Abstract
Background
Diabetic nephropathy (DN) is one of the most common complications of diabetes mellitus and is a major cause of end-stage kidney disease. Cordyceps sinensis (Cordyceps, Dong Chong Xia Cao) is a widely applied ingredient for treating patients with DN in China, while the molecular mechanisms remain unclear. This study is aimed at revealing the therapeutic mechanisms of Cordyceps in DN by undertaking a network pharmacology analysis.
Materials and Methods
In this study, active ingredients and associated target proteins of Cordyceps sinensis were obtained via Traditional Chinese Medicine Systems Pharmacology Database (TCMSP) and Swiss Target Prediction platform, then reconfirmed by using PubChem databases. The collection of DN-related target genes was based on DisGeNET and GeneCards databases. A DN-Cordyceps common target interaction network was carried out via the STRING database, and the results were integrated and visualized by utilizing Cytoscape software. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed to determine the molecular mechanisms and therapeutic effects of Cordyceps on the treatment of DN.
Results
Seven active ingredients were screened from Cordyceps, 293 putative target genes were identified, and 85 overlapping targets matched with DN were considered potential therapeutic targets, such as TNF, MAPK1, EGFR, ACE, and CASP3. The results of GO and KEGG analyses revealed that hub targets mainly participated in the AGE-RAGE signaling pathway in diabetic complications, TNF signaling pathway, PI3K-Akt signaling pathway, and IL-17 signaling pathway. These targets were correlated with inflammatory response, apoptosis, oxidative stress, insulin resistance, and other biological processes.
Conclusions
Our study showed that Cordyceps is characterized as multicomponent, multitarget, and multichannel. Cordyceps may play a crucial role in the treatment of DN by targeting TNF, MAPK1, EGFR, ACE, and CASP3 signaling and involved in the inflammatory response, apoptosis, oxidative stress, and insulin resistance.
1. Introduction
Diabetic nephropathy (DN) is featured as hyperglycemia, hyperfiltration, proteinuria, and progressive renal function decline, which can cause end-stage kidney disease [1]. It accounts for about 40% of chronic kidney disease worldwide and is undoubtedly a medical challenge worldwide [2], in terms of high incidence, multifactorial pathogenesis, and the absence of practical methods in the diagnosis and treatment [3]. Traditional Chinese medicine (TCM) guided by the unique theory provides an effective treatment of complex chronic diseases via multicomponent, multitarget, and multipathway [4]. In recent years, Chinese herb medicine has been commonly utilized to alleviate and reverse diabetes and its complications, such as DN in clinical practice and scientific researches, which has been considered a beneficial supplement of the drug therapy for DN [4]. Cordyceps, a traditional Chinese herbal medicine, is reported to have multiple health-promoting characteristics, including anti-inflammatory, anticancer, antidiabetic, analgesic, antioxidant, antiallergic, and antiobesity [5]. Also, Cordyceps has been reported to have broad pharmacological effects on DN by inhibiting the epithelial-mesenchymal transition, alleviating oxidative stress, repressing inflammation, modulating gut microbiota dysbiosis, and activating autophagy [6–9]. However, the pharmacological mechanisms of Cordyceps associated with DN only focus on a single chemical molecule. Accordingly, a comprehensive and systematic evaluation of the molecular mechanisms of Cordyceps on DN is indispensable.
This study is aimed at analyzing the pharmacological mechanisms of active ingredients of Cordyceps involved in the progression of DN via using the network pharmacology databases and biological analysis methods. It laid a stable foundation for further research on exploring pharmacological mechanisms of Cordyceps in treating DN. The study framework is showed in Figure 1.
Figure 1.
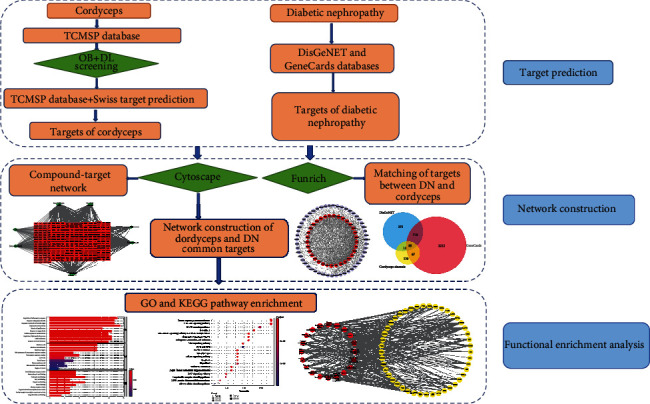
A flow chart based on an integration strategy of network pharmacology.
2. Materials and Methods
2.1. Establishment of Active Ingredients and Correlated Target Database
TCMSP (http://lsp.nwu.edu.cn/tcmsp.php), a systematic pharmacological platform that contains the relationships among herbal compounds, related targets, and diseases [10], was applied to identify the chemical constituents of Cordyceps. The active components were selected based on oral bioavailability (OB) and drug likeness (DL) values, and the ingredients were captured when the OB was ≥30% and the DL ≥ 0.18 (a screening threshold of TCMSP database) [10]. The chemical formulas of components were reconfirmed by PubChem (https://www.ncbi.nlm.nih.gov/pccompound) to double-check the final compounds of Cordyceps [11]. The targets associated with Cordyceps components were further identified based on the TCMSP database and Swiss Target Prediction (http://www.swisstargetprediction.ch.), a webserver to accurately identify the targets of active molecules [12]. The genes corresponding to the protein targets were obtained from the UniProt database.
2.2. Network Construction of Active Components-Potential Targets
A comprehensive network was constructed via using Cytoscape software to reflect the intricate relationship between active compounds and putative targets [13]. Nodes represent the components and targets, while edges reveal the interactions between components and targets (Figure 2).
Figure 2.
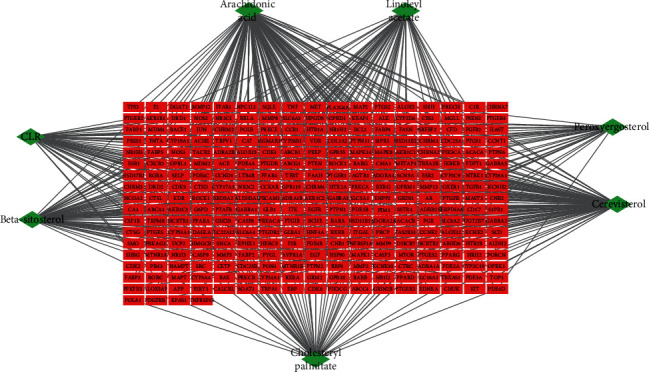
Compound-target network of putative targets and active Cordyceps ingredients. The green nodes represent the active components of Cordyceps, and nodes in red represent the corresponding targets of the ingredients.
