Abstract
Resection of brain tumors involving motor areas and pathways requires the identification and preservation of various cortical and subcortical structures involved in motor control at the time of the procedure, in order to maintain the patient's full motor capacities. The use of brain mapping techniques has now been integrated into clinical practice for many years, as they help the surgeon to identify the neural structures involved in motor functions. A common definition of motor function, as well as knowledge of its neural organization, has been continuously evolving, underlining the need for implementing intraoperative strategies at the time of the procedure. Similarly, mapping strategies have been subjected to continuous changes, enhancing the likelihood of preservation of full motor capacities. As a general rule, the motor mapping strategy should be as flexible as possible and adapted strictly to the individual patient and clinical context of the tumor. In this work, we present an overview of current knowledge of motor organization, indications for motor mapping, available motor mapping, and monitoring strategies, as well as their advantages and limitations. The use of motor mapping improves resection and outcomes in patients harboring tumors involving motor areas and pathways, and should be considered the gold standard in the resection of this type of tumor.
Keywords: Motor control, Brain tumors, Motor mapping techniques, Monitoring techniques, Motor area tumors, Glioma, Awake surgery, Oncological balance
ABBREVIATIONS
- 3D
three-dimensional
- CM
corticomotoneuronal
- CST
corticospinal tract
- CUSA
cavitron ultrasonic surgical aspirator
- DCS
direct cortical stimulation
- DES
direct electrical stimulation
- dPM
dorsal premotor
- ECOG
electrocorticography
- EEG
electroencephalogram
- EMG
electromyography
- EOR
extent of resection
- FLAIR
fluid-attenuated inversion-recovery
- fMRI
functional magnetic resonance imaging
- GTR
gross total resection
- HF
high-frequency
- hMT
hand-manipulation-task
- LF
low-frequency
- MEPs
motor evoked potentials
- MR
magnetic resonance
- MT
motor threshold
- PM
premotor
- RT
radiotherapy
- SLF
superior longitudinal fasciculus
- SMA
supplementary motor area
- SSEP
somatosensitive evoked potentials
- TCD
transcranial electrodes
- vPM
ventral premotor
The preservation of motor function has been considered a primary goal in neurosurgical oncology for many years.1-5 The occurrence of motor deficits severely impacts the patient's quality of life, as complete recovery is rarely obtained even when intensive rehabilitation is applied. Therefore, consistent efforts have been made to identify and preserve, as much as possible, patients’ motor abilities during surgery. Intraoperative neurophysiology is the most important surgical tool to identify neural cortical and subcortical structures involved in motor control.6 When direct electrical stimulation (DES) is applied over different brain areas involved in motor control, it can elicit motor responses7 or interfere with ongoing voluntary movements.8 To effectively perform motor mapping, the surgeon should be familiar with the basic physiological concepts and functional anatomy of the motor system and understand the neurosurgical armamentarium available to perform an effective intraoperative mapping, adapting the choice of tool to the clinical condition. By using a flexible approach, it is thus possible to extend the benefits of functional surgery to as many patients as possible.2,9 In the present review, we briefly describe the most recent models regarding functional organization of the motor system and discuss the main neurophysiological paradigms available in the theatre to perform motor mapping in most clinical conditions.
MOTOR SYSTEM ORGANIZATION
The current model of motor control organization has been formulated based on data obtained in neurophysiological studies on primates, functional neuroimaging research on human subjects,10-14 and from intraoperative data obtained during surgery for the removal of tumors.15,16 According to the most recent data, several cortical areas are involved in movement ideation, programming, execution, and control. Movements are organized in different levels of complexity according to hierarchical organization of the motor system.15,17,18 According to this view, the motor cortices control the spinal cord through various descending pathways, either indirect (through the brainstem or the cerebellum) or direct, which provides the final output from all CNS motor centers.14 The corticospinal tract (CST) is the direct pathway that controls skilled use of the extremities. It is made up of a lateral component descending contralaterally, which exerts contralateral control mainly over distal muscles; and also has a medial component descending ipsilaterally, exerting bilateral control over proximal and girdle muscles. Although indirect pathways exist across all species, they have progressively lost their importance during evolution, and the monosynaptic direct pathway has become the most relevant, connecting the motor cortex to motoneurons of many different muscles, particularly of the distal musculature (face, hand, feet).19 The CST has multiple areas of origin and its fibers have a range of axonal diameters; predominantly, these are very small (<1 μm), while very few have large diameters (the fast conducting). At least 50% of the CST originates from the primary motor cortex (M1).20,21 This direct connectivity between corticomotoneuronal (CM) and spinal motor neurons is restricted to primate distal limb muscles and is important for dexterous hand and digit movements. Viganò et al22,23 recently showed that, in the human M1 hand-knob, the CST is organized into 2 functional components, a more excitable caudal sector generating motor responses of short latency (referred to as the so-called “new-M1” in the primate), while the rostral sector (“old-M1” in the primate) generated motor responses of longer latency and lower excitability.
Rostral to M1, 2 main areas are involved in modulating motor responses: a premotor (PM) region (subdivided into ventral, vPM, and dorsal, dPM components)15,17 and the supplementary motor area (SMA).24,25 Posteriorly, in the parietal lobe, the primary sensory cortex, along with posterior parietal cortices, also contribute to sensory-motor control. PM areas in the human are thought to integrate multimodal sensory information in order to select and adapt the most appropriate motor task to perform a goal.17 This role is subserved by fibers connecting the posterior part of the frontal lobe and the superior and inferior parietal lobe, composed of the superior longitudinal fasciculus.10,26 In monkeys, the network connecting the anterior intraparietal area to area F5 (the “lateral grasping network”10) is involved in sensory-motor integration, coding visual information related to the properties of the object to grasp, in order to shape the hand before interaction with the object itself. A similar network has been proposed in humans, involving the supramarginal gyrus and vPM, connected by the third branch of the superior longitudinal fasciculus.26 The vPM is also connected with the second somatosensory area in the parietal operculum, which provides information regarding the ongoing movement to eventually modulate and adapt to the tactile properties of the grasp. Sensory information (visual, somatosensory, and proprioceptive) is also processed and integrated in the PM, which then generates and selects an appropriate motor program based on this information. This program is activated through projections with M1 and directly modulated by dense cortico-cortical connections.11,17 Moreover, PM descending fibers modulate muscle activation at the level of the spinal cord.16,17 Finally, the SMA-proper and preSMA, located on the mesial surface of the frontal lobe, are predominantly involved in movement initiation and the regulation of bilateral fine movements. These areas (particularly the SMA-proper) are primarily connected with the contralateral SMA through the corpus callosum and to M1 through U-shaped fibers. In addition, they have direct connections with the spinal cord and basal ganglia.
