Abstract
Background
Electronic medical records (EMRs) contain large amounts of rich clinical information. Developing EMR-based case definitions, also known as EMR phenotyping, is an active area of research that has implications for epidemiology, clinical care, and health services research.
Objective
This review aims to describe and assess the present landscape of EMR-based case phenotyping for the Charlson conditions.
Methods
A scoping review of EMR-based algorithms for defining the Charlson comorbidity index conditions was completed. This study covered articles published between January 2000 and April 2020, both inclusive. Embase (Excerpta Medica database) and MEDLINE (Medical Literature Analysis and Retrieval System Online) were searched using keywords developed in the following 3 domains: terms related to EMR, terms related to case finding, and disease-specific terms. The manuscript follows the Preferred Reporting Items for Systematic reviews and Meta-analyses extension for Scoping Reviews (PRISMA) guidelines.
Results
A total of 274 articles representing 299 algorithms were assessed and summarized. Most studies were undertaken in the United States (181/299, 60.5%), followed by the United Kingdom (42/299, 14.0%) and Canada (15/299, 5.0%). These algorithms were mostly developed either in primary care (103/299, 34.4%) or inpatient (168/299, 56.2%) settings. Diabetes, congestive heart failure, myocardial infarction, and rheumatology had the highest number of developed algorithms. Data-driven and clinical rule–based approaches have been identified. EMR-based phenotype and algorithm development reflect the data access allowed by respective health systems, and algorithms vary in their performance.
Conclusions
Recognizing similarities and differences in health systems, data collection strategies, extraction, data release protocols, and existing clinical pathways is critical to algorithm development strategies. Several strategies to assist with phenotype-based case definitions have been proposed.
Keywords: electronic medical records, Charlson comorbidity, EMR phenotyping, health services research
Introduction
Background
Recent advances in computational power, increased adoption of electronic medical records (EMRs), and the subsequent rise of big data analytics in health care have opened the door to precision medicine [1]. EMRs are systemized collections of patient health information and documentation, collected in real time and stored in a digital format. EMRs were originally designed to facilitate communication in support of clinical decision-making for individual patients and to improve the quality of care. Canada and other countries have heavily promoted EMR adoption [2,3]. Globally, EMR data have been used widely for secondary purposes, such as research.
Developing case definitions, a process known as phenotyping, has become an active area of research associated with EMRs. Establishing EMR data–based phenotyping is essential for setting up the operational framework toward pursuing precision medicine, which aims to tailor medical decisions and treatments to each patient in a timely manner. EMR phenotyping allows identification and surveillance of health conditions in a timely manner and can be integrated into existing clinical flows and infrastructure. Phenotyping comorbidities using EMR data have important implications on disease management. Comorbidity is a medical condition existing simultaneously with but independently from another condition in a patient. These diseases may be related to each other by some shared association [4]. The Charlson comorbidity index [4-6] is a measure that predicts 1-year mortality based on the presence or absence of specific chronic conditions. Typically, each condition is identified through the presence of specific International Classification of Diseases (ICD) codes and assigned a score depending on the risk of death. Scores are summed for each patient to provide a total score to predict mortality [7,8]. The Charlson [5] comorbidity algorithm is the most widely used comorbidity index at present and has demonstrated the importance of classifying conditions using health data [6,7], including risk adjustment analysis, developing patient safety indicators, and identifying specific disease cohorts for research and public health applications.
Objectives
Few reviews [9-12] have been published on developing EMR case definitions or phenotyping algorithms for selected chronic conditions, but none specifically cover all of the Charlson comorbidities. Furthermore, these articles narrowed their scope to specific perspectives [10] or specific settings (eg, inpatient or primary care only) [9,11]. These reviews report few studies utilizing natural language processing (NLP) or machine learning (ML), which emphasizes the importance of data science techniques (eg, deep learning) in the present health research. The primary objective of this study is to provide an overview of EMR-based phenotyping algorithms for the Charlson conditions. The secondary objective is to provide recommendations for health systems considering the adoption of EMR-based case phenotyping.
Methods
Article Screening
The methodology follows the guidelines recommended by the Preferred Reporting Items for Systematic Reviews and Meta-analysis Extension Protocols for Scoping Reviews (PRISMA-ScR) [13]. The Excerpta Medica database (Embase), and Medical Literature Analysis and Retrieval System Online (MEDLINE) databases were searched from January 2000 to April 2020 to identify peer-reviewed papers. The search strategy covered the following 3 domains: (1) terms related to EMRs, (2) terms related to case finding, and (3) disease-specific terms. We initially used validated clinical text descriptions from ICD-10 to derive search terms for selected conditions (Multimedia Appendix 1). Boolean algorithms were developed for each specific condition using the domain keywords (Multimedia Appendix 2). The cancer categories of metastatic cancer and malignant cancer were excluded, as there is already an existing review on this topic [11].
Manual screening was performed according to the following established study guidelines. Peer-reviewed journal papers were included if they were published between January 2000 and April 2020, written in English, involved human subjects and EMR, and were retrieved by the Boolean search algorithm for at least one Charlson condition. This review study focused only on case phenotyping using EMR data, and therefore, papers were excluded if they only involved administrative databases. Administrative data studies that linked EMR data were included. The presence of the Charlson conditions in each study, if reported, was defined by the presence of ICD-9 or ICD-10 codes stated in the manuscript. The full PRISMA flow diagram was created (Multimedia Appendix 3). The final search results were exported to a reference software (EndNote, Clarivate Inc) [14], and duplicates were removed.
Characterizing the Identified Literature
A data extraction form was developed. The extracted data components included article characteristics (year and country), health care type (eg, inpatient, outpatient, and emergency), specific name of the data source, whether diagnostic codes (eg, ICD) were used, types of EMR data (eg, structured, unstructured, or imaging), techniques (eg, epidemiology/biostatistics, ML, or NLP), and whether a validation methodology was employed. The extracted data types (categorical) were recoded as binary variables to indicate whether they were employed in the algorithm. The frequencies of the algorithms, EMR settings, and countries were calculated. The identified algorithms were substratified into the following 7 types in this review based on the types of data used: (1) diagnostic codes only; (2) codes and structured data (demographics, labs, and medications); (3) diagnostic codes and free-text data; (4) diagnostic codes, structured, and free-text data; (5) structured data only; (6) free-text data only; and (7) free-text and structured data. The detailed operational definitions of case definitions used in the identified studies were also extracted. The extracted data were summarized using frequencies and graphs where applicable. STATA 14 software (StataCorp LLC) [15] was used for statistical analysis. We further summarized the used data elements, disease context, data linkage, and validation of phenotyping algorithms using the extracted tables.
Results
Article Screening
After 1097 duplicates were removed, a total of 3691 abstracts were identified from the electronic databases. A total of 3402 abstracts were excluded based on the title and abstract screening, resulting in 289 full-text articles for full article review. Of these, 39 articles were excluded because they did not include any Charlson conditions, and 22 articles could not be retrieved, leading to the exclusion of 61 articles. The remaining 228 articles were considered eligible for this review and analyzed. References of eligible full articles were screened, and additional articles were identified for inclusion (n=46), leading to a total of 274 articles for qualitative synthesis. Articles covering multiple disease phenotypes were counted once per phenotype, leading to a total of 299 disease phenotyping algorithms. The PRISMA diagram depicting this process is shown in Multimedia Appendix 3.
Characteristics of the Identified Literature
The frequencies of the algorithms, EMR settings, and countries are shown in Table 1. The complete data extraction table is presented in Multimedia Appendix 4 [16-285]. A total of 274 articles representing 299 algorithms from 22 countries were identified in this review. The majority of this work was undertaken in the United States (181/299, 60.5%), followed by the United Kingdom (42/299, 14.0%) and Canada (15/299, 5.0%). Algorithm development has steadily increased over the years, with the majority of work published after 2016. The distributions of these algorithms by the year of publication and by country are shown in Figure 1. The breakdown of the disease areas of these algorithms is shown in Figure 2.
Table 1.
Descriptive summary of the 299 Charlson algorithms.
| Disease | Algorithm count | EMRa settings | Country | |||
| Inpatient | Inpatient and outpatient | Outpatient | Other | |||
| Myocardial infarction [16-38] | 23 | 16 | 3 | 4 | 0 |
|
| Congestive heart failure [19,39-75] | 38 | 22 | 1 | 14 | 1 |
|
| Peripheral vascular disease [19,76-89] | 15 | 6 | 1 | 7 | 1 |
|
| Cerebrovascular disease [19,47,57,78,83,90-107] | 23 | 14 | 0 | 9 | 0 |
|
| Hemiplegia and paraplegia | 0 | 0 | 0 | 0 | 0 | 0 |
| Dementia [19,84,108-130] | 25 | 13 | 1 | 10 | 1 |
|
| Chronic pulmonary disease [129,131-160] | 31 | 14 | 1 | 16 | 0 |
|
| Rheumatologic disease [161-185] | 25 | 15 | 1 | 9 | 0 |
|
| Peptic ulcer disease [186-189] | 4 | 3 | 0 | 1 | 0 |
|
| Diabetes [19,28,34,47,48,84,128,129,140,150,166,190-234] | 56 | 30 | 6 | 20 | 0 |
|
| Diabetes, with complications [57,235-242] | 9 | 6 | 1 | 2 | 0 |
|
| Renal disease [47,57,243-262] | 22 | 9 | 3 | 9 | 1 |
|
| Mild liver disease [189,263-276] | 15 | 11 | 3 | 0 | 1 |
|
| Moderate/severe liver disease [244,275-280] | 7 | 5 | 0 | 2 | 0 |
|
| HIV [137,281-285] | 6 | 4 | 2 | 0 | 0 | 6 United States |
aEMR: electronic medical record.
Figure 1.
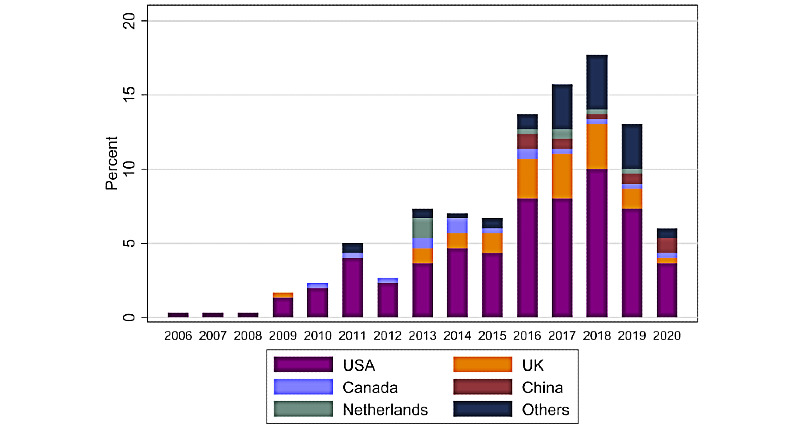
Distribution of published articles by country between January 2000 and April 2020.
Figure 2.
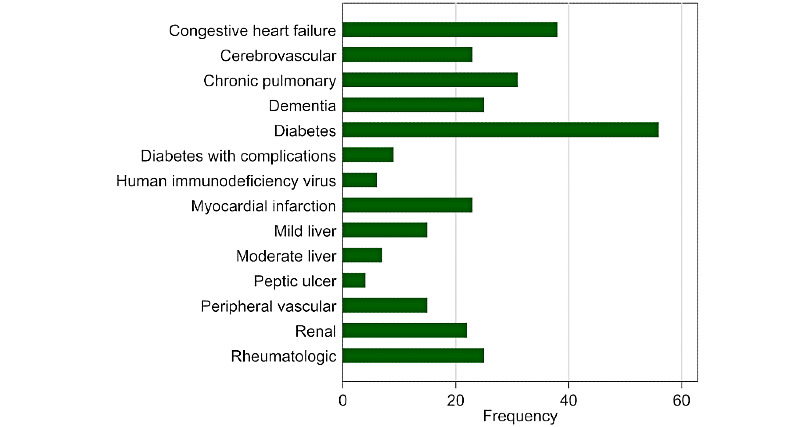
Distribution of electronic medical record data–based algorithms by Charlson disease area.
Table 2 provides a summary of the algorithm types used for each Charlson condition. The most common algorithm types were diagnostic codes and structured data (167/299, 55.9%), followed by diagnostic codes, structured and free-text data (51/299, 17.1%), and diagnostic codes only (40/299, 13.4%). Variations in the data sources used were observed based on disease context and data availability.
Table 2.
