Abstract
BACKGROUND
Cerebral cavernous angioma (CA) is a capillary microangiopathy predisposing more than a million Americans to premature risk of brain hemorrhage. CA with recent symptomatic hemorrhage (SH), most likely to re-bleed with serious clinical sequelae, is the primary focus of therapeutic development. Signaling aberrations in CA include proliferative dysangiogenesis, blood-brain barrier hyperpermeability, inflammatory/immune processes, and anticoagulant vascular domain. Plasma levels of molecules reflecting these mechanisms and measures of vascular permeability and iron deposition on magnetic resonance imaging are biomarkers that have been correlated with CA hemorrhage.
OBJECTIVE
To optimize these biomarkers to accurately diagnose cavernous angioma with symptomatic hemorrhage (CASH), prognosticate the risk of future SH, and monitor cases after a bleed and in response to therapy.
METHODS
Additional candidate biomarkers, emerging from ongoing mechanistic and differential transcriptome studies, would further enhance the sensitivity and specificity of diagnosis and prediction of CASH. Integrative combinations of levels of plasma proteins and characteristic micro-ribonucleic acids may further strengthen biomarker associations. We will deploy advanced statistical and machine learning approaches for the integration of novel candidate biomarkers, rejecting noncorrelated candidates, and determining the best clustering and weighing of combined biomarker contributions.
EXPECTED OUTCOMES
With the expertise of leading CA researchers, this project anticipates the development of future blood tests for the diagnosis and prediction of CASH to clinically advance towards precision medicine.
DISCUSSION
The project tests a novel integrational approach of biomarker development in a mechanistically defined cerebrovascular disease with a relevant context of use, with an approach applicable to other neurological diseases with similar pathobiologic features.
Keywords: Cavernous angioma with symptomatic hemorrhage (CASH), Cavernous angioma, Cerebral cavernous malformation (CCM), Symptomatic hemorrhage, Biomarkers, Machine learning, Plasma, QSM
Graphical Abstract
Graphical Abstract.
ABBREVIATIONS
- CA
cavernous angioma
- CASH
cavernous angioma with symptomatic hemorrhage
- CCM
Cerebral cavernous malformation
- COU
context of use
- DCEQP
dynamic contrast enhanced quantitative perfusion
- DE
differentially expressed
- EC
endothelial cell
- HMA
hemorrhagic microangiopathy
- miRNAs
micro-ribonucleic acids
- MRI
magnetic resonance imaging
- NVU
neuro-vascular unit
- qPCR
quantitative polymerase chain reaction
- QSM
quantitative susceptibility mapping
- SH
symptomatic hemorrhage
- TR
Trial readines
GENERAL INFORMATION
Protocol Title (ID#): Biomarkers of Cerebral Cavernous Angioma With Symptomatic Hemorrhage (CASH) (NIH/NINDS R01NS114552).
ClinicalTrials.gov Identifier: NCT04467489
Name and Address of the Sponsor/Funding Agency: NIH/NINDS R01NS114552 (2020-2025)
National Institute of Neurological Disorders and Stroke (NINDS)
National Institutes of Health (NIH)
9000 Rockville Pike
Bethesda, Maryland 20892
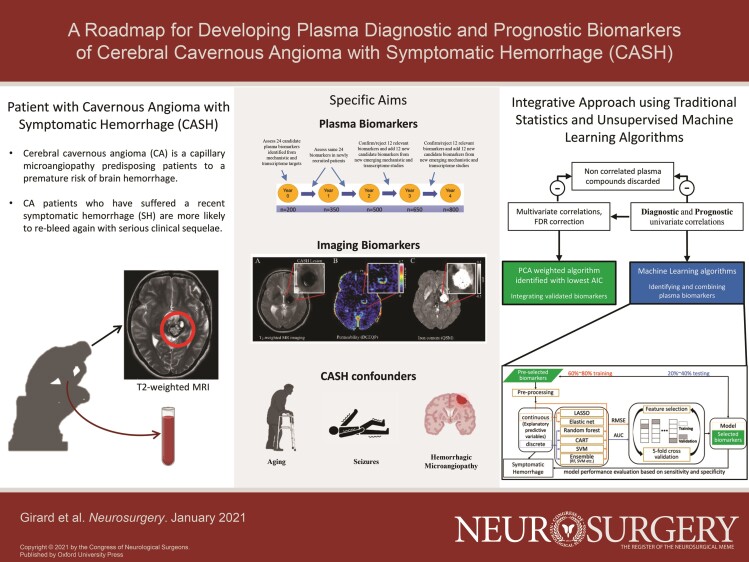
Cerebral Cavernous Angioma and the Problem of Symptomatic Hemorrhage
Cerebral cavernous angiomas (CAs) are enlarged, blood-filled capillary spaces, lined by “leaky” endothelium lacking mature angioarchitecture.1 CA affects >1000 000 Americans, usually sporadic, with a solitary lesion or a cluster of lesions associated with a developmental venous anomaly.2 The disease is familial in 20% to 30% of cases, exhibiting a Mendelian autosomal dominant inheritance with a heterozygous loss-of-function mutation in 1 of 3 cerebral cavernous malformation (CCM) genes, with multifocal lesions throughout the brain.3 CAs are histologically identical in familial and sporadic cases, and harbor endothelial cell (EC) somatic mutations in the same 3 genes.4-6
The natural history of CAs is generally benign, with <0.5% annual risk of clinically significant bleeding.7 However, once a lesion has manifested a symptomatic hemorrhage (SH), its untreated course is quite serious, with a 42% rate of recurrent bleeding within 5 yr.7-9 Cavernous angioma with symptomatic hemorrhage (CASH) is defined as imaging evidence of new lesional bleeding or hemorrhagic growth, in association with directly attributable symptoms.10 Trial readiness (TR) initiatives are underway, and repurposed drugs are at early stages of clinical evaluation aiming to stabilize the CASH lesion.11,12 The diagnosis of CASH can be uncertain, particularly when imaging studies are not performed in a timely fashion or are equivocal in relation to specific symptoms. In addition, future CASH remains largely unpredictable.
Context of Use and Definitions of Biomarkers
Biomarkers are defined with a context of use (COU), which describes their appropriate interpretation and application in medical product, development and regulatory review.13,14 A diagnostic biomarker detects the presence of a specific medical condition; a prognostic biomarker assesses the likelihood of progression, regression or recurrence of this medical condition; and a monitoring biomarker evaluates the progression or stability of medical conditions, or the effectiveness of an intervention over time.13
These biomarker categories are distinct from predictive biomarkers (identifying cases with an increased probability to develop an outcome upon exposure to certain medical intervention or an environmental factor), susceptibility/risk biomarkers (reflecting a probability of future development of a specific medical condition), or safety biomarkers (indicating or predicting toxicity or an adverse effect before or after a medical intervention).