INDICATIONS FOR MOTOR MAPPING AND PATIENT SELECTION
Motor mapping is required when approaching any tumors or lesions involving motor areas and pathways.4,6,27 Considering the previously described complex organization of the motor system, the use of motor mapping should not only be restricted to cases in which the main components (M1 and its fibers) are affected but should be tailored to the individual patient, tumor characteristic, and network.2,23,28 When the surgeon is planning to use a functional approach, a patient entering the outpatient clinic with a diagnosis of a tumor involving “motor pathways” must first be managed by identifying the motor network(s) involved and estimating the degree of functional reorganization achieved by the patient's surrounding brain.8,29 This information defines the feasibility of resection and the degree of tumor removal that can be achieved in each individual patient.9 The management must therefore include a detailed interview with the patient, alongside associated neurological and neuropsychological motor and cognitive evaluations. These data should be integrated with those obtained from neuroimaging, to design a comprehensive, patient-centered map of functional involvement and reorganization. This material is then used to inform the eventual decision to treat and preoperative planning for resection.9
Patient Interview and Neuropsychological Evaluation
The symptoms and duration of the clinical history are important variables that should be taken into account. A careful analysis of the semiology of the seizures will provide information on the progression as well as the current extension of the neoplasm, as well as precious information pertaining to the degree of functional reorganization achieved by the surrounding brain, due to the presence, growth, and extension of the neoplasm itself.9 The patient interview should be associated with the neurological examination. The percentage of patients with motor deficits before surgery ranges between 7.5% and 68.6% in different series (Table 1).2,6 The effect of steroid administration in improving motor deficits should also be considered. Other additional data that should be evaluated are the patient's educational level, their job and future career plans, current hobbies, and future pregnancy (in female patients).30 These data (referred to as patient needs) have to be carefully discussed with the patient when elaborating a tailored surgical strategy, particularly in the context of the permanent impact on quality of life that surgery may exert. With the neuropsychological assessment, the individual performance related to the functional reorganization of each patients is evaluated and used to design the intraoperative testing for awake mapping, when planned. A detailed motor system evaluation requires investigation of the patient's fine skilled movements, bilateral movements, and apraxia, to provide both qualitative and quantitative data.4,31
TABLE 1.
List of Representative Studies on the Use of Motor Mapping for Resection of Tumor Involving Motor Pathways
| Paper | Year | Number of patients | Location | % total resection | Preoperative deficit | Early deficit | Permanent deficit | Awake/asleep (%) | LF/HF |
|---|---|---|---|---|---|---|---|---|---|
| Keles et al, 200450 | 2004 | 294 | Motor pathways | n/a | 25.80% | 20.40% | 4.80% | Asleep | LF |
| Carrabba et al, 200751 | 2007 | 146 | Motor pathways | 94.4% | n/a | 10.9%-59.3% | 3.5%-6.5% | Asleep | HF |
| Nossek et al, 201152 | 2011 | 55 | Motor pathways | 71% | 49% | 16% | 12.7% | Asleep/awake | LF + HF |
| Zhu et al, 201253 | 2012 | 58 | Motor pathways | 69% | 20.70% | n/a | n/a | Asleep | LF |
| Seidel et al, 201341 | 2013 | 100 | Motor pathways | 71% | 44% | 30% | 5% | Asleep | HF |
| Shinoura et al, 201355 | 2013 | 102 | Motor pathways | 53% | 68.6% | 41.10% | 7.80% | Awake | LF |
| Bello et al, 20142 | 2014 | 591 | Motor pathways | 58.3%-84.2% | 12% | 59.5%-94.4% | 0%-75% | Asleep | HF + LF |
| Raabe et al, 201446 | 2014 | 69 | Motor pathways | 68% | 37% | 33% | 3% | Asleep | HF |
| Schucht et al, 201454 | 2014 | 72 | Motor pathways | 73% | 33% | 30% | 4% | Asleep | HF |
| Obermueller et al, 201556 | 2015 | 171 | Motor pathways | 70.60% | 39.30% | 14% | 12% | Asleep | HF |
| Shiban et al, 201544 | 2015 | 37 | Motor pathways | 75% | 32% | 14% | 0% | Asleep | HF |
| Shinoura et al, 201757 | 2017 | 61 | Only M1 | 41% | 83.6 | 39.30% | 10.44% | asleep/awake/asleep | LF |
| Eseonu et al, 201758 | 2017 | 58 | Motor pathways | 42%-62.9% | 56.80% | 38.7%-29.3% | 9.7%-11% | Alseep/awake | LF |
| Sanmillan et al, 201759 | 2017 | 33 | Motor pathways | 93.9% | 33% | 18.20% | 0% | Asleep/awake | HF/LF |
| Zuev et al, 201760 | 2017 | 65 | Motor pathways | 60% | 71% | 28% | 8% | Alseep/asleep-awake-asleep | HF |
| Plans et al, 201761 | 2017 | 92 | Motor pathways | n/a | n/a | 24% | 16% | Asleep | HF |
| Han et al, 201829 | 2018 | 702 | Motor pathways | n/a | 12.70% | 30% | 7% | Alseep/awake | LF |
| Magill et al, 201828 | 2018 | 49 | M1 only | 50.90% | 7.50% | 60.40% | 37.70% | Alseep/awake | LF |
| Moiyadi et al, 201839 | 2018 | 40 | Motor pathways | 70% | 30% | 25% | 0% | Asleep | HF |
| Rossi et al, 20188 | 2018 | 120 | Motor pathways | Mean > 90% | 0% (Selected) | 15% | 0% | Asleep/awake | HF/LF |
| Rossi et al, 201923 | 2019 | 102 | M1 only | 85% | 9.80% | 96% | 2% | Asleep | HF |
| Zelitzki et al, 201962 | 2019 | 85 | Motor pathways | 41.2 | 24.70% | 21% | 10.60% | Alseep/awake | LF |
The name of the first author, the year of publication, the number of patients enrolled, the area studied (M1: yes, M1 and others: no), rate of total resection (EOR > 95%), percentage of reported preoperative, immediate postoperative, and permanent motor deficit, the prevalent anesthesia regiment, and the type of stimulation used are reported.
NA = not applicable.
Imaging Workup
The imaging methods are divided into basic and advanced methods.
Basic methods: magnetic resonance standard evaluation
Basic methods consist of basal T1-weighted, postGd-T1-weighted sequences, and fluid-attenuated inversion-recovery (FLAIR) sequences, as well as standard diffusion images. Postgadolinium images identify tumor–vascular relationships, extension of the tumor mass in deep (perforating) vessel areas, and detect any areas of contrast enhancement that may represents foci of malignant transformation.2,9 The volumetric FLAIR (in lower grade) and the volumetric postcontrast-T1-weighted (in high-grade) sequences estimate the tumor volume,32 the extent and number of lobes affected, the deep brain structures and/or the corpus callosum involvement, the possible extension of tumor mass toward the contralateral hemisphere, the presence of any other distant sites, and the relationship between the tumor mass and sites/structures that can be anatomically defined as functional. All the information obtained from the standard magnetic resonance (MR) evaluation contributes to the general judgment of whether the tumor is operable, and the evaluation of the expected achievable extent of resection (EOR). From this point of view, considering the site, size, and extension of the tumor mass, the surgeon must imagine a three-dimensional (3D) map of the functional motor networks comprehensive of their cortical and subcortical components. This is required in order to plan the resection and develop the intraoperative testing that will be administered to the patients during the procedure. The use of advanced MR imaging methods can also be of some help for this purpose.33
Advanced magnetic resonance evaluation
On the cortical level, functional magnetic resonance imaging (fMRI) is generally used for visualizing motor functional sites. The degree of correlation between fMRI areas of activation in M1 and intraoperative findings is usually high (specificity > 80%) in cases with no or mild motor deficits, or limited M1 tumor infiltration. In cases of significant infiltration or previous treatments (surgery or radiotherapy (RT)), the correlation is instead less reliable (specificity < 35%).34 On the subcortical level, the visualization and reconstruction of subcortical bundles or tracts is generally carried out by tractography based on diffusion images.33,35 Various acquisition techniques and different algorithms of reconstruction have been used in different studies, which can obtain very different tract reconstructions (in terms of path or size) in the same individual patient. This can make the judgment of tumor proximity on the individual level quite problematic. However, in general, the information obtained with tractography can be quite useful for 3D reconstructions of tracts making up functional networks surrounding and involving the tumor, that the surgeon is likely to reach and encounter during the resection.36,37
This information may help in defining surgical planning, patient's position, and size of bone flap. However, a tract infiltration identified by the preoperative tractography should not be taken as a reason to stop the resection, because this technique cannot predict whether the tract is functional.