The Charlson algorithm types identified in this scoping review.
| Charlson condition | Algorithm type | ||||||
| Codes only | Codes and structured data | Codes and free-text data | Codes, structured, and free-text data | Structured data only | Free-text data only | Free-text and structured data | |
| Myocardial infarction (n=23, 7.7%)) | 7 | 10 | 0 | 4 | 0 | 2 | 0 |
| Congestive heart failure (n=38, 12.7%)) | 7 | 19 | 2 | 9 | 0 | 0 | 1 |
| Peripheral vascular disease (n=15, 5.0%) | 1 | 6 | 1 | 2 | 2 | 2 | 1 |
| Cerebrovascular disease (n=23, 7.7%) | 6 | 14 | 0 | 1 | 1 | 1 | 0 |
| Dementia (n=25, 8.4%) | 4 | 8 | 4 | 4 | 1 | 1 | 3 |
| Chronic pulmonary disease (n=31, 10.4%) | 3 | 17 | 3 | 3 | 0 | 4 | 1 |
| Rheumatologic disease (n=25, 8.4%) | 2 | 9 | 2 | 12 | 0 | 0 | 0 |
| Peptic ulcer disease (n=4, 1.3%) | 0 | 1 | 1 | 2 | 0 | 0 | 0 |
| Diabetes (n=56, 18.7%) | 6 | 41 | 1 | 6 | 1 | 0 | 1 |
| Diabetes with complications (n=9, 3.0%) | 1 | 8 | 0 | 0 | 0 | 0 | 0 |
| Renal disease (n=22, 7.4%) | 2 | 15 | 1 | 2 | 1 | 0 | 1 |
| Mild liver disease (n=15, 5.0%) | 1 | 11 | 0 | 2 | 0 | 0 | 1 |
| Moderate/severe liver disease (n=7, 2.3%) | 0 | 3 | 0 | 3 | 0 | 0 | 1 |
| HIV (n=6, 2.0%) | 0 | 5 | 0 | 1 | 0 | 0 | 0 |
| Combined (n=299, 100.0%) | 40 | 167 | 15 | 51 | 6 | 10 | 10 |
These algorithms were mostly developed either in primary care (103/299, 34.4%) or inpatient (168/299, 56.2%) settings. A total of 23 algorithms (23/299, 7.7%) used data sources from inpatient and outpatient EMR. This trend was consistent across the conditions assessed in this review. The United States had the highest algorithm count across most of the assessed conditions, followed by the United Kingdom, Canada, and other nations. Detailed information about the distribution of algorithms by disease, EMR setting, and country is shown in Table 1.
We abstracted study objectives and classified different purposes for which algorithms were developed for, as well as the setting of each study (Multimedia Appendix 4). Phenotyping algorithm development was not always the primary objective for the identified studies; sometimes, it was part of a larger process. The most commonly occurring objectives of the algorithms were (1) phenotyping algorithm development (193/299, 64.5%), (2) epidemiological analysis (70/299, 23.4%), and (3) predictive modeling (19/299, 6.4%). Other objectives included designing clinical decision support and implementation tools, genome analysis, and registry development. These objectives reflect the health system delivery and clinical practice contexts in which the studies were situated.
Data Elements: Structured Versus Unstructured
With regard to the EMR algorithms identified in this study, structured data most commonly consisted of demographics, diagnoses, procedures, vital signs, laboratory results, and medications. Structured data elements were the most common type of data employed by clinical rule–based algorithms and included basic demographics (eg, sex and age), medications, laboratory data, and diagnostic codes. A total of 233 out of 299 (77.9%) algorithms employed key laboratory diagnostic tests based on the present clinical practice.
These structured EMR components are typically available across EMR systems. Algorithms based on diagnostic codes and structured data were used primarily (213/299, 71.2%) for chronic conditions such as diabetes, where laboratory tests and medication may be necessary and sufficient for clinical decision-making. The use of diagnostic codes depended on the EMR setting (ie, outpatient or inpatient) and the health services jurisdiction (eg, United Kingdom vs United States vs Canada) where the work took place (Multimedia Appendix 4). Types of diagnostic codes identified included ICD-9, ICD-10, Read, Oxford Medical Information System, and International Classification of Primary Care (ICPC). ICD codes were used predominantly within inpatient settings (148/168, 88.1%). These basic structured data-based definitions were enhanced by incorporating unstructured data such as free text and imaging for designing classification algorithms (Table 2) for complicated chronic conditions. In summary, the disease context determined the data elements that were used.
Unstructured free-text data (eg, discharge summaries, consult notes, and nursing notes) were incorporated in approximately 86 out of 299 (28.8%) case phenotyping algorithms. NLP techniques were used to analyze such unstructured free-text data. Many studies used controlled medical terminologies, such as the Unified Medical Language System [286] and the Systematized Nomenclature of Medicine Clinical Terms [287], in the processing of clinical notes. Both terminologies can be used by medical researchers. Many studies also employed custom vocabularies developed in consultation with clinicians or had clinicians manually annotate the free-text data to obtain the reference standard. Variations in the processing of the unstructured data were also noted. NLP processing programs such as clinical Text Analysis and Knowledge Extraction System [288], MedTagger [289], or in-house programs were employed using one of the terminologies mentioned above. This data processing converted unstructured free-text data into structured data. The converted data are often combined with existing structured data for phenotyping and disease prediction using a wide range of techniques in epidemiology, statistics, and ML. Cox regression modeling was used for survival analysis, along with incidence and prevalence in epidemiological studies. Supervised learning classification algorithms such as Naive Bayes, support vector machines, logistic regression, and neural networks are commonly used in the ML studies. The manually annotated notes or reference standard obtained from the chart review provided labels for supervised ML.
Disease Context
Case phenotyping algorithms exhibited 2 distinct types of approaches: clinician-derived rule-based (ie, expert-driven) and data-driven approaches. Clinician-derived rule-based approaches for defining cases were based on clinical criteria dictated by guidelines or clinical practice. These rule-based methods are generally easy to interpret and are accepted as clinically relevant. However, criteria were inconsistent within and across multiple diseases even for the clinical rule-based case phenotyping, implying that the interpretation of algorithm results may depend on choices made during the algorithm development process [290]. Despite these variations, common structured data elements were identified in each disease discipline within each context of patient care. In contrast, data-driven approaches to defining cases use information extracted from available data to determine the disease status of the patient, often with improved performance (eg, sensitivity, positive predictive value [PPV], and F1 score) compared with baseline rule-based algorithms. For example, feeding all available free-text and laboratory data for congestive heart failure (CHF) into a prediction model can classify the CHF status [73]. One study employed principal component analysis [34]. However, the association between the predictor variables and outcomes is often difficult to ascertain, and the model may be difficult to interpret.
The algorithms used various EMR data elements depending on the clinical disease context. For each disease area, unique diagnostic methods or clinical data elements were observed. Diabetes was the most commonly identified disease in our literature search (56/299, 18.7%) and will be used as an example. Case phenotyping for diabetes had fewer data element variations compared with other diseases, and algorithms involved hemoglobin A1c (HbA1c), glucose levels, and fasting glucose as key laboratory tests and antidiabetic medications. Most diabetes algorithms did not define the severity of the disease but classified the conditions in terms of the presence or absence of type 1 or type 2 diabetes. Diabetes phenotyping studies designed patient cohort selection taking this into consideration. Developing phenotypes for identifying severe complications of diabetes did require additional data (ie, clinical narratives) and advanced methodological approaches (eg, NLP and ML), as structured data alone would not readily identify these unless diagnostic codes were included for such complications. EMR phenotypes for disease severity were sometimes developed, in the case of chronic conditions that have a widely accepted clinical severity definition. Using chronic kidney disease as an example, severity was defined according to the Kidney Disease Improving Global Outcomes [291] and the National Kidney Foundation [292] guidelines based on estimated glomerular filtration rate.
Data Linkage
A subset of phenotyping algorithms (30/299, 10.0%) linked EMR data to disease registries or genomics data. A total of 24 out of 299 (8.0%) algorithms linked clinical and health administrative databases. All data linkage occurred in studies that used diagnostic codes. The most commonly occurring diagnostic codes were ICD-9 and ICD-10, with some regional or national diagnostic codes (eg, Read codes among UK studies). The EMR administrative data linkage context appeared mostly within primary care data-based algorithms (14/24). The UK Clinical Practice Research Datalink was linked with Hospital Episode Statistics and other administrative data to primary care EMR. The most commonly linked inpatient care data came from the Electronic Medical Records and Genomics (eMERGE) consortium [293], which provided additional validation between clinical documentation and scientific (ie, genomic) observation. These data linkage studies were employed for epidemiological analyses (improved accuracy of incidence and prevalence estimates) of diseases at the population level [83,96,212].
Validity of Phenotyping Algorithms
Studies varied in their reporting metrics for the validity of case definition algorithms. Commonly reported metrics were sensitivity, specificity, positive predictive value, negative predictive value, accuracy, and F1 score. A total of 185 algorithms (185/299, 62.1%) employed chart review as the reference standard to calculate some of the aforementioned validation metrics. Of these 185 algorithms, 9 employed ML, 39 employed NLP, and 17 employed both ML and NLP. Of the 114 algorithms that did not conduct a chart review, 17 incorporated ML, 14 incorporated NLP, and 7 employed both ML and NLP techniques. Including free-text data as a data source in phenotyping algorithms tended to yield higher performance, with an average sensitivity of 0.906 (SD 0.110) and PPV of 0.913 (SD 0.120) when compared with studies that did not use free-text or ML (average sensitivity of 0.825 (SD 0.214) and average PPV of 0.853 (SD 0.174)). Incorporation of ML as part of the data-driven phenotyping also led to similar performance in sensitivity but weaker PPV, with an average sensitivity of 0.832 (SD 0.095) and average PPV of 0.633 (SD 0.358). In total, 59 out of 166 (35.5%) inpatient algorithms employed NLP, whereas 10 out of 93 (10.8%) primary care algorithms employed NLP. Among the works that used NLP, terminology standards were based on either Systematized Nomenclature of Medicine - Clinical Terms (SNOMED CT) or Unified Medical Language System (UMLS), although many developed their own in-house keywords. Coding standards within inpatients were based on either ICD-9 or ICD-10 depending on the timing of the study and the jurisdictions where each study took place. Similarly, primary care code standards also varied. For example, mostly Read or ICPC codes were used in the United Kingdom, whereas ICD codes were used in North America (United States and Canada). The additional data provide a specific list of ML techniques that were used in each study, if employed (Multimedia Appendix 4).
Discussion
EMR Phenotyping and Precision Medicine
Achieving precision medicine requires the right information to be delivered to the right personnel at the right time. Developing EMR data–based phenotypes and integrating them into existing health information systems is a pivotal step for building a learning health system. EMR phenotypes allow rapid detection of diseases and accelerate the delivery of information to clinicians who may need it to make informed clinical decisions, policymakers who may use them to obtain population information for making public health decisions, and health services organizations that may need such information for planning clinical operations or developing risk adjustment models for patient safety programs. The purposes of the case definitions identified in this review were largely achieving one of the stated objectives above.
EMR-based phenotype and algorithm development reflected the structure and data available within respective health systems. Diagnostic codes, such as ICD and present procedural terminology codes, are often used for billing purposes within inpatient and outpatient (ie, primary care) settings in certain countries (eg, the United States). These codes were also built into EMR systems (eg, problem lists). Consequently, these diagnostic codes were used extensively in algorithm development with the assumption that billing and problem list practices accurately reflect the provided care. In jurisdictional settings where ICD-based billing was not recorded directly in the EMR system during patient care (eg, inpatient care in Alberta), such assumptions could not be made and influenced the algorithm development process. Recognizing similarities and differences in data collection strategies, extraction, data release protocols, and existing clinical pathways is critical and will inform algorithm development strategies. ML and NLP techniques are increasingly being adopted in phenotyping algorithms. This is a testament to the fact that detailed records, available from free-text data, can assist with building high-performance classification algorithms.
Data Extraction, Validity, and Quality
Developing data-driven case finding algorithms is not feasible without electronic data [294]. However, EMR data are not always easy to work with [295], as they are primarily intended to support clinical practice rather than research. EMR settings influence data collection and extraction strategies. Inpatient facilities often set up electronic data warehouses where EMR data are collected into centralized repositories, including free-text data. Primary care settings, in contrast, have variations in their systems, and studies based on primary care data often only use more common data elements such as laboratory data and demographics for multisite studies. Free-text data are less available when compared with inpatient facilities. Primary care clinics, including specialist clinics, are privately operated in many jurisdictions, whereas inpatient care may be publicly or privately operated. These different entities may not always be required to share health data or may have different data management protocols. These considerations influenced the algorithm development process, and a stark contrast in the used data elements can be observed between algorithms developed in outpatient and inpatient settings. To mitigate some of these issues, researchers conducted data linkage between data sources to expand the scope of the available data.
In addition, significant changes in the terminology and coding standards and practices in EMRs have occurred and are actively occurring. This often makes it difficult or impossible to compare or share algorithms developed for different EMR systems using different coding standards (eg, ICD-9, ICD-10, Read, SNOMED RT, SNOMED CT, and MEDCIN for diagnostic codes). Furthermore, many investigators noted that their studies were based on data from a single center, as they did not have access to external EMR data outside of their own institution. Thus, the potential lack of generalizability was a limitation for some studies. However, algorithms developed using commonly available data elements were often externally validated in multiple studies. In particular, simpler algorithms involving diagnostic codes or laboratory data appeared to be externally validated more commonly. This trend was observed in diabetes and rheumatic conditions and occurred mostly in the United States.
Variation in reported metrics (eg, sensitivity, specificity, positive predictive value, negative predictive value, area under the receiver-operator characteristic curve, and F1 score) was observed in the identified literature. Standardized metrics used in health care should be reported, including sensitivity, specificity, positive predictive value, and negative predictive value. As there is a trade-off between sensitivity and positive predictive value and both are important, it is also useful to report the F1 score, which is the harmonic mean of these 2 quantities. In addition, as class imbalance is frequently a problem in the context of disease classification, with positive instances far less common than negative instances, studies are encouraged to report metrics that account for this, such as area under the precision-recall curve [296]. At present, there are no universally accepted EMR data quality assessment metrics available, although there are various proposed data quality assessment frameworks [297]. Data quality must be assessed based on the suitability of the data to achieve a specific research objective or downstream task. We discuss this later in the recommendations.
