Abstract
BACKGROUND
An early acute marker of long-term neurological outcome would be useful to help guide clinical decision making and therapeutic effectiveness after severe traumatic brain injury (TBI). We investigated the utility of the Disability Rating Scale (DRS) as early as 1 wk after TBI as a predictor of favorable 6-mo Glasgow Outcome Scale extended (GOS-E).
OBJECTIVE
To determine the predictability of a favorable 6-mo GOS-E using the DRS measured during weeks 1 to 4 of injury.
METHODS
The study is a sub analysis of patients enrolled in the Epo Severe TBI Trial (n = 200) to train and validate L1-regularized logistic regression models. DRS was collected at weeks 1 to 4 and GOS-E at 6 mo.
RESULTS
The average area under the receiver operating characteristic curve was 0.82 for the model with baseline demographic and injury severity variables and week 1 DRS and increased to 0.88 when including weekly DRS until week 4.
CONCLUSION
This study suggests that week 1 to 4 DRS may be predictors of favorable 6-mo outcome in severe TBI patients and thus useful both for clinical prognostication as well as surrogate endpoints for adaptive clinical trials.
Keywords: Traumatic brain injury, Closed head injury, Intracranial pressure, Disability Rating Scale, Prognostic
ABBREVIATIONS
- AUC
area under the ROC curve
- CT
computed tomography
- DRS
Disability Rating Scale
- ED
emergency department
- ER
emergency room
- GCS
Glasgow Coma Scale
- GOS
Glasgow Outcome Scale
- GOS-E
Glasgow Outcome Scale extended
- Hgb
hemoglobin
- ISS
Injury Severity Score
- OR
odds ratio
- RCT
randomized controlled trials
- ROC
receiver operating characteristic
- SD
standard deviation
- sTBI
severe traumatic brain injuries
- TBI
traumatic brain injury
Traumatic brain injury (TBI) is a major cause of death and disability in Americans under the age of 35 and the leading cause of premature death and disability worldwide.1,2 Several randomized controlled trials (RCT) aimed at improving outcomes in patients with severe traumatic brain injuries (sTBI) have failed to demonstrate treatment efficacy.3-8 Response-adaptive clinical trials and other advances in clinical trial methodology may help future studies by (a) randomizing participants to more promising treatments, thus increasing the power in multiarm studies, and (b) possibly dropping ineffective treatment arms. However, these types of studies rely on having an accurate early marker of long-term outcome that could capture treatment effectiveness and prognosis of long-term outcome. The Glasgow Outcome Scale (GOS) or extended version (GOS-E) at 6 mo after injury are the most common long-term outcome measures.
The search continues for an early acute marker of functional outcome that can help guide clinical decision making and therapeutic effectiveness. Many demographic and clinical variables have been assessed in studies with varying results.9,10 In mild TBI patients, demographic and clinical predictors and psychosocial factors in combination with preinjury mental health difficulties, education, and age have been described as important predictors for favorable recovery.11,12 Early neuroimaging, serum and/or cerebral spinal fluid biomarkers have been the latest focus of researchers in an attempt to identify early markers of functional outcome.10,13-15
Limitations of existing prediction models are well documented and include small sample sizes, complex modeling, and difficult to acquire physiological and biological markers.16
The Disability Rating Scale (DRS, Table 1) is an alternative outcome measure that can be assessed acutely in moderate and sTBI patients with good correlation to the GOS or GOS-E.17 We investigated the utility of the DRS as early as 1 wk after TBI as a predictor of 6-mo GOS-E. An early acute measure with already wide acceptance and validation has the potential to change the approach to clinical trials in sTBI patients.
TABLE 1.
