Abstract
BACKGROUND
Decline in neurocognitive functioning (NCF) often occurs following brain tumor resection. Functional connectomics have shown how neurologic insults disrupt cerebral networks underlying NCF, though studies involving patients with brain tumors are lacking.
OBJECTIVE
To investigate the impact of brain tumor resection upon the connectome and relationships with NCF outcome in the early postoperative period.
METHODS
A total of 15 right-handed adults with left perisylvian glioma underwent resting-state functional magnetic resonance imaging (rs-fMRI) and neuropsychological assessment before and after awake tumor resection. Graph theoretical analysis was applied to rs-fMRI connectivity matrices to calculate network properties. Network properties and NCF measures were compared across the pre- to postoperative periods with matched pairs Wilcoxon signed-rank tests. Associations between pre- to postoperative change in network and NCF measures were determined with Spearman rank-order correlations (ρ).
RESULTS
A majority of the sample showed postoperative decline on 1 or more NCF measures. Significant postoperative NCF decline was found across measures of verbal memory, processing speed, executive functioning, receptive language, and a composite index. Regarding connectomic properties, betweenness centrality and assortativity were significantly smaller postoperatively, and reductions in these measures were associated with better NCF outcomes. Significant inverse associations (ρ = −.51 to −.78, all P < .05) were observed between change in language, executive functioning, and learning and memory, and alterations in segregation, centrality, and resilience network properties.
CONCLUSION
Decline in NCF was common shortly following resection of glioma involving eloquent brain regions, most frequently in verbal learning/memory and executive functioning. Better postoperative outcomes accompanied reductions in centrality and resilience connectomic measures.
Keywords: Brain tumor, Glioma, Functional imaging, Connectomics, Neurocognitive function
ABBREVIATIONS
- COWA
controlled oral word association
- CTB COMP
Clinical Trial Battery Composite
- DR
delayed recall
- HVLT-R
Hopkins verbal learning test-revised
- Rec
delayed recognition
- MEG
magnetoencephalography
- MRI
magnetic resonance imaging
- NCF
neurocognitive functioning
- rs-fMRI
resting-state functional magnetic resonance imaging
- TMTA
trail making test part A
- TMTB
trail making test part B
- TR
total recall
Impairment of neurocognitive functioning (NCF) is ubiquitous in patients with brain tumors.1,2 However, patterns of NCF impairment vary considerably according to lesion size and location, along with other tumor, treatment, and patient characteristics, influencing the integrity of distributed cerebral networks.3-7 Our prior work identified neurosurgical tumor resection as an important contributor to NCF decline, particularly for patients harboring lesions in the language dominant (ie, usually left) hemisphere within the proximity of eloquent cortex (eg, perisylvian regions).8 Unfortunately, over 60% of patients with glioma may experience significant NCF decline following tumor resection, even when advanced planning and operative techniques are utilized.8,9
Over the past few decades, brain connectomics have matured into a well-established tool for interrogating the cerebral networks underlying NCF.10-12 Functional connectomic measures have been shown to be predictive of NCF in neurologic populations such as Alzheimer disease,13 as well as chemotherapy-related NCF decline in noncentral nervous system cancer.14 A number of studies have also described disruption of cerebral networks related to the presence of brain tumors (for a review, see Aerts et al5). However, very few studies have investigated alterations in the functional connectome associated with surgical tumor resection. The present study aims to confirm that NCF decline is common following resection of glioma involving left perisylvian brain regions, a population particularly vulnerable to poor NCF outcome,15,16 while also advancing understanding of the underlying mechanisms of NCF change through application of resting-state functional magnetic resonance imaging (MRI) (rs-fMRI) connectomics.
METHODS
Participants
Adult patients with newly identified brain lesions near left hemisphere eloquent language cortex (per Sawaya et al17) were prospectively recruited at the Brain and Spine Center of The University of Texas MD Anderson Cancer Center (MDACC). A sample of 15 patients was available for analyses. See Text, Supplemental Digital Content Methods section for full inclusion/exclusion criteria and detailed patient accrual information.
The MDACC Institutional Review Board approved the study and all patients provided written informed consent.