2.3. Selection of Potential DN Targets
The keyword “diabetic nephropathy” was inputted in the GeneCards (https://www.genecards.org/), a human gene compendium with information about genomics, proteomics, and transcriptomics [14], and DisGeNET (https://www.disgenet.org/home/), a comprehensive platform including one of the largest publicly accessible collections of genes, to search for DN-associated targets [15].
2.4. Screening Compound-Disease Overlapping Targets
The screened compound targets and disease targets were imported into Funrich, a software used mainly for functional enrichment and interaction network analysis of genes and proteins for analysis [16]. The common targets of compound-disease were obtained as the potential targets for further analysis (Figure 3).
Figure 3.
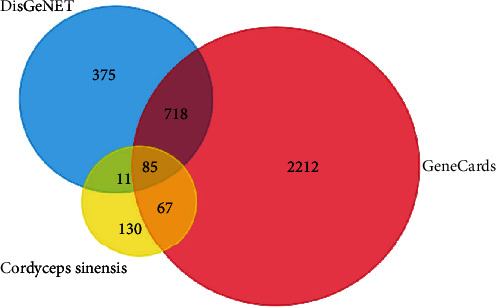
Overlapping target genes between DN and Cordyceps.
2.5. Network Construction of Compound-Disease Common Targets
A protein-protein interaction (PPI) network was obtained based on the STRING platform (https://string-db.org/), which covers nearly all functional interactions among the expressed proteins [17]. Target interaction information derived from the STRING database was imported into the Cytoscape (version 3.7.1; https://www.cytoscape.org/) software where the interaction information was integrated and analyzed.
2.6. GO and KEGG Pathway Enrichment Analyses
GO is the most comprehensive and widely used knowledgebase for the classification of gene functions, including the biological process (BP), molecular function (MF), and cell component (CC) [18]. KEGG (http://www.kegg.jp/) is an encyclopedia of genes and genomes; connecting genomic information to higher-order functional information to capture significantly enriched biological pathways is the major objective of the KEGG knowledgebase [19].
In our study, GO functional annotation and KEGG pathway enrichment analysis were carried out via using the ClusterProfiler package of R software, and P < 0.05 was employed as a screening threshold.
3. Results
3.1. Active Ingredients of Cordyceps
The active chemical components of Cordyceps were selected via the TCMSP databases, and 38 compounds were collected with the thresholds of OB ≥ 30% and DL ≥ 0.18 properties. Finally, 7 candidate ingredients were screened out for further study (Table 1).
Table 1.
Essential information about Cordyceps active components.
| Mol ID | Molecule name | OB (%) | DL |
|---|---|---|---|
| MOL001645 | Linoleyl acetate | 42.1 | 0.2 |
| MOL001439 | Arachidonic acid | 45.57 | 0.2 |
| MOL008999 | Cholesteryl palmitate | 31.05 | 0.45 |
| MOL000953 | CLR | 37.87 | 0.68 |
| MOL000358 | Beta-sitosterol | 36.91 | 0.75 |
| MOL008998 | Cerevisterol | 39.52 | 0.77 |
| MOL011169 | Peroxyergosterol | 44.39 | 0.82 |
3.2. Compound-Target Network Construction
In order to visualize the interaction relationship between the Cordyceps ingredients and corresponding targets, we established a compound-target network (Figure 2). By mapping 7 components to 293 potential targets, the network comprises 300 nodes and 500 edges, in which the red circles correspond to the putative targets and Cordyceps ingredients are in green. Chemical compound arachidonic acid, cerevisterol, beta-sitosterol, linoleyl acetate, cholesteryl palmitate, CLR, and peroxyergosterol correspond to 133, 100, 77, 57, 53, 49, and 31 targets, respectively. The results suggest that these 7 components probably serve significant therapeutic roles in DN.
3.3. Predicting DN-Related Targets
By retrieving the DisGeNET and GeneCards databases, results were integrated to obtain the DN-associated targets. As shown in Figure 3, the potential target genes in Cordyceps were matched to the DN-associated target gene by using the Funrich platform and was shown as a Venn diagram. Finally, 85 putative targets were selected according to the intersection of component targets and DN-related targets; one target was excluded because it had no interaction with other targets (Table 2).
Table 2.
Information of putative targets and topological attributes.