For these reasons, tractography, although useful for identifying a general map of the connectivity surrounding a tumor, may not be effective in estimating the EOR preoperatively at the individual patient level. These findings again indicate that both fMRI and tractography data, despite their important scientific roles, should not be relied upon by the surgeon in the decision-making process.38
INTRAOPERATIVE SETUP
When the surgeon decides on a surgical resection, at the time of surgical planning, the functional networks possibly encountered during the resection must be carefully pondered to select and optimize the set of intraoperative tests to administer. A comprehensive time-locked procedure should be elaborated in order to recognize and preserve any functional sites encountered during the procedure (which ultimately constitute the cortical/subcortical limits of resection).
The design of the approach, the estimated time that the patient should be kept awake, and the type of testing all depend on the location of the tumor, the number and type of networks involved, and the clinical and neurological condition of the patient.5,31 The type of mapping should be tailored upon patient's condition and needs. On this matter, there are no defined standards in the neurosurgical armamentarium, and the different teams applying brain mapping techniques adapt different approaches in term of intraoperative tests, neurophysiological stimulation strategies, monitor techniques, and, consequently, anesthesiology regimens (Table 2). Generally, in terms of brain mapping, these can be performed by using either standard or advanced techniques (Tables 2 and 3).31
TABLE 2.
Neurophysiological Properties of the Current Available Stimulation Paradigms, Most Used (and Recommended) Stimulation Intensity and Probes
| HF stimulation | LF stimulation | |
|---|---|---|
| Stimulation frequency | 250-500 Hz | 50-60 Hz |
| Pulse form | Monophasic | Mono- or biphasic |
| Pulse direction | ||
| CORTICAL | Anodal/positive | n/a |
| SUBCORTICAL | Cathodal/negative | |
| Duration of individual pulse phase | 300-500 μs (standard) Up to 800 μs (advanced) | 500 μs |
| Number of pulses | 5 (standard) 2-9 (advanced) | n/a |
| Common current intensity range and probes | ||
| ASLEEP | 5-15 mA | 7-16 mA |
| Monopolar/Bipolara probe | Bipolar probe (always) | |
| AWAKE | 2-7 mA | 2-7 mA |
| Monopolar/Bipolara probe | Bipolar probe (always) |
aBipolar probe with HF increases stimulus focality but requires increase in current intensity.
NA = not applicable.
TABLE 3.
Current Indications of Available Stimulation Paradigms According to the Tumor Location (and Circuits) To Be Investigated and Anesthesia Regimen Used
| Awake condition | |||||
|---|---|---|---|---|---|
| Asleep condition | Resting condition | During a motor task execution | |||
| Premotor and parietal tumors | |||||
| HF | LF | HF | LF | HF | LF |
| Effective limited risk of negativemapping | To consider risk of negative mapping | Efficacy and risk as for asleep condition | Efficacy and risk as for asleep condition | Effective (repetition rate increased to 3 Hz) | Effective working current established on vPM |
| M1 and M1 originating fibers | |||||
| HF | LF | HF | LF | HF | LF |
| Effective (change in pulses number andwidth possibly needed). Very limitedrisk of negative mapping. | Poorly effective high risk of negative mapping. Increased risk of intraoperative seizures. | Efficacy and risk as for asleep condition | Efficacy and risk as for asleep condition | Limited data | Limited data |
| Informative on distance from M1 fibers (1 mA = 1 mm rule) | Informative on distance from M1 fibers (1 mA = 1 mm rule) | ||||
| Increased risk of postoperative apraxia (damage to SLFIII) | Increased risk of intraoperative seizure | ||||
The efficacy and the limitations or risk for each condition is reported.
STANDARD MOTOR MAPPING
The ideal neurophysiological paradigm for motor mapping is one that works in all clinical conditions and is easy to use; mapping protocols should have a very low percentage of false positives or false negatives, and should also inform the surgeon as to the proximity of stimulation from the CST and, if feasible, which component of the CST the surgeon is encountering.2,7,27 In addition, in terms of monitoring, it should also provide on-line information relating to the functional integrity of the CST.39-41
For mapping, 2 paradigms have been extensively described in the literature: the low-frequency (LF) (or Penfield's technique1) and the train of stimuli (high-frequency, HF42). Total 2 types of probes are available, monopolar and bipolar. The neurophysiological differences between the 2 paradigms7,43 are beyond the scope of this review; hence, we will focus on the different clinical settings in which the 2 paradigms can be applied and their functional results. As a general rule, motor mapping should initially be used on the cortical level to identify motor sites, in order to identify a safe area to perform the corticectomy.2 Afterwards, it should be used on the subcortical level to define resection boundaries by identifying eloquent white matter fibers involved in motor control that should be preserved.27,29
Low-Frequency Stimulation Paradigm
The LF stimulation paradigm is characterized by a biphasic-square wave (0.5 ms of duration) delivered by a bipolar probe continuously every 20 ms, during the entire duration of the stimulation.7 When applied over M1 while the patient is at rest (the best condition for investigating M1), LF induces motor responses using a current intensity ranging between 2 and 7 mA when the patient is awake, and between 7 and 16 mA when the patient is asleep (Table 2). The motor response is detected either as an overt movement or by electromyography (EMG) recording.2 The advantage of using EMG is that it allows for the simultaneous monitoring of different body segments contra and ipsilaterally; additionally, EMG recordings allows for the correct interpretation of the evoked motor responses and detection of impeding seizures.2,7 When recording free running EMG, LF evoked responses are characterized by progressive motor unit recruitment dependent on the current intensity applied (Figure 1A), showing clear somatotopy along the motor cortex, with a prevalent response of extensors to flexors. When applied on the subcortical level, motor unit recruitment becomes tonic, with more muscles recruited (Figure 1B).
FIGURE 1.
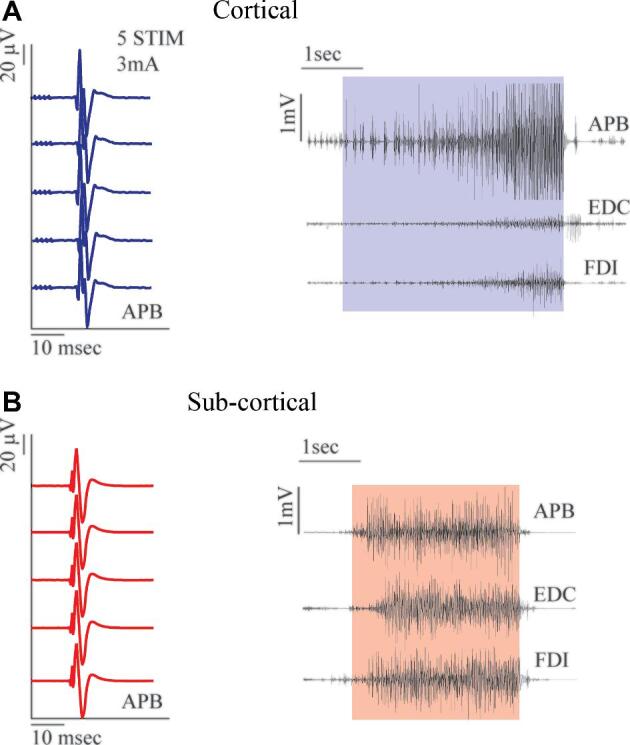
A, Left panel: Asleep surgery HF stimulation (4 mA, 5 pulses, 0.5-ms duration, 4-ms ISI, 1-Hz repetition rate, anodal, monopolar probe) on the primary motor cortex-hand knob region recruits MEPs in APB. Right panel: Asleep surgery LF stimulation (bipolar probe, 60 Hz, 4 mA) over the same hand-knob region of the primary motor cortex at threshold intensity evokes the somatotopic recruitment of different hand muscles (APB, EDC, FDI). The electrically induced EMG activity shows a progressive recruitment of motor units from the onset towards the end of the stimulus. B, Left panel: Asleep surgery. MEPs obtained in hand muscles (APB) by HF stimulation of the CST on the subcortical level (3 mA, 5 pulses, 0.5-ms duration, 4-ms ISI, 1-Hz repetition rate, cathodal, monopolar probe). Right panel: Asleep surgery LF subcortical stimulation (bipolar probe, 60 Hz, 6 mA). The EMG recruitment of different hand muscles (APB, EDC, FDI) is characterized by a tonic waveform. APB = abductor pollicis brevis, EDC = extensor digitorum communis, FDI = first dorsal interosseus.