Disability Rating Scale
| Category | Item | Scale |
|---|---|---|
| Arousal and awareness | Eye opening | 0 = Spontaneous; 1 = To speech and/or sensory stimulation; 2 = To pain; 3 = None |
| Best communication abilitya | 0 = Oriented; 1 = Confused; 2 = Inappropriate; 3 = Incomprehensible; 4 = None | |
| Best motor response | 0 = Obeying; 1 = Localizing; 2 = Withdrawing; 3 = Flexing; 4 = Extending; 5 = None | |
| Cognitive ability to handle self-care functions | Feeding | 0 = Complete; 1 = Partial; 2 = Minimal; 3 = None |
| Toileting | 0 = Complete; 1 = Partial; 2 = Minimal; 3 = None | |
| Grooming | 0 = Complete; 1 = Partial; 2 = Minimal; 3 = None | |
| Physical dependence on others | Level of functioning | 0 = Completely Independent; 1 = Independent in special environment; 2 = Mildly dependent; 3 = Moderately dependent; 4 = Markedly dependent; 5 = Totally dependent |
| Psychosocial adaptability | Employability | 0 = Not restricted; 1 = Selected jobs, competitive; 2 = Sheltered workshop, non-competitive; 3 = Not employable |
| Total DRS Score | Sum of scores: 0 to 29 (with 30 representing dead) |
aWith the DRS, if the patient cannot use voice because of tracheostomy or is aphasic or dysarthric or has vocal cord paralysis or voice dysfunction then estimate patient's best response and enter note under comments.
The DRS consists of 8 item scores from 4 categories and their sum where the level of disability ranges from no disability (score 0) to severe disability (score 29). A score of 30 is often used to denote those who have died.
METHODS
This research was completed in accordance with the Helsinki Declaration. In an RCT of sTBI patients (Epo Severe TBI trial), we collected DRS scores at 1, 2, 3, and 4 wk as well as structured-interview GOS-E scores at 6 mo after injury. The significance, design, and primary analytical procedures of the Epo sTBI clinical trial are reported in Robertson et al.3 In this study, 200 individuals ≥15 yr of age with a closed head injury admitted to 2 level 1 trauma centers were recruited within 6 h of injury. Exclusionary criteria included: (1) a Glasgow Coma Scale (GCS) of 3 and fixed dilated pupils, (2) penetrating head injury, (3) life-threatening systemic injuries, (4) pregnancy, (5) severe polytrauma, and (6) spinal cord injury. In a 2 × 2 factorial design, enrolled participants were randomly assigned to administration of Epo or placebo and to hemoglobin (Hgb) transfusion thresholds of 7 or 10 g/dL. Further details regarding the demographics and injury characteristics of this study population have been described in Robertson et al.3
The study was conducted under regulations for the Exception from Informed Consent for Emergency Research (21 CRF 50.24). The protocol was approved by the Food and Drug Administration and Institutional Review Boards at both clinical sites. When families were subsequently located and/or the patient recovered sufficiently to consent, they signed a consent form to continue to participate in the study.
Statistical Analyses
Subjects that had missing 6-mo GOS-E were omitted (n = 18). Potential predictor variables for the current study were selected a priori adhering to prespecified covariates that had been used in the final outcomes article for the Epo sTBI trial and included in the computation of the Injury Severity Score (ISS) and IMPACT prognostic scores. DRS scores were collected at 1, 2, 3, and 4 wk after injury and treated as continuous variables. The blinded outcome variable was GOS-E at 6 mo after injury, dichotomized into unfavorable if GOS-E was death, persistent vegetative state, or severe disability and favorable if GOS-E was moderate or good recovery. Other variables considered were all at baseline in the emergency room (ER) or during enrollment within 6 h of sTBI specified in the clinical trial: Gender, GCS sum (continuous and categorical versions), ISS, intubation in the ER, initial Marshall computed tomography (CT) scan category, mechanism of injury, hypoxia present, hypotension present, age, Hgb level in ER, glucose level in ER, number of pupils reactive, subarachnoid hemorrhage, extradural hematoma, surgery on admission, motor GCS, and the IMPACT lab model for risk of poor outcome. Details of the model fit and assessment are given in the Methods, Text, Supplemental Digital Content.
