Abstract
Background
Mobile health (mHealth) interventions can improve health by improving cardiovascular risk factors, but their adoption in care by physicians and patients is untapped. Few mHealth apps have been evaluated in clinical trials, and due to the fast pace of technological development, those previously evaluated are often outdated by the time trial results are available. Given the rapid pace of change in this field, it is not feasible to rigorously evaluate mHealth apps with current methodologies.
Objective
The overall aim of this pilot study was to test the feasibility of using a web research platform called Trial My App to conduct efficient and rigorous web-based randomized controlled trials (RCTs) of mHealth apps relevant to patients with cardiovascular risk factors by evaluating an app that targets hypertension.
Methods
For this study, 200 participants with suboptimally controlled hypertension will be recruited through advertisements in newsletters, media, and the internet, as well as through referrals from their health care providers. Screening, consent, randomization, and collection of patient-important health confidence and self-management ability outcomes will be conducted online through the Trial My App research platform. Participants will be randomized into 2 groups: 100 that will use an mHealth app for tracking hypertension and 100 that will be considered as an educational control. All participants will complete questionnaires at 0, 1, 3 and 6 months after enrolment. A substudy to validate the method of blood pressure readings and the consistency of data entered through Trial My App will be conducted with 40 participants.
Results
The development of the Trial My App web platform has been completed. The creation of survey instruments has been completed in collaboration with our patient partners and advisory board. Recruitment is expected to begin in the first quarter of 2021; data collection and analysis are expected to be completed approximately 1 year after study commencement. Results will be disseminated through conferences and publications. The primary outcomes of this study include the feasibility of conducting an RCT using the Trial My App platform by reporting recruitment, retention, and completion statistics. We will validate app-entered data with a standard 7-day home blood pressure measurement method. Lastly, the pilot, nonblinded RCT will assess the effectiveness of the mHealth app in improving the control of hypertension compared with the control of hypertension in the educational control group.
Conclusions
This study will determine if it is feasible to use the Trial My App web-based platform to evaluate the effectiveness of mHealth apps for patients with cardiovascular risk factors. As more mHealth apps are evaluated in RCTs, patients will be able to select apps that meet their needs and physicians will be able to make evidence-based recommendations to their patients for apps aimed at improving cardiovascular health.
Trial Registration
ClinicalTrials.gov NCT04528654; https://clinicaltrials.gov/ct2/show/NCT04528654
International Registered Report Identifier (IRRID)
PRR1-10.2196/26155
Keywords: mHealth, mobile health, hypertension, app, patient-oriented, feasibility, cardiovascular disease, internet-administered, randomized controlled trial
Introduction
Smartphones provide continuous connection to the internet and can run sophisticated software apps. Globally, over 3.5 billion individuals own a smartphone [1]. The delivery of health care interventions via mobile phones is known as mobile health (mHealth). In 2017, 86% of the Canadians surveyed owned a smartphone or tablet and 78% downloaded mHealth and other types of apps to these devices [2]. Of the 66% of Canadians who indicated that they self-track at least one aspect of their health, 40% used electronic devices to do so and 32% reported using at least one mHealth app to monitor their health [2,3]. Other authors have reported that up to 58% of the smartphone users have downloaded an mHealth app [4] and 3.6 billion health apps were projected to be downloaded in 2017 [5]. Since smartphones are used to track health and are often continuously carried by users, mHealth apps allow for frequent data collection and feedback for behaviors that affect health, and interventions can be deployed to many users at a relatively low cost [6].