RATIONALE AND BACKGROUND INFORMATION
Preliminary Studies: Mechanistic Rationale, Preclinical Studies, and Biomarker Validations
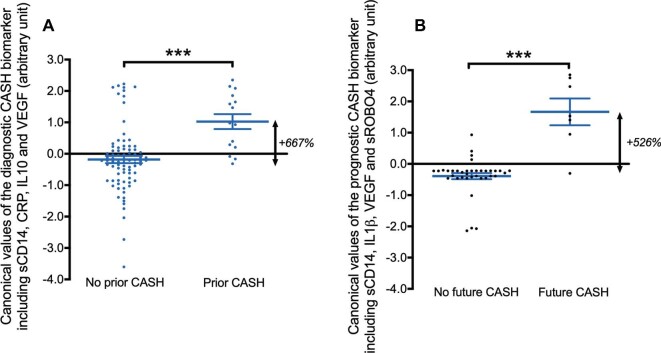
Resected human and murine CAs demonstrate a complex pathobiologic milieu, involving an interplay of inflammation, angiogenesis, loss of EC barrier function and extracellular matrix remodeling.15-25 These discoveries have motivated exploratory hypotheses of the associations between inflammatory states and the clinical behavior of CAs (Figure 1).26-34 We queried a broad panel of inflammatory and angiogenic putative biomarkers (Table 1),35-39 and first reported prognostic associations showing higher plasma levels of 4 biomarkers in cases who suffered a subsequent SH in the year following the plasma sampling.40 The best weighted combination of the levels of these molecules had an 86% sensitivity and 88% specificity in predicting a SH in the next year. Canonical values derived from this best model were 5X higher in patients who suffered a subsequent SH (Figure 2A).40
FIGURE 1.
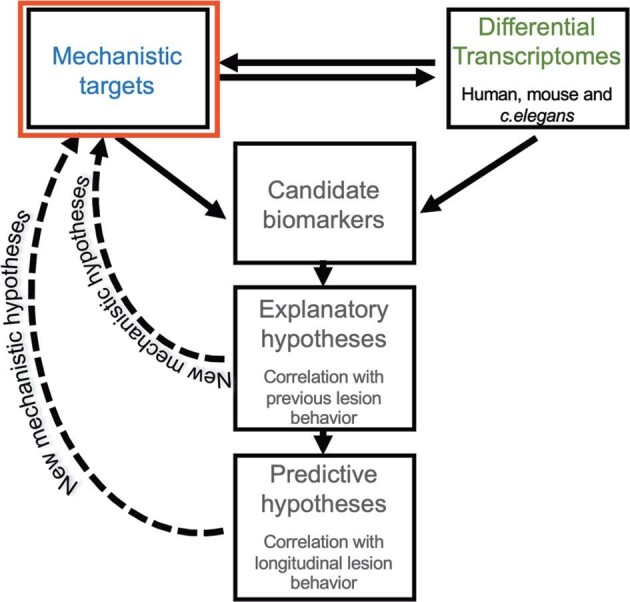
Conceptual workflow to study and validate candidate biomarkers of cerebral CASH. A panel of candidate biomarkers was defined via a systematic literature review of mechanistic and transcriptome studies reporting a role in the pathology of cerebral CAs and/or brain hemorrhage. Each biomarker candidate was then hypothesized to either have a diagnostic and/or prognostic association with SH.
TABLE 1.
Panel of Plasma Biomarker Molecules Selected for Pilot Studies, Based on Systematic Literature Review of Associations With CA Disease and Brain Hemorrhage
| Biomarker | Biological processes | References | Potential relation to CA and brain hemorrhagic diseases |
|---|---|---|---|
| 25-OH Vitamin D | Inflammation | Girard et al (2016)26 | Lower plasma levels of vitamin D in CA patients with a more aggressive disease course. |
| Gibson et al (2015)27 | Vitamin D supplementation reduces lesion burden in a preclinical CCM murine model. | ||
| CCL2/MCP-1 | Inflammation | Retta et al (2016)28 | Oxidative stress associated to chronic inflammation in hemorrhagic vascular diseases. |
| Keep et al (2014)29 | Increased CCL2/MCP-1 induces BBB permeability. | ||
| Luissint et al (2012)19 | CCL2/MCP-1 activates the RhoA/ROCK pathway. | ||
| C-reactive protein (CRP; binary and continuous) | Inflammation | Gao et al (2018)30 | CCM3 polymorphism is associated with increased CRP plasma levels. |
| Hsuchou et al (2012)20 | CRP increases BBB permeability. | ||
| Endoglin | AngiogenesisEndothelial permeabilityInflammation | Chen et al (2009)36 | Soluble endoglin induces cerebro-vascular remodeling and may promote sporadic AVM formation. |
| McAllister et al (1994)37 | Endoglin gene loss is causative of hereditary hemorrhagic telangiectasia. | ||
| Interferon gamma (IFNγ) | Endothelial permeabilityInflammation | Girard et al (2018)35 | Increased plasma levels of IFNγ in CA patients associated to an aggressive clinical course. |
| Ng et al (2015)38 | IFNγ increases endothelial permeability and causes cytoskeletal derangements. | ||
| Interleukins: | InflammationImmune responseAngiogenesis | Girard et al (2018)40 | Lower plasma levels of IL-6 and higher plasma levels of IL-1β in CA subjects who experienced clinical lesional activity within the year following the initial blood sample. |
| IL-1βIL-1βIL-2IL-6IL-8IL-10 | |||
| Girard et al (2018)35 | Increased plasma levels of IL-2, IL-1ß in CA associated to an aggressive clinical course. | ||
| Pawlikowska et al (2004)31 | Homozygosity for IL6–174G allele associated to greater risk of ICH in with AVM. | ||
| Leukocyte-EC Adhesion: | Inflammation | Lampugnani et al (2018)21 | Increased expression of ICAM-1 and VCAM-1 on ECs is a |
| VCAM1 | Endothelial permeability | marker of inflammation. | |
| ICAM1/CD54 | Robinson et al (1995)22 | Lower expression of ICAM1 and VCAM1 in CA lesions. | |
| Lipid Panel: | Inflammation | Shenkar et al (2017)23 | Simvastatin decreases ROCK activity in mature CCM lesions in mice. |
| HDLNon-HDLLDLTriglycerides | |||
| Girard et al (2016)26 | Lower levels of non-HDL cholesterol are associated with a more aggressive disease course. | ||
| Whitehead et al (2009)24 | Simvastatin rescues CCM phenotype in preclinical murine model. | ||
| Matrix metalloproteinases (MMPs):MMP2MMP9 | PermeabilityECM remodeling | Girard et al (2018)35Bicer et al (2010)32 | Plasma levels of MMP-2 and MMP-9 were respectively higher and lower in patients with previous seizure activity.Higher expression levels of MMP-2 and MMP-9 may explain CCMs subclinical hemorrhage. |
| Roundabout4 (ROBO4) | AngiogenesisEndothelial permeability | Girard et al (2018)40 | Greater plasma levels of ROBO4 in CA subjects who experienced clinical lesional activity within the year following the initial blood sample. |
| Wüstehube et al (2010)17 | CCM1 gene associated with a down-regulation of ROBO4 gene expression. | ||
| Jones et al (2008)39 | ROBO4 inhibits endothelial hyper-permeability and abnormal angiogenesis. | ||
| Soluble cluster of differentiation 14 (sCD14) | Inflammation | Girard et al (2018)40 | Lower plasma levels of sCD14 in CA subjects who experienced clinical lesional activity within the year following the initial blood sample. |
| Tang et al (2017)56 | Polymorphisms of CD14 that codes for the anchored membrane are associated with higher CCM lesion burden in familial CCM disease. | ||
| Choquet et al (2014)33 | CD14 polymorphism is associated with an increase susceptibility for high CCM lesions burden. | ||
| Tumor necrosis factors: | Inflammation | Girard et al (2018)35 | Increased TNFα plasma levels in CA patients associated to aggressive disease course. |
| TNFαTNF RI | |||
| Pawlikowska et al (2004)31 | Homozygosity for the TNFα-308 GG genotype is associated with a greater risk of ICH in brain AVM. | ||
| Vascular endothelial growth factor (VEGF) | AngiogenesisEndothelial permeability | Girard et al (2018)40Cunha et al (2017)25Jung et al (2003)34 | Lower plasma levels of VEGF in CA subjects who experienced clinical lesional activity within the year following the initial blood sample.VEGF is associated to vasculogenesis and endothelial permeability.Dynamic changes in VEGF during the clinical course of CCM. |
Arteriovenous malformation (AVM); blood brain barrier (BBB); extracellular matrix (ECM); High-density lipoprotein (HDL); intra-cerebral hemorrhage (ICH); low-density lipoprotein (LDL).