To fit each prediction model and subsequently get unbiased estimates of the accuracy, the data were split into a training (70%) and test set (30%), stratified on the outcome to obtain equal distribution of favorable 6-mo GOS-E in the training and test sets. We then fit 4 L1-regularized logistic regression models to the training set using the baseline variables and (1) week 1 DRS, (2) weeks 1 and 2 DRS, (3) weeks 1 to 3 DRS, and (4) weeks 1 to 4 DRS scores. The 4 models were then used to predict the favorable GOS-E score in the test set and receiver operating characteristic (ROC) analysis was used to compare the models. Patients were omitted prior to each training/test data separation and model fit if they died before that time period as their 6-mo outcome would already be known to be unfavorable GOS-E. There is no utility in assessing a prognostic algorithm on these subjects at that time point and keeping those that died in the sample would artificially inflate the model accuracy estimates. The remaining total used for model fit and prediction included 170, 164, 161, and 160 patients for the 4 models, respectively. We repeated the process 500 times and averaged the area under the ROC curves because random splits can lead to varying test set accuracies. To obtain the “best” model for future predictions, we used the same process to choose variables and coefficients with the whole dataset (training and test combined).
There were 3, 2, 9, and 8 values missing in week 1, 2, 3, and 4 DRS scores, respectively. These missing values were imputed using k-nearest neighbors (k = 10). All analyses were conducted using R version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
The distribution of baseline variables is displayed in Table 2. The patients were typical of an adult sTBI population in the US with the majority being young adults with a male predominance.18 Motor vehicle accidents were the most common mechanism of injury. A total of 32% had a mass lesion on their initial CT scan, and 30% went directly from the EC to the odds ratio (OR) for evacuation of the mass lesion.
TABLE 2.
Descriptive Statistics of Baseline and DRS Variables
| Variable | Overall (n = 182) | Unfavorable GOS-E score (n = 112) | Favorable GOS-E score (n = 70) |
|---|---|---|---|
| Age (yr), mean (SD) | 33.47 (13.48) | 36.66 (13.83) | 28.37 (11.22) |
| ISS, mean (SD) | 31.82 (8.57) | 32.20 (8.90) | 31.21 (8.06) |
| Motor component of GCS (at enrollment), mean (SD) | 3.57 (1.69) | 3.18 (1.78) | 4.20 (6.83) |
| Glasgow Coma Scale sum score (at enrollment), mean (SD) | 6.63 (2.68) | 6.00 (2.63) | 7.64 (2.44) |
| Glucose (emergency department [ED]), mmol/L, mean (SD) | 9.29 (3.84) | 9.56 (3.84) | 8.84 (3.83) |
| Hemoglobin (ED), g/dL, mean (SD) | 14.07 (1.93) | 13.78 (1.89) | 14.55 (11.22) |
| Gender, N (%) | |||
| Female | 24 (13.2) | 14 (12.5) | 10 (14.3) |
| Male | 158 (86.8) | 98 (87.5) | 60 (85.7) |
| Prehospital hypotension, N (%) | |||
| No | 159 (87.4) | 95 (84.8) | 64 (91.4) |
| Yes | 23 (12.6) | 17 (15.2) | 6 (8.6) |
| Prehospital hypoxia, N (%) | |||
| No | 145 (79.7) | 86 (76.8) | 59 (84.3) |
| Yes | 37 (20.3) | 26 (23.2) | 11 (15.7) |
| Pupil reactivity, N (%) | |||
| Both | 111 (61) | 57 (50.9) | 54 (77.1) |
| Neither | 50 (27.5) | 40 (35.7) | 10 (14.3) |
| 1 | 21 (11.5) | 15 (13.4) | 6 (8.6) |
| Subarachnoid hemorrhage, N (%) | |||
| No | 54 (29.7) | 24 (21.4) | 30 (42.9) |
| Yes | 128 (70.3) | 88 (78.6) | 40 (57.1) |