| No. | UniProt ID | Protein names | Gene names | Degree |
|---|---|---|---|---|
| 1 | P01375 | Tumor necrosis factor | TNF | 51 |
| 2 | P28482 | Mitogen-activated protein kinase 1 | MAPK1 | 45 |
| 3 | P01133 | Proepidermal growth factor | EGF | 45 |
| 4 | P00533 | Epidermal growth factor receptor | EGFR | 45 |
| 5 | P42574 | Caspase-3 | CASP3 | 45 |
| 6 | P04637 | Cellular tumor antigen p53 | TP53 | 44 |
| 7 | P35354 | Prostaglandin G/H synthase 2 | PTGS2 | 41 |
| 8 | P14780 | Matrix metalloproteinase-9 | MMP9 | 40 |
| 9 | P29474 | Nitric oxide synthase, endothelial | NOS3 | 40 |
| 10 | P05412 | Transcription factor AP-1 | JUN | 40 |
| 11 | P37231 | Peroxisome proliferator-activated receptor gamma | PPARG | 37 |
| 12 | P60484 | Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN | PTEN | 37 |
| 13 | P45983 | Mitogen-activated protein kinase 8 | MAPK8 | 36 |
| 14 | Q04206 | Transcription factor p65 | RELA | 35 |
| 15 | P35968 | Vascular endothelial growth factor receptor 2 | KDR | 33 |
| 16 | Q16539 | Mitogen-activated protein kinase 14 | MAPK14 | 33 |
| 17 | P08253 | 72 kDa type IV collagenase | MMP2 | 32 |
| 18 | P42345 | Serine/threonine-protein kinase mTOR | MTOR | 32 |
| 19 | P03372 | Estrogen receptor | ESR1 | 31 |
| 20 | P12821 | Angiotensin-converting enzyme | ACE | 28 |
| 21 | P01137 | Transforming growth factor beta-1 proprotein | TGFB1 | 27 |
| 22 | P16284 | Platelet endothelial cell adhesion molecule | PECAM1 | 27 |
| 23 | P42336 | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform | PIK3CA | 26 |
| 24 | P08254 | Stromelysin-1 | MMP3 | 25 |
| 25 | Q14790 | Caspase-8 | CASP8 | 25 |
| 26 | P09619 | Platelet-derived growth factor receptor beta | PDGFRB | 23 |
| 27 | Q00987 | E3 ubiquitin-protein ligase Mdm2 | MDM2 | 23 |
| 28 | P19438 | Tumor necrosis factor receptor superfamily member 1A | TNFRSF1A | 23 |
| 29 | P55211 | Caspase-9 | CASP9 | 22 |
| 30 | Q06124 | Tyrosine-protein phosphatase nonreceptor type 11 | PTPN11 | 22 |
| 31 | P41235 | Hepatocyte nuclear factor 4-alpha | HNF4A | 20 |
| 32 | P30556 | Type-1 angiotensin II receptor | AGTR1 | 20 |
| 33 | Q07869 | Peroxisome proliferator-activated receptor alpha | PPARA | 20 |
| 34 | P11511 | Aromatase | CYP19A1 | 16 |
| 35 | P35228 | Nitric oxide synthase, inducible | NOS2 | 16 |
| 36 | P49327 | Fatty acid synthase | FASN | 16 |
| 37 | O14920 | Inhibitor of nuclear factor kappa-B kinase subunit beta | IKBKB | 16 |
| 38 | P25116 | Proteinase-activated receptor 1 | F2R | 15 |
| 39 | P17252 | Protein kinase C alpha type | PRKCA | 15 |
| 40 | P07148 | Fatty acid-binding protein, liver | FABP1 | 15 |
| 41 | P15090 | Fatty acid-binding protein, adipocyte | FABP4 | 15 |
| 42 | P16109 | P-selectin | SELP | 14 |
| 43 | P11473 | Vitamin D3 receptor | VDR | 14 |
| 44 | P29350 | Tyrosine-protein phosphatase nonreceptor type 6 | PTPN6 | 14 |
| 45 | O95477 | Phospholipid-transporting ATPase ABCA1 | ABCA1 | 14 |
| 46 | P25101 | Endothelin-1 receptor | EDNRA | 13 |
| 47 | P55851 | Mitochondrial uncoupling protein 2 | UCP2 | 12 |
| 48 | P48736 | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform | PIK3CG | 12 |
| 49 | P05771 | Protein kinase C beta type | PRKCB | 12 |
| 50 | Q13464 | Rho-associated protein kinase 1 | ROCK1 | 12 |
| 51 | P20333 | Tumor necrosis factor receptor superfamily member 1B | TNFRSF1B | 12 |
| 52 | P15121 | Aldo-keto reductase family 1 member B1 | AKR1B1 | 11 |
| 53 | P21554 | Cannabinoid receptor 1 | CNR1 | 11 |
| 54 | P04629 | High affinity nerve growth factor receptor | NTRK1 | 11 |
| 55 | P22894 | Neutrophil collagenase | MMP8 | 11 |
| 56 | Q99814 | Endothelial PAS domain-containing protein 1 | EPAS1 | 10 |
| 57 | P10415 | Apoptosis regulator Bcl-2 | BCL2 | 10 |
| 58 | P32246 | C-C chemokine receptor type 1 | CCR1 | 8 |
| 59 | P11413 | Glucose-6-phosphate 1-dehydrogenase | G6PD | 8 |
| 60 | P08123 | Collagen alpha-2 | COL1A2 | 8 |
| 61 | P23219 | Prostaglandin G/H synthase 1 | PTGS1 | 8 |
| 62 | Q13133 | Oxysterols receptor LXR-alpha | NR1H3 | 8 |
| 63 | O75116 | Rho-associated protein kinase 2 | ROCK2 | 8 |
| 64 | Q03181 | Peroxisome proliferator-activated receptor delta | PPARD | 7 |
| 65 | P25105 | Platelet-activating factor receptor | PTAFR | 7 |
| 66 | P27169 | Serum paraoxonase/arylesterase 1 | PON1 | 7 |
| 67 | P17706 | Tyrosine-protein phosphatase nonreceptor type 2 | PTPN2 | 7 |
| 68 | O75469 | Nuclear receptor subfamily 1 group I member 2 | NR1I2 | 6 |
| 69 | P28223 | 5-Hydroxytryptamine receptor 2A | HTR2A | 6 |
| 70 | P12104 | Fatty acid-binding protein, intestinal | FABP2 | 6 |
| 71 | P02753 | Retinol-binding protein 4 | RBP4 | 5 |
| 72 | P39900 | Macrophage metalloelastase | MMP12 | 5 |
| 73 | P08235 | Mineralocorticoid receptor | NR3C2 | 5 |
| 74 | P11597 | Cholesteryl ester transfer protein | CETP | 5 |
| 75 | P07477 | Trypsin-1 | PRSS1 | 4 |
| 76 | Q07973 | 1,25-Dihydroxyvitamin D | CYP24A1 | 4 |
| 77 | P30542 | Adenosine receptor A1 | ADORA1 | 4 |
| 78 | P18054 | Polyunsaturated fatty acid lipoxygenase ALOX12 | ALOX12 | 4 |
| 79 | O00763 | Acetyl-CoA carboxylase 2 | ACACB | 4 |
| 80 | P04278 | Sex hormone-binding globulin | SHBG | 3 |
| 81 | P80365 | Corticosteroid 11-beta-dehydrogenase isozyme 2 | HSD11B2 | 3 |
| 82 | P13866 | Sodium/glucose cotransporter 1 | SLC5A1 | 2 |
| 83 | P35610 | Sterol O-acyltransferase 1 | SOAT1 | 1 |
| 84 | P05091 | Aldehyde dehydrogenase, mitochondrial | ALDH2 | 1 |
3.4. Common Target Network
85 putative genes correlated with DN were imported to the STRING database for analysis and network establishment. The interaction network was based on the selected targets with a medium confidence score of 0.400 (Figure 4). A total of 84 nodes and 767 edges were embodied, and the average node degree is 18.3 after analysis. The results were imported to the Cytoscape software for further analysis and network construction (Figure 5). In this figure, the edges represent the interaction between a pair of potential targets, while the nodes represent the targets, and the degree value indicates the intensity of target interaction.