High-Frequency Stimulation Paradigm
The HF paradigm is characterized by a monophasic-wave pulse 0.5 ms in duration, delivered as a train (interstimulus interval 2-4 ms), generally every second, either by a monopolar or bipolar probe.7 In the former, current travels between the probe and a reference electrode placed on the skull close to the motor trip; in the latter, it is delivered between the 2 balls of the probe. HF stimulation applied over M1 induces motor evoked potentials (MEPs), and an EMG machine is required for their detection (Figure 1A and 1B). The current intensity needed to evoke an MEP range between 2 and 7 mA in awake conditions and 5 to 15 mA in the asleep phase (Table 2).2 Morphology of the MEP response is similar irrespective of whether a response is evoked cortically or subcortically. In addition to qualitative information (the type of muscle recruited), HF stimulation provides quantitative data such as MEP amplitude, MEP morphology, and latency of the response from the stimulus. All these data help to correctly interpret the type of evoked responses obtained during stimulation and to perform the mapping.
Which Paradigm to Use
Both stimulation paradigms have been reported to be very effective in identifying motor areas and pathways, with variable rates of motor function preservation depending on the clinical context in which they are applied (Tables 2 and 3). In practical terms, when choosing the appropriate paradigm to apply, the key concept is the identification of the motor threshold (MT), ie, the lowest motor-evoked response induced by the lowest current intensity.2,7,43 The identification of the MT provides information as to the level of excitability of M1 as well as the distance between the site of stimulation and M1 (particularly when applied at the subcortical level).44
Low-frequency motor mapping
LF is particularly efficient in detecting motor responses when used in tumors with limited CST infiltration and sharp margins, occurring in patients with optimal seizure control and without significant preoperative motor deficits or previous treatments (low-risk tumors) (Table 3).2 Particular caution should be applied subcortically when LF is used in combination with a cavitron ultrasonic surgical aspirator (CUSA) (ultrasonic-aspirator), due to the occurrence of false negative responses, and in patients with a long history of seizures, previous motor deficits, or treatments.43,45 In the latter cases (high-risk tumors), LF may induce intraoperative seizures (ranging from 2% to 25% in different series) or result in false negative mapping (false negative results of stimulation), which lower the likelihood of performing a useful mapping, with the risk of surgery abortion (Tables 2 and 3).29 Therefore, while in low-risk tumors, the use of LF enables a very high rate of EOR (>90%); in difficult or high-risk tumors, the EOR drops <50%.2,7 False negative mapping differs from the concept of negative mapping, ie, a condition in which no motor responses are evoked when the current intensity is progressively increased during stimulation, because the site of stimulation is far away from functional/motor sites. Generally, a negative mapping is considered safe with LF when a current intensity of 16 mA in asleep conditions and 8 mA in awake conditions does not induce any motor response.29 When used on the subcortical level during motor pathway tumor resection, LF identifies motor sites in more than 40% of the cases, and this is associated with the risk of developing permanent motor deficits in 12% of cases. On the contrary, when no motor subcortical sites are identified, this risk drops to 3.2%.29 This figure could be explained either by the occurrence of ischemic insult, or by the physiological properties of the paradigm, which induces a motor responses only when the probe is in close vicinity of motor fibers.7
High-frequency motor mapping
HF stimulation delivered by a monopolar probe is highly efficient in most clinical conditions, regardless of previous deficits, treatment, long seizure history, high tumor infiltration and/or edema, CST infiltration, or anesthesia regimen (Table 3). HF is not influenced by the combined use of a CUSA.7 These data support the conclusion that HF is the most efficient paradigm for exciting M1 originating fibers, and when used subcortically, in the context of normal M1 excitability, it also provides information on the distance between the stimulation site and M1 fibers (1 m-1 mA rule).44 In this condition, motor fibers are found in most conditions, the current intensity is progressively reduced (starting from 10 mA to 12 mA) when nearing the CST, and when a subcortical MT of 3 mA is reached, the site of stimulation is very close to the CST, with a chance of inducing a permanent deficit below 2%.27,29,46 Of course, the subcortical MT should be adapted to the clinical context and varies according to clinical or radiological features in the group of “high-risk” motor tumors. In this setting, particularly in the case of difficult tumors (eg, with multiple previous treatments), the paradigm could be tuned to the clinical context with the use of flexible approaches such as the increase in the number of pulses (train of 7-9) and/or pulse width (500-800 ms). This allows for the elicitation of motor responses also in cases of failure of the standard protocol, making the mapping feasible with a very limited postoperative morbidity (<2%).23 In particular cases, when needed, the spatial resolution of the stimulation may also be increased by switching from the monopolar to bipolar probe, which has higher focality (Table 2). This is relevant in the case of tumors involving and extending toward M1, or to the SMA.23 In general, HF stimulation has been proven to be a more versatile and safe approach in comparison to LF, with less risk of intraoperative seizures (<2% even in difficult cases) and almost no chance of inducing (false) negative mapping. By using this approach, effective motor mapping is feasible in most clinical conditions, affording a very high degree of EOR (>90%) and very limited postoperative morbidity (2%). Morbidity is generally linked to the onset of ischemic events and is higher in the case of high-grade tumors.2,23,28
ADVANCED MOTOR MAPPING
The standard approach has been proven to be efficient in most tumors that involve M1 and its fibers (Tables 1-3). However, it has limitations. For example, when used in the case of tumors involving PM or parietal areas, preservation of M1 and its fibers is not enough to preserve motor performance. As reported by Rossi et al,8 a significant proportion of patients undergoing surgery under general anesthesia for tumors within a 2-cm distance from the central sulcus developed hand apraxia after surgery, despite the absence of new permanent motor deficits. Apraxia is a highly debilitating motor impairment that severely impacts the ability to perform highly-skilled motor movements, which is difficult to rehabilitate and impacts on the patient's quality of life.47 In fact, motor function requires a lot more than simple muscle contractions. We previously described the role of nonprimary motor areas in performing and adapting movements to the action's goal and the important role of subcortical neural networks in connecting these cortical areas.16 Hence, while M1 and its descending fibers can be identified by DES without the need of the patient's collaboration, structures involved in a higher level of motor programming and movement control (eg, grasping networks) can be mapped only in the awake setting, while the patient is performing an ongoing motor task (Figure 2).5 In this condition, the interferences generated by DES identify essential sites to be preserved on the cortical and subcortical level. There are 2 main motor tasks routinely used for this purpose.31 To investigate nonprimary motor areas, the simplest way is to ask to the patient to continuously perform a repetitive voluntary arm movement (flexion-extension) and to observe the interferences generated by DES during the task performance. This allows for the identification of cortical or subcortical sites that when stimulated cause an interruption of the ongoing movement.18,48 Recently, a more advanced intraoperative test, named the hand-manipulation-task (hMT) has been developed by Rossi et al. During this task, the patient is asked to perform a rotational movement with the hand, using a tool shaped like a screwdriver.8 DES is applied, causing several interferences with the ongoing movement when various cortical areas (S1, M1, vPM) or tracts (U-shaped fibers, superior longitudinal fasciculus (SLF)) are stimulated (Figure 3).15 The hMT, in comparison to the simple arm movement task, introduces the investigation of sensory-motor integration, a crucial component of motor control. Both tests (hMT or voluntary arm movement) have been successfully applied on the subcortical level to define limits of tumor resection and to preserve movement control.8,48 Both tasks can detect sites in the white matter of the frontal or parietal lobe, but at the moment, the full patterns of connectivity identified by DES during execution of these tasks is not known. One hypothesis is that they can detect a human homologue of the “lateral grasping network” described in monkeys.10,26 The clinical use of these tasks dramatically reduces the incidence of postoperative ideomotor apraxia (global incidence 2.5% when tasks are used in comparison with 50% when they are not applied) and the need for postoperative rehabilitation (50%-2%).8 This effect was particularly evident in patients with nondominant hemisphere tumors.8 Of interest, the use of these tasks did not influence overall EOR or the ability to achieve a complete tumor resection. Fibers subserving highly skilled motor functions (such as the SLFIII), although functionally relevant, are discrete and of small size.8,48
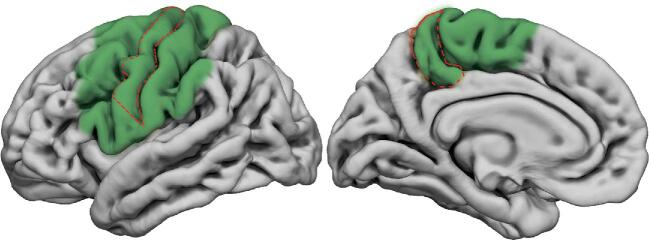
FIGURE 2.