Limitations
This study is not without limitations. First, it is possible that our search did not encompass all qualifying articles in the field. However, our search strategy was refined and improved by systematic review search experts and librarians, and we believe our search successfully captured a broad spectrum of articles on the Charlson conditions. Second, manual screening was carried out by one individual. The objectivity of the review may have been increased by including a second reviewer. Finally, our review did not discuss methods employed for assessing EMR data quality, which depends on the context and clinical application, and is a difficult concept to measure in general. To date, there is no universally accepted data quality metric developed for EMR data, and few of the papers in this review discuss whether or how data quality was assessed in their study. Further research is required to establish the scope of practice for EMR data quality assessment.
Recommendations on the Basis of Findings
Our review identified that case phenotyping algorithms depends on the health delivery system and disease context. We present a few observed strategies to assist with refining phenotype case definitions using the following key strategies: (1) understanding the health system structure and setting (eg, outpatient vs inpatient, coding practice) will provide a general sense of the type of EMR data that may be available; (2) considering data linkage can increase the scope of data available for algorithm development, it is important to recognize that data may not be standardized or comparable between different data sources. Additional data processing such as data recoding or data imputation may be needed; (3) identifying the relevant clinical and/or health services pathway and involving respective specialty physicians and other stakeholders as part of the algorithm development process can assist with knowledge translation; 4) employing a common data model (eg, observational medical outcomes partnership [298]) and using commonly available data elements to the possible extent can encourage widespread deployment and external validation. A common data model may differ between disease disciplines and health system areas; and (5) considering how to customize the algorithm to the needs of the end user. The needs are largely divided into clinical decision support through risk adjustment analysis, population-scale disease identification for public health initiatives, or developing methodologies to improve algorithm performance.
Health care is a unique environment, and a one-size-fits-all approach may not be appropriate. This review identified variations in EMR phenotyping, which were heavily influenced by the health care delivery setting and the disease context. To optimize performance, researchers should develop tailored algorithms that focus on the specific population of interest and the particular structure of the health system (eg, developing a primary care diabetes definition), while accounting for data issues such as variations in coding systems, clinical practice guidelines, and data quality. Once a locally developed algorithm is in place, health systems may consider implementing their case finding algorithms on standardized data models. This review identified several studies that either validated previously validated case definitions in a new setting or were refined to appropriately identify disease patients within a new setting. Having locally developed algorithms converted to standard data models will facilitate external validation and implementation, which can otherwise be a critical roadblock to the adoption of these algorithms, allowing for improved algorithm interoperability between health care systems.
The interoperability of algorithms across systems facilitates implementation within existing real-time clinical decision support systems. Easy access to developed code is also critical in validating and replicating published algorithms, after their computability has been confirmed. Analytical code and resources could be shared publicly (eg, on GitHub) to allow access for validation and implementation. The eMERGE consortium [293], CALIBER [299], and Canadian Primary Care Sentinel Surveillance Network [300], for example, have made their algorithms publicly available and have been widely adopted.
Conclusions
We assessed EMR-based phenotyping of the Charlson conditions in health care settings. The phenotyping algorithms were locally developed and tailored to the needs and objectives of the individual studies. The health system structure and disease context determined data availability and type. The disease context dictated the common data types used for algorithm development. NLP with free-text data was employed for complex diseases that were difficult to identify with algorithms using readily available structured data. Supervised ML was employed in phenotyping algorithms, where applicable, which worked with reference standards obtained from medical chart review. Studies are encouraged to report standard health system metrics and metrics that account for class imbalance. Locally developed algorithms were validated or refined for adoption in the new setting. Locally developed disease- and setting-specific algorithms could be translated into a common data model for easier interoperability of algorithms across systems. Integrating EMR phenotyping algorithms within a health system could lead to the development of a clinical decision support system that makes use of refined existing risk adjustment scoring for risk stratification in clinical point-of-care and inform the public health and health system decision-making process, thus, leading to learning health systems.
Abbreviations
- CHF
congestive heart failure
- eMERGE
Electronic Medical Records and Genomics
- EMR
electronic medical records
- ICD
International Classification of Diseases
- ICPC
International Classification of Primary Care
- ML
machine learning
- NLP
natural language processing
- PPV
positive predictive value
- PRISMA-ScR
Preferred Reporting Items for Systematic reviews and Meta-analyses extension for Scoping Reviews
Appendix
Developed search terms (Medical Subject Headings) for scoping literature review.
Embase and Medical Literature Analysis and Retrieval System Online search results of Charlson terms.
Preferred Reporting Items for Systematic reviews and Meta-analyses flow diagram.
Summary spreadsheet of identified articles between January 2000 and April 2020.
Footnotes
Conflicts of Interest: None declared.
References
- 1.Jameson JL, Longo DL. Precision medicine--personalized, problematic, and promising. N Engl J Med. 2015 Jun 04;372(23):2229–34. doi: 10.1056/NEJMsb1503104. [DOI] [PubMed] [Google Scholar]
- 2.Adler-Milstein J, Jha AK. HITECH Act Drove Large Gains In Hospital Electronic Health Record Adoption. Health Aff (Millwood) 2017 Aug 01;36(8):1416–1422. doi: 10.1377/hlthaff.2016.1651. [DOI] [PubMed] [Google Scholar]
- 3.Gagnon M, Payne-Gagnon J, Breton E, Fortin J, Khoury L, Dolovich L, Price D, Wiljer D, Bartlett G, Archer N. Adoption of Electronic Personal Health Records in Canada: Perceptions of Stakeholders. Int J Health Policy Manag. 2016 Jul 01;5(7):425–433. doi: 10.15171/ijhpm.2016.36. http://europepmc.org/abstract/MED/27694670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding health and health services. Ann Fam Med. 2009;7(4):357–63. doi: 10.1370/afm.983. http://www.annfammed.org/cgi/pmidlookup?view=long&pmid=19597174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 6.Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel J, Sundararajan V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011 Mar 15;173(6):676–82. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 7.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005 Nov;43(11):1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 8.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993 Oct;46(10):1075–9; discussion 1081. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]
- 9.McBrien KA, Souri S, Symonds NE, Rouhi A, Lethebe BC, Williamson TS, Garies S, Birtwhistle R, Quan H, Fabreau GE, Ronksley PE. Identification of validated case definitions for medical conditions used in primary care electronic medical record databases: a systematic review. J Am Med Inform Assoc. 2018 Nov 01;25(11):1567–1578. doi: 10.1093/jamia/ocy094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nissen F, Quint JK, Wilkinson S, Mullerova H, Smeeth L, Douglas IJ. Validation of asthma recording in electronic health records: a systematic review. Clin Epidemiol. 2017;9:643–656. doi: 10.2147/CLEP.S143718. doi: 10.2147/CLEP.S143718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang P, Garza M, Zozus M. Cancer Phenotype Development: A Literature Review. Stud Health Technol Inform. 2019;257:468–472. [PubMed] [Google Scholar]
- 12.Xu J, Rasmussen LV, Shaw PL, Jiang G, Kiefer RC, Mo H, Pacheco JA, Speltz P, Zhu Q, Denny JC, Pathak J, Thompson WK, Montague E. Review and evaluation of electronic health records-driven phenotype algorithm authoring tools for clinical and translational research. J Am Med Inform Assoc. 2015 Dec;22(6):1251–60. doi: 10.1093/jamia/ocv070. http://europepmc.org/abstract/MED/26224336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, Hempel S, Akl EA, Chang C, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson MG, Garritty C, Lewin S, Godfrey CM, Macdonald MT, Langlois EV, Soares-Weiser K, Moriarty J, Clifford T, Tunçalp �, Straus SE. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018 Oct 02;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 14.EndNote Version X8 . https://endnote.com. Philadelphia, PA: Clarivate; 2013. [Google Scholar]
- 15.STATA SSR14. College Station, TX: StataCorp LLC; 2015. https://www.stata.com. [Google Scholar]
- 16.Ammann EM, Schweizer ML, Robinson JG, Eschol JO, Kafa R, Girotra S, Winiecki SK, Fuller CC, Carnahan RM, Leonard CE, Haskins C, Garcia C, Chrischilles EA. Chart validation of inpatient ICD-9-CM administrative diagnosis codes for acute myocardial infarction (AMI) among intravenous immune globulin (IGIV) users in the Sentinel Distributed Database. Pharmacoepidemiol Drug Saf. 2018 Apr;27(4):398–404. doi: 10.1002/pds.4398. http://europepmc.org/abstract/MED/29446185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ando T, Ooba N, Mochizuki M, Koide D, Kimura K, Lee SL, Setoguchi S, Kubota K. Positive predictive value of ICD-10 codes for acute myocardial infarction in Japan: a validation study at a single center. BMC Health Serv Res. 2018 Dec 26;18(1):895. doi: 10.1186/s12913-018-3727-0. https://bmchealthservres.biomedcentral.com/articles/10.1186/s12913-018-3727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Backenroth D, Chase H, Friedman C, Wei Y. Using Rich Data on Comorbidities in Case-Control Study Design with Electronic Health Record Data Improves Control of Confounding in the Detection of Adverse Drug Reactions. PLoS One. 2016;11(10):e0164304. doi: 10.1371/journal.pone.0164304. https://dx.plos.org/10.1371/journal.pone.0164304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bent-Ennakhil N, Cécile Périer M, Sobocki P, Gothefors D, Johansson G, Milea D, Empana J. Incidence of cardiovascular diseases and type-2-diabetes mellitus in patients with psychiatric disorders. Nord J Psychiatry. 2018 Oct;72(7):455–461. doi: 10.1080/08039488.2018.1463392. [DOI] [PubMed] [Google Scholar]