FIGURE 2.
The diagnostic and prognostic biomarker for CASH developed by traditional statistics, and validated by ML methods. A, The canonical values estimated with the diagnostic CASH biomarker (−3.37*[sCD14] + 1.47*[ C-Reactive Protein] – 0.36*[vascular endothelial growth factor [VEGF] – 0.57*[ interleukin [IL]-10]) were 6-fold greater in subjects who experienced a SH in the year prior to plasma sample collection (P < .001). The diagnostic CASH biomarker was able to distinguish prior CASH patients with a sensitivity of 80% and specificity of 77%. B, The canonical values calculated using the prognostic CASH biomarker (−0.135*[sCD14] + 7.73*[IL-1β] − 0.775*[VEGF] + 0.658*[sROBO4]) were 5-fold higher (P < .001) in cases who experienced a SH in the year following the plasma sampling. The prognostic CASH biomarker was able to distinguish patients with 86% sensitivity and 88% specificity. ***P < .001
We have also queried the same plasma molecules for diagnostic sensitivity and specificity of SH in the year prior to plasma sample collection.41 The best diagnostic biomarker was a weighted combination of 4 molecules that showed excellent discrimination from healthy controls (Figure 2B). Two of the molecules were common, and two others respectively distinguished the diagnostic and prognostic panels. These results were validated with machine learning (ML) approaches.41
Micro-Ribonucleic Acids in Peripheral Plasma Reflect CASH Cases
Micro-ribonucleic acids (miRNAs) are small noncoding RNA molecules with roles in mRNA silencing and post-transcriptional inhibition of gene expression.42 miRNAs, isolated from various body fluids, including plasma,43 have been shown to rescue endothelial phenotype44 and to inhibit vasculogenesis.45 We identified 13 differentially expressed (DE) miRNAs in the plasma of CASH subjects (Table 2).41 Integrative analysis between those miRNAs and DE genes in lesional CASH neuro-vascular units (NVUs) showed that interleukin-10 receptor subunit alpha is the putative target of hsa-miT-185-5. We had also identified decreased plasma levels of interleukin-10 in CASH subjects.
TABLE 2.
Differently Expressed miRNAs in the Plasma of CASH vs non-CASH Subjects
| miRNA | Log2 (fold change) | P value | P value (FDR corrected) |
|---|---|---|---|
| hsa-miR-363-3p | 4.275 | .000 | .003 |
| hsa-miR-486-5p | 2.900 | .000 | .003 |
| hsa-miR-15a-5p | 3.490 | .000 | .003 |
| hsa-miR-25-3p | 2.704 | .000 | .003 |
| hsa-miR-106b-3p | 2.975 | .000 | .006 |
| hsa-miR-16-2-3p | 5.263 | .000 | .007 |
| hsa-miR-183-5p | 2.985 | .000 | .007 |
| hsa-miR-16-5p | 2.426 | .000 | .007 |
| hsa-miR-185-5p | 2.813 | .000 | .012 |
| hsa-miR-501-3p | 1.923 | .001 | .032 |
| hsa-miR-181a-5p | 1.183 | .002 | .036 |
| hsa-miR-532-5p | 5.468 | .002 | .036 |
| hsa-mir-7641-2-3p_novel | 7.484 | .002 | .037 |
False discovery rate, FDR
Republished with permission of American Society for Clinical Investigation, from JCI Insight, Biomarkers of CASH, Lyne et al, 4(12), 201941; permission conveyed through Copyright Clearance Center, Inc.
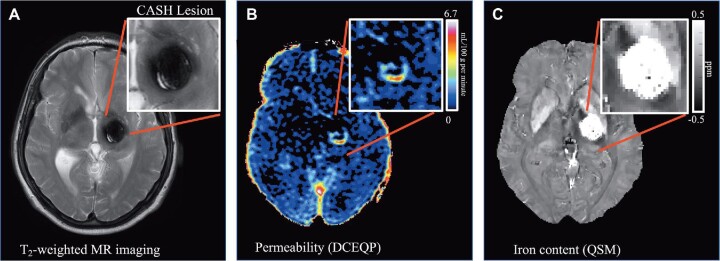
Imaging Biomarkers of Vascular Permeability and Iron Accumulation as Monitoring Biomarkers of Hemorrhage in CAs
Our group has implemented novel magnetic resonance imaging (MRI) applications assessing iron deposition in human CAs using quantitative susceptibility mapping (QSM) and dynamic contrast enhanced quantitative perfusion (DCEQP), reflecting postulated vascular hyper-permeability (Figure 3).46-50 These were not suitable as diagnostic or prognostic biomarkers. Since both measures increased significantly in CAs manifesting interval SH during longitudinal follow-up, with highly sensitive and specific thresholds,50 they were proposed as monitoring biomarkers of lesional bleeding and responses to therapy.51
FIGURE 3.
In-Vivo MRI biomarkers of CASH from an illustrative case. A, Conventional MRI T2-weighted image of a CASH lesion. The adjudicated definition of CASH requires imaging evidence of new lesional bleeding or hemorrhagic growth, in association with directly attributable symptoms. CASH clinical behavior has been correlated with measures of B, vascular permeability using DCEQP (color scale of Ki units in ml/100g/min), and C, iron deposition using QSM(grey scale in ppm). The protocols for acquiring and post-processing DCEQP and QSM are performed in the context of the CASH TR project at initial enrollment, and at the end of 1- and 2-yr follow-up.
Critical Knowledge Gaps
(1) It is unknown if the plasma biomarkers are relevant to disease subgroups. (2) Multisite validation is needed to assess the impact of any analytic and batch errors, and potential referral and enrollment biases. (3) Any approach to identify the best biomarkers should allow the integration and testing of novel candidates.17,52-60 (4) It would be important to know if the MRI biomarkers can be reflected by blood tests, facilitating and potentially enhancing the monitoring of CASH lesions. (5) It is important to assess the same biomarkers in non-CA controls, particularly in the aging human brain, with and without hemorrhagic microangiopathy (HMA), and in association with seizures.
STUDY GOALS AND OBJECTIVES
Specific Aim 1. Plasma Molecules as Diagnostic and Prognostic Biomarkers of CASH
Rationale and Hypotheses to be Tested
Novel candidate molecules (Figure 1, Table 3), and DE plasma miRNAs (Table 2) emerged from recent mechanistic and transcriptome discoveries. Hypotheses include (1) incorporating these novel candidate plasma proteins and miRNAs will enhance the sensitivity and specificity of plasma biomarkers identified in pilot studies, (2) diagnostic and prognostic biomarkers are applicable in relation to sex, age and clinically relevant disease subgroups, and in cases recruited at multiple sites.