Neurocognitive Assessment
All patients underwent standard of care neuropsychological evaluations within 2 wk prior to tumor resection and again within 12 wk after neurosurgical resection but prior to initiation of radiation or chemotherapy. Table 1 lists the neuropsychological tests by domain included in the battery. All tests represent well-validated measures with adequate test-retest reliability.18 When available, alternate forms were utilized at postsurgical evaluation to minimize practice effects. NCF test scores were standardized using normative data19-24 stratified by patient age, in addition to gender and level of education when appropriate, and converted to z-scores (M = 0, SD = 1). Additionally, a derived composite was calculated, referred to as the Clinical Trial Battery Composite (CTB COMP). The CTB COMP represents the mean of z-scores from controlled oral word association (COWA), Trail making test part A (TMTA), Trail making test part B (TMTB), Hopkins Verbal Learning Test-Revised (HVLT-R) total recall (TR), HVLT-R delayed recall (DR), and HVLT-R delayed recognition (Rec).25,26 Differences between pre- and postsurgical z-scores of −0.99 to + 0.99 were considered stable. Positive changes of +1.00 or greater were considered improved. Negative changes ranging from −1.00 to −1.99 were described as mildly declined, changes of −2.00 to −2.99 moderately declined, and −3.00 or lower severely declined.
TABLE 1.
Neurocognitive Measures Grouped by Principal Domain
| Domain | Test | Abbreviation |
|---|---|---|
| Attention | WAIS-IV digit span | Digit span |
| WAIS-IV arithmetic | Arithmetic | |
| Learning and memory | HVLT-R total recall | HVLT-R TR |
| HVLT-R delayed recall | HVLT-R DR | |
| HVLT-R delayed recognition | HVLT-R Rec | |
| Processing speed | WAIS-IV coding | Coding |
| WAIS-IV symbol search | Symbol search | |
| Trail making test part A | TMTA | |
| Executive function | Trail making test part B | TMTB |
| WAIS-IV similarities | Similarities | |
| MAE controlled oral word association | COWA | |
| Semantic (animal) fluency | Animals | |
| Language | MAE token test | Token |
| Boston naming test naming | Naming | |
| Visuospatial function | WAIS-IV block design | Block design |
| Clinical Trial Battery Composite | HVLT-R, TMT, COWA | CTB COMP |
WAIS-IV, Wechsler Adult Intelligence Scale-Fourth Edition; MAE, Multilingual Aphasia Examination.
Neuroimaging
MRI Acquisition and Processing
Each patient underwent MRI of the brain (anatomical and functional) within 10 d before neurosurgery and again within 6 wk following tumor resection but prior to initiation of radiation or chemotherapy. Resting-state fMRI was processed in MATLAB (Mathworks, Natick, Massachusetts) via Statistical Parametric Mapping (SPM 12, Wellcome Department of Cognitive Neurology, London, United Kingdom) with the Brain Connectivity Toolbox27 and Group Independent component analysis of fMRI Toolbox (GIFT).28 Data were segmented to regions of interest from the Automated Anatomical Labeling (AAL) atlas29 after normalization to the Montreal Neurological Institute (MNI) space using the DARTEL toolbox.30 From derived connectivity matrices, networks were constructed via application of graph theory, yielding unweighted graphs with nodes representing brain regions and edges representing the functional relationships between them. See Text, Supplemental Digital Content Methods section for detailed image acquisition and processing information.
Network Analysis
Graph theoretical connectomic properties were calculated at the whole brain level using the Brain Connectivity Toolbox,27 including measures of integration (global efficiency and characteristic path length), segregation (modularity, clustering coefficient, and local efficiency), centrality (betweenness centrality), and resilience (assortativity).5,31,32 Prior work indicates that network measures have good to excellent test-retest reliability.33 For detailed descriptions of network connectivity measures and their mathematical representations, see Rubinov and Sporns.12
Statistical Analysis
Because of sample size restrictions and potential violations of normality assumptions, nonparametric inferential statistical analyses were performed. Standardized NCF z-scores and network metrics were compared across the pre- to postoperative period with matched pairs Wilcoxon signed-rank tests. As recommended by Cohen,34 effect sizes of nonparametric comparisons were quantified with the r statistic, calculated by dividing the absolute (positive) test statistic (Z) by the square root of the number of pairs. Associations between pre- to postoperative NCF z-score change and network metric change were determined with Spearman rank-order correlations (ρ). All statistical analyses were performed with SPSS 24.0 (IBM Corp, Armonk, New York). Given the exploratory nature of the investigation, two-sided tests were used with an unadjusted significance level of P ≤ .05.
RESULTS
Descriptive Characteristics
Sample characteristics are presented in Table 2. Surgical techniques involved awake craniotomy with direct cortical stimulation language mapping and image guided (MRI and/or ultrasound) microsurgical tumor resection for all patients. Although there was greater variability in time from surgery to postoperative NCF assessment as compared to the surgery to imaging interval (see Table 2), about 75% of the sample had postoperative NCF testing within 10 d of postoperative imaging sessions, suggesting relatively good alignment of neuropsychological testing and neuroimaging studies.
TABLE 2.