Health behaviors, including smoking, inactivity, and poor diet, are the major contributors to cardiovascular disease [7]. The American Heart Association has endorsed provider and patient self-management of cardiovascular disease risk factors as an effective form of secondary prevention [8]. Though the field of mHealth is in its infancy, early studies have shown that apps can improve health-related behaviors and reduce cardiovascular risk factors, largely through knowledge translation, by improving adherence to or by uptake of medications and behaviors that are known to be effective [9]. Randomized controlled trials (RCTs) report that apps can help patients lose weight [10-14], quit smoking [15], and increase physical activity [16]. An RCT of lifestyle-focused text messages reduced low-density lipoprotein cholesterol levels, systolic blood pressure, and BMI in patients with coronary heart disease [17], and a systematic review of RCTs of home blood pressure telemonitoring showed reduced systolic and diastolic blood pressure [18,19]. Thus, current data suggest that mHealth interventions can improve health by improving such cardiovascular risk factors, but their adoption in health care by physicians and patients is minimal. The 2014 National Physician Survey of licensed Canadian physicians reported that 83% did not recommend mobile apps to their patients, although 72% of the general practitioners and 53% of the specialists referred patients to websites and 50% used mobile apps such as e-textbooks or calculators in their practices [20]. We recently conducted a needs assessment survey of 113 physicians, which showed that over half of the physicians recommended apps to their patients despite their lack of clinical evidence. Many physicians in the survey indicated that they rely on personal opinions and patient recommendations to support their recommendations of apps despite wanting to choose apps that have a higher level of evidence such as RCTs or expert panel reviews. Our needs assessment also indicated that physicians do not recommend apps because they are not aware of them or do not have time to review their clinical efficacy. Without proper testing and evaluation, gaps in app development, including lack of expert involvement, poor user input validation, lack of evidence base, and poor quality of information, may pose clinical risks and safety harms to consumers [21]. With the growing interest in mHealth to monitor patient health, it is imperative that physicians stay informed and minimize their medical-legal risks by recommending apps with proven efficacy.
Despite this desire for better evidence, only a minority of the mHealth apps available in web-based app stores and commonly downloaded by patients have been evaluated in clinical trials, and due to the fast pace of technological development, those that have been evaluated are often outdated by the time trial results are available. Of the top 100 grossing health and fitness apps, researchers found that none had been formally evaluated in clinical studies [22]. Multiple reviews have highlighted the lack of quality research evidence on the efficacy of apps [9,23-25]. mHealth research has several unique challenges contributing to this problem. Chiefly, technology is rapidly progressing and equally rapid techniques for evaluating such technology are needed. Testing must be cost-efficient, given the limited funding for evaluating mHealth interventions compared with pharmacological interventions or medical devices [9]. These constraints limit the evidence base and the incorporation of apps in patient care.
The rapid proliferation of smartphone technology provides untapped potential to improve the efficient conduct of such research. Using internet-enabled devices to perform research can potentially (1) accelerate large-scale enrolment by contacting and screening potential participants who do not frequently interact with the health system through clinics or hospitals and (2) reduce costs and improve participation by allowing frequent and inexpensive data collection directly from participants. With the rapid development of internet-connected and smartphone-connected consumer devices that can collect biometric data, smartphones also have the potential to collect objective, real-time data directly from patients [26].
Methods
Overview
We have developed an innovative research approach using a web-based platform called Trial My App, which is designed to perform efficient trials of apps relevant to patients with cardiovascular risk factors. In our initial phase, we engaged an advisory board of patients to codevelop criteria for app and trial outcome selection, which will be used to support further deployment of Trial My App. The patients used apps for goal setting, decision-making, information sharing, and empowerment in managing their health. From these themes, we derived a series of survey questions to determine if an app was meeting these outcomes, and we included this survey in the pilot trial. Content analysis by the advisory board also indicated that patients considered a number of favorable technical factors when selecting their mobile apps: (1) relevant feedback on progress, (2) security, (3) low cost, (4) customizability, (5) usability, (6) information credibility, (7) multifunctional app integration, and (8) interdevice compatibility. The undesired features were as follows: (1) unreliable technology, (2) distraction, (3) collection of personal information, and (4) learning curve. These criteria were applied to shortlist apps for hypertension, and through discussion with the research team and patient partners, we identified Sphygmo BP as the intervention app for this pilot trial (Multimedia Appendix 1). Sphygmo BP was created in partnership with the University of Alberta to help patients with hypertension to self-manage their blood pressure. The app tracks and averages blood pressure, glucose levels, weight, temperature, respiratory rate, and oxygen saturation. It also includes educational components to facilitate better patient awareness of management strategies for blood pressure and is designed to facilitate patient-physician communication through telemonitoring.
Objectives
The primary objective of the study is to test the feasibility of conducting an RCT of an mHealth hypertension-tracking app using the Trial My App platform. The secondary objective is to test if the use of the Sphygmo BP app reduces blood pressure in patients with suboptimally controlled hypertension when compared with the use of a website with information on hypertension. It would be valuable for patients and physicians to know whether the use of the app is likely to result in reductions in blood pressure that have been shown to reduce clinical outcomes.
Study Design
This is a pilot, nonblinded parallel-group RCT, comparing the use of a hypertension app versus an education control in participants with suboptimally controlled hypertension. Outcomes include feasibility, clinical, and patient-important endpoints.