- 20.Bjerking LH, Hansen KW, Madsen M, Jensen JS, Madsen JK, Sørensen R, Galatius S. Use of diagnostic coronary angiography in women and men presenting with acute myocardial infarction: a matched cohort study. BMC Cardiovasc Disord. 2016 Jun 01;16:120. doi: 10.1186/s12872-016-0248-9. https://bmccardiovascdisord.biomedcentral.com/articles/10.1186/s12872-016-0248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coloma PM, Valkhoff VE, Mazzaglia G, Nielsson MS, Pedersen L, Molokhia M, Mosseveld M, Morabito P, Schuemie MJ, van der Lei J, Sturkenboom M, Trifirò G, EU-ADR Consortium Identification of acute myocardial infarction from electronic healthcare records using different disease coding systems: a validation study in three European countries. BMJ Open. 2013 Jun 20;3(6) doi: 10.1136/bmjopen-2013-002862. http://bmjopen.bmj.com/cgi/pmidlookup?view=long&pmid=23794587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cross DS, McCarty CA, Steinhubl SR, Carey DJ, Erlich PM. Development of a multi-institutional cohort to facilitate cardiovascular disease biomarker validation using existing biorepository samples linked to electronic health records. Clin Cardiol. 2013 Aug;36(8):486–91. doi: 10.1002/clc.22146. doi: 10.1002/clc.22146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Findlay I, Morris T, Zhang R, McCowan C, Shield S, Forbes B, McConnachie A, Mangion K, Berry C. Linking hospital patient records for suspected or established acute coronary syndrome in a complex secondary care system: a proof-of-concept e-registry in National Health Service Scotland. Eur Heart J Qual Care Clin Outcomes. 2018 Jul 01;4(3):155–167. doi: 10.1093/ehjqcco/qcy007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FitzHenry F, Murff HJ, Matheny ME, Gentry N, Fielstein EM, Brown SH, Reeves RM, Aronsky D, Elkin PL, Messina VP, Speroff T. Exploring the frontier of electronic health record surveillance: the case of postoperative complications. Med Care. 2013 Jul;51(6):509–16. doi: 10.1097/MLR.0b013e31828d1210. http://europepmc.org/abstract/MED/23673394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Floyd JS, Blondon M, Moore KP, Boyko EJ, Smith NL. Validation of methods for assessing cardiovascular disease using electronic health data in a cohort of Veterans with diabetes. Pharmacoepidemiol Drug Saf. 2016 May;25(4):467–71. doi: 10.1002/pds.3921. http://europepmc.org/abstract/MED/26555025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldstein BA, Assimes T, Winkelmayer WC, Hastie T. Detecting clinically meaningful biomarkers with repeated measurements: An illustration with electronic health records. Biometrics. 2015 Jul;71(2):478–86. doi: 10.1111/biom.12283. http://europepmc.org/abstract/MED/25652566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrett E, Bhaskaran K, Timmis A, Denaxas S, Hemingway H, Smeeth L. Association between clinical presentations before myocardial infarction and coronary mortality: a prospective population-based study using linked electronic records. Eur Heart J. 2014 Oct 14;35(35):2363–71. doi: 10.1093/eurheartj/ehu286. http://europepmc.org/abstract/MED/25038774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hivert M, Grant RW, Shrader P, Meigs JB. Identifying primary care patients at risk for future diabetes and cardiovascular disease using electronic health records. BMC Health Serv Res. 2009 Sep 22;9:170. doi: 10.1186/1472-6963-9-170. https://bmchealthservres.biomedcentral.com/articles/10.1186/1472-6963-9-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahler SA, Lenoir KM, Wells BJ, Burke GL, Duncan PW, Case LD, Herrington DM, Diaz-Garelli J, Futrell WM, Hiestand BC, Miller CD. Safely Identifying Emergency Department Patients With Acute Chest Pain for Early Discharge. Circulation. 2018 Nov 27;138(22):2456–2468. doi: 10.1161/CIRCULATIONAHA.118.036528. http://europepmc.org/abstract/MED/30571347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manemann SM, Gerber Y, Chamberlain AM, Dunlay SM, Bell MR, Jaffe AS, Weston SA, Killian JM, Kors J, Roger VL. Acute coronary syndromes in the community. Mayo Clin Proc. 2015 May;90(5):597–605. doi: 10.1016/j.mayocp.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murff HJ, FitzHenry F, Matheny ME, Gentry N, Kotter KL, Crimin K, Dittus RS, Rosen AK, Elkin PL, Brown SH, Speroff T. Automated identification of postoperative complications within an electronic medical record using natural language processing. JAMA. 2011 Aug 24;306(8):848–55. doi: 10.1001/jama.2011.1204. [DOI] [PubMed] [Google Scholar]
- 32.Persell SD, Dunne AP, Lloyd-Jones DM, Baker DW. Electronic health record-based cardiac risk assessment and identification of unmet preventive needs. Med Care. 2009 May;47(4):418–24. doi: 10.1097/MLR.0b013e31818dce21. [DOI] [PubMed] [Google Scholar]
- 33.Reynolds K, Go AS, Leong TK, Boudreau DM, Cassidy-Bushrow AE, Fortmann SP, Goldberg RJ, Gurwitz JH, Magid DJ, Margolis KL, McNeal CJ, Newton KM, Novotny R, Quesenberry CP, Rosamond WD, Smith DH, VanWormer JJ, Vupputuri S, Waring SC, Williams MS, Sidney S. Trends in Incidence of Hospitalized Acute Myocardial Infarction in the Cardiovascular Research Network (CVRN) Am J Med. 2017 Mar;130(3):317–327. doi: 10.1016/j.amjmed.2016.09.014. http://europepmc.org/abstract/MED/27751900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song Wenyu, Huang Hailiang, Zhang Cheng-Zhong, Bates David W, Wright Adam. Using whole genome scores to compare three clinical phenotyping methods in complex diseases. Sci Rep. 2018 Jul 27;8(1):11360. doi: 10.1038/s41598-018-29634-w. doi: 10.1038/s41598-018-29634-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tien M, Kashyap R, Wilson GA, Hernandez-Torres V, Jacob AK, Schroeder DR, Mantilla CB. Retrospective Derivation and Validation of an Automated Electronic Search Algorithm to Identify Post Operative Cardiovascular and Thromboembolic Complications. Appl Clin Inform. 2015;6(3):565–76. doi: 10.4338/ACI-2015-03-RA-0026. http://europepmc.org/abstract/MED/26448798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torabi A, Cleland JGF, Sherwi N, Atkin P, Panahi H, Kilpatrick E, Thackray S, Hoye A, Alamgir F, Goode K, Rigby A, Clark AL. Influence of case definition on incidence and outcome of acute coronary syndromes. Open Heart. 2016;3(2):e000487. doi: 10.1136/openhrt-2016-000487. https://openheart.bmj.com/lookup/pmidlookup?view=long&pmid=28123755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang N, Li T, Du Q. Risk factors of upper gastrointestinal hemorrhage with acute coronary syndrome. Am J Emerg Med. 2019 Apr;37(4):615–619. doi: 10.1016/j.ajem.2018.06.055. [DOI] [PubMed] [Google Scholar]
- 38.Zheng J, Yarzebski J, Ramesh BP, Goldberg RJ, Yu H. Automatically Detecting Acute Myocardial Infarction Events from EHR Text: A Preliminary Study. AMIA Annu Symp Proc. 2014;2014:1286–93. http://europepmc.org/abstract/MED/25954440. [PMC free article] [PubMed] [Google Scholar]
- 39.Bielinski SJ, Pathak J, Carrell DS, Takahashi PY, Olson JE, Larson NB, Liu H, Sohn S, Wells QS, Denny JC, Rasmussen-Torvik LJ, Pacheco JA, Jackson KL, Lesnick TG, Gullerud RE, Decker PA, Pereira NL, Ryu E, Dart RA, Peissig P, Linneman JG, Jarvik GP, Larson EB, Bock JA, Tromp GC, de Andrade M, Roger VL. A Robust e-Epidemiology Tool in Phenotyping Heart Failure with Differentiation for Preserved and Reduced Ejection Fraction: the Electronic Medical Records and Genomics (eMERGE) Network. J Cardiovasc Transl Res. 2015 Dec;8(8):475–83. doi: 10.1007/s12265-015-9644-2. http://europepmc.org/abstract/MED/26195183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blecker S, Katz SD, Horwitz LI, Kuperman G, Park H, Gold A, Sontag D. Comparison of Approaches for Heart Failure Case Identification From Electronic Health Record Data. JAMA Cardiol. 2016 Dec 01;1(9):1014–1020. doi: 10.1001/jamacardio.2016.3236. http://europepmc.org/abstract/MED/27706470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bosch L, Assmann P, de Grauw WJC, Schalk BWM, Biermans MCJ. Heart failure in primary care: prevalence related to age and comorbidity. Prim Health Care Res Dev. 2019 Jul 29;20:e79. doi: 10.1017/S1463423618000889. http://europepmc.org/abstract/MED/31868152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bosco-Lévy P, Duret S, Picard F, Dos Santos P, Puymirat E, Gilleron V, Blin P, Chatellier G, Looten V, Moore N. Diagnostic accuracy of the International Classification of Diseases, Tenth Revision, codes of heart failure in an administrative database. Pharmacoepidemiol Drug Saf. 2019 Feb;28(2):194–200. doi: 10.1002/pds.4690. [DOI] [PubMed] [Google Scholar]
- 43.Byrd RJ, Steinhubl SR, Sun J, Ebadollahi S, Stewart WF. Automatic identification of heart failure diagnostic criteria, using text analysis of clinical notes from electronic health records. Int J Med Inform. 2014 Dec;83(12):983–92. doi: 10.1016/j.ijmedinf.2012.12.005. http://europepmc.org/abstract/MED/23317809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi E, Schuetz A, Stewart WF, Sun J. Using recurrent neural network models for early detection of heart failure onset. J Am Med Inform Assoc. 2017 Dec 01;24(2):361–370. doi: 10.1093/jamia/ocw112. http://europepmc.org/abstract/MED/27521897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai W, Brisimi TS, Adams WG, Mela T, Saligrama V, Paschalidis IC. Prediction of hospitalization due to heart diseases by supervised learning methods. Int J Med Inform. 2015 Mar;84(3):189–97. doi: 10.1016/j.ijmedinf.2014.10.002. http://europepmc.org/abstract/MED/25497295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evans RS, Benuzillo J, Horne BD, Lloyd JF, Bradshaw A, Budge D, Rasmusson KD, Roberts C, Buckway J, Geer N, Garrett T, Lappé DL. Automated identification and predictive tools to help identify high-risk heart failure patients: pilot evaluation. J Am Med Inform Assoc. 2016 Sep;23(5):872–8. doi: 10.1093/jamia/ocv197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frigaard M, Rubinsky A, Lowell L, Malkina A, Karliner L, Kohn M, Peralta CA. Validating laboratory defined chronic kidney disease in the electronic health record for patients in primary care. BMC Nephrol. 2019 Jan 03;20(1):3. doi: 10.1186/s12882-018-1156-2. https://www.biomedcentral.com/1471-2369/20/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gini R, Schuemie MJ, Mazzaglia G, Lapi F, Francesconi P, Pasqua A, Bianchini E, Montalbano C, Roberto G, Barletta V, Cricelli I, Cricelli C, Dal Co G, Bellentani M, Sturkenboom M, Klazinga N. Automatic identification of type 2 diabetes, hypertension, ischaemic heart disease, heart failure and their levels of severity from Italian General Practitioners' electronic medical records: a validation study. BMJ Open. 2016 Dec 09;6(12):e012413. doi: 10.1136/bmjopen-2016-012413. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=27940627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huusko J, Purmonen T, Toppila I, Lassenius M, Ukkonen H. Real-world clinical diagnostics of heart failure patients with reduced or preserved ejection fraction. ESC Heart Fail. 2020 Jul;7(3):1039–1048. doi: 10.1002/ehf2.12665. doi: 10.1002/ehf2.12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jonnalagadda SR, Adupa AK, Garg RP, Corona-Cox J, Shah SJ. Text Mining of the Electronic Health Record: An Information Extraction Approach for Automated Identification and Subphenotyping of HFpEF Patients for Clinical Trials. J Cardiovasc Transl Res. 2017 Jul;10(3):313–321. doi: 10.1007/s12265-017-9752-2. [DOI] [PubMed] [Google Scholar]
- 51.Kaspar M, Fette G, Güder G, Seidlmayer L, Ertl M, Dietrich G, Greger H, Puppe F, Störk S. Underestimated prevalence of heart failure in hospital inpatients: a comparison of ICD codes and discharge letter information. Clin Res Cardiol. 2018 Oct;107(9):778–787. doi: 10.1007/s00392-018-1245-z. http://europepmc.org/abstract/MED/29667017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koudstaal S, Pujades-Rodriguez M, Denaxas S, Gho JMIH, Shah AD, Yu N, Patel RS, Gale CP, Hoes AW, Cleland JG, Asselbergs FW, Hemingway H. Prognostic burden of heart failure recorded in primary care, acute hospital admissions, or both: a population-based linked electronic health record cohort study in 2.1 million people. Eur J Heart Fail. 2017 Sep;19(9):1119–1127. doi: 10.1002/ejhf.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kurgansky KE, Schubert P, Parker R, Djousse L, Riebman JB, Gagnon DR, Joseph J. Association of pulse rate with outcomes in heart failure with reduced ejection fraction: a retrospective cohort study. BMC Cardiovasc Disord. 2020 Feb 26;20(1):92. doi: 10.1186/s12872-020-01384-6. https://bmccardiovascdisord.biomedcentral.com/articles/10.1186/s12872-020-01384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindmark K, Boman K, Olofsson M, Törnblom M, Levine A, Castelo-Branco A, Schlienger R, Bruce Wirta S, Stålhammar J, Wikström G. Epidemiology of heart failure and trends in diagnostic work-up: a retrospective, population-based cohort study in Sweden. Clin Epidemiol. 2019;11:231–244. doi: 10.2147/CLEP.S170873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Magnusson P, Palm A, Branden E, Mörner S. Misclassification of hypertrophic cardiomyopathy: validation of diagnostic codes. Clin Epidemiol. 2017;9:403–410. doi: 10.2147/CLEP.S139300. doi: 10.2147/CLEP.S139300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panahiazar M, Taslimitehrani V, Pereira N, Pathak J. Using EHRs and Machine Learning for Heart Failure Survival Analysis. Stud Health Technol Inform. 2015;216:40–4. http://europepmc.org/abstract/MED/26262006. [PMC free article] [PubMed] [Google Scholar]
- 57.Navaneethan SD, Jolly SE, Schold JD, Arrigain S, Saupe W, Sharp J, Lyons J, Simon JF, Schreiber MJ, Jain A, Nally JV. Development and validation of an electronic health record-based chronic kidney disease registry. Clin J Am Soc Nephrol. 2011 Jan;6(1):40–9. doi: 10.2215/CJN.04230510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ng K, Steinhubl SR, deFilippi C, Dey S, Stewart WF. Early Detection of Heart Failure Using Electronic Health Records: Practical Implications for Time Before Diagnosis, Data Diversity, Data Quantity, and Data Density. Circ Cardiovasc Qual Outcomes. 2016 Nov;9(6):649–658. doi: 10.1161/CIRCOUTCOMES.116.002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pakhomov S, Weston SA, Jacobsen SJ, Chute CG, Meverden R, Roger VL. Electronic medical records for clinical research: application to the identification of heart failure. Am J Manag Care. 2007 Jun;13(6 Part 1):281–8. https://www.ajmc.com/journals/issue/2007/2007-06-vol13-n6-pt1/jun07-2488p281-288. [PubMed] [Google Scholar]