Demographic and Clinical Characteristics
| N = 15 | |
|---|---|
| Age, yr | |
| M (SD) | 44.6 (14.9) |
| % Female | 53 |
| % White | 87 |
| % Right hand dominant | 100 |
| Education, yr | |
| M (SD) | 16.0 (2.7) |
| Seizure history, % yes | 80 |
| AED at preop scan, % yes | 87 |
| AED at postop scan, % yes | 93 |
| Steroid at preop scan, % yes | 53 |
| Steroid at postop scan, % yes | 53 |
| Preop scan to resection, d | |
| M (SD) | 2.3 (2.2) |
| Resection to postop scan, d | |
| M (SD) | 17.1 (6.4) |
| Preop NCF testing to resection, d | |
| M (SD) | 2.8 (2.1) |
| Resection to postop NCF testing, d | |
| M (SD) | 33.2 (27.1) |
| Hemisphere lesion, % | |
| Left | 100 |
| Lesion location, % | |
| Frontal | 47 |
| Temporal | 40 |
| Parietal | 13 |
| Lesion volume | |
| Preop FLAIR, cm3 | |
| M (SD) | 61.4 (37.1) |
| Postop FLAIR, cm3 | |
| M (SD) | 10.7 (13.6) |
| Residual FLAIR, % | |
| M (SD) | 15.9 (15.4) |
| Extent of resection, % | |
| Gross total | 47 |
| Near total | 20 |
| Subtotal | 33 |
| Awake craniotomy, % yes | 100 |
| Prior intervention, % | |
| None | 33 |
| Biopsy | 67 |
| Tumor grade, % | |
| II | 53 |
| III | 7 |
| IV | 40 |
| Histology, % | |
| Glioblastoma | 40 |
| Astrocytoma | 47 |
| Oligodendroglioma | 13 |
AED, antiepileptic drug.
Neurocognitive Functioning
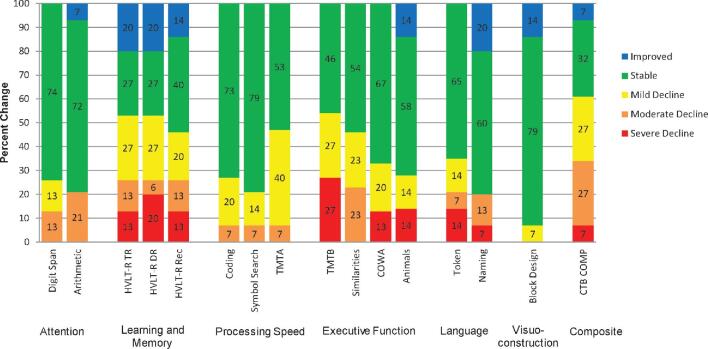
Decline appeared greatest and most frequently on HVLT-R TR, HVLT-R DR, and TMTB (Figure 1). About 60% of the sample exhibited decline on CTB COMP and 87% of the sample showed at least mild decline (z-score change ≤ −1.00) on 1 or more individual measures, with 53% showing decline on 5 or more measures. On average, significant postoperative NCF decline with large effect sizes was found across 6 of the 14 measures and CTB COMP (Table 3). Regarding executive functioning, NCF performances significantly decreased on TMTB, Similarities, and COWA. Significant decline was also observed in language comprehension (Token) and processing speed (TMTA). Regarding memory, the group exhibited significant decline on HVLT-R Rec, though declines on HVLT-R TR and HVLT-R DR also trended toward significance. The CTB COMP significantly declined from the pre- to postoperative period and showed the greatest effect size of all outcome measures. Neurocognitive change was not significantly associated with tumor type, pre- or postoperative lesion volumes, extent of resection, or antiepileptic and steroid medication use.
FIGURE 1.
Rates and magnitude of pre- to postoperative neurocognitive change by measure Note, N = 15 for all but arithmetic (N = 14), symbol search (N = 14), similarities (N = 13), animals (N = 14), token (N = 14), and block design (N = 14).
TABLE 3.