Eligibility Criteria
The inclusion criteria are as follows: (1) age over 18 years, (2) diagnosis of hypertension, (3) interested in using an app for hypertension management, (4) access to a smartphone with internet connection, and (5) access to a blood pressure monitoring device (in home or community setting, eg, pharmacy). The exclusion criteria are as follows: (1) participant-reported blood pressure within target (target range for patients with diabetes is systolic blood pressure <130 mm Hg and diastolic blood pressure <80 mm Hg; for those without diabetes, target range is systolic blood pressure <140 mm Hg and diastolic blood pressure <90 mm Hg according to Hypertension Canada guidelines [27]) within the 2 weeks prior to enrolment, (2) emergent hypertensive concerns (potential participants with systolic blood pressure ≥180 mm Hg or diastolic blood pressure ≥120 mm Hg will be advised to seek medical attention and will be excluded), (3) current use of a mobile app for hypertension management, (4) living outside of Canada, (5) pregnancy, and (6) unwillingness or inability to give informed consent.
Recruitment
The primary study site will be the Health Information Research Unit at McMaster University in Hamilton, Ontario. A combination of passive and active recruitment strategies will be used. A variety of recruitment materials will be distributed by a research assistant throughout the community and outpatient or specialty clinic waiting rooms. These materials include videos, papers, and web-based posters/postcards, emails, as well as posts advertising on social media. Social media recruitment on Facebook, Twitter, and Google Network will consist of general posts and targeted ads using Facebook Ads Manager. Partner newsletters and websites include Hamilton Academy of Medicine, McMaster Institute for Research on Aging, McMaster Okanagan Charter, and RSearch. We will also engage clinicians and their administrative staff within the McMaster Department of Medicine, Hamilton Health Sciences outpatient clinics, Queen Square Family Health, and other community, primary, and specialty clinics in Ontario to identify potential participants and invite them to register with Trial My App to determine if they want to participate in the trial. Snowball sampling through participants and personal networks may also be used. Interested candidates will be provided a link or a quick response code to access the website within the marketing materials. They could also contact our research assistant through a dedicated Trial My App email account if they prefer the initial contact by email or phone.
All trial stages, including screening, consent, randomization, and collection of clinical and patient-important outcomes data, will be performed virtually using the Trial My App platform. This phase includes a substudy to validate the web-based collection of patient data. Ethics approval of the substudy will be sought separately. Participants will be asked to register on the Trial My App site with an email and a password. The informed consent form is in Multimedia Appendix 2. Once they have electronically consented to using the web app, they will complete a user profile questionnaire and be screened for participation in the pilot trial in a subsequent survey. Multimedia Appendix 3 contains all user baseline, screening, and follow-up questionnaires. If participants meet inclusion criteria, they will be asked to electronically consent to take part in the pilot trial and provide data at 0, 1, 3, and 6 months. The participant flow is shown in Figure 1. The diagram of the app flow is shown in Figure 2.
Figure 1.
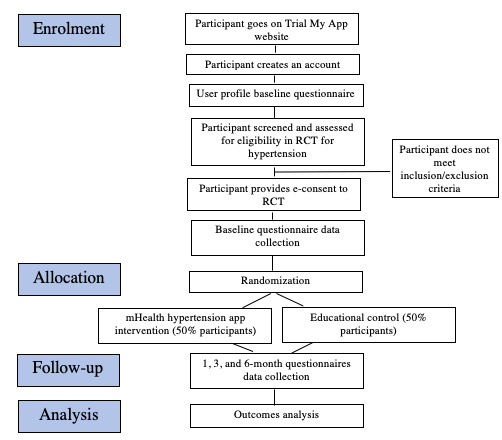
Participant flow diagram. RCT: Randomized controlled trial; mHealth: mobile health.
Figure 2.
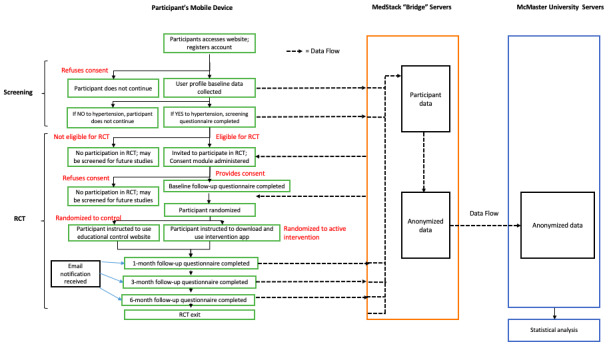
Trial My App web flow diagram. RCT: randomized controlled trial.