- 60.Patel YR, Robbins JM, Kurgansky KE, Imran T, Orkaby AR, McLean RR, Ho Y, Cho K, Michael Gaziano J, Djousse L, Gagnon DR, Joseph J. Development and validation of a heart failure with preserved ejection fraction cohort using electronic medical records. BMC Cardiovasc Disord. 2018 Jun 28;18(1):128. doi: 10.1186/s12872-018-0866-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pike MM, Decker PA, Larson NB, St Sauver JL, Takahashi PY, Roger VL, Rocca WA, Miller VM, Olson JE, Pathak J, Bielinski SJ. Improvement in Cardiovascular Risk Prediction with Electronic Health Records. J Cardiovasc Transl Res. 2016 Jun;9(3):214–222. doi: 10.1007/s12265-016-9687-z. http://europepmc.org/abstract/MED/26960568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rasmy L, Wu Y, Wang N, Geng X, Zheng WJ, Wang F, Wu H, Xu H, Zhi D. A study of generalizability of recurrent neural network-based predictive models for heart failure onset risk using a large and heterogeneous EHR data set. J Biomed Inform. 2018 Aug;84:11–16. doi: 10.1016/j.jbi.2018.06.011. https://linkinghub.elsevier.com/retrieve/pii/S1532-0464(18)30117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shameer K, Johnson K, Yahi A. Predictive Modeling of Hospital Readmission Rates Using Electronic Medical Record-Wide Machine Learning: A Case-Study Using Mount Sinai Heart Failure Cohort. Pacific Symposium on Biocomputing;Pacific Symposium on Biocomputing. 2016:22. doi: 10.1142/9789813207813_0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stålhammar J, Stern L, Linder R, Sherman S, Parikh R, Ariely R, Deschaseaux C, Wikström G. The burden of preserved ejection fraction heart failure in a real-world Swedish patient population. J Med Econ. 2014 Jan;17(1):43–51. doi: 10.3111/13696998.2013.848808. [DOI] [PubMed] [Google Scholar]
- 65.Sun J, Hu J, Luo D, Markatou M, Wang F, Edabollahi S, Steinhubl SE, Daar Z, Stewart WF. Combining knowledge and data driven insights for identifying risk factors using electronic health records. AMIA Annu Symp Proc. 2012;2012:901–10. http://europepmc.org/abstract/MED/23304365. [PMC free article] [PubMed] [Google Scholar]
- 66.Taslimitehrani V, Dong G, Pereira NL, Panahiazar M, Pathak J. Developing EHR-driven heart failure risk prediction models using CPXR(Log) with the probabilistic loss function. J Biomed Inform. 2016 Apr;60:260–9. doi: 10.1016/j.jbi.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thomas IC, Nishimura M, Ma J, Dickson SD, Alshawabkeh L, Adler E, Maisel A, Criqui MH, Greenberg B. Clinical Characteristics and Outcomes of Patients With Heart Failure and Methamphetamine Abuse. J Card Fail. 2020 Mar;26(3):202–209. doi: 10.1016/j.cardfail.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 68.Tison GH, Chamberlain AM, Pletcher MJ, Dunlay SM, Weston SA, Killian JM, Olgin JE, Roger VL. Identifying heart failure using EMR-based algorithms. Int J Med Inform. 2018 Dec;120:1–7. doi: 10.1016/j.ijmedinf.2018.09.016. http://europepmc.org/abstract/MED/30409334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vijayakrishnan R, Steinhubl SR, Ng K, Sun J, Byrd RJ, Daar Z, Williams BA, deFilippi C, Ebadollahi S, Stewart WF. Prevalence of heart failure signs and symptoms in a large primary care population identified through the use of text and data mining of the electronic health record. J Card Fail. 2014 Jul;20(7):459–64. doi: 10.1016/j.cardfail.2014.03.008. http://europepmc.org/abstract/MED/24709663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Luo J, Hao S, Xu H, Shin AY, Jin B, Liu R, Deng X, Wang L, Zheng L, Zhao Y, Zhu C, Hu Z, Fu C, Hao Y, Zhao Y, Jiang Y, Dai D, Culver DS, Alfreds ST, Todd R, Stearns F, Sylvester KG, Widen E, Ling XB. NLP based congestive heart failure case finding: A prospective analysis on statewide electronic medical records. Int J Med Inform. 2015 Dec;84(12):1039–47. doi: 10.1016/j.ijmedinf.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 71.Wang Y, Ng K, Byrd R. Early detection of heart failure with varying prediction windows by structured and unstructured data in electronic health records. Conference proceedings : ;Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference. 2015:2530–2533. doi: 10.1109/embc.2015.7318907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu J, Roy J, Stewart WF. Prediction modeling using EHR data: challenges, strategies, and a comparison of machine learning approaches. Med Care. 2010 Jun;48(6 Suppl):S106–13. doi: 10.1097/MLR.0b013e3181de9e17. [DOI] [PubMed] [Google Scholar]
- 73.Xu Y, Lee S, Martin E, D'souza AG, Doktorchik CTA, Jiang J, Lee S, Eastwood CA, Fine N, Hemmelgarn B, Todd K, Quan H. Enhancing ICD-Code-Based Case Definition for Heart Failure Using Electronic Medical Record Data. J Card Fail. 2020 Jul;26(7):610–617. doi: 10.1016/j.cardfail.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 74.Yang X, Gong Y, Waheed N, March K, Bian J, Hogan WR, Wu Y. Identifying Cancer Patients at Risk for Heart Failure Using Machine Learning Methods. AMIA Annu Symp Proc. 2019;2019:933–941. http://europepmc.org/abstract/MED/32308890. [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang R, Ma S, Shanahan L, Munroe J, Horn S, Speedie S. Discovering and identifying New York heart association classification from electronic health records. BMC Med Inform Decis Mak. 2018 Jul 23;18(Suppl 2):48. doi: 10.1186/s12911-018-0625-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Afzal N, Mallipeddi VP, Sohn S, Liu H, Chaudhry R, Scott CG, Kullo IJ, Arruda-Olson AM. Natural language processing of clinical notes for identification of critical limb ischemia. Int J Med Inform. 2018 Mar;111:83–89. doi: 10.1016/j.ijmedinf.2017.12.024. https://linkinghub.elsevier.com/retrieve/pii/S1386-5056(17)30475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Afzal N, Sohn S, Abram S, Scott CG, Chaudhry R, Liu H, Kullo IJ, Arruda-Olson AM. Mining peripheral arterial disease cases from narrative clinical notes using natural language processing. J Vasc Surg. 2017 Jun;65(6):1753–1761. doi: 10.1016/j.jvs.2016.11.031. https://linkinghub.elsevier.com/retrieve/pii/S0741-5214(16)31844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Archangelidi O, Pujades-Rodriguez M, Timmis A, Jouven X, Denaxas S, Hemingway H. Clinically recorded heart rate and incidence of 12 coronary, cardiac, cerebrovascular and peripheral arterial diseases in 233,970 men and women: A linked electronic health record study. Eur J Prev Cardiol. 2018 Sep;25(14):1485–1495. doi: 10.1177/2047487318785228. [DOI] [PubMed] [Google Scholar]
- 79.Arruda-Olson AM, Afzal N, Priya Mallipeddi V, Said A, Moussa Pacha H, Moon S, Chaudhry AP, Scott CG, Bailey KR, Rooke TW, Wennberg PW, Kaggal VC, Oderich GS, Kullo IJ, Nishimura RA, Chaudhry R, Liu H. Leveraging the Electronic Health Record to Create an Automated Real-Time Prognostic Tool for Peripheral Arterial Disease. J Am Heart Assoc. 2018 Dec 04;7(23):e009680. doi: 10.1161/JAHA.118.009680. https://www.ahajournals.org/doi/10.1161/JAHA.118.009680?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Caleyachetty R, Thomas GN, Toulis KA, Mohammed N, Gokhale KM, Balachandran K, Nirantharakumar K. Metabolically Healthy Obese and Incident Cardiovascular Disease Events Among 3.5 Million Men and Women. J Am Coll Cardiol. 2017 Oct 19;70(12):1429–1437. doi: 10.1016/j.jacc.2017.07.763. https://linkinghub.elsevier.com/retrieve/pii/S0735-1097(17)39050-2. [DOI] [PubMed] [Google Scholar]
- 81.Daskivich T, Abedi G, Kaplan S. Electronic Health Record Problem Lists: Accurate Enough for Risk Adjustment? Am J Manag Care. 2018;24(1):A. [PubMed] [Google Scholar]
- 82.Emdin CA, Anderson SG, Callender T, Conrad N, Salimi-Khorshidi G, Mohseni H, Woodward M, Rahimi K. Usual blood pressure, peripheral arterial disease, and vascular risk: cohort study of 4.2 million adults. BMJ. 2015 Oct 29;351:h4865. doi: 10.1136/bmj.h4865. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=26419648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.George J, Rapsomaniki E, Pujades-Rodriguez M, Shah AD, Denaxas S, Herrett E, Smeeth L, Timmis A, Hemingway H. How Does Cardiovascular Disease First Present in Women and Men? Incidence of 12 Cardiovascular Diseases in a Contemporary Cohort of 1,937,360 People. Circulation. 2015 Oct 06;132(14):1320–8. doi: 10.1161/CIRCULATIONAHA.114.013797. http://europepmc.org/abstract/MED/26330414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kho AN, Pacheco JA, Peissig PL, Rasmussen L, Newton KM, Weston N, Crane PK, Pathak J, Chute CG, Bielinski SJ, Kullo IJ, Li R, Manolio TA, Chisholm RL, Denny JC. Electronic medical records for genetic research: results of the eMERGE consortium. Sci Transl Med. 2011 Apr 20;3(79):79re1. doi: 10.1126/scitranslmed.3001807. http://stm.sciencemag.org/cgi/pmidlookup?view=long&pmid=21508311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kullo IJ, Shameer K, Jouni H, Lesnick TG, Pathak J, Chute CG, de Andrade M. The ATXN2-SH2B3 locus is associated with peripheral arterial disease: an electronic medical record-based genome-wide association study. Front Genet. 2014;5:166. doi: 10.3389/fgene.2014.00166. doi: 10.3389/fgene.2014.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Man A, Zhu Y, Zhang Y, Dubreuil M, Rho YH, Peloquin C, Simms RW, Choi HK. The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Ann Rheum Dis. 2013 Jul;72(7):1188–93. doi: 10.1136/annrheumdis-2012-202007. http://europepmc.org/abstract/MED/22904260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ross EG, Jung K, Dudley JT, Li L, Leeper NJ, Shah NH. Predicting Future Cardiovascular Events in Patients With Peripheral Artery Disease Using Electronic Health Record Data. Circ Cardiovasc Qual Outcomes. 2019 Mar;12(3):e004741. doi: 10.1161/CIRCOUTCOMES.118.004741. http://europepmc.org/abstract/MED/30857412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Savova GK, Fan J, Ye Z, Murphy SP, Zheng J, Chute CG, Kullo IJ. Discovering peripheral arterial disease cases from radiology notes using natural language processing. AMIA Annu Symp Proc. 2010 Nov 13;2010:722–6. http://europepmc.org/abstract/MED/21347073. [PMC free article] [PubMed] [Google Scholar]
- 89.Wolfson J, Vock DM, Bandyopadhyay S, Kottke T, Vazquez-Benitez G, Johnson P, Adomavicius G, O'Connor PJ. Use and Customization of Risk Scores for Predicting Cardiovascular Events Using Electronic Health Record Data. J Am Heart Assoc. 2017 May 24;6(4) doi: 10.1161/JAHA.116.003670. https://www.ahajournals.org/doi/10.1161/JAHA.116.003670?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ammann EM, Leira EC, Winiecki SK, Nagaraja N, Dandapat S, Carnahan RM, Schweizer ML, Torner JC, Fuller CC, Leonard CE, Garcia C, Pimentel M, Chrischilles EA. Chart validation of inpatient ICD-9-CM administrative diagnosis codes for ischemic stroke among IGIV users in the Sentinel Distributed Database. Medicine (Baltimore) 2017 Dec;96(52):e9440. doi: 10.1097/MD.0000000000009440. doi: 10.1097/MD.0000000000009440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bell S, Daskalopoulou M, Rapsomaniki E, George J, Britton A, Bobak M, Casas JP, Dale CE, Denaxas S, Shah AD, Hemingway H. Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: population based cohort study using linked health records. BMJ. 2017 Mar 22;356:j909. doi: 10.1136/bmj.j909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Castro VM, Dligach D, Finan S, Yu S, Can A, Abd-El-Barr M, Gainer V, Shadick NA, Murphy S, Cai T, Savova G, Weiss ST, Du R. Large-scale identification of patients with cerebral aneurysms using natural language processing. Neurology. 2017 Jan 10;88(2):164–168. doi: 10.1212/WNL.0000000000003490. http://europepmc.org/abstract/MED/27927935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Esteban S, Rodríguez Tablado M, Ricci RI, Terrasa S, Kopitowski K. A rule-based electronic phenotyping algorithm for detecting clinically relevant cardiovascular disease cases. BMC Res Notes. 2017 Jul 14;10(1):281. doi: 10.1186/s13104-017-2600-2. https://bmcresnotes.biomedcentral.com/articles/10.1186/s13104-017-2600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garg R, Oh E, Naidech A, Kording K, Prabhakaran S. Automating Ischemic Stroke Subtype Classification Using Machine Learning and Natural Language Processing. J Stroke Cerebrovasc Dis. 2019 Jul;28(7):2045–2051. doi: 10.1016/j.jstrokecerebrovasdis.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 95.Gon Y, Kabata D, Yamamoto K, Shintani A, Todo K, Mochizuki H, Sakaguchi M. Validation of an algorithm that determines stroke diagnostic code accuracy in a Japanese hospital-based cancer registry using electronic medical records. BMC Med Inform Decis Mak. 2017 Dec 04;17(1):157. doi: 10.1186/s12911-017-0554-x. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-017-0554-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gulliford MC, Charlton J, Ashworth M, Rudd AG, Toschke AM, eCRT Research Team Selection of medical diagnostic codes for analysis of electronic patient records. Application to stroke in a primary care database. PLoS One. 2009 Oct 24;4(9):e7168. doi: 10.1371/journal.pone.0007168. https://dx.plos.org/10.1371/journal.pone.0007168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Imran TF, Posner D, Honerlaw J, Vassy JL, Song RJ, Ho Y, Kittner SJ, Liao KP, Cai T, O'Donnell CJ, Djousse L, Gagnon DR, Gaziano JM, Wilson PW, Cho K. A phenotyping algorithm to identify acute ischemic stroke accurately from a national biobank: the Million Veteran Program. Clin Epidemiol. 2018;10:1509–1521. doi: 10.2147/CLEP.S160764. doi: 10.2147/CLEP.S160764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kivimäki M, Batty GD, Singh-Manoux A, Britton A, Brunner EJ, Shipley MJ. Validity of Cardiovascular Disease Event Ascertainment Using Linkage to UK Hospital Records. Epidemiology. 2017 Sep;28(5):735–739. doi: 10.1097/EDE.0000000000000688. http://europepmc.org/abstract/MED/28570383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kogan E, Twyman K, Heap J, Milentijevic D, Lin JH, Alberts M. Assessing stroke severity using electronic health record data: a machine learning approach. BMC Med Inform Decis Mak. 2020 Jan 08;20(1):8. doi: 10.1186/s12911-019-1010-x. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-019-1010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kreuger AL, Middelburg RA, Beckers EAM, de Vooght KMK, Zwaginga JJ, Kerkhoffs JH, van der Bom JG. The identification of cases of major hemorrhage during hospitalization in patients with acute leukemia using routinely recorded healthcare data. PLoS One. 2018;13(8):e0200655. doi: 10.1371/journal.pone.0200655. https://dx.plos.org/10.1371/journal.pone.0200655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ljubisavljevic S, Milosevic V, Stojanov A, Ljubisavljevic M, Dunjic O, Zivkovic M. Identification of clinical and paraclinical findings predictive for headache occurrence during spontaneous subarachnoid hemorrhage. Clin Neurol Neurosurg. 2017 Jul;158:40–45. doi: 10.1016/j.clineuro.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 102.Ni Y, Alwell K, Moomaw CJ, Woo D, Adeoye O, Flaherty ML, Ferioli S, Mackey J, De Los Rios La Rosa F, Martini S, Khatri P, Kleindorfer D, Kissela BM. Towards phenotyping stroke: Leveraging data from a large-scale epidemiological study to detect stroke diagnosis. PLoS One. 2018;13(2):e0192586. doi: 10.1371/journal.pone.0192586. http://dx.plos.org/10.1371/journal.pone.0192586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Øie LR, Madsbu MA, Giannadakis C, Vorhaug A, Jensberg H, Salvesen �, Gulati S. Validation of intracranial hemorrhage in the Norwegian Patient Registry. Brain Behav. 2018 Feb;8(2):e00900. doi: 10.1002/brb3.900. doi: 10.1002/brb3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oostema JA, Konen J, Chassee T, Nasiri M, Reeves MJ. Clinical predictors of accurate prehospital stroke recognition. Stroke. 2015 Jun;46(6):1513–7. doi: 10.1161/STROKEAHA.115.008650. [DOI] [PubMed] [Google Scholar]
- 105.Pouwels KB, Voorham J, Hak E, Denig P. Identification of major cardiovascular events in patients with diabetes using primary care data. BMC Health Serv Res. 2016 May 02;16:110. doi: 10.1186/s12913-016-1361-2. https://bmchealthservres.biomedcentral.com/articles/10.1186/s12913-016-1361-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weinstein R, Ess K, Sirdar B, Song S, Cutting S. Primary Intraventricular Hemorrhage: Clinical Characteristics and Outcomes. J Stroke Cerebrovasc Dis. 2017 May;26(5):995–999. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.114. [DOI] [PubMed] [Google Scholar]
- 107.Wheater E, Mair G, Sudlow C, Alex B, Grover C, Whiteley W. A validated natural language processing algorithm for brain imaging phenotypes from radiology reports in UK electronic health records. BMC Med Inform Decis Mak. 2019 Sep 09;19(1):184. doi: 10.1186/s12911-019-0908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Amra S, O'Horo JC, Singh TD, Wilson GA, Kashyap R, Petersen R, Roberts RO, Fryer JD, Rabinstein AA, Gajic O. Derivation and validation of the automated search algorithms to identify cognitive impairment and dementia in electronic health records. J Crit Care. 2017 Feb;37:202–205. doi: 10.1016/j.jcrc.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 109.Anzaldi LJ, Davison A, Boyd CM, Leff B, Kharrazi H. Comparing clinician descriptions of frailty and geriatric syndromes using electronic health records: a retrospective cohort study. BMC Geriatr. 2017 Dec 25;17(1):248. doi: 10.1186/s12877-017-0645-7. https://bmcgeriatr.biomedcentral.com/articles/10.1186/s12877-017-0645-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Barnes DE, Zhou J, Walker RL, Larson EB, Lee SJ, Boscardin WJ, Marcum ZA, Dublin S. Development and Validation of eRADAR: A Tool Using EHR Data to Detect Unrecognized Dementia. J Am Geriatr Soc. 2020 Jan;68(1):103–111. doi: 10.1111/jgs.16182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Boustani M, Perkins AJ, Khandker RK, Duong S, Dexter PR, Lipton R, Black CM, Chandrasekaran V, Solid CA, Monahan P. Passive Digital Signature for Early Identification of Alzheimer's Disease and Related Dementia. J Am Geriatr Soc. 2020 Mar;68(3):511–518. doi: 10.1111/jgs.16218. [DOI] [PubMed] [Google Scholar]
- 112.Corradi JP, Chhabra J, Mather JF, Waszynski CM, Dicks RS. Analysis of multi-dimensional contemporaneous EHR data to refine delirium assessments. Comput Biol Med. 2016 Aug 01;75:267–74. doi: 10.1016/j.compbiomed.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 113.Ernecoff NC, Wessell KL, Gabriel S, Carey TS, Hanson LC. A Novel Screening Method to Identify Late-Stage Dementia Patients for Palliative Care Research and Practice. J Pain Symptom Manage. 2018 Apr;55(4):1152–1158.e1. doi: 10.1016/j.jpainsymman.2017.12.480. http://europepmc.org/abstract/MED/29288881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ford E, Rooney P, Oliver S, Hoile R, Hurley P, Banerjee S, van Marwijk H, Cassell J. Identifying undetected dementia in UK primary care patients: a retrospective case-control study comparing machine-learning and standard epidemiological approaches. BMC Med Inform Decis Mak. 2019 Dec 02;19(1):248. doi: 10.1186/s12911-019-0991-9. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-019-0991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Halpern R, Seare J, Tong J, Hartry A, Olaoye A, Aigbogun MS. Using electronic health records to estimate the prevalence of agitation in Alzheimer disease/dementia. Int J Geriatr Psychiatry. 2019 Mar;34(3):420–431. doi: 10.1002/gps.5030. http://europepmc.org/abstract/MED/30430642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jaakkimainen RL, Bronskill SE, Tierney MC, Herrmann N, Green D, Young J, Ivers N, Butt D, Widdifield J, Tu K. Identification of Physician-Diagnosed Alzheimer's Disease and Related Dementias in Population-Based Administrative Data: A Validation Study Using Family Physicians' Electronic Medical Records. J Alzheimers Dis. 2016 Aug 10;54(1):337–49. doi: 10.3233/JAD-160105. [DOI] [PubMed] [Google Scholar]
- 117.Kharrazi H, Anzaldi LJ, Hernandez L, Davison A, Boyd CM, Leff B, Kimura J, Weiner JP. The Value of Unstructured Electronic Health Record Data in Geriatric Syndrome Case Identification. J Am Geriatr Soc. 2018 Aug;66(8):1499–1507. doi: 10.1111/jgs.15411. [DOI] [PubMed] [Google Scholar]
- 118.Lewis G, Werbeloff N, Hayes JF, Howard R, Osborn DPJ. Diagnosed depression and sociodemographic factors as predictors of mortality in patients with dementia. Br J Psychiatry. 2018 Aug;213(2):471–476. doi: 10.1192/bjp.2018.86. http://europepmc.org/abstract/MED/29898791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McCoy TH, Han L, Pellegrini AM, Tanzi RE, Berretta S, Perlis RH. Stratifying risk for dementia onset using large-scale electronic health record data: A retrospective cohort study. Alzheimers Dement. 2020 Mar;16(3):531–540. doi: 10.1016/j.jalz.2019.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Perera G, Pedersen L, Ansel D, Alexander M, Arrighi HM, Avillach P, Foskett N, Gini R, Gordon MF, Gungabissoon U, Mayer M, Novak G, Rijnbeek P, Trifirò G, van der Lei J, Visser PJ, Stewart R. Dementia prevalence and incidence in a federation of European Electronic Health Record databases: The European Medical Informatics Framework resource. Alzheimers Dement. 2018 Feb;14(2):130–139. doi: 10.1016/j.jalz.2017.06.2270. https://linkinghub.elsevier.com/retrieve/pii/S1552-5260(17)32523-2. [DOI] [PubMed] [Google Scholar]
- 121.Pham TM, Petersen I, Walters K, Raine R, Manthorpe J, Mukadam N, Cooper C. Trends in dementia diagnosis rates in UK ethnic groups: analysis of UK primary care data. Clin Epidemiol. 2018;10:949–960. doi: 10.2147/CLEP.S152647. doi: 10.2147/CLEP.S152647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ponjoan A, Garre-Olmo J, Blanch J, Fages E, Alves-Cabratosa L, Martí-Lluch R, Comas-Cufí M, Parramon D, García-Gil M, Ramos R. How well can electronic health records from primary care identify Alzheimer's disease cases? Clin Epidemiol. 2019;11:509–518. doi: 10.2147/CLEP.S206770. doi: 10.2147/CLEP.S206770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ponjoan A, Garre-Olmo J, Blanch J, Fages E, Alves-Cabratosa L, Martí-Lluch R, Comas-Cufí M, Parramon D, Garcia-Gil M, Ramos R. Epidemiology of dementia: prevalence and incidence estimates using validated electronic health records from primary care. Clin Epidemiol. 2019;11:217–228. doi: 10.2147/CLEP.S186590. doi: 10.2147/CLEP.S186590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pujades-Rodriguez M, Assi V, Gonzalez-Izquierdo A, Wilkinson T, Schnier C, Sudlow C, Hemingway H, Whiteley WN. The diagnosis, burden and prognosis of dementia: A record-linkage cohort study in England. PLoS One. 2018;13(6):e0199026. doi: 10.1371/journal.pone.0199026. https://dx.plos.org/10.1371/journal.pone.0199026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Reuben DB, Hackbarth AS, Wenger NS, Tan ZS, Jennings LA. An Automated Approach to Identifying Patients with Dementia Using Electronic Medical Records. J Am Geriatr Soc. 2017 Mar;65(3):658–659. doi: 10.1111/jgs.14744. [DOI] [PubMed] [Google Scholar]
- 126.Sommerlad A, Perera G, Mueller C, Singh-Manoux A, Lewis G, Stewart R, Livingston G. Hospitalisation of people with dementia: evidence from English electronic health records from 2008 to 2016. Eur J Epidemiol. 2019 Jul;34(6):567–577. doi: 10.1007/s10654-019-00481-x. http://europepmc.org/abstract/MED/30649705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van Bussel EF, Richard E, Arts DL, Nooyens ACJ, Coloma PM, de Waal MWM, van den Akker M, Biermans MCJ, Nielen MMJ, van Boven K, Smeets H, Matthews FE, Brayne C, Busschers WB, van Gool WA, Moll van Charante EP. Dementia incidence trend over 1992-2014 in the Netherlands: Analysis of primary care data. PLoS Med. 2017 Mar;14(3):e1002235. doi: 10.1371/journal.pmed.1002235. https://dx.plos.org/10.1371/journal.pmed.1002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wei W, Teixeira PL, Mo H, Cronin RM, Warner JL, Denny JC. Combining billing codes, clinical notes, and medications from electronic health records provides superior phenotyping performance. J Am Med Inform Assoc. 2016 Apr;23(e1):e20–7. doi: 10.1093/jamia/ocv130. http://europepmc.org/abstract/MED/26338219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Williamson T, Green ME, Birtwhistle R, Khan S, Garies S, Wong ST, Natarajan N, Manca D, Drummond N. Validating the 8 CPCSSN case definitions for chronic disease surveillance in a primary care database of electronic health records. Ann Fam Med. 2014 Jul;12(4):367–72. doi: 10.1370/afm.1644. http://www.annfammed.org/cgi/pmidlookup?view=long&pmid=25024246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wu C, Kuo C, Su C, Wang S, Dai H. Using text mining to extract depressive symptoms and to validate the diagnosis of major depressive disorder from electronic health records. J Affect Disord. 2020 Jan 01;260:617–623. doi: 10.1016/j.jad.2019.09.044. [DOI] [PubMed] [Google Scholar]
- 131.Afzal Z, Engelkes M, Verhamme KMC, Janssens HM, Sturkenboom MCJM, Kors JA, Schuemie MJ. Automatic generation of case-detection algorithms to identify children with asthma from large electronic health record databases. Pharmacoepidemiol Drug Saf. 2013 Aug;22(8):826–33. doi: 10.1002/pds.3438. [DOI] [PubMed] [Google Scholar]