| Epidural hematoma, N (%) | |||
| No | 153 (84.1) | 101 (90.2) | 52 (74.3) |
| Yes | 29 (15.9) | 11 (9.8) | 18 (25.7) |
| Surgery on admission, N (%) | |||
| No | 127 (69.8) | 67 (59.8) | 60 (85.7) |
| Yes | 55 (30.2) | 45 (40.2) | 10 (14.3) |
| Intubated in, N (%) | |||
| Field/OR/other | 43 (23.6) | 28 (25.0) | 15 (21.4) |
| ER | 139 (76.4) | 84 (75.0) | 55 (78.6) |
| Glasgow Coma Scale score (at enrollment), N (%) | |||
| GCS > 8 | 40 (22.0) | 20 (17.9) | 20 (28.6) |
| GCS 6 to 8 | 79 (43.4) | 40 (35.7) | 39 (55.7) |
| GCS 3 to 5 | 63 (34.6) | 52 (46.4) | 11 (15.7) |
| Marshall CT scan (ED), diffuse injury category, N (%) | |||
| Mass lesion (evacuated/unevacuated) | 59 (32.4) | 49 (43.8) | 10 (14.3) |
| Diffuse injury 2 | 82 (45.1) | 36 (32.1) | 46 (65.7) |
| Diffuse injury 3 | 41 (22.5) | 27 (24.1) | 14 (20.0) |
| Mechanism of injury, N (%) | |||
| Assault | 19 (10.4) | 17 (15.2) | 2 (2.9) |
| Fall/jump | 26 (14.3) | 17 (15.2) | 9 (12.9) |
| Motor vehicle | 107 (58.8) | 59 (52.7) | 48 (68.6) |
| Motorcycle | 26 (14.3) | 15 (13.4) | 11 (15.7) |
| Other | 4 (2.2) | 4 (3.6) | 0 (0.0) |
| DRS at week 1, mean (SD) | 22.56 (6.53) | 25.20 (4.70) | 18.26 (6.83) |
| DRS at week 2, mean (SD) | 20.11 (7.65) | 23.59 (5.59) | 14.38 (7.14) |
| DRS at week 3, mean (SD) | 18.16 (8.35) | 22.30 (6.25) | 11.11 (6.59) |
| DRS at week 4, mean (SD) | 16.82 (9.03) | 21.56 (6.96) | 9.05 (6.20) |
Table 3 displays the shrunken coefficients from L1-regularized logistic regression models with 6-mo favorable GOS-E outcome score using the combined training and test sets. The IMPACT score, age, presence of an epidural hematoma, and surgery on admission were important in all 4 models. The DRS scores were also selected as important variables by the L1-regularization, highlighting the predictive power of DRS. Not surprisingly, the week 4 DRS score used in the model was considered as the most predictive one.
TABLE 3.
Shrunken Coefficients for the Variables Chosen From L1-Regularized Logistic Regression Models Predicting 6-mo favorable GOS
| Variable | Model 1 (baseline + 1 wk DRS) | Model 2 (baseline + 1 and 2 wk DRS) | Model 3 (baseline + 1, 2, and 3 wk DRS) | Model 4 (baseline + 1, 2, 3, and 4 wk DRS) |
|---|---|---|---|---|
| (Intercept) | 2.882 | 2.719 | 3.452 | 3.285 |
| IMPACT probability of poor GOS score (lab model) | −0.024 | −0.022 | −0.016 | −0.017 |
| Age (yr) | −0.030 | −0.009 | −0.022 | −0.021 |
| ISS | 0 | 0 | 0 | 0 |
| Epidural hematoma | 0.597 | 0.100 | 0.396 | 0.370 |
| Motor component of GCS (at enrollment) | 0 | 0 | 0 | 0 |
| Glasgow Coma Scale score (at enrollment), continuous | 0.022 | 0 | 0 | 0 |
| Glasgow Coma Scale score (at enrollment), categorized | ||||
| GCS > 8, reference | – | – | – | – |
| GCS 6 to 8 | 0 | 0 | 0 | 0 |
| GCS 3 to 5 | 0 | 0 | 0 | 0 |
| Marshall CT scan (ED) | ||||
| Mass lesion (evacuated/unevacuated, reference) | – | – | – | – |
| Diffuse injury 2 | 0.323 | 0 | 0 | 0 |
| Diffuse injury 3 | 0 | 0 | 0 | 0 |
| Glucose (ED), mmol/L | 0 | 0 | 0 | 0 |
| Hemoglobin (ED), g/dL | 0 | 0 | 0 | 0 |
| Male | −0.039 | 0 | 0 | 0 |
| Prehospital hypotension | 0 | 0 | 0 | 0 |
| Prehospital hypoxia | 0 | 0 | 0 | 0 |
| Intubated in ER | 0 | 0 | 0 | 0 |
| Mechanism of injury | ||||
| Assault (reference) | – | – | – | – |
| Fall/jump | 0 | 0 | 0 | 0 |
| Motor vehicle | 0 | 0 | 0 | 0 |
| Motorcycle | 0.286 | 0 | 0.271 | 0.590 |
| Other | −0.366 | 0 | 0 | 0 |
| Pupil reactivity | ||||