Pre- and Postoperative Neurocognitive Performances
| Preoperative | Postoperative | |||||
|---|---|---|---|---|---|---|
| Domain and test | N | z-Score Mdn (IQR) | N | z-Score Mdn (IQR) | P a | Effect Sizeb |
| Attention | ||||||
| Digit span | 15 | −0.33 (1.33) | 15 | −0.33 (2.00) | .178 | .35 |
| Arithmetic | 15 | −0.33 (1.34) | 14 | −0.50 (2.00) | .322 | .26 |
| Learning and memory | ||||||
| HVLT-R TR | 15 | −1.79 (1.79) | 15 | −2.41 (2.89) | .053 | .50 |
| HVLT-R DR | 15 | −1.94 (3.60) | 15 | −2.88 (4.09) | .052 | .50 |
| HVLT-R Rec | 15 | −1.00 (1.82) | 15 | −2.71 (2.73) | .035 | .54 |
| Processing speed | ||||||
| Coding | 15 | −0.33 (1.66) | 15 | −0.67 (2.33) | .065 | .48 |
| Symbol Search | 14 | −0.33 (2.67) | 14 | −1.00 (2.33) | .171 | .37 |
| TMTA | 15 | 0.39 (1.15) | 15 | −0.30 (2.22) | .020 | .60 |
| Executive function | ||||||
| TMTB | 15 | −0.20 (1.68) | 15 | −1.10 (4.95) | .020 | .60 |
| Similarities | 15 | 0.00 (0.67) | 13 | −0.67 (1.83) | .013 | .69 |
| COWA | 15 | −1.14 (1.75) | 15 | −2.10 (2.26) | .013 | .64 |
| Animals | 15 | −1.30 (2.30) | 14 | −1.85 (2.73) | .181 | .36 |
| Language | ||||||
| Token | 15 | 0.44 (1.05) | 14 | −0.44 (2.15) | .008 | .71 |
| Naming | 15 | −1.60 (1.10) | 15 | −1.30 (2.50) | .699 | .10 |
| Visuospatial function | ||||||
| Block Design | 15 | 0.00 (1.33) | 14 | −0.17 (1.42) | .645 | .12 |
| CTB COMP | 15 | −1.24 (1.60) | 15 | −2.30 (1.95) | .005 | .72 |
See Table 1 for test name abbreviations.
aPre- and postoperative z-scores compared with matched pairs Wilcoxon signed-rank tests.
bEffect size (r) calculated as the absolute (positive) standardized test statistic (Z) divided by the square root of the number of pairs.
Bolded; significant difference, P ≤ .05.
Functional Connectomics
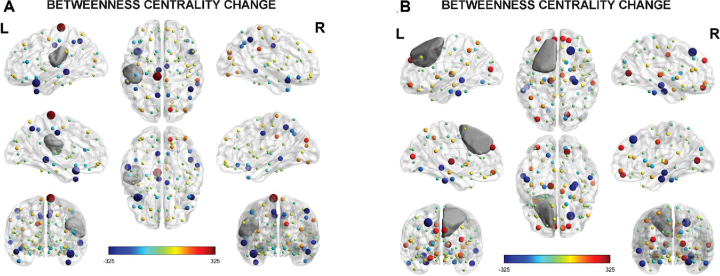
Significant reductions with large effect sizes were found in betweenness centrality and assortativity (Table 4). Change in connectomic properties was not significantly associated with tumor type, pre- or postoperative lesion volume, extent of resection, or antiepileptic and steroid medication use. Figure 2 depicts differing case examples visualizing changes in pre- and postoperative betweenness centrality graphs. Change in assortativity was not visualized, as the property represents a global metric and cannot be interpreted or meaningfully visualized on a nodal basis.
TABLE 4.
Pre- and Postoperative Connectomic Properties
| Preoperative | Postoperative | |||
|---|---|---|---|---|
| N = 15 | Mdn (IQR) | Mdn (IQR) | P b | Effect sizec |
| Integration | ||||
| Global efficiencya | 0.531 (0.023) | 0.533 (0.026) | .733 | .09 |
| Characteristic path lengtha | 2.192 (0.122) | 2.169 (0.094) | .233 | .31 |
| Segregation | ||||
| Modularity | 0.338 (0.070) | 0.342 (0.093) | .955 | .01 |
| Clustering coefficient | 0.576 (0.037) | 0.576 (0.050) | .691 | .10 |
| Local efficiency | 0.758 (0.018) | 0.745 (0.049) | .496 | .18 |
| Centrality | ||||
| Betweenness centrality | 104.444 (9.667) | 99.800 (12.133) | .031 | .56 |
| Resilience | ||||
| Assortativity | 0.293 (0.132) | 0.210 (0.131) | .027 | .57 |
Connectivity matrix thresholded to density of 0.2.
aExcluding infinite path lengths.
bPre- and postoperative z-scores compared with matched pairs Wilcoxon signed-rank tests.
cEffect size (r) calculated as the absolute (positive) standardized test statistic (Z) divided by the square root of the number of pairs.
Bolded; significant difference, P ≤ .05.
FIGURE 2.