Figure 4.
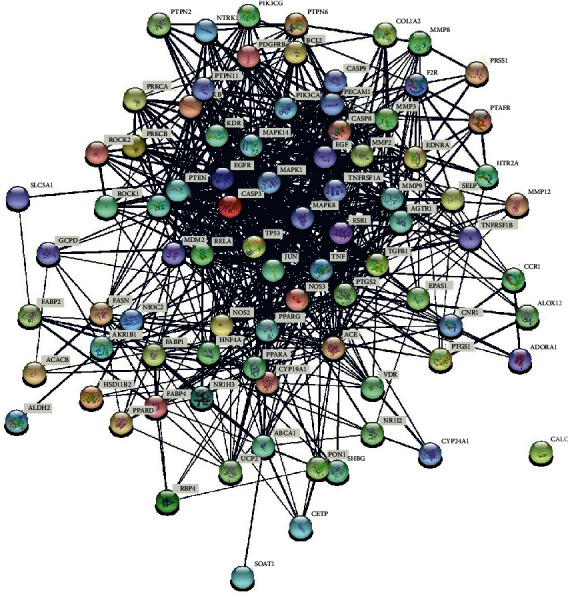
PPI network of overlapping targets between DN and Cordyceps. Each circular node represents a protein target, and the 3D structure in the circular nodes shows the protein spatial structure. The lines among different nodes represent the association among potential protein targets, while the width of lines was according to the action intensity.
Figure 5.
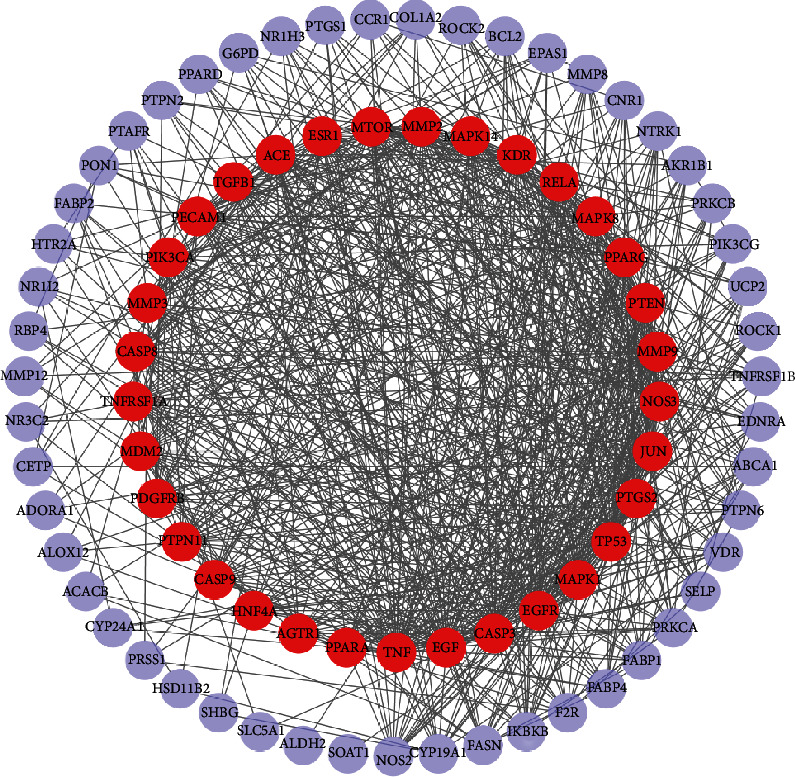
PPI network integration and analysis. The red nodes display the most potential targets in treating DN, which are greater than the average degree. Also, the nodes in purple show the potential targets under the average degree. Furthermore, the density of lines among inner nodes indicates the interaction relationship between different protein targets.
3.5. GO and KEGG Pathway Enrichment Analyses
After using the ClusterProfiler package for pathway analysis, a total of 1843 biological processes were enriched. The top 10 remarkably enriched BP terms were selected for analysis, including regulation of inflammatory response, response to lipopolysaccharide, response to molecule of bacterial origin, response to nutrient levels, muscle cell proliferation, response to oxygen levels, cellular response to chemical stress, regulation of lipid metabolic process, and lipid localization. Besides, 32 cell components were enriched, and the top 10 entries were screened, consisting of membrane raft, membrane microdomain, membrane region, RNA polymerase II transcription regulator complex, transcription regulator complex, caveola, vesicle lumen, plasma membrane raft, mitochondrial outer membrane, and region of cytosol. Furthermore, a total of 118 molecular functions were enriched; the top 10 entries were selected, containing steroid hormone receptor activity, monocarboxylic acid-binding, carboxylic acid-binding, fatty acid-binding, organic acid-binding, steroid binding, nuclear receptor activity, ligand-activated transcription factor activity, nuclear receptor transcription coactivator activity, and long-chain fatty acid-binding. These processes are of great significance to further understand the curative mechanism of Cordyceps on the treatment of DN. The results of the GO analysis are illustrated in Figure 6.
Figure 6.
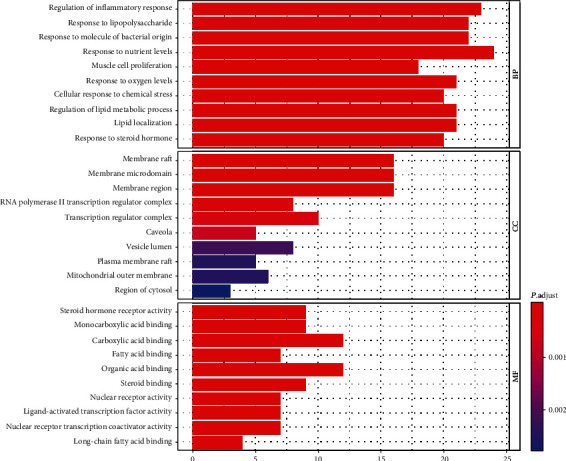
Enriched GO terms for BP, MF, and CC of putative targets of active components of Cordyceps. The color of the bar is displayed in a gradient from red to blue according to the ascending order of the P value, while the length of the bar is arranged according to the ascending order of the number of gene counts.