Template of lateral and mesial surface of the left hemisphere showing in green the cortical areas where the use of motor task in awake condition is recommended to assess high skilled motor movement. Primary motor area (M1) is highlighted with a dotted red line.
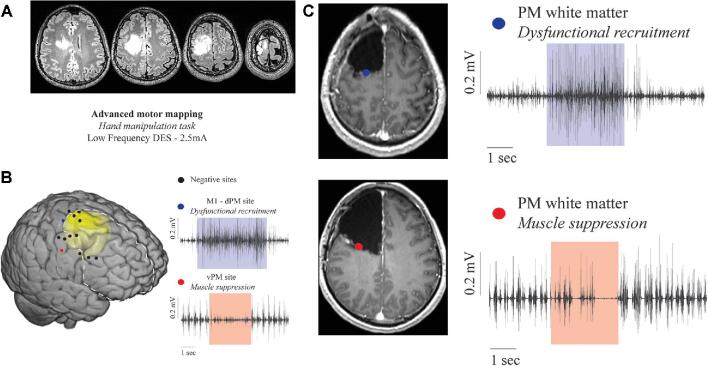
FIGURE 3.
Representative case of a 39-yr-old male with a previous history of focal seizures presented with a low-grade tumor involving the right prefrontal area. The patient was operated under asleep-awake-asleep anesthesia. The hMT was performed on the cortical and subcortical levels to assess the praxis network. A, Preoperative axial FLAIR pictures. B, MNI template in which the tumor volume was reported in yellow. Cortical sites tested during hMT with LF (3 mA, bipolar probe) are reported. Sites where no interferences were evoked are represented with black dots. The only site where dysfunctional recruitment was induced is reported as a blue dot (the EMG trace recorded from APB shows modification of the pattern of muscle activation during stimulation). The only site where a muscle suppression was induced is reported as a red dot (the EMG trace recorded from APB shows a significant reduction in motor unit recruitment). The light blue and red boxes highlight the stimulus application. C, Subcortical stimulation with LF during hMT identifies the posterior subcortical boundaries of resection. The upper panel shows a site where dysfunctional recruitment was induced (blue dot superimposed on an axial T1-post gadolinium postoperative MR) (EMG trace of APB showing modification of muscle pattern of activation). The lower panel shows a site where muscle suppression was induced (red dot superimposed on an axial T1-post gadolinium postoperative MR) (EMG trace of APB showing an almost complete suppression of muscle activation).
A second limitation of the standard approach regards the resection of tumors involving or originating within M1. These tumors were considered not amenable to surgical resections for many years, due to the associated high rate of postoperative morbidity. Consequently, a limited approach consisting of a biopsy was generally proposed. There are very few data available on the use of LF stimulation in this type of tumor surgery (Tables 1-3). LF works mainly in awake conditions and identifies cortical motor sites in around 90% of cases.2 Similarly, on the subcortical level, LF identifies sites evoking a motor response in more than 73% of cases. Gross total resection (GTR) is obtained in 50% of the cases, with a 10% chance of severe permanent deficits.2 In this setting, LF works particularly well in cases of tumors originating from the PM cortices invading M1 when growing posteriorly. LF is efficient in identifying the posterior margin of the resection represented by M1 fibers. However, when the tumor originates inside M1, particularly in cases not reaching the surface, LF evokes motor responses in multiple cortical sites, preventing the identification of a safe entry zone in the precentral gyrus, resulting in mapping abortion. In the same setting, HF stimulation provides a more versatile approach.23 The standard paradigm (train of 5) can identify, in most cases, a safe entry zone on the cortical level and the margin of resection is improved, affording a GTR in more than 73% of patients with a very low morbidity (<2%). The standard paradigm works better in tumors reaching the surface, with sharp margins that show contrast enhancement.23 In cases of patients who received previous treatments (particularly RT), the standard paradigm can, however, result in (false) negative mapping, even when a high current intensity is applied (>25 mA). In these cases, to obtain a reliable mapping on the cortical and subcortical level, an increase in the train (train of 7 or 9) or pulse width (500-800 u μs) may be needed (Table 2). This affords a high rate of complete resection (>90%) and a low postoperative morbidity (<2%).23 In the case of diffuse tumors (lower-grade setting) without any sign of enhancement or of deep-seated tumors, a combined standard and reduced train (1 or 2 pulses) approach is recommended to achieve a reliable mapping.23 In fact, while the standard HF technique (5 pulses) often evokes MEPs in most of the explored cortical sites, the reduction in the number of stimuli (2 or 1) allows for the definition of negative cortical areas inside the precentral gyrus (usually located in the rostral sector of M1) that can be safely used as a cortical entry point to reach and resect the tumor.23 The same strategy is also applied on the subcortical level to extend the resection towards the CST. This combined approach enables the resection to be extended (GTR > 88%), with a low morbidity (<2%). Overall, these data indicate HF stimulation is highly versatile and is able to reach reliable mapping in most clinical conditions.
