food and Drug Administration. Vaccines and Related Biological Products Advisory Committee meeting—December 17, 2020—FDA briefing document—Moderna COVID-19 vaccine. Accessed December 15, 2020. https://www.fda.gov/media/144434/download [Google Scholar]
- 9.Rasmussen SA, Watson AK, Kennedy ED, Broder KR, Jamieson DJ. Vaccines and pregnancy: past, present, and future. Semin Fetal Neonatal Med 2014;19:161–9. doi: 10.1016/j.siny.2013.11.014 [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Local reactions, systemic reactions, adverse events, and serious adverse events: Pfizer-BioNTech COVID-19 vaccine. Accessed December 13, 2020. https://www.cdc.gov/vaccines/covid-19/info-by-manufacturer/pfizer/reactogenicity.html [Google Scholar]
- 11.Graham JM., Jr Update on the gestational effects of maternal hyperthermia. Birth Defects Res 2020;112:943–52. doi: 10.1002/bdr2.1696 [DOI] [PubMed] [Google Scholar]
- 12.Masarwa R, Levine H, Gorelik E, Reif S, Perlman A, Matok I. Prenatal exposure to acetaminophen and risk for attention deficit hyperactivity disorder and autistic spectrum disorder: a systematic review, meta-analysis, and meta-regression analysis of cohort studies. Am J Epidemiol 2018;187:1817–27. doi: 10.1093/aje/kwy086 [DOI] [PubMed] [Google Scholar]
- 13.Saleh E, Moody MA, Walter EB. Effect of antipyretic analgesics on immune responses to vaccination. Hum Vaccin Immunother 2016;12:2391–402. doi: 10.1080/21645515.2016.1183077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CNN. FDA is “comfortable” with Pfizer/BioNTech vaccine's safety for 16- and 17-year-olds. Accessed December 13, 2020. https://www.cnn.com/world/live-news/coronavirus-pandemic-vaccine-updates-12-12-20-intl/h_2af381c74dea46a804eac838994a983c [Google Scholar]
- 15.Centers for Disease Control and Prevention. Interim clinical considerations for use of Pfizer-BioNTech COVID-19 vaccine. Accessed December 14, 2020. https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/clinical-considerations.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fcovid-19%2Finfo-by-manufacturer%2Fpfizer%2Fclinical-considerations.html [Google Scholar]
- 16.Society for Maternal-Fetal Medicine. Society for Maternal-Fetal Medicine (SMFM) statement: SARS-CoV-2 vaccination in pregnancy. Accessed December 13, 2020. https://www.smfm.org/publications/339-society-for-maternal-fetal-medicine-smfm-statement-sars-cov-2-vaccination-in-pregnancy [Google Scholar]
- 17.American College of Obstetricians and Gynecologists. Vaccinating pregnant and lactating patients against COVID-19: practice advisory—December 2020. Accessed December 13, 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19 [Google Scholar]
- 18.Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1641–7. doi: 10.15585/mmwr.mm6944e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vivanti AJ, Vauloup-Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun 2020;11:3572. doi: 10.1038/s41467-020-17436-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodworth KR, Olsen EO, Neelam V, Lewis EL, Galang RR, Oduyebo T, et al. Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy—SET-NET, 16 jurisdictions, March 29–October 14, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1635–40. doi: 10.15585/mmwr.mm6944e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. COVID-19 (coronavirus disease): people with certain medical conditions. Accessed December 13, 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html [Google Scholar]
- 22.U.S. Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine EUA letter of authorization. Accessed December 13, 2020. https://www.fda.gov/media/144412/download [Google Scholar]
- 23.U.S. Food and Drug Administration. FDA takes additional action in fight against COVID-19 by issuing emergency use authorization for second COVID-19 vaccine. Accessed December 19, 2020. https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid [Google Scholar]