Visualization of pre- to postoperative change in betweenness centrality for case examples. A, The graph depicts betweenness centrality nodal change for a patient with left frontoparietal glioblastoma. Betweenness centrality appeared reduced (M change = −20.02) at postoperative follow-up as represented by a majority of cooler colored nodes. Regarding NCF, the patient exhibited largely stable postoperative functioning (CTB COMP z-score change = 0.13) with improvement in verbal memory and naming aspects of language. B, The graph depicts betweenness centrality nodal change for a patient with left superior frontal grade II astrocytoma. Betweenness centrality appeared increased (M change = 18.62) postoperatively, as indicated by a majority of warmer colored nodes. Cognitively, broad declines were noted postoperatively (CTB COMP z-score change = −2.10), most severely within the domains of verbal fluency, verbal memory, and processing speed. Note, graphs represent the difference between postoperative and preoperative betweenness centrality of the nodes. Cooler colors represent postoperative decrease in betweenness centrality from preoperative levels and warmer colors represent postoperative increase. Node size represents the absolute difference between postoperative and preoperative betweenness centrality, linearly normalized between the minimum and maximum node size. Grey area represents lesion overlay.
Associations Between Alterations in Connectomic Properties and Neurocognitive Change
A number of large magnitude associations were observed between pre- to postoperative changes in connectomic properties and NCF performances. Regarding language measures, change in Token was significantly inversely associated with change in clustering coefficient [ρ(12) = −.55, P = .043]. For executive functioning, change in COWA was significantly inversely associated with change in assortativity [ρ(13) = −.53, P = .041] and trended toward a significant inverse relationship with change in clustering coefficient [ρ(13) = −.51, P = .054]. Regarding learning and memory, change in HVLT-R TR was significantly associated with change in betweenness centrality [ρ(12) = −.78, P = .001], and change in HVLT-R DR was significantly inversely associated with change in assortativity [ρ(13) = −.58, P = .023] and change in betweenness centrality [ρ(13) = −.60, P = .017]. Change in HVLT-R Rec was significantly associated with change in local efficiency [ρ(13) = −.54, P = .036]. Additionally, change in CTB COMP was significantly inversely associated with change in betweenness centrality [ρ(15) = -.64, P = .010]. Consistent with these results, the patient depicted in Panel A of Figure 2 exhibited longitudinal cognitive improvement or stability corresponding to the postoperative decreases in betweenness centrality, whereas the patient in Panel B showed broad cognitive decline associated with the increased postoperative betweenness centrality.
DISCUSSION
Key Results
A majority of patients exhibited NCF decline shortly following tumor resection, most frequently and severely in the domains of verbal learning and memory and executive functioning, though auditory comprehension also significantly worsened. A minority of patients exhibited substantial improvement in NCF following surgery. The breadth and frequency of postoperative decline is somewhat greater than other reports,35,36 though this likely relates, at least in part, to the particular population investigated in the present study. Patients included harbored lesions in left perisylvian regions near eloquent cortex, which convey particular risk of postoperative NCF impairment. Indeed, the present findings are consistent with our prior work8 and that of others,37 which document a similar frequency and severity of NCF decline in the near postoperative period following resection of glioma near eloquent regions.
Regarding connectomic properties, betweenness centrality and assortativity showed significant and large reductions following surgery. Interestingly, large inverse associations were noted between changes in numerous connectomic properties and various NCF domains, most strongly with executive functioning, learning and memory, and comprehension. Additionally, the NCF composite was significantly inversely associated with betweenness centrality. These associations imply that patients with the least postoperative decrease in connectomic properties, particularly hubness and segregation, tend to exhibit poorer NCF outcomes. Although variability exists, similar inverse associations between connectomic properties and NCF have been reported in magnetoencephalography (MEG) and rs-fMRI connectivity studies involving various neurologic populations.31,38,39 With validation of these associations, predictive models may be developed to identify patients and surgical approaches that optimize postoperative functional connectivity, which, in turn, may convey greater preservation of NCF.
Interpretation
Although our results indicated that a majority of patients with eloquent glioma exhibit NCF decline following resection, it should be noted that postoperative imaging occurred about 2 to 3 wk following resection, with neuropsychological testing around 1 mo postsurgically. As such, it is possible that some patients may have exhibited NCF improvement spontaneously and/or with rehabilitative therapies in the mo following resection. A recent systematic review regarding NCF outcomes in patients with eloquent glioma reported that nearly 80% of patients exhibited decline in at least one NCF domain in the near postoperative phase, consistent with our results.37 However, findings were mixed at longer-term follow-up (3-6 mo), with some studies showing improvement and others reporting progressive worsening. Aside from timing of assessment, other factors likely contributing to variability in study outcomes include differing criteria for impairment, surgical techniques utilized (eg, awake craniotomy, language mapping, image guidance, intraoperative MRI, etc), patient inclusion criteria, disease progression, and adjuvant therapies. Further longitudinal follow-up with rs-fMRI, neuropsychological testing, and well-characterized neurosurgical procedures would help clarify factors contributing to NCF outcomes and recovery trajectories, furthering understanding of which patients are most likely to harbor the most persistent deficits.