- 132.Akgün KM, Sigel K, Cheung K, Kidwai-Khan F, Bryant AK, Brandt C, Justice A, Crothers K. Extracting lung function measurements to enhance phenotyping of chronic obstructive pulmonary disease (COPD) in an electronic health record using automated tools. PLoS One. 2020;15(1):e0227730. doi: 10.1371/journal.pone.0227730. https://dx.plos.org/10.1371/journal.pone.0227730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Almoguera B, Vazquez L, Mentch F, Connolly J, Pacheco JA, Sundaresan AS, Peissig PL, Linneman JG, McCarty CA, Crosslin D, Carrell DS, Lingren T, Namjou-Khales B, Harley JB, Larson E, Jarvik GP, Brilliant M, Williams MS, Kullo IJ, Hysinger EB, Sleiman PMA, Hakonarson H. Identification of Four Novel Loci in Asthma in European American and African American Populations. Am J Respir Crit Care Med. 2017 Mar 15;195(4):456–463. doi: 10.1164/rccm.201604-0861OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Asche C, Said Q, Joish V, Hall CO, Brixner D. Assessment of COPD-related outcomes via a national electronic medical record database. Int J Chron Obstruct Pulmon Dis. 2008;3(2):323–6. doi: 10.2147/copd.s1857. https://www.dovepress.com/articles.php?article_id=1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Borlée F, Yzermans CJ, Krop E, Aalders B, Rooijackers J, Zock J, van Dijk CE, Maassen CBM, Schellevis F, Heederik D, Smit LAM. Spirometry, questionnaire and electronic medical record based COPD in a population survey: Comparing prevalence, level of agreement and associations with potential risk factors. PLoS One. 2017;12(3):e0171494. doi: 10.1371/journal.pone.0171494. https://dx.plos.org/10.1371/journal.pone.0171494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cave AJ, Davey C, Ahmadi E, Drummond N, Fuentes S, Kazemi-Bajestani SMR, Sharpe H, Taylor M. Development of a validated algorithm for the diagnosis of paediatric asthma in electronic medical records. NPJ Prim Care Respir Med. 2016 Dec 24;26:16085. doi: 10.1038/npjpcrm.2016.85. http://europepmc.org/abstract/MED/27882997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Crothers K, Rodriguez CV, Nance RM, Akgun K, Shahrir S, Kim J, W Soo Hoo G, Sharafkhaneh A, Crane HM, Justice AC. Accuracy of electronic health record data for the diagnosis of chronic obstructive pulmonary disease in persons living with HIV and uninfected persons. Pharmacoepidemiol Drug Saf. 2019 Feb;28(2):140–147. doi: 10.1002/pds.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.DiSantostefano RL, Sampson T, Le HV, Hinds D, Davis KJ, Bakerly ND. Risk of pneumonia with inhaled corticosteroid versus long-acting bronchodilator regimens in chronic obstructive pulmonary disease: a new-user cohort study. PLoS One. 2014;9(5):e97149. doi: 10.1371/journal.pone.0097149. https://dx.plos.org/10.1371/journal.pone.0097149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hsu J, Pacheco JA, Stevens WW, Smith ME, Avila PC. Accuracy of phenotyping chronic rhinosinusitis in the electronic health record. Am J Rhinol Allergy. 2014;28(2):140–4. doi: 10.2500/ajra.2014.28.4012. http://europepmc.org/abstract/MED/24717952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kadhim-Saleh A, Green M, Williamson T, Hunter D, Birtwhistle R. Validation of the diagnostic algorithms for 5 chronic conditions in the Canadian Primary Care Sentinel Surveillance Network (CPCSSN): a Kingston Practice-based Research Network (PBRN) report. J Am Board Fam Med. 2013;26(2):159–67. doi: 10.3122/jabfm.2013.02.120183. http://www.jabfm.org/cgi/pmidlookup?view=long&pmid=23471929. [DOI] [PubMed] [Google Scholar]
- 141.Kurmi OP, Vaucher J, Xiao D, Holmes MV, Guo Y, Davis KJ, Wang C, Qin H, Turnbull I, Peng P, Bian Z, Clarke R, Li L, Chen Y, Chen Z. Validity of COPD diagnoses reported through nationwide health insurance systems in the People's Republic of China. Int J Chron Obstruct Pulmon Dis. 2016;11:419–30. doi: 10.2147/COPD.S100736. doi: 10.2147/COPD.S100736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee TM, Tu K, Wing LL, Gershon AS. Identifying individuals with physician-diagnosed chronic obstructive pulmonary disease in primary care electronic medical records: a retrospective chart abstraction study. NPJ Prim Care Respir Med. 2017 May 15;27(1):34. doi: 10.1038/s41533-017-0035-9. http://europepmc.org/abstract/MED/28507288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liang H, Tsui BY, Ni H, Valentim CCS, Baxter SL, Liu G, Cai W, Kermany DS, Sun X, Chen J, He L, Zhu J, Tian P, Shao H, Zheng L, Hou R, Hewett S, Li G, Liang P, Zang X, Zhang Z, Pan L, Cai H, Ling R, Li S, Cui Y, Tang S, Ye H, Huang X, He W, Liang W, Zhang Q, Jiang J, Yu W, Gao J, Ou W, Deng Y, Hou Q, Wang B, Yao C, Liang Y, Zhang S, Duan Y, Zhang R, Gibson S, Zhang CL, Li O, Zhang ED, Karin G, Nguyen N, Wu X, Wen C, Xu J, Xu W, Wang B, Wang W, Li J, Pizzato B, Bao C, Xiang D, He W, He S, Zhou Y, Haw W, Goldbaum M, Tremoulet A, Hsu C, Carter H, Zhu L, Zhang K, Xia H. Evaluation and accurate diagnoses of pediatric diseases using artificial intelligence. Nat Med. 2019 Mar;25(3):433–438. doi: 10.1038/s41591-018-0335-9. [DOI] [PubMed] [Google Scholar]
- 144.Nissen F, Morales DR, Mullerova H, Smeeth L, Douglas IJ, Quint JK. Validation of asthma recording in the Clinical Practice Research Datalink (CPRD) BMJ Open. 2017 Aug 11;7(8):e017474. doi: 10.1136/bmjopen-2017-017474. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=28801439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nissen F, Morales DR, Mullerova H, Smeeth L, Douglas IJ, Quint JK. Concomitant diagnosis of asthma and COPD: a quantitative study in UK primary care. Br J Gen Pract. 2018 Dec;68(676):e775–e782. doi: 10.3399/bjgp18X699389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pacheco JA, Avila PC, Thompson JA, Law M, Quraishi JA, Greiman AK, Just EM, Kho A. A highly specific algorithm for identifying asthma cases and controls for genome-wide association studies. AMIA Annu Symp Proc. 2009 Dec 14;2009:497–501. [PMC free article] [PubMed] [Google Scholar]
- 147.Pennington AF, Strickland MJ, Freedle KA, Klein M, Drews-Botsch C, Hansen C, Darrow LA. Evaluating early-life asthma definitions as a marker for subsequent asthma in an electronic medical record setting. Pediatr Allergy Immunol. 2016 Sep;27(6):591–6. doi: 10.1111/pai.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rothnie KJ, Chandan JS, Goss HG, Müllerová H, Quint JK. Validity and interpretation of spirometric recordings to diagnose COPD in UK primary care. Int J Chron Obstruct Pulmon Dis. 2017;12:1663–1668. doi: 10.2147/COPD.S133891. doi: 10.2147/COPD.S133891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rothnie KJ, Müllerová H, Hurst JR, Smeeth L, Davis K, Thomas SL, Quint JK. Validation of the Recording of Acute Exacerbations of COPD in UK Primary Care Electronic Healthcare Records. PLoS One. 2016;11(3):e0151357. doi: 10.1371/journal.pone.0151357. http://dx.plos.org/10.1371/journal.pone.0151357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schulz S, Seddig T, Hanser S, Zaiss A, Daumke P. Checking coding completeness by mining discharge summaries. Stud Health Technol Inform. 2011;169:594–8. [PubMed] [Google Scholar]
- 151.Seol HY, Rolfes MC, Chung W, Sohn S, Ryu E, Park MA, Kita H, Ono J, Croghan I, Armasu SM, Castro-Rodriguez JA, Weston JD, Liu H, Juhn Y. Expert artificial intelligence-based natural language processing characterises childhood asthma. BMJ Open Resp Res. 2020 Feb 04;7(1):e000524. doi: 10.1136/bmjresp-2019-000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sohn S, Wang Y, Wi C, Krusemark EA, Ryu E, Ali MH, Juhn YJ, Liu H. Clinical documentation variations and NLP system portability: a case study in asthma birth cohorts across institutions. J Am Med Inform Assoc. 2017 Nov 30; doi: 10.1093/jamia/ocx138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sohn S, Wi C, Wu ST, Liu H, Ryu E, Krusemark E, Seabright A, Voge GA, Juhn YJ. Ascertainment of asthma prognosis using natural language processing from electronic medical records. J Allergy Clin Immunol. 2018 Jun;141(6):2292–2294.e3. doi: 10.1016/j.jaci.2017.12.1003. http://europepmc.org/abstract/MED/29438770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sperrin M, Webb DJ, Patel P, Davis KJ, Collier S, Pate A, Leather DA, Pimenta JM. Chronic obstructive pulmonary disease exacerbation episodes derived from electronic health record data validated using clinical trial data. Pharmacoepidemiol Drug Saf. 2019 Oct;28(10):1369–1376. doi: 10.1002/pds.4883. http://europepmc.org/abstract/MED/31385428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sundaresan AS, Schneider G, Reynolds J, Kirchner HL. Identifying Asthma Exacerbation-Related Emergency Department Visit Using Electronic Medical Record and Claims Data. Appl Clin Inform. 2018 Jul;9(3):528–540. doi: 10.1055/s-0038-1666994. http://www.thieme-connect.com/DOI/DOI?10.1055/s-0038-1666994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vazquez Guillamet R, Ursu O, Iwamoto G, Moseley PL, Oprea T. Chronic obstructive pulmonary disease phenotypes using cluster analysis of electronic medical records. Health Informatics J. 2018 Dec;24(4):394–409. doi: 10.1177/1460458216675661. https://journals.sagepub.com/doi/10.1177/1460458216675661?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed. [DOI] [PubMed] [Google Scholar]
- 157.Wi C, Sohn S, Ali M, Krusemark E, Ryu E, Liu H, Juhn YJ. Natural Language Processing for Asthma Ascertainment in Different Practice Settings. J Allergy Clin Immunol Pract. 2018;6(1):126–131. doi: 10.1016/j.jaip.2017.04.041. http://europepmc.org/abstract/MED/28634104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wi C, Sohn S, Rolfes MC, Seabright A, Ryu E, Voge G, Bachman KA, Park MA, Kita H, Croghan IT, Liu H, Juhn YJ. Application of a Natural Language Processing Algorithm to Asthma Ascertainment. An Automated Chart Review. Am J Respir Crit Care Med. 2017 Aug 15;196(4):430–437. doi: 10.1164/rccm.201610-2006OC. http://europepmc.org/abstract/MED/28375665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wu ST, Sohn S, Ravikumar KE, Wagholikar K, Jonnalagadda SR, Liu H, Juhn YJ. Automated chart review for asthma cohort identification using natural language processing: an exploratory study. Ann Allergy Asthma Immunol. 2013 Dec;111(5):364–9. doi: 10.1016/j.anai.2013.07.022. http://europepmc.org/abstract/MED/24125142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zeng QT, Goryachev S, Weiss S, Sordo M, Murphy SN, Lazarus R. Extracting principal diagnosis, co-morbidity and smoking status for asthma research: evaluation of a natural language processing system. BMC Med Inform Decis Mak. 2006;6:30. doi: 10.1186/1472-6947-6-30. http://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/1472-6947-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Barnado A, Casey C, Carroll RJ, Wheless L, Denny JC, Crofford LJ. Developing Electronic Health Record Algorithms That Accurately Identify Patients With Systemic Lupus Erythematosus. Arthritis Care Res (Hoboken) 2017 May;69(5):687–693. doi: 10.1002/acr.22989. http://europepmc.org/abstract/MED/27390187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Carroll RJ, Eyler AE, Denny JC. Naïve Electronic Health Record phenotype identification for Rheumatoid arthritis. AMIA Annu Symp Proc. 2011;2011:189–96. [PMC free article] [PubMed] [Google Scholar]
- 163.Carroll RJ, Thompson WK, Eyler AE, Mandelin AM, Cai T, Zink RM, Pacheco JA, Boomershine CS, Lasko TA, Xu H, Karlson EW, Perez RG, Gainer VS, Murphy SN, Ruderman EM, Pope RM, Plenge RM, Kho AN, Liao KP, Denny JC. Portability of an algorithm to identify rheumatoid arthritis in electronic health records. J Am Med Inform Assoc. 2012 Jun;19(e1):e162–9. doi: 10.1136/amiajnl-2011-000583. http://europepmc.org/abstract/MED/22374935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cote J, Berger A, Kirchner LH, Bili A. Low vitamin D level is not associated with increased incidence of rheumatoid arthritis. Rheumatol Int. 2014 Oct;34(10):1475–9. doi: 10.1007/s00296-014-3019-x. [DOI] [PubMed] [Google Scholar]