Sample Size
In the feasibility study, at least 80% of the participants in each group who successfully complete the final questionnaire within 1 year of trial start will be considered as the primary feasibility outcome. To estimate a completion rate of 80% in each group within a margin of error of 8% and with a confidence interval of 95%, we estimated a sample size of at least 100 participants in each group, and 200 participants in total is required. With a sample size of 200 participants in total (ie, 100 per group), we will have 80% power to detect a reduction in blood pressure of 8 mm Hg, which is considered a minimally clinically important difference. Assuming an 18 mm Hg standard deviation in systolic blood pressure, to detect an 8 mm Hg difference between groups with a power of 80% and a type I error of 5%, we would ideally require 81 participants in each arm [28]. Accounting for a 20% dropout rate, we aim to enroll 100 participants in each arm. We recognize that the selected app may not result in such a large difference in the systolic blood pressure, but the calculation is to guide our recruitment targets. If a difference in the blood pressure is found, we will be able to inform patients and physicians that this app may have a major impact on clinical outcomes such as heart attack, stroke, and congestive heart failure.
Intervention
After screening and baseline questionnaires, participants will be randomized using a web-based blocked randomization list of 4, 6, or 8 block sizes and a 1:1 allocation ratio. The intervention group will be instructed to download the Sphygmo BP app via a link provided within the Trial My App. The control group will receive a link to the Heart and Stroke foundation website, which includes information on hypertension management and measuring blood pressure [29]. All participants are expected to continue to receive usual care by their physician, including any anti-hypertensive medication and lifestyle changes.
Data Collection
At 1, 3, and 6 months after enrollment into the RCT, Trial My App will send email reminders with a link to follow-up questionnaires to all participants to assess self-reported blood pressure and several patient-reported outcomes. Completing follow-up will be defined as answering the questionnaire within 7 days of receipt of the questionnaire notification via email. To encourage participation and reduce attrition, participants will receive a Can $10 (US $1=Can $1.27) electronic gift card for each completed assessment and an additional Can $10 gift card if they complete all 4 follow-up assessments.
Outcomes
Feasibility Outcomes
Our primary goal at this stage is to determine whether the Trial My App platform can be used to conduct RCTs evaluating mHealth apps by the ability to complete an adequately powered RCT of least 80 participants in each group that successfully complete the study and the 6-month questionnaire within an arbitrarily defined reasonable time frame of 12 months. The remaining feasibility outcomes and associated indicators are shown in Table 1.
Table 1.
Feasibility outcomes and indicators.
| Outcome | Definition | Indicator | Minimum required sample size |
| Participation completion | Number of participants who successfully complete the final questionnaire within 1 year of trial start | 80% out of total randomized participants in each group | 100 participants per group is needed to achieve a margin of error of 8% with a 95% confidence level |
| Eligibility | Proportion of participants who sign up that meet eligibility criteria | At least 50% of responses to baseline and screening questionnaires are eligible | 200 participants in total to achieve a margin of error of 7% with a 95% CI |
| Recruitment | Number of eligible participants recruited and consented | At least 50% of target sample size of 200 randomized within 6 months | 200 participants in total to achieve a margin of error of 7% with a 95% CI |
| Retention | Proportion of withdrawal and dropouts after recruitment | Less than 20% of the participants lost to 6-month follow-up | 200 participants in total to achieve a margin of error of 5.5% with a 95% CI |
| Outcome acceptability | Follow-up questionnaire completion rates | 70% of the questionnaires that are submitted within 7 days of notification reminder | 200 participants in total to achieve a margin of error of 6.3% with a 95% CI |
| Intervention acceptability | Frequency of app usage in the intervention group | Answers to frequency of use and features used questions in follow-up questionnaires | N/Aa |
| Appropriateness of data collection processes | Completeness of data | 50% of the questionnaires completed and less than 20% of the missing response rates in each questionnaire | N/A |
aN/A: not applicable.
Pilot Efficacy Outcomes
The secondary objective of the RCT is to conduct a trial comparing the intervention hypertension app with a control group. The main outcome is clinical changes in blood pressure based on self-reported answers in the questionnaires. The remaining patient-reported outcomes and their associated indicators from baseline to 6 months are shown in Table 2. Adherence to hypertension self-care behaviors will be scored using the validated H-SCALE (Hypertension Self-Care Activity Level Effects) [30] and health care self-efficacy will be scored using the validated Health Confidence Score [31]. Similar outcome measures (eg, medication adherence, diet, physical activity) have been used in other studies of hypertension apps and measure hypertension-specific self-efficacy scores [32].