Interpretation of the reductions in connectomic properties is not entirely clear and very little work has examined alterations in rs-fMRI connectomic properties following brain tumor resection. Of the few studies examining rs-fMRI connectivity changes associated with brain tumor resection, results are mixed, with indication of both increased and decreased efficiency metrics following surgical tumor resection.40 Despite this, prior investigations utilizing MEG provide potential insight.38,41,42 Increased MEG functional connectivity has been documented in patients with brain tumors at baseline as compared to healthy controls, suggesting pathological hyperconnectivity associated with the presence of the lesion.38,41 Additionally, theta band connectivity appears to decrease following neurosurgical tumor resection, thought to be related to normalization of the hyperconnectivity.42 It is possible that the reductions in centrality and assortativity observed in the present study reflect partial normalization of pathological connectivity via surgery. However, it should be noted that if reduction in postoperative connectomic properties does indeed reflect some normalization of hyperconnectivity, such normalization appears partial at best and other factors are likely to contribute to NCF outcomes given that a majority of patients continue to exhibit NCF decline and/or persistent impairment in the postoperative period.
Limitations
It is acknowledged that the accuracy of the hyperconnectivity and normalization hypothesis cannot be conclusively determined via this study given the lack of a healthy control comparison group needed to establish the presence of preoperative hyperconnectivity in the patient group. An additional limitation involves the inclusion of tumors with differing histologies and grades, as patterns of network disturbances appear to differ across tumor types.39,42 A sizeable proportion of patients also had biopsies prior to tumor resection and before their preoperative neuropsychological testing and rs-fMRI sessions. However, sensitivity analyses comparing the NCF and connectomics of those with and without prior biopsies were conducted and no significant differences were found, suggesting that biopsy did not significantly influence the data. It is also acknowledged that preoperative and intraoperative mapping studies (eg, fMRI, DTI, and direct cortical stimulation) and surgical techniques utilized might have impacted surgical planning and resection, with consequent effect upon NCF and connectomic changes.
With these considerations in mind, further work should strive to incorporate multiple functional imaging modalities and intraoperative mapping data with a more homogeneous patient population while also including a well-matched group of controls to better examine the preoperative hyperconnectivity and postoperative normalization hypothesis. Characterization of surgical and perioperative complications would also provide a more nuanced understanding of etiological contributors to alterations in NCF and functional connectomic properties. Additionally, although our study involves a sample size comparable to other longitudinal functional neuroimaging studies, future investigations would benefit from larger samples to increase power, allow correction for multiple comparisons, and enable more sophisticated analytic approaches. A larger sample would also enable analyses which may identify baseline patient, clinical, tumor, and imaging characteristics predictive of NCF and connectomic change.
Generalizability
Given the selected sample, the findings of the present study may not generalize to the glioma population as a whole. Despite this, results may be considered largely representative of an important subgroup of glioma patients particularly vulnerable to postsurgical NCF decline. Imaging processing methods may also impact generalizability, as choice of parcellation scheme represents a potential source of error with numerous anatomical templates existing.43,44 Fortunately, differences in parcellation scheme are unlikely to significantly alter the results of this study as measures of network segregation, integration, and small-world topology have been found to be robust to differences in scheme utilized.45 Another limitation of using group-level anatomical parcellation is the presence of lesions. Not only could there be neuroplastic reorganization of the functional anatomy, but the lesion could also confound the spatial normalization essential for atlas based parcellation. Despite this, advanced spatial normalization techniques such as DARTEL (as used in the present study) have been shown to overcome some of the limitations of older and more vulnerable methods such as cost-function masking.46
CONCLUSION
Decline in NCF was common shortly following resection of glioma involving eloquent brain regions and functional brain connectomic changes attributable to surgical tumor resection may explain some of the postoperative NCF changes, as better outcomes accompanied reductions in centrality and resilience connectomic measures. This work represents a first step toward establishing the clinical relevance of rs-fMRI and functional connectomics to neurosurgical management of brain tumors. By establishing links between alterations in functional networks and NCF outcome following brain tumor resection, this study provides support for the potential of rs-fMRI and connectomics to inform, and with further development, potentially predict outcome following brain tumor resection. By doing so, surgical approaches may be further individualized and patient NCF and quality of life better preserved.