In terms of KEGG analysis, a total of 118 pathways were obtained. The top 20 significantly enriched pathways were screened out based on the threshold of P < 0.05 (Figure 7). The results indicated that these genes were mainly associated with the inflammatory signaling pathway, apoptosis, oxidative stress, and insulin signaling pathway, including AGE-RAGE signaling pathway in diabetic complications, TNF signaling pathway, apoptosis, IL-17 signaling pathway, PI3K-Akt signaling pathway, and insulin resistance. The results prove that Cordyceps may alleviate DN by regulating insulin resistance, apoptosis, and inflammatory reaction.
Figure 7.
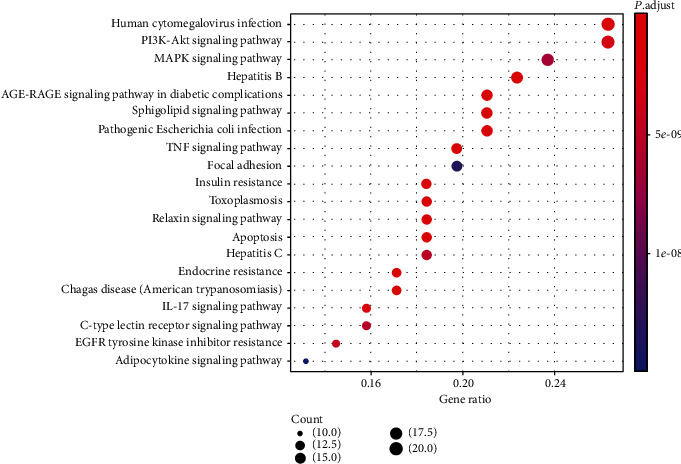
KEGG pathway analysis of potential targets of Cordyceps. The top 20 pathways are selected following the criteria of P < 0.05. The longitudinal axis represents the name of different pathways, and the transverse axis shows the number of enriched genes. Besides, the dot size represents the proportion of the number of enriched genes to the total number of genes, which is according to the descending proportion value. And the color of the dot is displayed in a gradient from red to blue according to the ascending order of the P value.
To more intuitively demonstrate which pathway each target is involved in, the target pathway data captured from KEGG analysis was uploaded into Cytoscape software for constructing a network graph of target and pathway (Figure 8).
Figure 8.
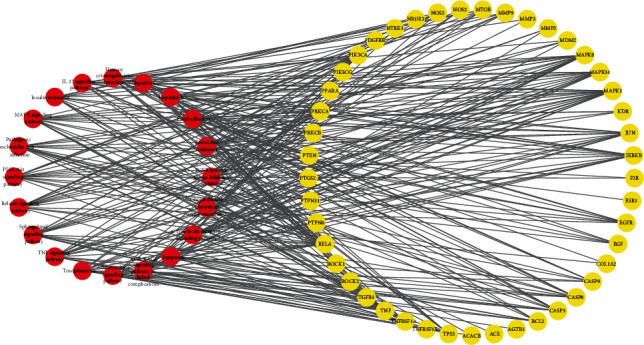
Pathway-target interaction network. Red circles indicate pathways which have interactions with potential targets, while the yellow circles represent putative targets.
4. Discussion
DN, featured as high incidence, multifactorial pathogenesis, and absence of practical methods for diagnosis and treatment, is undoubtedly a medical challenge worldwide. The etiology of DN is multifactorial; with hyperglycemia, oxidative stress, and advanced glycation end products (AGE) as the leading factors, chronic inflammation and infiltrated immune cells in renal tissue are considered to be the common pathological consequences [20, 21]. Despite current improving therapies, there is still a considerable residual risk of DN onset and progression [22]. Some relevant studies highlight new perspectives of TCM for delaying DN progression and strengthen the therapeutic rationale for TCM on the treatment of DN [23, 24]. Cordyceps contains several active ingredients which affect multiple targets and pathways in the progression of DN and has been used as an adjuvant on the treatment of DN in China for a long time [7, 25]. In this study, we selected 7 active ingredients from Cordyceps based on the OB and DL, including linoleyl acetate, arachidonic acid, cholesteryl palmitate, CLR, beta-sitosterol, cerevisterol, and peroxyergosterol. Some compounds of Cordyceps were reported to have the effect on ameliorating endocrine and metabolic disorders during the development of DN [26–29]. For instance, it was reported that beta-sitosterol protects the expression of insulin signaling molecules through activating insulin receptor and glucose transporter 4 in the adipose tissue with a high-fat diet, which can slow the development of DN [29]. Arachidonic acid is a strong inducer of insulin secretion and it can attenuate DN by inhibiting the TGF-β/Smad signaling pathway [26, 30]. In addition, arachidonic acid also can facilitate the production of anti-inflammatory lipoxins which were reported to improve insulin sensitivity and may prevent the development of diabetes [27]. Besides, several arachidonic acid metabolites, including PGE2, PGI2, and LXA4, PGE2 and PGI2 can alleviate insulin resistance and improve insulin sensitivity of pancreatic cells [28]; LXA4 can inhibit the production of IL-6, TNF-α, and ROS, thus alleviating inflammation, and has an antidiabetic effect [31, 32].
We found that many targets can be regulated by multiple compounds from the compound-target network, such as CYP19A1, NOS2, NR1H3, SHBG, and PTGS2. When it comes to core targets, TNF (degree = 51), MAPK1 (degree = 51), EGFR (degree = 45), and CASP3 (degree = 45) played an important role in the process of Cordyceps in DN treatment. TNF and MAPK1 are correlated with inflammation response and deterioration of renal function [33, 34]. CASP3 is known to have an important role in the promotion of apoptotic cell death [35]. AG1478 can block EGFR signaling and inhibit oxidative stress and endoplasmic reticulum stress markers in diabetic mice [36]. In addition, inhibition of EGFR activation is associated with improved DN and insulin resistance in type 2 diabetes mouse models [37]. Therefore, the candidate targets are mainly enriched for oxidative stress, insulin resistance, apoptosis, and inflammation.
We also selected 85 common targets between the components of Cordyceps and DN for performing GO enrichment, consisting of biological processes, molecular function, and cellular components, which is aimed at predicting the mechanism of Cordyceps in treating DN. We found that the candidate targets are involved in multiple biological processes, such as regulation of inflammatory response, response to lipopolysaccharide, response to nutrient levels, response to oxygen levels, cellular response to chemical stress, regulation of lipid metabolic process, and lipid localization. The active targets such as TNF, PPARG, MAPK1, EGFR, and TGF-β1 mainly participate in the biological processes of inflammatory response, oxidative stress, and lipid metabolic process [36, 38, 39]. Thus, the molecular processes of several targets are relatively consistent with the pathogenesis and mechanism of clinical DN. In addition, cellular components constitute membrane raft, membrane microdomain, membrane region, RNA polymerase II transcription regulator complex, and transcription regulator complex. It indirectly illustrates the complexity of the pathogenesis of DN and its damage to several cellular components, and Cordyceps may have a function in regulating these cellular components, eventually improving DN. Besides, molecular functions are mainly enriched in steroid hormone-related activity and steroid-binding, and many studies reported that steroid hormones were closely related to DN in patients with diabetes [40, 41]. It reveals that Cordyceps might target steroid hormone in DN treatment.