TABLE 3.
Proposed Candidate Biomarkers (Panel 1)
| Biomarkers to be assessed by ELISA assays | Mechanistic justification or preliminary validation | Differential transcriptomes (P value, FDR corrected) |
|---|---|---|
| C-Reactive Protein (CRP) | Increased CRP plasma levels in CA patients after SH; belongs to the weighted combined diagnostic biomarker.41 | .001a (FCGR2B) |
| Interleukin-1β (IL-1β) | Higher plasma levels of IL-1β in CA subjects who experienced a SH within the year following the initial blood sample; belongs to the weighted combined prognostic biomarker.40 | .048a |
| Lower plasma levels of IL-1β in CA patients after SH.41 | ||
| Interleukin-10 (IL-10) | Lower plasma levels of IL-10 in CA patients after SH; belongs to the weighted combined diagnostic biomarker.41 | .013a |
| Roundabout4 (ROBO4) | Greater plasma levels of ROBO4 in CA subjects who experienced SH within the year following the initial blood sample; belongs to the weighted combined prognostic biomarker.40 | .187a |
| Soluble cluster of differentiation 14 (sCD14) | Lower plasma levels of sCD14 in CA patients after SH; belongs to the weighted combined diagnostic biomarker.41 | .025a |
| Lower plasma levels of sCD14 in CA subjects who experienced SH within the year following the initial blood sample; belongs to the weighted combined prognostic biomarker.40 | ||
| Vascular endothelial growth factor (VEGF) | Lower plasma levels of VEGF in CA patients after SH; belongs to the weighted combined diagnostic biomarker.41 | .009a (VEGFA) |
| Lower plasma levels of VEGF in CA subjects who experienced SH within the year following the initial blood sample; belongs to the weighted combined prognostic biomarker.40 | .007a (FLT1) | |
| ADAM metallopeptidase with thrombospondin type 1 motif 4 (ADAMTS4) | Endothelial TLR4 and the microbiome drive CAs | 2.3 × 10−4a |
| ADAM metallopeptidase with thrombospondin type 1 motif 5 (ADAMTS5) | CAs arise from endothelial gain of MEKK3-KLF2/4 signaling.56,57 | 2.1 × 10−3a |
| Angiopoietin-1 | Endothelial exocytosis of angiopoietin-2 resulting from CCM3 deficiency contributes to CAs.54 | 1.09 × 10−17a |
| Angiopoietin-2 | ||
| Lipopolysaccharide binding protein (LBP) | LBP inhibits LPS bio-activity in-vivo and in-vitro; increased LBP plasma levels in healthy non-CA subjects vs CA cases.67 | N.A. |
| Interleukins (IL-) | IL-4, -7, -16, - 21 and -33 were strong contenders in differential transcriptome of laser capture microdissected human CA neurovascular units.52 | .02a |
| IL-7 | 2.9 × 10−4a | |
| IL-16 | 0.02a | |
| IL-21 | 4.9 × 10−3a | |
| IL-33 | 0.01a | |
| Thrombomodulin | Thrombomodulin increased in human CA lesions and higher plasma levels in CA patients.60 | 9.64 × 10−14b |
| Thrombospondin-1 | Thrombospondin1 replacement prevents CAs.58 | 3.19 × 10−3b |
| Thrombospondin-2 | 5.1 × 10−4a | |
| Tissue plasminogen activator (tPA) | Central nervous system hemorrhage in CAs associated with greater expression of endothelial protein C receptor.60 | N.A. |
Cerebral angioma (CA); enzyme-linked immunosorbent assay (ELISA); lipopolysaccharide (LPS); Not Applicable (N.A.).
aData from laser capture microdissected neurovascular units from human resected CA lesions in comparison to human normal brain.
bData from mouse brain microvascular ECs with and without induced Ccm gene loss.
Objective
To test whether individual and combined levels of candidate plasma proteins and miRNAs can be associated with diagnosis of CASH (cross-sectional) and can predict/prognosticate future SH (longitudinal).
Specific Aim 2. Imaging vs Plasma as Monitoring Biomarkers in CASH
Rationale and Hypotheses to be Tested
Increases in mean lesional QSM iron content and DCEQP permeability were recorded in longitudinal follow-up of CAs, with sensitive/specific thresholds during 1-yr epochs associated with clinically significant SH.50 We hypothesize that change in the plasma biomarkers of prospective monitoring of CASH cases can reflect or complement changes in the MRI measures during 1-yr epochs.
Objective
To assess whether changes in QSM and DCEQP used as monitoring biomarkers after SH are reflected by changes in plasma biomarkers and miRNAs. We leverage imaging biomarkers data already being acquired in the ongoing CASH TR project to correlate with plasma biomarkers being assessed in Specific Aim 1.
Specific Aim 3. Plasma Biomarkers in Non-CA Subjects
Rationale and Hypotheses to be Tested
CA, the aging NVU, and potentially HMA share phenotypic signatures and signaling aberrations reflecting blood-brain barrier disruption and hemorrhage.61-63 Epilepsy also presents a potential confounder with CA biomarkers.35,64,65 We hypothesize that plasma levels of biomarkers being evaluated in CASH diagnostic and prognostic questions are different in paired comparisons between non-CA young and older subjects, with and without seizures and HMA on MRI.
Objective
To assess the plasma biomarkers in non-CA young and older subjects, with and without seizures or HMA, clarify potential confounders in the context of clinical use, and motivate novel hypotheses for broader applications.
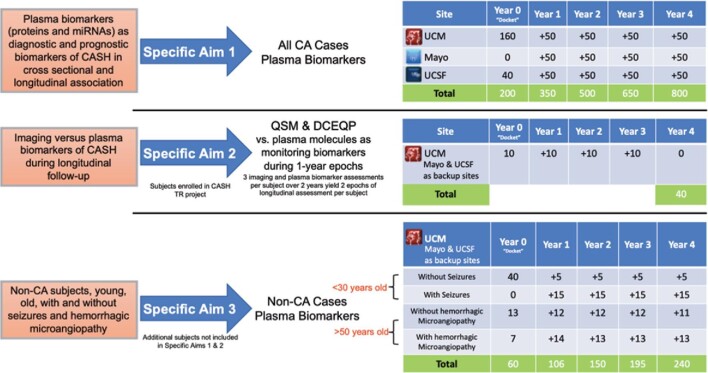
STUDY DESIGN
This is an observational cohort study with no intervention (Figure 4). The inclusion/exclusion criteria for each Specific Aim are presented in Supplemental Table 1.
FIGURE 4.
Description of each Specific Aims 1 to 3, with proposed case numbers already at hand from pilot studies at each site at Year 0. Additional CA cases shall be recruited at each site, with total target enrollments for Years 1 to 4. Forty subjects enrolled in Specific Aim 1, who are also part of CASH TR project, are used in Specific Aim 2. Together, Specific Aims 1 and 2 shall include 800 subjects. Specific Aim 3 shall include 240 additional subjects. Lagging enrollments and follow-ups are completed in Year 5. Sites include University of Chicago Medicine (UCM), Mayo Clinic at Rochester (Mayo) and University of California San Francisco (UCSF). Imaging includes QSM and DCEQP.