Table 2.
Efficacy outcomes and indicators.
| Outcome, definitions | Indicator | |
| Clinical assessment | ||
| Difference in mean change in blood pressure from baseline to 6 months between groups | Statistically significant difference in mean change in systolic blood pressure measurements (defined as P<.05 using a Pearson test) | |
| Proportion of patients at their recommended blood pressure | Blood pressure measurements compared to standard ranges | |
| Self-management ability | ||
| Difference in mean change of self-managing behaviors | H-SCALEa score and statistically significant correlations with blood pressure at 95% CI | |
| Difference in mean change in feelings of self-efficacy | Frequency distribution and mean of Health Confidence Score at 95% CI | |
| Patient-reported outcomes | ||
|
|
Descriptive analysis of patient-oriented experiences between groups at baseline and 6 months | Agreeability with goal setting, decision making, sharing data, and empowerment statements in questionnaires developed from the advisory board themes (at 95% CI) |
aH-SCALE: Hypertension Self-Care Activity Level Effects.
Statistical Analysis
Descriptive statistical analysis will be performed on the data set using appropriate statistical methods to measure feasibility. Efficacy outcomes will be compared between the intervention and control groups by using logistic regression.
Ethics Approval
This study has received approval from the Hamilton Integrated Research Ethics Board, #8039. This is a minimal risk study and the subject matter is not likely to be distressing to participants. Participation in this study may be inconvenient, taking about 10-20 minutes to complete at each timepoint (baseline, 1, 3, and 6 months). In the event of possible emotional distress, participants will be able to discontinue the use of the web app. Participant identifiers will be replaced with a code number; therefore, the data that researchers will access are not identifiable. As the intervention app is designed to track blood pressure to facilitate management and communication and participants will continue to receive usual care, no other harms are foreseen. Participants will be asked to complete an electronic consent form. The research team will have access to the final trial data set and ensure that all privacy policies are strictly maintained. Data will be securely stored on a MedStack server built into the Trial My App platform. MedStack is a health data privacy compliance automation platform that builds, measures, and actively manages compliance and provides secure, flexible, and single-tenant cloud infrastructure tailored to Trial My App. Medstack complies with Ontario’s Personal Health Information Privacy Act legislation. The information collected will be anonymized and encrypted before transferring to a secure server and firewall-protected network on a password-protected computer located at the Health Information Research Unit at McMaster University.
Results
Trial Progress
The development of the Trial My App web platform has been completed with a software developer and has undergone functionality and remote usability testing to uncover technical bugs and improve the design. The creation of survey instruments has been completed in collaboration with our patient partners and advisory board. Recruitment is expected to begin in the first quarter of 2021; data collection and analysis are expected to be completed approximately 1 year after study commencement. Dissemination of results will occur through conferences and publications.
Patient Engagement Strategy
Two patients with lived experience of cardiovascular diseases are collaborating as partners on the research team and additional patients serve on an advisory board overseeing the development of Trial My App. They have identified criteria for selecting apps to evaluate in future RCTs and aided in developing outcomes that are relevant to patients managing their cardiovascular risk factors with apps. Key contributions of our patient partners include joining bimonthly meetings with the research team to discuss project planning, developing questions for and taking part in the advisory group, testing the usability of Trial My App, reviewing and contributing to publications and other knowledge translation outputs, and contributing to production and circulation of recruitment materials. Any required training on these skills is provided by the research team.
Discussion
To our knowledge, this study is the first of its kind to create a web-based platform to conduct RCTs of mHealth apps for cardiovascular risk. A limitation of this methodology is the collection of self-report data as it is subject to several response biases, including social desirability, recall, or measurement error biases. The research team will include an additional substudy to measure the concordance of self-reported blood pressure measurements submitted via the Trial My App web app and the reference standard of 7-day average home blood pressure measurements [33]. A subgroup of participants will measure their blood pressure by using identical automatic home blood pressure monitoring devices 4 times daily for 1 week. The research team anticipates that participants will use the apps to varying degrees to help them manage their health, as they would normally; assessing these elements are beyond the scope of the study. The investigators expect that the pilot findings will demonstrate the feasibility of gathering valid patient-reported outcomes via web-based questionnaires that can be applied more broadly to other clinical studies. The findings of this trial may inform the evaluation of other mHealth apps for other conditions at a relatively low cost and more quickly than using traditional RCT methods. These results will also provide useful information for app developers who are interested in testing their apps for clinical effectiveness as well as patients and clinicians who are interested in incorporating effective mHealth apps into their care.