| Both (Reference) | – | – | – | – |
| Neither | −0.210 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 |
| Subarachnoid hemorrhage | 0 | 0 | 0 | 0 |
| Surgery on admission | −0.126 | −0.204 | −0.374 | −0.358 |
| Week 1 DRS | −0.107 | 0 | 0 | 0 |
| Week 2 DRS | NA | −0.100 | 0 | 0 |
| Week 3 DRS | NA | NA | −0.149 | −0.001 |
| Week 4 DRS | NA | NA | NA | −0.159 |
These coefficients makeup the actual prediction model using the usual logistic regression equation:
logit (Prob(GOS = favorable)) =2.882 – 0.024 × (IMPACT score) – 0.030 × age + 0.597 × (epidural hematoma) + 0.022 × (GCS sum) + 0.323 × (diffuse injury 2 on Marshall CT scan) – 0.039 × male +0.286 × (motorcycle injury) –0.366 × (other mechanism of injury) – 0.210 × (neither pupil reactive) – 0.126 × (surgery on admission) – 0.107(week 1 DRS). To get the probability, we use the formula  , where the summation is the right side of the equation just above.
, where the summation is the right side of the equation just above.
The 4 models had quite good accuracies for the test sets, with an average Area under the ROC curve (AUC) (over the 500 randomly selected test sets) ranging from 0.822 for the model with baseline + week 1 DRS to 0.881 when including weeks 1 to 4 DRS (Table 4, Figure 1). Models 1 and 2 had similar accuracies and then the accuracy increased for models 3 and 4. The model with weeks 1 to 4 DRS was well calibrated with intercept 0.04 and slope 0.96 (P = .98).
TABLE 4.
Accuracies of L1-Regularized Logistic Regression Prediction Models for Outcome 6-mo Favorable GOS-E on 500 Random Test Sets.
| Model | Average AUC | SD of AUC |
|---|---|---|
| Baseline | 0.801 | 0.05 |
| Baseline + 1 wk DRS | 0.822 | 0.05 |
| Baseline + 1 and 2 wk DRS | 0.836 | 0.05 |
| Baseline + 1, 2, and 3 wk DRS | 0.873 | 0.04 |
| Baseline + 1, 2, 3, and 4 wk DRS | 0.881 | 0.04 |
AUC, area under the ROC curve; DRS, Disability Rating Scale
FIGURE 1.

Distribution of the area under the ROC curves of L1-regularized logistic regression with outcome 6-mo favorable GOS-E using selected baseline variables and DRS at week 1 (model 1), weeks 1 to 2 (model 2), weeks 1 to 3 (model 3), and weeks 1 to 4 (model 4), predicted on 500 random test sets.
Table 5 and Figure 2 display the area under the ROC curves for the individual DRS component score (unadjusted for any other variables) at weeks 1 to 4 to predict a favorable 6-mo GOS-E score. Spearman's rank correlations between DRS components and ordinal 6-mo GOS-E are detailed in Table 5.
TABLE 5.
Spearman's Rank Correlations (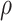 ) Between DRS Components at Weeks 1 to 4 and 6-mo GOS-E (Nondichotomized) Outcome
) Between DRS Components at Weeks 1 to 4 and 6-mo GOS-E (Nondichotomized) Outcome

|
Area under the ROC curve for the DRS components predicting favorable 6-mo GOS-E. DRS component data are not available for those who died at each week thus these individuals were excluded from the calculation. At week 1, feeding, communication, and motor scores were the most predictive components. At week 2, grooming, communication, and feeding were most predictive. At week 3, feeding, grooming, and motor components were most predictive. By week 4, feeding, grooming, and level of functioning dominated other components.
FIGURE 2.
Area under the ROC curve for the individual DRS components at week 1 (top left), week 2 (top right), week 3 (bottom left), and week 4 (bottom right) to predict favorable 6-mo GOS-E.