To perform a reliable cortical mapping and identify a safe cortical entry zone, the assessment of the cortical Motor Threshold is crucial; subcortically, fundamental is the estimation of the minimal distance between the probe and the functional motor fibers. It is preferable, when this advanced approach is applied, to use general anesthesia, allowing a stable cortical excitability needed for the reliable evaluation of electrophysiolgical quantitative parameters (ie, MEP amplitude-current intensity), which are generally quite variable in awake condition. Moreover, HF stimulation is less ictogenic, even in patients with altered cortical excitability.23
MAPPING AND MONITORING
The ideal motor mapping protocol should also incorporate monitoring, ie, the availability of online information as to the functional integrity of the CST. Mapping alone is not enough to preserve motor functions, because postoperative deficits may develop due to damage of motor pathways, generally upward of the site of stimulation, occurring due to ischemic events.40 Monitoring during motor mapping incorporates electroencephalogram (EEG) and electrocorticography (ECOG), as well as MEP and somatosensitive evoked potentials (SSEP) monitoring. Although considered by some groups as optional, EEG and ECOG are used to evaluate the status of anesthesia and to titrate the level of anesthetics to the cortical excitability. To deliver reliable mapping, it is important that EEG and ECOG activity are continuous, avoiding any burst suppression. This reduces the chance of negative mapping (due to reduced cortical excitability) and the need to apply high-current intensities. The use of MEPs allows for continuous evaluation of the functionality of motor pathways, and they are very sensitive to vascular damage.49 Often, the use of MEPs is associated with that of SSEPs, which provide additional information as to the maintenance of good vascular supply. MEP monitoring can be obtained either by the use of transcranial electrodes (TCD) or by the use of cortical strips placed over M1 (direct cortical stimulation, DCS). TCD offers the opportunity to record MEPs from the beginning of surgery to the end, and to monitor both hemispheres.39,40 According to the montage in use (C2-C1 or C3-C4), MEPs from the lower or upper limb are monitored. It is generally recommended not to apply too high a current intensity to reduce the risk of stimulating too deep, missing the upper portion of the motor pathway. DCS stimulates directly through a cortical strip placed over M1, uses lower current intensities, and evokes MEPs from dura opening to closure. MEP monitoring is predictive of motor outcome. The occurrence of reversible or irreversible loss of MEPs during the procedure is associated with a high chance (80%) of developing motor deficits and poor motor outcome. There is no consensus on the type of alterations predictive of motor pathway injury. Any change in MEP amplitude (<50%), increase in current intensity, or change in latency has been related to possible irreversible damage on the pathway and should be referred to the surgeon during the procedure in order to undertake the proper measure to reduce the risk. However, particularly in the case of insular tumor surgery, changes in MEP recording may occur suddenly (in less than 3 s) and at the end of the resection time or during closure, highlighting the current difficulties in establishing a hierarchical series of predictive changes.41 In any case, the availability of such information enables all measures to be taken in a timely manner to reduce the functional impact of ischemic events, which improves outcome. Median and tibial nerve SSEPs are used to either indicate cortical or subcortical ischemia affecting the somatosensory cortex or lemniscal pathways. During supratentorial surgeries, a 50% decrease in amplitude or increase in latency of the cortical potential is commonly used as a warning criterion. A special application is the technique of SSEP phase reversal, ie, the recording of an inversed cortical SSEP potential over M1, to determine the central sulcus. Many surgical teams that are experienced with cortical mapping do not use this methodology, as they find direct cortical mapping providing more precise information.7,27 Nevertheless, SSEP phase reversal is an excellent tool that is of considerable help in cases of distorted anatomy or inconclusive mapping results.
ANESTHESIA
A crucial point is to decide whether to perform the motor mapping under general anesthesia or in awake conditions. Unfortunately, no conclusive data are available on this matter. Pure motor mapping can in fact be safely and efficiently performed under general anesthesia, albeit with the limitations previously described. Nowadays, the decision to perform motor mapping under awake anesthesia or in the asleep setting is based mostly on the surgeon's experience and preference. As a point of good practice, if the patient is in good neurological conditions, shows no sign of motor deficit or apraxia, and is harboring a tumor within 2 cm from the central sulcus, the adoption of advanced mapping in awake conditions should be considered, to fully maintain high skilled movements (ie, to also preserve the lateral grasping network).8 However, the choice of the strategy should be tuned to the patient conditions, needs, and type of tumors (volume, extension, and contrast enhancement). When the surgeon decides that patient collaboration is needed, the use of an asleep-awake-asleep strategy can help.48 This technique decreases the patient's discomfort during the opening and closing phases and also, by ensuring an adequate control of arterial and venous blood pressure, facilitates hemostasis in the conclusive phase. Importantly, this technique allows to constrain the time needed for mapping to the clinical context: a long cortical/subcortical mapping in patients highly cooperative with good clinical and neurological conditions or, conversely, a limited, focused mapping when degraded neurological or general (age, anxiety) conditions prevent a long awake phase.
LIMITATIONS AND CONCLUSIONS
The use of motor mapping improves resection and outcomes in patients with tumors involving motor pathways, and it should be considered to be the gold standard. The type of strategy should be tuned to the clinical context and the surgeon should be aware in each individual case of the possible network(s) involved and choose the appropriate mapping strategy according to the patient and tumor features. While the standard approach, particularly with LF stimulation, requires the availability of a stimulator and of a trained anesthesiologist only, the use of HF stimulation and of advanced approaches necessitates the use of an advanced intraoperative machine, the presence of a neuropsychologist, a neurophysiologist or neurophysiology well-trained technician, and of a neurosurgeon trained in neurophysiology (the 2 latter figures for correct stimulation choice and evoked responses interpretation). This, thus, introduces problems of cost and volume load.
Funding
This study did not receive any funding or financial support.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Contributor Information
Marco Rossi, Neurosurgery , Department of Oncology and Hemato-Oncology, Università degli Studi di Milano, Milano, Italy.
Tommaso Sciortino, Neurosurgery , Department of Oncology and Hemato-Oncology, Università degli Studi di Milano, Milano, Italy.
Marco Conti Nibali, Neurosurgery , Department of Oncology and Hemato-Oncology, Università degli Studi di Milano, Milano, Italy.
Lorenzo Gay, Neurosurgery , Department of Oncology and Hemato-Oncology, Università degli Studi di Milano, Milano, Italy.
Luca Viganò, Neurosurgery , Department of Oncology and Hemato-Oncology, Università degli Studi di Milano, Milano, Italy.
Guglielmo Puglisi, Neurosurgery , Department of Oncology and Hemato-Oncology, Università degli Studi di Milano, Milano, Italy; Laboratory of Motor Control, Department of Biotechnology and Translational Medicine, Università degli Studi di Milano Milano, Italy.
Antonella Leonetti, Neurosurgery , Department of Oncology and Hemato-Oncology, Università degli Studi di Milano, Milano, Italy; Laboratory of Motor Control, Department of Biotechnology and Translational Medicine, Università degli Studi di Milano Milano, Italy.
Henrietta Howells, Laboratory of Motor Control, Department of Biotechnology and Translational Medicine, Università degli Studi di Milano Milano, Italy.
Luca Fornia, Laboratory of Motor Control, Department of Biotechnology and Translational Medicine, Università degli Studi di Milano Milano, Italy.
Gabriella Cerri, Laboratory of Motor Control, Department of Biotechnology and Translational Medicine, Università degli Studi di Milano Milano, Italy.
Marco Riva, Neurosurgery , Department of Oncology and Hemato-Oncology, Università degli Studi di Milano, Milano, Italy.
Lorenzo Bello, Neurosurgery , Department of Oncology and Hemato-Oncology, Università degli Studi di Milano, Milano, Italy.