METHODOLOGY
Specific Aim 1. Plasma Molecules as Diagnostic and Prognostic Biomarkers of CASH
Subjects and Methods
We will recruit 800 consecutive subjects, including 200 from pilot studies whose plasma samples and clinical data are in hand. After Year 2 of the study, a cohort of 500 shall be available for interim analyses using the proposed biomarker Panel 1, while all 800 subjects will be available after Year 4 to test the enhanced biomarker Panel 2 (Figure 4).
Candidate Biomarkers
In all, 12 plasma proteins thus far studied had significant diagnostic or prognostic univariate correlations with SH.40,41 These 12 will be studied along with 12 novel biomarker candidates that emerged from recent mechanistic discoveries (Table 3).54,56-58,60,66,67 We will reject non-correlated molecules using TS or less contributive molecules using ML, and add additional candidates in the second half of the study (Panel 2) based on discoveries in the next 2 to 3 yr.
Circulating miRNA Discovery and Quantitative Polymerase Chain Reaction Assays
Plasma aliquots from 100 subjects will identify DE miRNAs associated with prior SH (diagnostic), and new bleeding within a year (prognostic) by small RNA-Seq sequencing. The top 5 diagnostic and prognostic miRNAs identified shall be assayed by real time quantitative polymerase chain reaction (qPCR) for plasma levels in the larger cohorts subjected to plasma protein biomarker studies. Plasma qPCR levels of those miRNAs shall be correlated with SH individually, and in integrative combinations with queried plasma proteins.
Data Analyses Plan
Plasma biomarker levels shall be correlated with a history of SH in the prior year, as the primary diagnostic outcome, and correlated with new SH occurrence during the subsequent year as the primary prognostic outcome, adjudicated by clinical and imaging review. These shall be assessed at 1 yr of follow-up during clinical visit, or by follow-up contact and imaging studies if SH is suspected.
Additional analyses for diagnostic and prognostic outcomes shall be conducted in prespecified subgroups of sex, age (<30 and >50 yr), sporadic vs familial genotype, brainstem lesion location, epilepsy (≥1 seizure in the prior year, with/without medications), and site of enrollment. A subgroup analysis of prior SH in the year before enrollment (CASH at enrollment) shall also be conducted for prognostic outcome.
Exploratory analyses shall correlate the biomarkers (individual and combined) with the number of months since SH within the prior year, and the number of months until subsequent SH in the following year.
Plasma samples selected for analyses shall include all SH cases (adjudicated SH within a year prior to enrollment for diagnostic questions, and within 1 yr after enrollment for prognostic questions), and an equal number of plasma samples for biomarker testing among the larger group of non-SH cases in the enrolled cohort, best matching (1:1) SH cases for sex, age, sporadic/familial genotype, epilepsy and brainstem lesion location.
Specific Aim 2. Imaging vs Plasma as Monitoring Biomarkers in CASH
Subjects and Methods
Plasma samples shall be collected from 40 subjects enrolled in the CASH TR project at UCM, in conjunction with their planned imaging at baseline, at 1- and 2-yr follow-ups (Supplemental Figures 1, 2 and 3).
Data Analyses
We shall create a binary variable depending on whether the percent change in mean lesional QSM or DCEQP during 1-yr epochs exceeds previously defined respective thresholds (+5.8% QSM and +39.6% for DCEQP),50 significantly associated with SH at high sensitivity and specificity. We will then assess the association of the binary variable with changes in plasma protein and miRNA qPCR levels being assessed in Specific Aim 1. We will also have the opportunity to assess the association between the changes in plasma protein and miRNA qPCR levels, and mean lesional QSM or DCEQP as a continuous variable.
Specific Aim 3. Plasma Biomarkers in non-CA Subjects
Subjects and Methods
At UCM, 240 non-CA subjects shall be recruited, with plasma collection, protein and miRNA assessments as per Specific Aim 1.
Data Analyses
The data analysis plan will follow the same procedures in Specific Aim 1 comparing plasma protein and miRNA levels (each separate and combined), between 2 respective cohorts (young/old without HMA, young with/without seizures, and old with/without HMA). Refer to Supplemental Digital Content for more Methods forSpecific Aims 1-3.
DISCUSSION
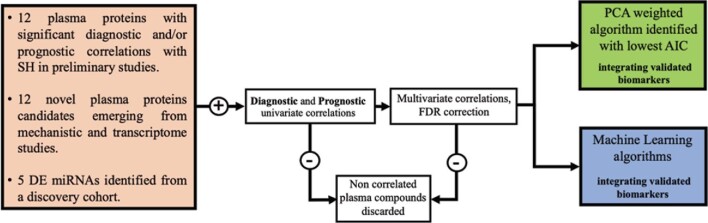
This proposal describes a novel integrative approach of selecting and combining plasma protein and DE miRNA biomarker candidates, and rejecting noncorrelated biomarkers (Figure 5). Biomarker associations also carry fundamental mechanistic implications, and identifying putative gene targets of miRNAs associated with CASH will generate new hypotheses that can aid in understanding factors impacting SH.
FIGURE 5.
Conceptual framework to define a biomarker of CASH. The proposal describes a novel integrative approach to develop a diagnostic and prognostic biomarker of CASH. The 12 plasma proteins that showed in preliminary studies significant diagnostic and/or prognostic univariate correlations with SH will be kept in the analysis pipelines. We will integrate up to 12 additional plasma proteins (that emerged from recent mechanistic and transcriptome discoveries), and 5 differently expressed plasma miRNAs (identified in a discovery cohort). The compounds showing no diagnostic or prognostic associations will be discarded. The best weighted diagnostic and prognostic combinations will be then derived with the plasma compounds that show univariate associations using traditional statistics and unsupervised ML approaches. FDR, false discovery rate; AIC, Akaike information criterion.
A blood test would be advantageous over more complex and expensive imaging techniques, and would prompt development for a novel COU.
Biomarker links to aging, HMA and seizures in non-CA subjects would identify potential confounders and motivate novel hypotheses about biomarker roles in those settings. Our results may not be generalizable to more or less severe cases than those enrolled, or address other potential confounders. An important practical implication would define normal ranges for the respective biomarkers in non-CA subjects. This will help establish positive and negative explanatory/prognostic thresholds needed for clinical application.
TRIAL STATUS
The study is expected to commence on July 1, 2020.
SAFETY CONSIDERATIONS
The study involves no more than minimal risks.
FOLLOW-UP
All 3 sites are committed to yearly follow-up of all CA subjects, per current care standards, including follow-up surveillance imaging if new symptoms arise. For Specific Aim 1, 1-yr after enrollment, the medical records are queried for any evidence of new SH (this may be gleaned by telephone contact, with a request of confirmatory MRI report/compact disc, if a clinical visit is not logged). This is also done after a second year of follow-up in cases in Specific Aim 2, as also required in the CASH TR project. Follow-up is not required for cases enrolled in Specific Aim 3.