DISCUSSION
Key Results
Many studies have focused on identifying predictors of functional outcome based on baseline (eg, demographic, injury characteristics, biomarkers, and neuroimaging) as well as postbaseline (eg, GCS and ICP) variables.19,20 However, markers or predictors are still needed to help guide clinical decision making and serve as a surrogate for functional outcome. We hypothesized that DRS in the first few weeks after injury, independent of baseline variables at the time of injury, would be a good predictor of 6-mo outcome, providing objective data to support clinical and prognostic decision making in the early or acute care setting.
The DRS, already validated as a longitudinal outcome scale applicable in acute and rehabilitation settings, may be an ideal early predictor of 6-mo outcome. The DRS is considered an alternative outcome to GOS or GOS-E and is frequently collected as a secondary endpoint in current clinical trials.8,21-24 The DRS has some attractive features as it can be performed by a wide range of clinicians, can be used in acute and rehabilitation settings as well as in a longitudinal fashion to measure improvement or decline.25,26 Our data show the correlation of the DRS and 6-mo GOS-E scores and with stepwise improvement in prediction from 1 to 4 wk. An early sTBI study used DRS scores at week 2 and week 4 to impute the 6-mo outcome, reporting that DRS at 4 wk had 87.2% accuracy to classify subjects into favorable or unfavorable 6-mo GOS, although the methodology to estimate this accuracy was not presented.8 Furthermore, the majority of published DRS outcome studies involve scores at 3 mo or later. The potential strength of using a DRS score from an earlier timepoint may allow for longitudinal measurements that can continue from the acute phase through rehabilitation as well as use as an early response in adaptive design trials.25
Severely injured patients often have difficulties with arousal and awareness immediately after TBI and some for a long time afterwards. Interestingly, the DRS eye opening AUCs ranked lower compared to the 2 other essentially-GCS components in all weeks. Communication (evaluated differently than the verbal item on the GCS; Table 1) and motor scores had higher AUCs than eye opening at all weeks. Among the cognitive aspects of feeding, toileting, and grooming, the toileting sequence is the simplest followed by feeding and grooming. This might in part explain why the DRS toileting component was not as predictive of GOS as feeding and grooming at any week. Those 2 items remained highly predictive through week 4. Level of functioning demands a higher stage of recovery than the previous items and it might not be expected to be among the most predictive AUC value until the end of week 4 or later. Employability involves the highest level of functioning so it would not be expected to be very predictive until after 4 wk post injury. Some of the jumps in the ranks throughout the 4 wk for items may also be a result of randomness and differences in the omission of those who had already died up until each time point.
Several other studies have been investigating early predictors of neurological outcome. Models using baseline variables had accuracy on validation data sets of 0.77 to 0.796.9,27 When adding physiological data and injury severity during the first 24 h after injury, accuracy on validation data sets was 0.85 to 0.86.9 In this study, the accuracy increased to 0.88 in validation data by using the DRS at weeks 1 to 4. It is expected that the accuracy could potentially increase more by including other physiological or injury severity data post baseline up until each week. This can be explored in future studies.
Response adaptive designs have the potential to make clinical trials more flexible by utilizing results accumulating in the trial to modify the trial's course in accordance with prespecified rules. Trials with an adaptive design are often more efficient, informative, and ethical than trials with a traditional fixed design because they often make better use of resources such as time and money, and might require fewer participants.28,29 While the oncology field has more quickly trialed adaptive designs, TBI research has limited exposure to adaptive trials. The HOBIT trial is currently enrolling and utilizes an adaptive design with the 6-mo GOS-E as its outcome and 6-mo marker for adaptation.30 The importance of a short-term surrogate for the GOS and GOS-E cannot be understated in a regulated treatment space environment reliant on RCT for new therapy introduction. Identification of acute measures that can predict global 6-mo outcomes may allow for early identification of ineffective therapies, possibly leading to reduced costs associated with clinical trials. Additionally, an early surrogate endpoint could be used in Bayesian adaptive trials to modify the randomization probabilities to the study arms based on previous subjects’ surrogate outcomes, leading to more desirable odds of being randomized to a treatment that seems most promising. This study provides 4 models that incorporate week 1 to 4 DRS scores as predictors of favorable 6-mo GOS-E scores with the possibility to identify promising and failing therapies in the exploratory and confirmatory phases of clinical trial design.