REFERENCES
- 1. Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60(4):389-443. [Google Scholar]
- 2. Bello L, Riva M, Fava Eet al. Tailoring neurophysiological strategies with clinical context enhances resection and safety and expands indications in gliomas involving motor pathways. Neuro Oncol. 2014;16(8):1110-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hervey-Jumper SL, Berger MS. Maximizing safe resection of low- and high-grade glioma. J Neurooncol. 2016;130(2):269-282. [DOI] [PubMed] [Google Scholar]
- 4. Duffau H, Mandonnet E. The “onco-functional balance” in surgery for diffuse low-grade glioma: integrating the extent of resection with quality of life. Acta Neurochir (Wien). 2013;155(6):951-957. [DOI] [PubMed] [Google Scholar]
- 5. Rossi M, Sani S, Nibali MC, Fornia L, Bello L, Byrne RW. Mapping in low-grade glioma surgery. Neurosurg Clin N Am. 2019;30(1):55-63. [DOI] [PubMed] [Google Scholar]
- 6. De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol. 2012;30(20):2559-2565. [DOI] [PubMed] [Google Scholar]
- 7. Szelényi A, Senft C, Jardan Met al. Intra-operative subcortical electrical stimulation: a comparison of two methods. Clin Neurophysiol. 2011;122(7):1470-1475. [DOI] [PubMed] [Google Scholar]
- 8. Rossi M, Fornia L, Puglisi Get al. Assessment of the praxis circuit in glioma surgery to reduce the incidence of postoperative and long-term apraxia: a new intraoperative test. J Neurosurg. 2018;130(1):17-27. [DOI] [PubMed] [Google Scholar]
- 9. Rossi Marco, Ambrogi Federico, Lorenzo Gay MGet al. Is supratotal resection achievable in low-grade gliomas? Feasibility, putative factors, safety and functional outcome. published online:2019. J Neurosurg. (doi:10.3171/2019.2.JNS183408). [DOI] [PubMed] [Google Scholar]
- 10. Borra E, Gerbella M, Rozzi S, Luppino G. The macaque lateral grasping network: a neural substrate for generating purposeful hand actions. Neurosci Biobehav Rev. 2017;75:65-90. [DOI] [PubMed] [Google Scholar]
- 11. Cerri G, Shimazu H, Maier MA, Lemon RN. Facilitation from ventral premotor cortex of primary motor cortex outputs to macaque hand muscles. J Neurophysiol. 2003;90(2):832-842. [DOI] [PubMed] [Google Scholar]
- 12. Rizzolatti G, Cattaneo L, Fabbri-Destro M, Rozzi S. Cortical mechanisms underlying the organization of goal-directed actions and mirror neuron-based action understanding. Physiol Rev. 2014;94(2):655-706. [DOI] [PubMed] [Google Scholar]
- 13. Ferri S, Peeters R, Nelissen K, Vanduffel W, Rizzolatti G, Orban GA. A human homologue of monkey F5c. Neuroimage. 2015;111:251-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lemon RN. What drives corticospinal output? F1000 Biol Rep. 2010;2:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Viganò L, Fornia L, Rossi Met al. Anatomo-functional characterisation of the human “Hand-Knob”: a direct electrophysiological study. Cortex. 2018;113:239-254. [DOI] [PubMed] [Google Scholar]
- 16. Fornia L, Ferpozzi V, Montagna Met al. Functional characterization of the left ventrolateral premotor cortex in humans: a direct electrophysiological approach. Cereb Cortex. 2018;28(1):167-183. [DOI] [PubMed] [Google Scholar]
- 17. Fornia L, Rossi M, Rabuffetti Met al. Direct electrical stimulation of premotor areas: different effects on hand muscle activity during object manipulation. Cereb Cortex. 2019;28(1):167-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Desmurget M, Richard N, Beuriat PAet al. Selective inhibition of volitional hand movements after stimulation of the dorsoposterior parietal cortex in humans. Curr Biol. 2018;28(20):3303-3309.e3. [DOI] [PubMed] [Google Scholar]
- 19. Maier MA, Kirkwood PA, Brochier T, Lemon RN. Responses of single corticospinal neurons to intracortical stimulation of primary motor and premotor cortex in the anesthetized macaque monkey. J Neurophysiol. 2013;109(12):2982-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Firmin L, Field P, Maier MAet al. Axon diameters and conduction velocities in the macaque pyramidal tract. J Neurophysiol. 2014;112(6):1229-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kraskov A, Baker S, Soteropoulos D, Kirkwood P, Lemon R. The corticospinal discrepancy: where are all the slow pyramidal tract neurons? Cereb Cortex. 2019;29(9):3977-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Geyer S, Ledberg A, Schleicher Aet al. Two different areas within the primary motor cortex of man. Nature. 1996;382(6594):805-807. doi:10.1038/382805a0. [DOI] [PubMed] [Google Scholar]
- 23. Rossi M, Conti Nibali M, Viganò Let al. Resection of tumors within the primary motor cortex using high-frequency stimulation: oncological and functional efficiency of this versatile approach based on clinical conditions. published online:2019. J Neurosurg. (doi:10.3171/2019.5.jns19453) [DOI] [PubMed] [Google Scholar]
- 24. Kalaska JF, Scott SH, Cisek P, Sergio LE. Cortical control of reaching movements. Curr Opin Neurobiol. 1997;7(6):849-859. [DOI] [PubMed] [Google Scholar]
- 25. Dea M, Hamadjida A, Elgbeili G, Quessy S, Dancause N. Different patterns of cortical inputs to subregions of the primary motor cortex hand representation in cebus apella. Cereb Cortex. 2016;26(4):1747-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Borra E, Luppino G. Large-scale temporo–parieto–frontal networks for motor and cognitive motor functions in the primate brain. Cortex. 2019;118:19-37. [DOI] [PubMed] [Google Scholar]
- 27. Schucht P, Seidel K, Jilch A, Beck J, Raabe A. A review of monopolar motor mapping and a comprehensive guide to continuous dynamic motor mapping for resection of motor eloquent brain tumors. Neurochirurgie. 2017;63(3):175-180. [DOI] [PubMed] [Google Scholar]
- 28. Magill ST, Han SJ, Li J, Berger MS. Resection of primary motor cortex tumors: feasibility and surgical outcomes. J Neurosurg. 2017;129(October):1-12. [DOI] [PubMed] [Google Scholar]
- 29. Han SJ, Morshed RA, Troncon Iet al. Subcortical stimulation mapping of descending motor pathways for perirolandic gliomas: assessment of morbidity and functional outcome in 702 cases. J Neurosurg. 2018;131(1):201-208. [DOI] [PubMed] [Google Scholar]
- 30. Puglisi G, Sciortino T, Rossi Met al. Preserving executive functions in nondominant frontal lobe glioma surgery: an intraoperative tool. J Neurosurg. 2018;131(2):474-480. [DOI] [PubMed] [Google Scholar]
- 31. Rossi M, Nibali MC, Torregrossa F, Bello L, Grasso G. Innovation in neurosurgery: the concept of cognitive mapping. World Neurosurg. 2019;131:364-370. [DOI] [PubMed] [Google Scholar]
- 32. Van den Bent MJ, Wefel JS, Schiff Det al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583-593. [DOI] [PubMed] [Google Scholar]
- 33. Conti Nibali M, Rossi M, Sciortino Tet al. Preoperative surgical planning of glioma. Limitations and reliability of fMRI and DTI tractography. J Neurosurg Sci. 2018;63(2):127-134. [DOI] [PubMed] [Google Scholar]
- 34. Castellano A, Cirillo S, Bello L, Riva M, Falini A. Functional MRI for surgery of gliomas. Curr Treat Options Neurol. 2017;19(10):34. [DOI] [PubMed] [Google Scholar]