DATA MANAGEMENT AND STATISTICAL ANALYSIS
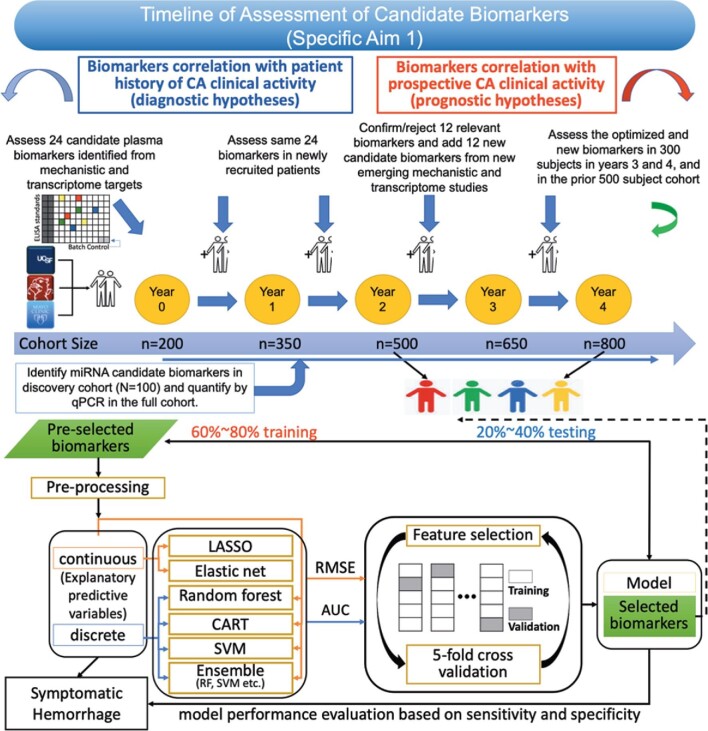
The ML analysis plan (Figure 6), sample size calculations, as well as ML simulations for each of the three Specific Aims, are presented in the Supplemental Digital Content. Appropriate and complete statistical modeling and ML implementation of data collected is imperative to the planning, development, and completion of this proposal (Supplemental Tables 2-5). The most critical component is the accurate assessment of the enrollment rates of subjects at the three sites, as well as the rates of data missingness and the prevalence of SH in our diagnostic and prognostic cohorts. Estimates of data missingness are smaller in diagnostic correlations, given the simple clinical data elements. We therefore projected that a total sample size of 773 would accommodate the lowest estimates of SH prevalence (15% in the diagnostic samples) and highest estimates of data missingness rate (20% in the diagnostic samples; Supplemental Tables 2A). For the more powerful prognostic biomarker, much smaller sample sizes are needed to achieve the same level of significance and statistical power, with prognostic SH rate <10% and data missingness >40% (n = 270; Supplemental Table 2B).
FIGURE 6.
Proposed data analyses plan using traditional statistics and ML approaches. Previous biomarker projects on uncommon diseases have been hindered by small sample sizes limiting the power to query relevant subgroups. We here recruit plan to recruit 800 subjects from established CA centers and consortia, including 200 already enrolled in pilot studies, whose plasma samples and clinical data are in hand (“docket cases”). We will deploy supervised ML algorithms, in addition to traditional statistics, to define the best clustering and weighing of combined diagnostic and prognostic biomarker of SH in CA patients. LASSO, least absolute shrinkage and selection operator; CART, Classification & Regression Trees; SVM, support-vector machines; RF, relative frequency: RMSE, root mean square error; AUC, area under the curve.
QUALITY ASSURANCE
UCM shall be the Data Coordinating Center. The investigative teams at UCSF and Mayo shall assist with data adjudication.
EXPECTED OUTCOMES OF THE STUDY
Our goal is to develop blood tests, potentially reflecting or complementing complex and more expensive imaging assessments, to better diagnose, predict and monitor CASH. Associations of any CASH biomarkers with age, HMA or seizures in non-CA subjects would likely reflect shared mechanisms.
DURATION OF THE PROJECT
We project enrolling 1040 cases over 4 yr. Completion of lagging enrollments and follow-up data analyses are planned in Year 5.
PROJECT MANAGEMENT
We propose recruiting a large cohort from leading centers already collaborating in CA research and applying harmonized data elements. UCM includes senior scientists who conducted pilot studies. The investigative teams at UCSF and Mayo have an exceptional track record in CA patient recruitment, clinical data elements and risk stratification. They shall assist with analysis and interpretation.
ETHICS
UCM will be identified as the governing central Institutional Review Board (IRB). IRB authorization and informed patient consent shall be requested at the three sites to expand ongoing approved biomarker studies.
Funding
This study was funded by National Institute of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS).
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Contributor Information
Romuald Girard, Neurovascular Surgery Program, Department of Surgery, Section of Neurosurgery, University of Chicago Medicine and Biological Sciences, Chicago, Illinois.
Yan Li, Neurovascular Surgery Program, Department of Surgery, Section of Neurosurgery, University of Chicago Medicine and Biological Sciences, Chicago, Illinois; Bioinformatics core, Center for Research Informatics, University of Chicago, Chicago, Illinois.
Agnieszka Stadnik, Neurovascular Surgery Program, Department of Surgery, Section of Neurosurgery, University of Chicago Medicine and Biological Sciences, Chicago, Illinois.
Robert Shenkar, Neurovascular Surgery Program, Department of Surgery, Section of Neurosurgery, University of Chicago Medicine and Biological Sciences, Chicago, Illinois.
Nicholas Hobson, Neurovascular Surgery Program, Department of Surgery, Section of Neurosurgery, University of Chicago Medicine and Biological Sciences, Chicago, Illinois.
Sharbel Romanos, Neurovascular Surgery Program, Department of Surgery, Section of Neurosurgery, University of Chicago Medicine and Biological Sciences, Chicago, Illinois.
Abhinav Srinath, Neurovascular Surgery Program, Department of Surgery, Section of Neurosurgery, University of Chicago Medicine and Biological Sciences, Chicago, Illinois.
Thomas Moore, Neurovascular Surgery Program, Department of Surgery, Section of Neurosurgery, University of Chicago Medicine and Biological Sciences, Chicago, Illinois.
Rhonda Lightle, Neurovascular Surgery Program, Department of Surgery, Section of Neurosurgery, University of Chicago Medicine and Biological Sciences, Chicago, Illinois.
Abdallah Shkoukani, Neurovascular Surgery Program, Department of Surgery, Section of Neurosurgery, University of Chicago Medicine and Biological Sciences, Chicago, Illinois.
Amy Akers, Angioma Alliance, Norfolk, Virginia.
Timothy Carroll, Department of Diagnostic Radiology, The University of Chicago Medicine and Biological Sciences, Chicago, Illinois.
Gregory A Christoforidis, Department of Diagnostic Radiology, The University of Chicago Medicine and Biological Sciences, Chicago, Illinois.
James I Koenig, National Institute of Neurological Disorders and Stroke, Bethesda, Maryland.
Cornelia Lee, Angioma Alliance, Norfolk, Virginia.
Kristina Piedad, Neurovascular Surgery Program, Department of Surgery, Section of Neurosurgery, University of Chicago Medicine and Biological Sciences, Chicago, Illinois.
Steven M Greenberg, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts.
Helen Kim, Department of Anesthesia & Perioperative Care, University of California at San Francisco, San Francisco, California.
Kelly D Flemming, Department of Neurology, Mayo Clinic, Rochester, Minnesota.
Yuan Ji, Department of Public Health Sciences, University of Chicago, Chicago, Illinois.
Issam A Awad, Neurovascular Surgery Program, Department of Surgery, Section of Neurosurgery, University of Chicago Medicine and Biological Sciences, Chicago, Illinois.