Acknowledgments
The project is funded by a grant from the Canadian Institutes of Health Research. The funding agency does not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Any opinions expressed are only those of the authors and do not necessarily represent the views of any of their affiliated institutions. The authors acknowledge the comments of the peer reviewers from the funding agency in improving the quality of this protocol.
Abbreviations
- mHealth
mobile health
- RCT
randomized controlled trial
Appendix
Description of the blood pressure management app used for the study.
Information and consent form.
Study instruments.
Peer review report.
Footnotes
Authors' Contributions: CL, VB, and IG conceived the original study idea. CL is the principal investigator and drafted the manuscript. CL is the grant holder. VB, IG, JV, JDS, MG, and EV provided clinical and feasibility testing expertise. JM provided statistical expertise. MB and our advisory board provided help with the development of questionnaires and patient-relevant outcomes. ZM (ZLTechnovation) provided expertise in the development of the web platform. All authors contributed to the review of the study protocol and approved the final manuscript.
Conflicts of Interest: VB reports honoraria and an educational grant from Pfizer, honoraria from Bayer, and loan of devices from Apple for research purposes.
References
- 1.Statista Number of smartphone users worldwide 2014-2020. 2018. [2018-09-07]. https://www.statista.com/statistics/330695/number-of-smartphone-users-worldwide/
- 2.Paré G, Bourget C. Diffusion of smart devices for health in Canada. 2017. [2018-09-07]. https://www.infoway-inforoute.ca/en/component/edocman/resources/reports/benefits-evaluation/3366-the-diffusion-of-smart-devices-for-health-in-canada-study-final-report.
- 3.Paré Guy, Leaver C, Bourget C. Diffusion of the digital health self-tracking movement in Canada: Results of a national survey. J Med Internet Res. 2018 May 02;20(5):e177. doi: 10.2196/jmir.9388. https://www.jmir.org/2018/5/e177/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krebs P, Duncan DT. Health app use among US mobile phone owners: A national survey. JMIR Mhealth Uhealth. 2015 Nov 04;3(4):e101. doi: 10.2196/mhealth.4924. https://mhealth.jmir.org/2015/4/e101/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Research2Guidance; 2017. [2018-09-07]. 325,000 mobile health apps available in 2017 - Android now the leading mHealth platform. https://research2guidance.com/325000-mobile-health-apps-available-in-2017/ [Google Scholar]
- 6.Neubeck L, Lowres N, Benjamin EJ, Freedman SB, Coorey G, Redfern J. The mobile revolution--using smartphone apps to prevent cardiovascular disease. Nat Rev Cardiol. 2015 Jun;12(6):350–60. doi: 10.1038/nrcardio.2015.34. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L, INTERHEART Study Investigators Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 8.Allegrante JP, Wells MT, Peterson JC. Interventions to Support Behavioral Self-Management of Chronic Diseases. Annu Rev Public Health. 2019 Apr 01;40:127–146. doi: 10.1146/annurev-publhealth-040218-044008. http://europepmc.org/abstract/MED/30601717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke LE, Ma J, Azar KMJ, Bennett GG, Peterson ED, Zheng Y, Riley W, Stephens J, Shah SH, Suffoletto B, Turan TN, Spring B, Steinberger J, Quinn CC. Current science on consumer use of mobile health for cardiovascular disease prevention: A scientific statement from the American Heart Association. Circulation. 2015 Sep 22;132(12):1157–213. doi: 10.1161/CIR.0000000000000232. http://europepmc.org/abstract/MED/26271892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Napolitano MA, Hayes S, Bennett GG, Ives AK, Foster GD. Using Facebook and text messaging to deliver a weight loss program to college students. Obesity (Silver Spring) 2013 Jan;21(1):25–31. doi: 10.1002/oby.20232. doi: 10.1002/oby.20232. [DOI] [PubMed] [Google Scholar]