Using network pharmacological analysis and performing KEGG enrichment, we found that these Cordyceps components may relieve the symptoms of DN through the action of targets in various signaling pathways and multiple biological processes, including AGE-RAGE signaling pathway, TNF signaling pathway, apoptosis, IL-17 signaling pathway, PI3K-Akt signaling pathway, and insulin resistance. Activation of the receptor for AGEs or RAGE (receptor for advanced glycation end-products) is associated with the development of DN [42], which evokes oxidative stress and chronic inflammation in renal tissues, ending up in losses in kidney function by activating various intracellular signalings like PI3K/Akt/mTOR, NF-κB, MAPK/ERK, and TGF-β/Smad [43–45]. Additionally, it is believed that reactive oxygen species (ROS) can regulate PI3K/Akt/mTOR signaling and play an essential role in the development of DN, including epithelial-mesenchymal transition (EMT). During EMT, epithelial cells lose their primary epithelial properties, such as epithelial- (E-) cadherin, while acquiring characteristics typical of mesenchymal cells such as α-SMA, ending up with renal interstitial fibrosis [46]. Moreover, PI3K/Akt/mTOR signaling can promote high glucose-induced podocyte apoptosis, which contributes to the pathogenesis of DN [47].
TNF signaling is characterized as a well-known inflammatory cytokine associated with renal injury [48]. Once stimulated by TNF-α, NF-κB moves from the cytoplasm to the nucleus and activates the transcription of VCAM-1, ICAM-1, IL-6, and IL-8, which will result in endothelial inflammatory and DN pathological process acceleration [49]. Furthermore, NF-κB can be activated by several cytokines, which in turn induces the production of TNF-α, resulting in diabetic renal damage [50].
Insulin resistance is a critical process and one of the main symptoms in the initiation and progression of DN, which is closely related to microalbuminuria [51, 52]. High levels of insulin can cause insulin receptor degradation and drive early podocyte insulin resistance, and both the insulin receptor and nephrin are needed for full insulin sensitivity of podocytes. Thus, it explains why individuals with nephropathy caused by type 2 diabetes are commonly hyperinsulinaemic in the early stage of their disease [53]. Moreover, there are lots of mediators of insulin resistance that participate in driving renal function decline, including TGF-β1, blood pressure, inflammation, TNF-α, IL-6, and oxidative stress [54–56].
Therefore, these results of network pharmacological analyses not only verify that our screened targets are consistent with previous literature reports but also indicate that Cordyceps play a significant therapeutic role in DN by regulating several signaling pathways, including inflammatory response, insulin resistance, oxidative stress, apoptosis, and other pathways with unclear mechanisms. It will provide a novel methodology for further study of the therapeutic mechanism of Cordyceps in alleviating DN.
5. Conclusion
In summary, our network pharmacological analyses have shown that Cordyceps play an indispensable supplementary role in the treatment of DN, which is consistent with previous studies. Moreover, the biological functions of active chemical molecules and their corresponding targets of Cordyceps analyzed by network pharmacological methods provide a unique and innovative path for the study of TCM and further reveal the molecular biological mechanism of Cordyceps in treating DN. However, in vivo and in vitro experiments should be undertaken to validate the relationship between key targets and pathways of Cordyceps for the treatment of DN. Despite the limitations of this study, the results of this study provide new evidence and theoretical basis which will be used in subsequent theoretical and clinical research studies of Cordyceps.
Acknowledgments
This work was supported by the Science and Technology Department of Sichuan Province (grant number: 20YYJC4065).
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
Yan Li and Lei Wang contributed equally to this work. All authors have contributed to this study and approve its submission.
References
- 1.Sagoo M. K., Gnudi L. Diabetic nephropathy: an overview. Methods in Molecular Biology. 2020;2067:3–7. doi: 10.1007/978-1-4939-9841-8_1. [DOI] [PubMed] [Google Scholar]
- 2.Raval N., Kumawat A., Kalyane D., Kalia K., Tekade R. K. Understanding molecular upsets in diabetic nephropathy to identify novel targets and treatment opportunities. Drug Discovery Today. 2020;25(5):862–878. doi: 10.1016/j.drudis.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Fu H., Liu S., Bastacky S. I., Wang X., Tian X. J., Zhou D. Diabetic kidney diseases revisited: a new perspective for a new era. Mol Metab. 2019;30:250–263. doi: 10.1016/j.molmet.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun G.-d., Li C.-y., Cui W.-p., et al. Review of herbal traditional Chinese medicine for the treatment of diabetic nephropathy. Journal Diabetes Research. 2016;2016, article 5749857:1–18. doi: 10.1155/2016/5749857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olatunji O. J., Tang J., Tola A., Auberon F., Oluwaniyi O., Ouyang Z. The genus Cordyceps: an extensive review of its traditional uses, phytochemistry and pharmacology. Fitoterapia. 2018;129:293–316. doi: 10.1016/j.fitote.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Yang J., Dong H., Wang Y., et al. Cordyceps cicadae polysaccharides ameliorated renal interstitial fibrosis in diabetic nephropathy rats by repressing inflammation and modulating gut microbiota dysbiosis. International Journal of Biological Macromolecules. 2020;163:442–456. doi: 10.1016/j.ijbiomac.2020.06.153. [DOI] [PubMed] [Google Scholar]
- 7.Dong Z., Sun Y., Wei G., Li S., Zhao Z. A nucleoside/nucleobase-rich extract from Cordyceps sinensis inhibits the epithelial-mesenchymal transition and protects against renal fibrosis in diabetic nephropathy. Molecules. 2019;24(22):p. 4119. doi: 10.3390/molecules24224119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen D. D., Xu R., Zhou J. Y., et al. Cordyceps militaris polysaccharides exerted protective effects on diabetic nephropathy in mice via regulation of autophagy. Food & Function. 2019;10(8):5102–5114. doi: 10.1039/C9FO00957D. [DOI] [PubMed] [Google Scholar]