- 35. Potgieser ARE, Wagemakers M, Van Hulzen ALJ, De Jong BM, Hoving EW, Groen RJM. The role of diffusion tensor imaging in brain tumor surgery: a review of the literature. Clin Neurol Neurosurg. 2014;124:51-58. [DOI] [PubMed] [Google Scholar]
- 36. Bello L, Gambini A, Castellano Aet al. Motor and language DTI fiber tracking combined with intraoperative subcortical mapping for surgical removal of gliomas. Neuroimage. 2008;39(1):369-82. [DOI] [PubMed] [Google Scholar]
- 37. Castellano A, Bello L, Michelozzi Cet al. Role of diffusion tensor magnetic resonance tractography in predicting the extent of resection in glioma surgery. Neuro Oncol. 2012;14(2):192-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Duffau H. The dangers of magnetic resonance imaging diffusion tensor tractography in brain surgery. World Neurosurg. 2014;81(1):56-58. [DOI] [PubMed] [Google Scholar]
- 39. Moiyadi A, Velayutham P, Shetty Pet al. Combined motor evoked potential monitoring and subcortical dynamic mapping in motor eloquent tumors allows safer and extended resections. World Neurosurg. 2018;120:e259-e268. [DOI] [PubMed] [Google Scholar]
- 40. Abboud T, Schwarz C, Westphal M, Martens T. A comparison between threshold criterion and amplitude criterion in transcranial motor evoked potentials during surgery for supratentorial lesions. J Neurosurg. 2019;131(3):740-749. [DOI] [PubMed] [Google Scholar]
- 41. Seidel K, Beck J, Stieglitz L, Schucht P, Raabe A. The warning-sign hierarchy between quantitative subcortical motor mapping and continuous motor evoked potential monitoring during resection of supratentorial brain tumors. J Neurosurg. 2013;118(2):287-296. [DOI] [PubMed] [Google Scholar]
- 42. Taniguchi M, Cedzich C, Taniguchi M, Cedzich C, Schramm J. Modification of cortical stimulation for motor evoked potentials under general anesthesia: technical description. Neurosurgery. 1993;32(2):219-226. [DOI] [PubMed] [Google Scholar]
- 43. Szelényi A, Bello L, Duffau Het al. Intraoperative electrical stimulation in awake craniotomy: methodological aspects of current practice. Neurosurg Focus. 2010;28(2):E7. [DOI] [PubMed] [Google Scholar]
- 44. Shiban E, Krieg SM, Haller Bet al. Intraoperative subcortical motor evoked potential stimulation: how close is the corticospinal tract? J Neurosurg. 2015;123(3):711-720. [DOI] [PubMed] [Google Scholar]
- 45. Carrabba G, Mandonnet E, Fava Eet al. Transient inhibition of motor function induced by the cavitron ultrasonic surgical aspirator during brain mapping. Neurosurgery. 2008;63(1):E178-E179. [DOI] [PubMed] [Google Scholar]
- 46. Raabe A, Beck J, Schucht P, Seidel K. Continuous dynamic mapping of the corticospinal tract during surgery of motor eloquent brain tumors: evaluation of a new method. J Neurosurg. 2014;120(5):1015-1024. [DOI] [PubMed] [Google Scholar]
- 47. Goldenberg G. Apraxia and the parietal lobes. Neuropsychologia. 2009;47(6):1449-1459. [DOI] [PubMed] [Google Scholar]
- 48. Almairac F, Herbet G, Moritz-Gasser S, Duffau H. Parietal network underlying movement control: disturbances during subcortical electrostimulation. Neurosurg Rev. 2014;37(3):513-516. [DOI] [PubMed] [Google Scholar]
- 49. MacDonald DB, Skinner S, Shils J, Yingling C. Intraoperative motor evoked potential monitoring - a position statement by the American society of neurophysiological monitoring. Clin Neurophysiol. 2013;124(12):2291-2316. [DOI] [PubMed] [Google Scholar]
- 50. Keles GE, Lundin DA, Lamborn KR, Chang EF, Ojemann G, Berger MS. Intraoperative subcortical stimulation mapping for hemispherical perirolandic gliomas located within or adjacent to the descending motor pathways: evaluation of morbidity and assessment of functional outcome in 294 patients. J Neurosurg. 2004;100(3):369-375. [DOI] [PubMed] [Google Scholar]
- 51. Carrabba G, Fava E, Giussani Cet al. Cortical and subcortical motor mapping in rolandic and perirolandic glioma surgery: impact on postoperative morbidity and extent of resection. J Neurosurg Sci. 2007;51(2):45-51. [PubMed] [Google Scholar]
- 52. Nossek E, Korn A, Shahar Tet al. Intraoperative mapping and monitoring of the corticospinal tracts with neurophysiological assessment and 3-dimensional ultrasonography-based navigation. J Neurosurg. 2011;114(3):738-746. [DOI] [PubMed] [Google Scholar]
- 53. Zhu FP, Wu JS, Song YYet al. Clinical application of motor pathway mapping using diffusion tensor imaging tractography and intraoperative direct subcortical stimulation in cerebral glioma surgery: a prospective cohort study. Neurosurgery. 2012;71(6):1170-1183. [DOI] [PubMed] [Google Scholar]
- 54. Schucht P, Seidel K, Beck Jet al. Intraoperative monopolar mapping during 5-ALA-guided resections of glioblastomas adjacent to motor eloquent areas: evaluation of resection rates and neurological outcome. Neurosurg Focus. 2014;37(6):E16. [DOI] [PubMed] [Google Scholar]
- 55. Shinoura N, Midorikawa A, Yamada Ret al. Awake craniotomy for brain lesions within and near the primary motor area: a retrospective analysis of factors associated with worsened paresis in 102 consecutive patients. Surg Neurol Int. 2013;4:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Obermueller T, Schaeffner M, Gerhardt J, Meyer B, Ringel F, Krieg SM. Risks of postoperative paresis in motor eloquently and non-eloquently located brain metastases. BMC Cancer. 2014. (doi:10.1186/1471-2407-14-21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shinoura N, Midorikawa A, Yamada Ret al. Operative strategies during awake surgery affect deterioration of paresis a month after surgery for brain lesions in the primary motor area. J Neurol Surg A Cent Eur Neurosurg. 2017;78(4):368-373. [DOI] [PubMed] [Google Scholar]
- 58. Eseonu CI, Rincon-Torroella J, ReFaey Ket al. Awake craniotomy vs craniotomy under general anesthesia for perirolandic gliomas: evaluating perioperative complications and extent of resection. Neurosurgery. 2017;81(3):481-489. [DOI] [PubMed] [Google Scholar]
- 59. Sanmillan JL, Fernández-Coello A, Fernández-Conejero I, Plans G, Gabarrós A. Functional approach using intraoperative brain mapping and neurophysiological monitoring for the surgical treatment of brain metastases in the central region. J Neurosurg. 2017;126(3):698-707. [DOI] [PubMed] [Google Scholar]
- 60. Zuev AA, Korotchenko EN, Ivanova DS, Pedyash NV., Teplykh BA. Surgical treatment of eloquent brain area tumors using neurophysiological mapping of the speech and motor areas and conduction tracts. Zh Vopr Nejrokhir Im NN Burdenko. 2017;81(1):39-50. [DOI] [PubMed] [Google Scholar]
- 61. Plans G, Fernández-Conejero I, Rifà-Ros X, Fernández-Coello A, Rosselló A, Gabarrós A. Evaluation of the high-frequency monopolar stimulation technique for mapping and monitoring the corticospinal tract in patients with supratentorial gliomas. A proposal for intraoperative management based on neurophysiological data analysis in a series of 92 patients. Clin Neurosurg. 2017;81(4):585-594. [DOI] [PubMed] [Google Scholar]
- 62. Zelitzki R, Korn A, Arial E, Ben-Harosh C, Ram Z, Grossman R. Comparison of motor outcome in patients undergoing awake vs general anesthesia surgery for brain tumors located within or adjacent to the motor pathways. Clin Neurosurg. 2019;81(4):585-594. [DOI] [PubMed] [Google Scholar]