- 11.Patrick K, Raab F, Adams MA, Dillon L, Zabinski M, Rock CL, Griswold WG, Norman GJ. A text message-based intervention for weight loss: randomized controlled trial. J Med Internet Res. 2009;11(1):e1. doi: 10.2196/jmir.1100. http://www.jmir.org/2009/1/e1/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shuger SL, Barry VW, Sui X, McClain A, Hand GA, Wilcox S, Meriwether RA, Hardin JW, Blair SN. Electronic feedback in a diet- and physical activity-based lifestyle intervention for weight loss: a randomized controlled trial. Int J Behav Nutr Phys Act. 2011 May 18;8:41. doi: 10.1186/1479-5868-8-41. https://ijbnpa.biomedcentral.com/articles/10.1186/1479-5868-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spring Bonnie, Duncan Jennifer M, Janke E Amy, Kozak Andrea T, McFadden H Gene, DeMott Andrew, Pictor Alex, Epstein Leonard H, Siddique Juned, Pellegrini Christine A, Buscemi Joanna, Hedeker Donald. Integrating technology into standard weight loss treatment: a randomized controlled trial. JAMA Intern Med. 2013 Jan 28;173(2):105–11. doi: 10.1001/jamainternmed.2013.1221. http://europepmc.org/abstract/MED/23229890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner-McGrievy GM, Campbell MK, Tate DF, Truesdale KP, Bowling JM, Crosby L. Pounds Off Digitally study: a randomized podcasting weight-loss intervention. Am J Prev Med. 2009 Oct;37(4):263–9. doi: 10.1016/j.amepre.2009.06.010. http://europepmc.org/abstract/MED/19765496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev. 2016 Apr 10;4:CD006611. doi: 10.1002/14651858.CD006611.pub4. http://europepmc.org/abstract/MED/27060875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coughlin SS, Whitehead M, Sheats JQ, Mastromonico J, Smith S. A review of smartphone applications for promoting physical activity. Jacobs J Community Med. 2016;2(1) http://europepmc.org/abstract/MED/27034992. [PMC free article] [PubMed] [Google Scholar]
- 17.Santo K, Hyun K, de Keizer L, Thiagalingam A, Hillis GS, Chalmers J, Redfern J, Chow CK. The effects of a lifestyle-focused text-messaging intervention on adherence to dietary guideline recommendations in patients with coronary heart disease: an analysis of the TEXT ME study. Int J Behav Nutr Phys Act. 2018 May 23;15(1):45. doi: 10.1186/s12966-018-0677-1. https://ijbnpa.biomedcentral.com/articles/10.1186/s12966-018-0677-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gandhi S, Chen S, Hong L, Sun K, Gong E, Li C, Yan LL, Schwalm J. Effect of mobile health interventions on the secondary prevention of cardiovascular disease: Systematic review and meta-analysis. Can J Cardiol. 2017 Feb;33(2):219–231. doi: 10.1016/j.cjca.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Omboni S, Gazzola T, Carabelli G, Parati G. Clinical usefulness and cost effectiveness of home blood pressure telemonitoring: meta-analysis of randomized controlled studies. J Hypertens. 2013 Mar;31(3):455–467. doi: 10.1097/HJH.0b013e32835ca8dd. [DOI] [PubMed] [Google Scholar]
- 20.The College of Family Physicians of Canada. Canadian Medical Association. The Royal College of Physicians and Surgeons of Canada 2014 National Physician Survey. 2014. [2020-05-12]. http://nationalphysiciansurvey.ca/wp-content/uploads/2014/10/2014-ByProvince-ElectronicToolsinClinicalPractice-EN.pdf.