- 9.Wang X., Qin A., Xiao F., et al. N6-(2-Hydroxyethyl)-adenosine fromCordyceps cicadaeprotects against diabetic kidney disease via alleviation of oxidative stress and inflammation. Journal of Food Biochemistry. 2019;43(2, article e12727) doi: 10.1111/jfbc.12727. [DOI] [PubMed] [Google Scholar]
- 10.Ru J., Li P., Wang J., et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. Journal of Cheminformatics. 2014;6(1):p. 13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S. Getting the most out of PubChem for virtual screening. Expert Opin Drug Discov. 2016;11(9):843–855. doi: 10.1080/17460441.2016.1216967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gfeller D., Grosdidier A., Wirth M., Daina A., Michielin O., Zoete V. SwissTargetPrediction: a web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014;42:W32–W38. doi: 10.1093/nar/gku293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doncheva N. T., Morris J. H., Gorodkin J., Jensen L. J. Cytoscape StringApp: network analysis and visualization of proteomics data. Journal of Proteome Research. 2018;18(2):623–632. doi: 10.1021/acs.jproteome.8b00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stelzer G., Rosen N., Plaschkes I., et al. The GeneCards suite: from gene data mining to disease genome sequence analyses. Current Protocols in Bioinformatics. 2016;54:1.30.1–1.30.33. doi: 10.1002/cpbi.5. [DOI] [PubMed] [Google Scholar]
- 15.Piñero J., Bravo À., Queralt-Rosinach N., et al. DisGeNET: a comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Research. 2017;45(D1):D833–d839. doi: 10.1093/nar/gkw943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pathan M., Keerthikumar S., Ang C. S., et al. FunRich: an open access standalone functional enrichment and interaction network analysis tool. Proteomics. 2015;15(15):2597–2601. doi: 10.1002/pmic.201400515. [DOI] [PubMed] [Google Scholar]
- 17.Szklarczyk D., Gable A. L., Lyon D., et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Research. 2019;47(D1):D607–d613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Gene Ontology Resource. 20 years and still GOing strong. Nucleic Acids Research. 2019;47(D1):D330–d338. doi: 10.1093/nar/gky1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanehisa M., Furumichi M., Tanabe M., Sato Y., Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Research. 2017;45(D1):D353–d361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hickey F. B., Martin F. Role of the immune system in diabetic kidney disease. Current Diabetes Reports. 2018;18(4):p. 20. doi: 10.1007/s11892-018-0984-6. [DOI] [PubMed] [Google Scholar]
- 21.Tesch G. H. Diabetic nephropathy - is this an immune disorder? Clinical Science (London, England) 2017;131(16):2183–2199. doi: 10.1042/CS20160636. [DOI] [PubMed] [Google Scholar]
- 22.Alicic R. Z., Rooney M. T., Tuttle K. R. Diabetic kidney disease: challenges, progress, and possibilities. Clinical Journal of the American Society of Nephrology. 2017;12(12):2032–2045. doi: 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H. Y., Pan H. C., Chen Y. C., et al. Traditional Chinese medicine use is associated with lower end-stage renal disease and mortality rates among patients with diabetic nephropathy: a population-based cohort study. BMC Complementary and Alternative Medicine. 2019;19(1):p. 81. doi: 10.1186/s12906-019-2491-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen D., Tan R. Z., Zhao C. Y., et al. Astragalus mongholicus Bunge and Panax notoginseng (Burkill) F.H. Chen formula for renal injury in diabetic nephropathy-in vivo and in vitro evidence for autophagy regulation. Frontiers in Pharmacology. 2020;11:p. 732. doi: 10.3389/fphar.2020.00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao K., Gao Q., Zong C., Ge L., Liu J. Cordyceps sinensis prevents contrast-induced nephropathy in diabetic rats: its underlying mechanism. International Journal of Clinical and Experimental Pathology. 2018;11(12):5571–5580. [PMC free article] [PubMed] [Google Scholar]
- 26.Sonnweber T., Pizzini A., Nairz M., Weiss G., Tancevski I. Arachidonic acid metabolites in cardiovascular and metabolic diseases. International Journal of Molecular Sciences. 2018;19(11):p. 3285. doi: 10.3390/ijms19113285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gundala N. K. V., Naidu V. G. M., Das U. N. Amelioration of streptozotocin-induced type 2 diabetes mellitus in Wistar rats by arachidonic acid. Biochemical and Biophysical Research Communications. 2018;496(1):105–113. doi: 10.1016/j.bbrc.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Luo P., Wang M. H. Eicosanoids, β-cell function, and diabetes. Prostaglandins & Other Lipid Mediators. 2011;95(1-4):1–10. doi: 10.1016/j.prostaglandins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ponnulakshmi R., Shyamaladevi B., Vijayalakshmi P., Selvaraj J. In silico and in vivo analysis to identify the antidiabetic activity of beta sitosterol in adipose tissue of high fat diet and sucrose induced type-2 diabetic experimental rats. Toxicology Mechanisms and Methods. 2019;29(4):276–290. doi: 10.1080/15376516.2018.1545815. [DOI] [PubMed] [Google Scholar]
- 30.Chen G., Wang P., Zhao G., et al. Cytochrome P450 epoxygenase CYP2J2 attenuates nephropathy in streptozotocin-induced diabetic mice. Prostaglandins & Other Lipid Mediators. 2011;96(1-4):63–71. doi: 10.1016/j.prostaglandins.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das U. N. Arachidonic acid and lipoxin A4 as possible endogenous anti-diabetic molecules. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2013;88(3):201–210. doi: 10.1016/j.plefa.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Börgeson E., Johnson A. M. F., Lee Y. S., et al. Lipoxin A4 attenuates obesity-induced adipose inflammation and associated liver and kidney disease. Cell Metabolism. 2015;22(1):125–137. doi: 10.1016/j.cmet.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navarro J. F., Mora-Fernández C. The role of TNF-alpha in diabetic nephropathy: pathogenic and therapeutic implications. Cytokine & Growth Factor Reviews. 2006;17(6):441–450. doi: 10.1016/j.cytogfr.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Geng X. Q., Ma A., He J. Z., et al. Ganoderic acid hinders renal fibrosis via suppressing the TGF-β/Smad and MAPK signaling pathways. Acta Pharmacologica Sinica. 2020;41(5):670–677. doi: 10.1038/s41401-019-0324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang B., Jain S., Ashra S. Y., Furness P. N., Nicholson M. L. Apoptosis and caspase-3 in long-term renal ischemia/reperfusion injury in rats and divergent effects of immunosuppressants. Transplantation. 2006;81(10):1442–1450. doi: 10.1097/01.tp.0000209412.77312.69. [DOI] [PubMed] [Google Scholar]