Funding
The research reported in this publication was supported by The University of Texas MD Anderson Cancer Center Diagnostic Imaging Clinical Research Committee (to Dr Liu) and the National Institute of Nursing Research of the National Institutes of Health under Award Number R01NR014195 (to Dr Wefel). The content is solely the responsibility of the authors and does not necessarily represent the official views of The University of Texas MD Anderson Cancer Center or the National Institutes of Health.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Notes
This work was previously presented as a rapid oral report at the 7th meeting of the Intraoperative Imaging Society in Houston, Texas, on March 16, 2019.
Contributor Information
Kyle R Noll, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Henry S Chen, Department of Imaging Physics, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Jeffrey S Wefel, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas; Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Vinodh A Kumar, Department of Neuroradiology, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Ping Hou, Department of Imaging Physics, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Sherise D Ferguson, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Ganesh Rao, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Jason M Johnson, Department of Neuroradiology, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Donald F Schomer, Department of Neuroradiology, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Dima Suki, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Sujit S Prabhu, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Ho-Ling Liu, Department of Imaging Physics, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Supplemental Digital Content. Text. The Supplemental Digital Content expands upon the Methods provided.
REFERENCES
- 1. Tucha O, Smely C, Preier M, Lange KW. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery. 2000;47(2):324-333. [DOI] [PubMed] [Google Scholar]
- 2. Habets EJ, Kloet A, Walchenbach Ret al. Tumour and surgery effects on cognitive functioning in high-grade glioma patients. Acta Neurochir. 2014;156(8):1451-1459. [DOI] [PubMed] [Google Scholar]
- 3. Wefel JS, Noll KR, Rao G, Cahill DP. Neurocognitive function varies by IDH1 genetic mutation status in patients with malignant glioma prior to surgical resection. Neuro Oncol. 2016;18(12):1656-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wefel JS, Noll KR, Scheurer ME. Neurocognitive functioning and genetic variation in patients with primary brain tumours. Lancet Oncol. 2016;17(3):e97-e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aerts H, Fias W, Caeyenberghs K, Marinazzo D. Brain networks under attack: robustness properties and the impact of lesions. Brain. 2016;139(12):3063-3083. [DOI] [PubMed] [Google Scholar]
- 6. Fox ME, King TZ.. Functional connectivity in adult brain tumor patients: a systematic review. Brain Connect. 2018;8(7):381-397. [DOI] [PubMed] [Google Scholar]
- 7. Hadjiabadi DH, Pung L, Zhang Jet al. Brain tumors disrupt the resting-state connectome. Neuroimage Clin. 2018;18:279-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Noll KR, Weinberg JS, Ziu Met al. Neurocognitive changes associated with surgical resection of left and right temporal lobe glioma. Neurosurgery. 2015;77(5):777-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Noll K, Sabsevitz D, Prabhu S, Wefel J. Neuropsychology in the neurosurgical management of primary brain tumors. In: Pearson C, Ecklund-Johnson E, Gale S, eds. Neurosurgical Neuropsychology. San Diego, CA: Academic Press; 2019:157-183. [Google Scholar]
- 10. Fornito A, Zalesky A, Breakspear M. The connectomics of brain disorders. Nat Rev Neurosci. 2015;16(3):159-172. [DOI] [PubMed] [Google Scholar]
- 11. Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537-541. [DOI] [PubMed] [Google Scholar]
- 12. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059-1069. [DOI] [PubMed] [Google Scholar]
- 13. Brier MR, Thomas JB, Fagan AMet al. Functional connectivity and graph theory in preclinical alzheimer's disease. Neurobiol Aging. 2014;35(4):757-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kesler SR, Rao A, Blayney DWet al. Predicting long-term cognitive outcome following breast cancer with pre-treatment resting state fMRI and random forest machine learning. Front Hum Neurosci. 2017;11:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu AS, Witgert ME, Lang FFet al. Neurocognitive function before and after surgery for insular gliomas. J Neurosurg. 2011;115(6):1115-1125. [DOI] [PubMed] [Google Scholar]
- 16. Satoer D, Vork J, Visch-Brink Eet al. Cognitive functioning early after surgery of gliomas in eloquent areas. J Neurosurg. 2012;117(5):831-838. [DOI] [PubMed] [Google Scholar]
- 17. Sawaya R, Hammoud M, Schoppa Det al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42(5):1044-1055. [DOI] [PubMed] [Google Scholar]
- 18. Strauss E, Sherman EM, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed New York, NY: Oxford University Press; 2006. [Google Scholar]