Limitations
This study has several limitations that should be noted. (1) The data used for the models were from a single trial of 200 subjects conducted at 2 sites in Houston, Texas, and these results should be validated in other populations. The Epo sTBI trial enrolled 52% Hispanic and 22% Black, reflecting a diverse Houston population. (2) The sample size used to train and validate the model was not a very large sample. Our repeated splitting of the training and test sets was used to estimate the variance in the test set AUC (standard deviation (SD) = 0.04-0.05). This highlights the variability associated with the test set sample size as well as the random splitting into training and test sets. (3) We used a single type of statistical model for the prediction, L1-regularized logistic regression. There are many types of statistical and algorithmic models that could have been used and none dominate all others in all situations. We chose this particular model because our goal was to use a model that was simple to interpret and easy for other research groups to incorporate. (4) The models include some variables that may not be part of the usual clinical assessment for TBI (eg, Marshall CT score and some components of the IMPACT score). (5) We did not have longer outcomes than 6-mo and thus this work is limited in predicting ultimate recovery.
CONCLUSION
Week 1 to 4 DRS may be predictors of 6-mo outcome in sTBI patients and thus useful both for clinical prognostication as well as surrogate endpoints for adaptive clinical trials. Further studies to validate these models in various sTBI populations are warranted.
Funding
This study was supported by the National Institute of Neurological Disorders and Stroke (grant #P01-NS38660).
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Contributor Information
Jose-Miguel Yamal, Department of Biostatistics and Data Science, The University of Texas School of Public Health, Houston, Texas.
Imoigele P Aisiku, Department of Emergency Medicine, Harvard Medical School/Brigham and Women's Hospital, Boston, Massachusetts.
H Julia Hannay, Department of Psychology and Texas Institute for Measurement Evaluation and Statistics (TIMES), University of Houston, Houston Texas.
Frances A Brito, Department of Biostatistics and Data Science, The University of Texas School of Public Health, Houston, Texas.
Claudia S Robertson, Department of Neurosurgery, Baylor College of Medicine, Houston, Texas.
Supplemental Digital Content. Methods, Text. The Supplemental Digital Content expands on the statistical model fit and assessment.
REFERENCES
- 1. Maas AI, Menon DK, Adelson PDet al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12):987-1048. [DOI] [PubMed] [Google Scholar]
- 2. Coronado VG, McGuire LC, Sarmiento Ket al. Trends in traumatic brain injury in the US and the public health response: 1995-2009. J Saf Res. 2012;43(4):299-307. [DOI] [PubMed] [Google Scholar]
- 3. Robertson CS, Hannay HJ, Yamal Jet al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: a randomized clinical trial. JAMA. 2014;312(1):36-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wright DW, Yeatts SD, Silbergleit Ret al. Very early administration of progesterone for acute traumatic brain injury. N Engl J Med. 2014;371(26):2457-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vanderploeg RD, Schwab K, Walker WCet al. Rehabilitation of traumatic brain injury in active duty military personnel and veterans: defense and veterans brain injury center randomized controlled trial of two rehabilitation approaches. Arch Phys Med Rehabil. 2008;89(12):2227-2238. [DOI] [PubMed] [Google Scholar]
- 6. Zhao S, Yu A, Wang X, Gao X, Chen J. Post-injury treatment of 7, 8-dihydroxyflavone promotes neurogenesis in the hippocampus of the adult mouse. J Neurotrauma. 2016;33(22):2055-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clifton GL, Miller ER, Choi SCet al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344(8):556-563. [DOI] [PubMed] [Google Scholar]
- 8. Clifton GL, Valadka A, Zygun Det al. Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): a randomised trial. Lancet Neurol. 2011;10(2):131-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rubin ML, Yamal J, Chan W, Robertson CS. Prognosis of six-month Glasgow Outcome Scale in severe traumatic brain injury using hospital admission characteristics, injury severity characteristics, and physiological monitoring during the first day post-injury. J Neurotrauma. 2019;36(16):2417-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Irimia A, Wang B, Aylward SRet al. Neuroimaging of structural pathology and connectomics in traumatic brain injury: toward personalized outcome prediction. NeuroImage Clin. 2012;1(1):1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van der Naalt J, Timmerman ME, de Koning MEet al. Early predictors of outcome after mild traumatic brain injury (UPFRONT): an observational cohort study. Lancet Neurol. 2017;16(7):532-540. [DOI] [PubMed] [Google Scholar]