- 36.Xu Z., Zhao Y., Zhong P., et al. EGFR inhibition attenuates diabetic nephropathy through decreasing ROS and endoplasmic reticulum stress. Oncotarget. 2017;8(20):32655–32667. doi: 10.18632/oncotarget.15948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z., Li Y., Overstreet J. M., et al. Inhibition of epidermal growth factor receptor activation is associated with improved diabetic nephropathy and insulin resistance in type 2 diabetes. Diabetes. 2018;67(9):1847–1857. doi: 10.2337/db17-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Araújo L. S., Torquato B. G. S., da Silva C. A., et al. Renal expression of cytokines and chemokines in diabetic nephropathy. BMC Nephrology. 2020;21(1):p. 308. doi: 10.1186/s12882-020-01960-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu R., Bo H., Huang S. Association between the PPARG gene polymorphism and the risk of diabetic nephropathy: a meta-analysis. Genetic Testing and Molecular Biomarkers. 2012;16(5):429–434. doi: 10.1089/gtmb.2011.0242. [DOI] [PubMed] [Google Scholar]
- 40.Maric C., Forsblom C., Thorn L., Wadén J., Groop P. H., FinnDiane Study Group Association between testosterone, estradiol and sex hormone binding globulin levels in men with type 1 diabetes with nephropathy. Steroids. 2010;75(11):772–778. doi: 10.1016/j.steroids.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grossmann M., Thomas M. C., Panagiotopoulos S., et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. The Journal of Clinical Endocrinology and Metabolism. 2008;93(5):1834–1840. doi: 10.1210/jc.2007-2177. [DOI] [PubMed] [Google Scholar]
- 42.Wadén J. M., Dahlström E. H., Elonen N., et al. Soluble receptor for AGE in diabetic nephropathy and its progression in Finnish individuals with type 1 diabetes. Diabetologia. 2019;62(7):1268–1274. doi: 10.1007/s00125-019-4883-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong J.-N., Li W.-W., Wang L.-L., et al. Jiangtang decoction ameliorate diabetic nephropathy through the regulation of PI3K/Akt-mediated NF-κB pathways in KK-Ay mice. Chinese Medicine. 2017;12(1):p. 13. doi: 10.1186/s13020-017-0134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yiu W. H., Wong D. W. L., Wu H. J., et al. Kallistatin protects against diabetic nephropathy in db/db mice by suppressing AGE-RAGE-induced oxidative stress. Kidney International. 2016;89(2):386–398. doi: 10.1038/ki.2015.331. [DOI] [PubMed] [Google Scholar]
- 45.Xu D., Kyriakis J. M. Phosphatidylinositol 3’-kinase-dependent activation of renal mesangial cell Ki-Ras and ERK by advanced glycation end products. The Journal of Biological Chemistry. 2003;278(41):39349–39355. doi: 10.1074/jbc.M302771200. [DOI] [PubMed] [Google Scholar]
- 46.Lu Q., Wang W. W., Zhang M. Z., et al. ROS induces epithelial-mesenchymal transition via the TGF-β1/PI3K/Akt/mTOR pathway in diabetic nephropathy. Experimental and Therapeutic Medicine. 2019;17(1):835–846. doi: 10.3892/etm.2018.7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X. M., Yao M., Liu S. X., Hao J., Liu Q. J., Gao F. Interplay between the Notch and PI3K/Akt pathways in high glucose-induced podocyte apoptosis. American Journal of Physiology. Renal Physiology. 2014;306(2):F205–F213. doi: 10.1152/ajprenal.90005.2013. [DOI] [PubMed] [Google Scholar]
- 48.Navarro J. F., Mora C., Muros M., Garcia J. Urinary tumour necrosis factor- excretion independently correlates with clinical markers of glomerular and tubulointerstitial injury in type 2 diabetic patients. Nephrology, Dialysis, Transplantation. 2006;21(12):3428–3434. doi: 10.1093/ndt/gfl469. [DOI] [PubMed] [Google Scholar]
- 49.Liang J., Yuan S., Wang X., et al. Attenuation of pristimerin on TNF-α-induced endothelial inflammation. International Immunopharmacology. 2020;82:p. 106326. doi: 10.1016/j.intimp.2020.106326. [DOI] [PubMed] [Google Scholar]
- 50.Caamaño J., Hunter C. A. NF-kappaB family of transcription factors: central regulators of innate and adaptive immune functions. Clinical Microbiology Reviews. 2002;15(3):414–429. doi: 10.1128/CMR.15.3.414-429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parvanova A. I., Trevisan R., Iliev I. P., et al. Insulin resistance and microalbuminuria: a cross-sectional, case-control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes. 2006;55(5):1456–1462. doi: 10.2337/db05-1484. [DOI] [PubMed] [Google Scholar]
- 52.Sarafidis P. A., Ruilope L. M. Insulin resistance, microalbuminuria, and chronic kidney disease. Current Hypertension Reports. 2008;10(4):249–251. doi: 10.1007/s11906-008-0046-6. [DOI] [PubMed] [Google Scholar]
- 53.Lay A. C., Hurcombe J. A., Betin V. M. S., et al. Prolonged exposure of mouse and human podocytes to insulin induces insulin resistance through lysosomal and proteasomal degradation of the insulin receptor. Diabetologia. 2017;60(11):2299–2311. doi: 10.1007/s00125-017-4394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karalliedde J., Gnudi L. Diabetes mellitus, a complex and heterogeneous disease, and the role of insulin resistance as a determinant of diabetic kidney disease. Nephrology, Dialysis, Transplantation. 2016;31(2):206–213. doi: 10.1093/ndt/gfu405. [DOI] [PubMed] [Google Scholar]
- 55.Shoelson S. E., Lee J., Goldfine A. B. Inflammation and insulin resistance. The Journal of Clinical Investigation. 2006;116(7):1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gnudi L. Cellular and molecular mechanisms of diabetic glomerulopathy. Nephrology, Dialysis, Transplantation. 2012;27(7):2642–2649. doi: 10.1093/ndt/gfs121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