- 21.Akbar S, Coiera E, Magrabi F. Safety concerns with consumer-facing mobile health applications and their consequences: a scoping review. J Am Med Inform Assoc. 2020 Feb 01;27(2):330–340. doi: 10.1093/jamia/ocz175. http://europepmc.org/abstract/MED/31599936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng Y, Wong SS. Trend and features of top 100 grossing health and fitness iPhone apps. FASEB J. 2014;28(1):5. doi: 10.1096/fasebj.28.1_supplement.1028.5. [DOI] [Google Scholar]
- 23.Byambasuren O, Sanders S, Beller E, Glasziou P. Prescribable mHealth apps identified from an overview of systematic reviews. NPJ Digit Med. 2018;1:12. doi: 10.1038/s41746-018-0021-9. doi: 10.1038/s41746-018-0021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKay FH, Cheng C, Wright A, Shill J, Stephens H, Uccellini M. Evaluating mobile phone applications for health behaviour change: A systematic review. J Telemed Telecare. 2018 Jan;24(1):22–30. doi: 10.1177/1357633X16673538. [DOI] [PubMed] [Google Scholar]
- 25.Parati G, Torlasco C, Omboni S, Pellegrini D. Smartphone Applications for Hypertension Management: a Potential Game-Changer That Needs More Control. Curr Hypertens Rep. 2017 Jun;19(6):48. doi: 10.1007/s11906-017-0743-0. [DOI] [PubMed] [Google Scholar]
- 26.Bhavnani SP, Narula J, Sengupta PP. Mobile technology and the digitization of healthcare. Eur Heart J. 2016 May 07;37(18):1428–38. doi: 10.1093/eurheartj/ehv770. http://europepmc.org/abstract/MED/26873093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hypertension Canada Treatment of hypertension in association with diabetes mellitus. 2015. [2020-05-12]. https://guidelines.hypertension.ca/prevention-treatment/hypertension-with-diabetes/
- 28.Charrois TL, McAlister FA, Cooney D, Lewanczuk R, Kolber MR, Campbell NR, Rosenthal M, Houle SK, Tsuyuki RT. Improving hypertension management through pharmacist prescribing; the rural Alberta clinical trial in optimizing hypertension (Rural RxACTION): trial design and methods. Implement Sci. 2011 Aug 11;6:94. doi: 10.1186/1748-5908-6-94. https://implementationscience.biomedcentral.com/articles/10.1186/1748-5908-6-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heart and Stroke Foundation of Canada High blood pressure. 2020. [2020-05-11]. https://www.heartandstroke.ca/heart/risk-and-prevention/condition-risk-factors/high-blood-pressure.
- 30.Warren-Findlow J, Seymour RB. Prevalence rates of hypertension self-care activities among African Americans. J Natl Med Assoc. 2011 Jun;103(6):503–12. doi: 10.1016/s0027-9684(15)30365-5. http://europepmc.org/abstract/MED/21830634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benson T, Potts HWW, Bark P, Bowman C. Development and initial testing of a Health Confidence Score (HCS) BMJ Open Qual. 2019;8(2):e000411. doi: 10.1136/bmjoq-2018-000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Persell SD, Peprah YA, Lipiszko D, Lee JY, Li JJ, Ciolino JD, Karmali KN, Sato H. Effect of Home Blood Pressure Monitoring via a Smartphone Hypertension Coaching Application or Tracking Application on Adults With Uncontrolled Hypertension: A Randomized Clinical Trial. JAMA Netw Open. 2020 Mar 02;3(3):e200255. doi: 10.1001/jamanetworkopen.2020.0255. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2020.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rabi DM, McBrien KA, Sapir-Pichhadze R, Nakhla M, Ahmed SB, Dumanski SM, Butalia S, Leung AA, Harris KC, Cloutier L, Zarnke KB, Ruzicka M, Hiremath S, Feldman RD, Tobe SW, Campbell TS, Bacon SL, Nerenberg KA, Dresser GK, Fournier A, Burgess E, Lindsay P, Rabkin SW, Prebtani APH, Grover S, Honos G, Alfonsi JE, Arcand J, Audibert F, Benoit G, Bittman J, Bolli P, Côté Anne-Marie, Dionne J, Don-Wauchope A, Edwards C, Firoz T, Gabor JY, Gilbert RE, Grégoire Jean C, Gryn SE, Gupta M, Hannah-Shmouni F, Hegele RA, Herman RJ, Hill MD, Howlett JG, Hundemer GL, Jones C, Kaczorowski J, Khan NA, Kuyper LM, Lamarre-Cliche M, Lavoie KL, Leiter LA, Lewanczuk R, Logan AG, Magee LA, Mangat BK, McFarlane PA, McLean D, Michaud A, Milot A, Moe GW, Penner SB, Pipe A, Poppe AY, Rey E, Roerecke M, Schiffrin EL, Selby P, Sharma M, Shoamanesh A, Sivapalan P, Townsend RR, Tran K, Trudeau L, Tsuyuki RT, Vallée Michel, Woo V, Bell AD, Daskalopoulou SS. Hypertension Canada's 2020 Comprehensive Guidelines for the Prevention, Diagnosis, Risk Assessment, and Treatment of Hypertension in Adults and Children. Can J Cardiol. 2020 May;36(5):596–624. doi: 10.1016/j.cjca.2020.02.086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of the blood pressure management app used for the study.
Information and consent form.
Study instruments.
Peer review report.