Supplemental Digital Content. A Roadmap for Developing Plasma Diagnostic and Prognostic Biomarkers of Cerebral CASH. This includes Supplemental Methods, Supplemental Statistics and Simulations, as well as 3 Supplemental Figures, 5 Supplemental Tables and Supplemental References.
REFERENCES
- 1.Robinson JR Jr, Awad IA, Masaryk TJ, Estes ML. Pathological heterogeneity of angiographically occult vascular malformations of the brain. Neurosurgery. 1993;33(4):547-554. [DOI] [PubMed] [Google Scholar]
- 2.Abdulrauf SI, Kaynar MY, Awad IA. A comparison of the clinical profile of cavernous malformations with and without associated venous malformations. Neurosurgery. 1999;44(1):41-47. [DOI] [PubMed] [Google Scholar]
- 3.Gault J, Sain S, Hu LJ, Awad IA. Spectrum of genotype and clinical manifestations in cerebral cavernous malformations. Neurosurgery. 2006;59(6):1278-1285. [DOI] [PubMed] [Google Scholar]
- 4.Akers AL, Johnson E, Steinberg GK, Zabramski JM, Marchuk DA. Biallelic somatic and germline mutations in cerebral cavernous malformations (CCMs): evidence for a two-hit mechanism of CCM pathogenesis. Hum Mol Genet. 2009;18(5):919-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gault J, Shenkar R, Recksiek P, Awad IA. Biallelic somatic and germ line CCM1 truncating mutations in a cerebral cavernous malformation lesion. Stroke. 2005;36(4):872-874. [DOI] [PubMed] [Google Scholar]
- 6.McDonald DA, Shi C, Shenkar Ret al. Lesions from patients with sporadic cerebral cavernous malformations harbor somatic mutations in the CCM genes: evidence for a common biochemical pathway for CCM pathogenesis. Hum Mol Genet. 2014;23(16):4357-4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akers A, Al-Shahi Salman R, Awad IAet al. Synopsis of guidelines for the clinical management of cerebral cavernous malformations: consensus recommendations based on systematic literature review by the angioma alliance scientific advisory board clinical experts panel. Neurosurgery. 2017;80(5):665-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Shahi Salman R, Hall JM, Horne MAet al. Untreated clinical course of cerebral cavernous malformations: a prospective, population-based cohort study. Lancet Neurol. 2012;11(3):217-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horne MA, Flemming KD, Su ICet al. Clinical course of untreated cerebral cavernous malformations: a meta-analysis of individual patient data. Lancet Neurol. 2016;15(2):166-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Shahi Salman R, Berg MJ, Morrison L, Awad IA, Angioma Alliance Scientific Advisory B. Hemorrhage from cavernous malformations of the brain: definition and reporting standards. Angioma alliance scientific advisory board. Stroke. 2008;39(12):3222-3230. [DOI] [PubMed] [Google Scholar]
- 11.Polster SP, Cao Y, Carroll Tet al. Trial readiness in cavernous angiomas with symptomatic hemorrhage (CASH). Neurosurgery. 2019;84(4):954-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polster SP, Stadnik A, Akers ALet al. Atorvastatin treatment of cavernous angiomas with symptomatic hemorrhage exploratory proof of concept (AT CASH EPOC) trial. Neurosurgery. 2019;85(6):843-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amur S, LaVange L, Zineh I, Buckman-Garner S, Woodcock J. Biomarker qualification: toward a multiple stakeholder framework for biomarker development, regulatory acceptance, and utilization. Clin Pharmacol Ther. 2015;98(1):34-46. [DOI] [PubMed] [Google Scholar]
- 14.Amur SG, Sanyal S, Chakravarty AGet al. Building a roadmap to biomarker qualification: challenges and opportunities. Biomark Med. 2015;9(11):1095-1105. [DOI] [PubMed] [Google Scholar]
- 15.Shi C, Shenkar R, Zeineddine HAet al. B-Cell depletion reduces the maturation of cerebral cavernous malformations in murine models. J Neuroimmune Pharmacol. 2016;11(2):369-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brutsch R, Liebler SS, Wustehube Jet al. Integrin cytoplasmic domain-associated protein-1 attenuates sprouting angiogenesis. Circ Res. 2010;107(5):592-601. [DOI] [PubMed] [Google Scholar]
- 17.Wustehube J, Bartol A, Liebler SSet al. Cerebral cavernous malformation protein CCM1 inhibits sprouting angiogenesis by activating DELTA-NOTCH signaling. Proc Natl Acad Sci. 2010;107(28):12640-12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noshiro S, Mikami T, Kataoka-Sasaki Yet al. Co-expression of tissue factor and IL-6 in immature endothelial cells of cerebral cavernous malformations. J Clin Neurosci. 2017;37:83-90. [DOI] [PubMed] [Google Scholar]
- 19.Luissint AC, Artus C, Glacial F, Ganeshamoorthy K, Couraud PO. Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids Barriers CNS. 2012;9(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsuchou H, Kastin AJ, Mishra PK, Pan W. C-reactive protein increases BBB permeability: implications for obesity and neuroinflammation. Cell Physiol Biochem. 2012;30(5):1109-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lampugnani MG, Dejana E, Giampietro C. Vascular endothelial (VE)-Cadherin, endothelial adherens junctions, and vascular disease. Cold Spring Harb Perspect Biol. 2018;10(10):a029322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson JR Jr, Awad IA, Zhou P, Barna BP, Estes ML. Expression of basement membrane and endothelial cell adhesion molecules in vascular malformations of the brain: preliminary observations and working hypothesis. Neurol Res. 1995;17(1):49-58. [DOI] [PubMed] [Google Scholar]
- 23.Shenkar R, Shi C, Austin Cet al. RhoA kinase inhibition with fasudil versus simvastatin in murine models of cerebral cavernous malformations. Stroke. 2017;48(1):187-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitehead KJ, Chan AC, Navankasattusas Set al. The cerebral cavernous malformation signaling pathway promotes vascular integrity via rho GTPases. Nat Med. 2009;15(2):177-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunha SI, Magnusson PU, Dejana E, Lampugnani MG. Deregulated TGF-beta/BMP signaling in vascular malformations. Circ Res. 2017;121(8):981-999. [DOI] [PubMed] [Google Scholar]
- 26.Girard R, Khanna O, Shenkar Ret al. Peripheral plasma vitamin d and non-HDL cholesterol reflect the severity of cerebral cavernous malformation disease. Biomark Med. 2016;10(3):255-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibson CC, Zhu W, Davis CTet al. Strategy for identifying repurposed drugs for the treatment of cerebral cavernous malformation. Circulation. 2015;131(3):289-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Retta SF, Glading AJ.. Oxidative stress and inflammation in cerebral cavernous malformation disease pathogenesis: two sides of the same coin. Int J Biochem Cell Biol. 2016;81(Pt B):254-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keep RF, Zhou N, Xiang J, Andjelkovic AV, Hua Y, Xi G. Vascular disruption and blood-brain barrier dysfunction in intracerebral hemorrhage. Fluids Barriers CNS. 2014;11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Y, Zhao Z, Yang Let al. Arsenic exposure assists ccm3 genetic polymorphism in elevating blood pressure. Oncotarget. 2018;9(4):4915-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pawlikowska L, Tran MN, Achrol ASet al. Polymorphisms in genes involved in inflammatory and angiogenic pathways and the risk of hemorrhagic presentation of brain arteriovenous malformations. Stroke. 2004;35(10):2294-2300. [DOI] [PubMed] [Google Scholar]