- 12. Roy D, Peters ME, Everett ADet al. Loss of consciousness and altered mental state as predictors of functional recovery within 6 months following mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2020;32(2):132-138. [DOI] [PubMed] [Google Scholar]
- 13. Raheja A, Sinha S, Samson Net al. Serum biomarkers as predictors of long-term outcome in severe traumatic brain injury: analysis from a randomized placebo-controlled phase II clinical trial. J Neurosurg. 2016;125(3):631-641. [DOI] [PubMed] [Google Scholar]
- 14. Nwachuku EL, Puccio AM, Adeboye A, Chang Y, Kim J, Okonkwo DO. Time course of cerebrospinal fluid inflammatory biomarkers and relationship to 6-month neurologic outcome in adult severe traumatic brain injury. Clin Neurol Neurosurg. 2016;149(Oct):1-5. [DOI] [PubMed] [Google Scholar]
- 15. Osier ND, Conley YP, Okonkwo DO, Puccio AM. Variation in candidate traumatic brain injury biomarker genes are associated with gross neurological outcomes after severe traumatic brain injury. J Neurotrauma. 2018;35(22):2684-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Walker WC, Stromberg KA, Marwitz JHet al. Predicting long-term global outcome after traumatic brain injury: development of a practical prognostic tool using the traumatic brain injury model systems national database. J Neurotrauma. 2018;35(14):1587-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rappaport M, Hall KM, Hopkins K, Belleza T, Cope DN. Disability Rating Scale for severe head trauma: coma to community. Arch Phys Med Rehabil. 1982;63(3):118-123. [PubMed] [Google Scholar]
- 18. Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002-2006. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- 19. Maas AI, Lingsma HF, Roozenbeek B. Predicting outcome after traumatic brain injury. In: Handbook of Clinical Neurology. Vol. 128 New York, Oxford: Elsevier; 2015:455-474. [DOI] [PubMed] [Google Scholar]
- 20. Chesnut RM, Temkin N, Carney Net al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012;367(26):2471-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jennett B, Bond M.. Assessment of outcome after severe brain damage: a practical scale. Lancet North Am Ed. 1975;305(7905):480-484. [DOI] [PubMed] [Google Scholar]
- 22. Wilson JL, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15(8):573-585. [DOI] [PubMed] [Google Scholar]
- 23. McCauley SR, Wilde EA, Moretti Pet al. Neurological Outcome Scale for traumatic brain injury: III. criterion-related validity and sensitivity to change in the NABIS hypothermia-II clinical trial. J Neurotrauma. 2013;30(17):1506-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilde EA, Whiteneck GG, Bogner Jet al. Recommendations for the use of common outcome measures in traumatic brain injury research. Arch Phys Med Rehabil. 2010;91(11):1650-1660.e17. [DOI] [PubMed] [Google Scholar]
- 25. Benoit JS, Hannay HJ, Yamal J, Francis DJ, Aisiku I, Robertson C. Longitudinal changes in Disability Rating Scale scores: a secondary analysis among patients with severe TBI enrolled in the Epo clinical trial. J Int Neuropsychol Soc. 2019;25(3):293-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dams-O’Connor K, Pretz C, Billah T, Hammond FM, Harrison-Felix C. Global outcome trajectories after TBI among survivors and nonsurvivors: a national institute on disability and rehabilitation research traumatic brain injury model systems study. J Head Trauma Rehabil. 2015;30(4):E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Steyerberg EW, Mushkudiani N, Perel Pet al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5(8):e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shih WJ Plan to be flexible: a commentary on adaptive designs. Biom J. 2006;48(4):656-659. [DOI] [PubMed] [Google Scholar]
- 29. Pallmann P, Bedding AW, Choodari-Oskooei Bet al. Adaptive designs in clinical trials: why use them, and how to run and report them. BMC med. 2018;16(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gajewski BJ, Berry SM, Barsan WGet al. Hyperbaric oxygen brain injury treatment (HOBIT) trial: a multifactor design with response adaptive randomization and longitudinal modeling. Pharm Stat. 2016;15(5):396-404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