- 19. Wechsler D Wechsler Adult Intelligence Scale–Fourth Edition (WAIS–IV). San Antonio, TX: NCS Pearson; 2008. [Google Scholar]
- 20. Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins verbal learning test revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12(1):43-55. [Google Scholar]
- 21. Tombaugh TN Trail making test a and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203-214. [DOI] [PubMed] [Google Scholar]
- 22. Ruff RM, Light RH, Parker SB, Levin HS. Benton controlled oral word association test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11(4):329-338. [PubMed] [Google Scholar]
- 23. Benton A, Hamsher K, Sivan A. Multilingual Aphasia Examination Manual. Iowa City, IA: AJA Associates, Inc.; 2000. [Google Scholar]
- 24. Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Lutz, FL: Psychological Assessment Resources, Inc.; 2004. [Google Scholar]
- 25. Armstrong TR, Wefel JS, Wang Met al. Net clinical benefit analysis of radiation therapy oncology group 0525: a phase III trial comparing conventional adjuvant temozolomide with dose-intensive temozolomide in patients with newly diagnosed glioblastoma. J Clin Oncol. 2013;31(32):4076-4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wefel JS, Vardy J, Ahles T, Schagen SB. International cognition and cancer task force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703-708. [DOI] [PubMed] [Google Scholar]
- 27. Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125-141. [DOI] [PubMed] [Google Scholar]
- 28. Rachakonda S, Egolf E, Correa N, Calhoun V. Group ICA of fMRI toolbox (GIFT) manual. http://www.nitrc.org/docman/view.php/55/295/v1.3d Accessed November 1, 2019. [Google Scholar]
- 29. Tzourio‐Mazoyer N, Landeau B, Papathanassiou Det al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273-289. [DOI] [PubMed] [Google Scholar]
- 30. Ashburner J A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95-113. [DOI] [PubMed] [Google Scholar]
- 31. Agosta F, Galantucci S, Valsasina Pet al. Disrupted brain connectome in semantic variant of primary progressive aphasia. Neurobiol Aging. 2014;35(11):2646-2655. [DOI] [PubMed] [Google Scholar]
- 32. Bahrami N, Seibert TM, Karunamuni Ret al. Altered network topology in patients with primary brain tumors after fractionated radiotherapy. Brain Connect. 2017;7(5):299-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Welton T, Kent DA, Auer DP, Dineen RA. Reproducibility of graph-theoretic brain network metrics: a systematic review. Brain Connect. 2015;5(4):193-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cohen J Statistical Power Analysis for the Behavioral Sciences. 2nd ed Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 35. Talacchi A, Santini B, Savazzi S, Gerosa M. Cognitive effects of tumour and surgical treatment in glioma patients. J Neurooncol. 2011;103(3):541-549. [DOI] [PubMed] [Google Scholar]
- 36. Barzilai O, Moshe SB, Sitt Ret al. Improvement in cognitive function after surgery for low-grade glioma. J Neurosurg. 2018;130(2):426-434. [DOI] [PubMed] [Google Scholar]
- 37. Satoer D, Visch-Brink E, Dirven C, Vincent A. Glioma surgery in eloquent areas: can we preserve cognition? Acta Neurochir. 2016;158(1):35-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bosma I, Douw L, Bartolomei Fet al. Synchronized brain activity and neurocognitive function in patients with low-grade glioma: a magnetoencephalography study. Neuro Oncol. 2008;10(5):734-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Derks J, Reijneveld JC, Douw L. Neural network alterations underlie cognitive deficits in brain tumor patients. Curr Opin Oncol. 2014;26(6):627-633. [DOI] [PubMed] [Google Scholar]
- 40. Huang Q, Zhang R, Hu Xet al. Disturbed small-world networks and neurocognitive function in frontal lobe low-grade glioma patients. PLoS One. 2014;9(4):e94095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bartolomei F, Bosma I, Klein Met al. Disturbed functional connectivity in brain tumour patients: evaluation by graph analysis of synchronization matrices. Clin Neurophysiol. 2006;117(9):2039-2049. [DOI] [PubMed] [Google Scholar]
- 42. Douw L, Baayen H, Bosma Iet al. Treatment-related changes in functional connectivity in brain tumor patients: a magnetoencephalography study. Exp Neurol. 2008;212(2):285-290. [DOI] [PubMed] [Google Scholar]
- 43. Shattuck DW, Mirza M, Adisetiyo Vet al. Construction of a 3D probabilistic atlas of human cortical structures. Neuroimage. 2008;39(3):1064-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fan L, Li H, Zhuo Jet al. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb Cortex. 2016;26(8):3508-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arslan S, Ktena SI, Makropoulos Aet al. Human brain mapping: a systematic comparison of parcellation methods for the human cerebral cortex. Neuroimage. 2018;170:5-30. [DOI] [PubMed] [Google Scholar]
- 46. Ripollés P, Marco-Pallarés J, de Diego-Balaguer Ret al. Analysis of automated methods for spatial normalization of lesioned brains. Neuroimage. 2012;60(2):1296-1306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.