- 32.Bicer A, Guclu B, Ozkan Aet al. Expressions of angiogenesis associated matrix metalloproteinases and extracellular matrix proteins in cerebral vascular malformations. J Clin Neurosci. 2010;17(2):232-236. [DOI] [PubMed] [Google Scholar]
- 33.Choquet H, Pawlikowska L, Nelson Jet al. Polymorphisms in inflammatory and immune response genes associated with cerebral cavernous malformation type 1 severity. Cerebrovasc Dis. 2014;38(6):433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung KH, Chu K, Jeong SW, Park HK, Bae HJ, Yoon BW. Cerebral cavernous malformations with dynamic and progressive course: correlation study with vascular endothelial growth factor. Arch Neurol. 2003;60(11):1613-1618. [DOI] [PubMed] [Google Scholar]
- 35.Girard R, Zeineddine HA, Fam MDet al. Plasma biomarkers of inflammation reflect seizures and hemorrhagic activity of cerebral cavernous malformations. Transl Stroke Res. 2018;9(1):34-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Hao Q, Kim Het al. Soluble endoglin modulates aberrant cerebral vascular remodeling. Ann Neurol. 2009;66(1):19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McAllister KA, Grogg KM, Johnson DWet al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994;8(4):345-351. [DOI] [PubMed] [Google Scholar]
- 38.Ng CT, Fong LY, Sulaiman MRet al. Interferon-gamma increases endothelial permeability by causing activation of p38 MAP kinase and actin cytoskeleton alteration. J Interferon Cytokine Res. 2015;35(7):513-522. [DOI] [PubMed] [Google Scholar]
- 39.Jones CA, London NR, Chen Het al. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat Med. 2008;14(4):448-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Girard R, Zeineddine HA, Koskimaki Jet al. Plasma biomarkers of inflammation and angiogenesis predict cerebral cavernous malformation symptomatic hemorrhage or lesional growth. Circ Res. 2018;122(12):1716-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyne SB, Girard R, Koskimaki Jet al. Biomarkers of cavernous angioma with symptomatic hemorrhage. JCI Insight. 2019;4(12):e128577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2019;20(1):21-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson JR, Zhu J, Kilari D, Wang L. Applications of extracellular RNAs in oncology. Mol Diagn Ther. 2017;21(1):1-11. [DOI] [PubMed] [Google Scholar]
- 44.Ferreira R, Santos T, Amar Aet al. MicroRNA-18a improves human cerebral arteriovenous malformation endothelial cell function. Stroke. 2014;45(1):293-297. [DOI] [PubMed] [Google Scholar]
- 45.Huang J, Song J, Qu Met al. MicroRNA-137 and microRNA-195* inhibit vasculogenesis in brain arteriovenous malformations. Ann Neurol. 2017;82(3):371-384. [DOI] [PubMed] [Google Scholar]
- 46.Mikati AG, Tan H, Shenkar Ret al. Dynamic permeability and quantitative susceptibility: related imaging biomarkers in cerebral cavernous malformations. Stroke. 2014;45(2):598-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shenkar R, Shi C, Rebeiz Tet al. Exceptional aggressiveness of cerebral cavernous malformation disease associated with PDCD10 mutations. Genet Med. 2015;17(3):188-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mikati AG, Khanna O, Zhang Let al. Vascular permeability in cerebral cavernous malformations. J Cereb Blood Flow Metab. 2015;35(10):1632-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hart BL, Taheri S, Rosenberg GA, Morrison LA. Dynamic contrast-enhanced MRI evaluation of cerebral cavernous malformations. Transl Stroke Res. 2013;4(5):500-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Girard R, Fam MD, Zeineddine HAet al. Vascular permeability and iron deposition biomarkers in longitudinal follow-up of cerebral cavernous malformations. J Neurosurg. 2017;127(1):102-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeineddine HA, Girard R, Cao Yet al. Quantitative susceptibility mapping as a monitoring biomarker in cerebral cavernous malformations with recent hemorrhage. J Magn Reson Imaging. 2018;47(4):1133-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koskimaki J, Girard R, Li Yet al. Comprehensive transcriptome analysis of cerebral cavernous malformation across multiple species and genotypes. JCI Insight. 2019;4(3):e126167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koskimaki J, Zhang D, Li Yet al. Transcriptome clarifies mechanisms of lesion genesis versus progression in models of ccm3 cerebral cavernous malformations. Acta Neuropathol Commun. 2019;7(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou HJ, Qin L, Zhang Het al. Endothelial exocytosis of angiopoietin-2 resulting from CCM3 deficiency contributes to cerebral cavernous malformation. Nat Med. 2016;22(9):1033-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maddaluno L, Rudini N, Cuttano Ret al. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature. 2013;498(7455):492-496. [DOI] [PubMed] [Google Scholar]
- 56.Tang AT, Choi JP, Kotzin JJet al. Endothelial TLR4 and the microbiome drive cerebral cavernous malformations. Nature. 2017;545(7654):305-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Z, Tang AT, Wong WYet al. Cerebral cavernous malformations arise from endothelial gain of MEKK3-KLF2/4 signalling. Nature. 2016;532(7597):122-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lopez-Ramirez MA, Fonseca G, Zeineddine HAet al. Thrombospondin1 (TSP1) replacement prevents cerebral cavernous malformations. J Exp Med. 2017;214(11):3331-3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Niida Y, Inoue M, Ozaki M, Takase E. Human malformation syndromes of defective GLI: opposite phenotypes of 2q14.2 (GLI2) and 7p14.2 (GLI3) microdeletions and a GLIA/R balance model. Cytogenet Genome Res. 2017;153(2):56-65. [DOI] [PubMed] [Google Scholar]
- 60.Lopez-Ramirez MA, Pham A, Girard Ret al. Cerebral cavernous malformations form an anticoagulant vascular domain in humans and mice. Blood. 2019;133(3):193-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krouwer VJ, Hekking LH, Langelaar-Makkinje M, Regan-Klapisz E, Post JA. Endothelial cell senescence is associated with disrupted cell-cell junctions and increased monolayer permeability. Vascular cell. 2012;4(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elahy M, Jackaman C, Mamo JCet al. Blood-brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun Ageing. 2015;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bake S, Friedman JA, Sohrabji F. Reproductive age-related changes in the blood brain barrier: expression of IgG and tight junction proteins. Microvasc Res. 2009;78(3):413-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rossi B, Angiari S, Zenaro E, Budui SL, Constantin G. Vascular inflammation in central nervous system diseases: adhesion receptors controlling leukocyte-endothelial interactions. J Leukoc Biol. 2011;89(4):539-556. [DOI] [PubMed] [Google Scholar]
- 65.Fabene PF, Navarro Mora G, Martinello Met al. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat Med. 2008;14(12):1377-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Avraham-Davidi I, Ely Y, Pham VNet al. ApoB-containing lipoproteins regulate angiogenesis by modulating expression of VEGF receptor 1. Nat Med. 2012;18(6):967-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Polster SP, Sharma A, Tanes Cet al. Permissive microbiome characterizes human subjects with a neurovascular disease cavernous angioma. Nat Commun. 2020;11(1):2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.