- 165.de Abreu MM, Maiorano AC, Tedeschi SK, Yoshida K, Lin T, Solomon DH. Outcomes of lupus and rheumatoid arthritis patients with primary dengue infection: A seven-year report from Brazil. Semin Arthritis Rheum. 2018 Apr;47(5):749–755. doi: 10.1016/j.semarthrit.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 166.Escudié J, Rance B, Malamut G, Khater S, Burgun A, Cellier C, Jannot A. A novel data-driven workflow combining literature and electronic health records to estimate comorbidities burden for a specific disease: a case study on autoimmune comorbidities in patients with celiac disease. BMC Med Inform Decis Mak. 2017 Oct 29;17(1):140. doi: 10.1186/s12911-017-0537-y. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-017-0537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ford E, Carroll J, Smith H, Davies K, Koeling R, Petersen I, Rait G, Cassell J. What evidence is there for a delay in diagnostic coding of RA in UK general practice records? An observational study of free text. BMJ Open. 2016 Jun 28;6(6):e010393. doi: 10.1136/bmjopen-2015-010393. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=27354069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ford E, Nicholson A, Koeling R, Tate A, Carroll J, Axelrod L, Smith HE, Rait G, Davies KA, Petersen I, Williams T, Cassell JA. Optimising the use of electronic health records to estimate the incidence of rheumatoid arthritis in primary care: what information is hidden in free text? BMC Med Res Methodol. 2013 Aug 21;13:105. doi: 10.1186/1471-2288-13-105. https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/1471-2288-13-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jamian L, Wheless L, Crofford LJ, Barnado A. Rule-based and machine learning algorithms identify patients with systemic sclerosis accurately in the electronic health record. Arthritis Res Ther. 2019 Dec 30;21(1):305. doi: 10.1186/s13075-019-2092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jorge A, Castro VM, Barnado A, Gainer V, Hong C, Cai T, Cai T, Carroll R, Denny JC, Crofford L, Costenbader KH, Liao KP, Karlson EW, Feldman CH. Identifying lupus patients in electronic health records: Development and validation of machine learning algorithms and application of rule-based algorithms. Semin Arthritis Rheum. 2019 Aug;49(1):84–90. doi: 10.1016/j.semarthrit.2019.01.002. http://europepmc.org/abstract/MED/30665626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kronzer VL, Wang L, Liu H, Davis JM, Sparks JA, Crowson CS. Investigating the impact of disease and health record duration on the eMERGE algorithm for rheumatoid arthritis. J Am Med Inform Assoc. 2020 May 01;27(4):601–605. doi: 10.1093/jamia/ocaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liao KP, Cai T, Gainer V, Goryachev S, Zeng-treitler Q, Raychaudhuri S, Szolovits P, Churchill S, Murphy S, Kohane I, Karlson EW, Plenge RM. Electronic medical records for discovery research in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2010 Aug;62(8):1120–7. doi: 10.1002/acr.20184. doi: 10.1002/acr.20184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lin C, Karlson EW, Canhao H, Miller TA, Dligach D, Chen PJ, Perez RNG, Shen Y, Weinblatt ME, Shadick NA, Plenge RM, Savova GK. Automatic prediction of rheumatoid arthritis disease activity from the electronic medical records. PLoS One. 2013;8(8):e69932. doi: 10.1371/journal.pone.0069932. https://dx.plos.org/10.1371/journal.pone.0069932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Muller S, Hider SL, Raza K, Stack RJ, Hayward RA, Mallen CD. An algorithm to identify rheumatoid arthritis in primary care: a Clinical Practice Research Datalink study. BMJ Open. 2015 Dec 23;5(12):e009309. doi: 10.1136/bmjopen-2015-009309. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=26700281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Murray SG, Avati A, Schmajuk G, Yazdany J. Automated and flexible identification of complex disease: building a model for systemic lupus erythematosus using noisy labeling. J Am Med Inform Assoc. 2019 Jan 01;26(1):61–65. doi: 10.1093/jamia/ocy154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nicholson A, Ford E, Davies KA, Smith HE, Rait G, Tate AR, Petersen I, Cassell J. Optimising use of electronic health records to describe the presentation of rheumatoid arthritis in primary care: a strategy for developing code lists. PLoS One. 2013;8(2):e54878. doi: 10.1371/journal.pone.0054878. https://dx.plos.org/10.1371/journal.pone.0054878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nielen MMJ, Ursum J, Schellevis FG, Korevaar JC. The validity of the diagnosis of inflammatory arthritis in a large population-based primary care database. BMC Fam Pract. 2013 Jul 07;14:79. doi: 10.1186/1471-2296-14-79. https://bmcfampract.biomedcentral.com/articles/10.1186/1471-2296-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nikiphorou E, de Lusignan S, Mallen CD, Khavandi K, Bedarida G, Buckley CD, Galloway J, Raza K. Cardiovascular risk factors and outcomes in early rheumatoid arthritis: a population-based study. Heart. 2020 Mar 24; doi: 10.1136/heartjnl-2019-316193. http://heart.bmj.com/cgi/pmidlookup?view=long&pmid=32209618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Partington RJ, Muller S, Helliwell T, Mallen CD, Abdul Sultan A. Incidence, prevalence and treatment burden of polymyalgia rheumatica in the UK over two decades: a population-based study. Ann Rheum Dis. 2018 Dec;77(12):1750–1756. doi: 10.1136/annrheumdis-2018-213883. [DOI] [PubMed] [Google Scholar]
- 180.Redd D, Frech TM, Murtaugh MA, Rhiannon J, Zeng QT. Informatics can identify systemic sclerosis (SSc) patients at risk for scleroderma renal crisis. Comput Biol Med. 2014 Oct;53:203–5. doi: 10.1016/j.compbiomed.2014.07.022. http://europepmc.org/abstract/MED/25168254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ritchie MD, Denny JC, Crawford DC, Ramirez AH, Weiner JB, Pulley JM, Basford MA, Brown-Gentry K, Balser JR, Masys DR, Haines JL, Roden DM. Robust replication of genotype-phenotype associations across multiple diseases in an electronic medical record. Am J Hum Genet. 2010 Apr 09;86(4):560–72. doi: 10.1016/j.ajhg.2010.03.003. http://linkinghub.elsevier.com/retrieve/pii/S0002-9297(10)00146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Turner CA, Jacobs AD, Marques CK, Oates JC, Kamen DL, Anderson PE, Obeid JS. Word2Vec inversion and traditional text classifiers for phenotyping lupus. BMC Med Inform Decis Mak. 2017 Aug 22;17(1):126. doi: 10.1186/s12911-017-0518-1. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-017-0518-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Verma A, Basile AO, Bradford Y, Kuivaniemi H, Tromp G, Carey D, Gerhard GS, Crowe JE, Ritchie MD, Pendergrass SA. Phenome-Wide Association Study to Explore Relationships between Immune System Related Genetic Loci and Complex Traits and Diseases. PLoS One. 2016;11(8):e0160573. doi: 10.1371/journal.pone.0160573. https://dx.plos.org/10.1371/journal.pone.0160573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang L, Rastegar-Mojarad M, Ji Z, Liu S, Liu K, Moon S, Shen F, Wang Y, Yao L, Davis Iii JM, Liu H. Detecting Pharmacovigilance Signals Combining Electronic Medical Records With Spontaneous Reports: A Case Study of Conventional Disease-Modifying Antirheumatic Drugs for Rheumatoid Arthritis. Front Pharmacol. 2018;9:875. doi: 10.3389/fphar.2018.00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhou S, Fernandez-Gutierrez F, Kennedy J, Cooksey R, Atkinson M, Denaxas S, Siebert S, Dixon WG, O'Neill TW, Choy E, Sudlow C, UK Biobank Follow-upOutcomes Group. Brophy S. Defining Disease Phenotypes in Primary Care Electronic Health Records by a Machine Learning Approach: A Case Study in Identifying Rheumatoid Arthritis. PLoS One. 2016;11(5):e0154515. doi: 10.1371/journal.pone.0154515. http://dx.plos.org/10.1371/journal.pone.0154515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gill JM, Mainous AG, Koopman RJ, Player MS, Everett CJ, Chen YX, Diamond JJ, Lieberman MI. Impact of EHR-based clinical decision support on adherence to guidelines for patients on NSAIDs: a randomized controlled trial. Ann Fam Med. 2011;9(1):22–30. doi: 10.1370/afm.1172. http://www.annfammed.org/cgi/pmidlookup?view=long&pmid=21242557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Salmasian H, Freedberg DE, Abrams JA, Friedman C. An automated tool for detecting medication overuse based on the electronic health records. Pharmacoepidemiol Drug Saf. 2013 Feb;22(2):183–9. doi: 10.1002/pds.3387. http://europepmc.org/abstract/MED/23233423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shelat VG, Ahmed S, Chia CLK, Cheah YL. Strict Selection Criteria During Surgical Training Ensures Good Outcomes in Laparoscopic Omental Patch Repair (LOPR) for Perforated Peptic Ulcer (PPU) Int Surg. 2015 Mar;100(2):370–5. doi: 10.9738/INTSURG-D-13-00241.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Singh B, Singh A, Ahmed A, Wilson GA, Pickering BW, Herasevich V, Gajic O, Li G. Derivation and validation of automated electronic search strategies to extract Charlson comorbidities from electronic medical records. Mayo Clin Proc. 2012 Sep;87(9):817–24. doi: 10.1016/j.mayocp.2012.04.015. http://europepmc.org/abstract/MED/22958988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ahmad FS, Chan C, Rosenman MB, Post WS, Fort DG, Greenland P, Liu KJ, Kho AN, Allen NB. Validity of Cardiovascular Data From Electronic Sources: The Multi-Ethnic Study of Atherosclerosis and HealthLNK. Circulation. 2017 Oct 26;136(13):1207–1216. doi: 10.1161/CIRCULATIONAHA.117.027436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chi GC, Li X, Tartof SY, Slezak JM, Koebnick C, Lawrence JM. Validity of ICD-10-CM codes for determination of diabetes type for persons with youth-onset type 1 and type 2 diabetes. BMJ Open Diabetes Res Care. 2019;7(1):e000547. doi: 10.1136/bmjdrc-2018-000547. https://drc.bmj.com/cgi/pmidlookup?view=long&pmid=30899525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Coleman N, Halas G, Peeler W, Casaclang N, Williamson T, Katz A. From patient care to research: a validation study examining the factors contributing to data quality in a primary care electronic medical record database. BMC Fam Pract. 2015 Feb 05;16:11. doi: 10.1186/s12875-015-0223-z. https://bmcfampract.biomedcentral.com/articles/10.1186/s12875-015-0223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Crawford AG, Cote C, Couto J, Daskiran M, Gunnarsson C, Haas K, Haas S, Nigam SC, Schuette R. Prevalence of obesity, type II diabetes mellitus, hyperlipidemia, and hypertension in the United States: findings from the GE Centricity Electronic Medical Record database. Popul Health Manag. 2010 Jul;13(3):151–61. doi: 10.1089/pop.2009.0039. [DOI] [PubMed] [Google Scholar]
- 194.Esteban S, Rodríguez Tablado M, Peper F, Mahumud YS, Ricci RI, Kopitowski K, Terrasa S. Development and Validation of Various Phenotyping Algorithms for Diabetes Mellitus Using Data from Electronic Health Records. Stud Health Technol Inform. 2017;245:366–369. [PubMed] [Google Scholar]
- 195.Gjelsvik B, Tran AT, Berg TJ, Bakke �, Mdala I, Nøkleby K, Cooper JG, Claudi T, Løvaas KF, Thue G, Sandberg S, Jenum AK. Exploring the relationship between coronary heart disease and type 2 diabetes: a cross-sectional study of secondary prevention among diabetes patients. BJGP Open. 2019 May;3(1):bjgpopen18X101636. doi: 10.3399/bjgpopen18X101636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Harris SB, Glazier RH, Tompkins JW, Wilton AS, Chevendra V, Stewart MA, Thind A. Investigating concordance in diabetes diagnosis between primary care charts (electronic medical records) and health administrative data: a retrospective cohort study. BMC Health Serv Res. 2010 Dec 23;10:347. doi: 10.1186/1472-6963-10-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Henderson J, Barnett S, Ghosh A, Pollack AJ, Hodgkins A, Win KT, Miller GC, Bonney A. Validation of electronic medical data: Identifying diabetes prevalence in general practice. Health Inf Manag. 2019 Jan;48(1):3–11. doi: 10.1177/1833358318798123. [DOI] [PubMed] [Google Scholar]
- 198.Ho ML, Lawrence N, van Walraven C, Manuel D, Keely E, Malcolm J, Reid RD, Forster AJ. The accuracy of using integrated electronic health care data to identify patients with undiagnosed diabetes mellitus. J Eval Clin Pract. 2012 Jul;18(3):606–11. doi: 10.1111/j.1365-2753.2011.01633.x. [DOI] [PubMed] [Google Scholar]
- 199.Kadhim-Saleh A, Green M, Williamson T, Hunter D, Birtwhistle R. Validation of the diagnostic algorithms for 5 chronic conditions in the Canadian Primary Care Sentinel Surveillance Network (CPCSSN): a Kingston Practice-based Research Network (PBRN) report. J Am Board Fam Med. 2013;26(2):159–67. doi: 10.3122/jabfm.2013.02.120183. http://www.jabfm.org/cgi/pmidlookup?view=long&pmid=23471929. [DOI] [PubMed] [Google Scholar]
- 200.Ke C, Stukel TA, Luk A, Shah BR, Jha P, Lau E, Ma RCW, So W, Kong AP, Chow E, Chan JCN. Development and validation of algorithms to classify type 1 and 2 diabetes according to age at diagnosis using electronic health records. BMC Med Res Methodol. 2020 Feb 24;20(1):35. doi: 10.1186/s12874-020-00921-3. https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/s12874-020-00921-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kho AN, Hayes MG, Rasmussen-Torvik L, Pacheco JA, Thompson WK, Armstrong LL, Denny JC, Peissig PL, Miller AW, Wei W, Bielinski SJ, Chute CG, Leibson CL, Jarvik GP, Crosslin DR, Carlson CS, Newton KM, Wolf WA, Chisholm RL, Lowe WL. Use of diverse electronic medical record systems to identify genetic risk for type 2 diabetes within a genome-wide association study. J Am Med Inform Assoc. 2012;19(2):212–8. doi: 10.1136/amiajnl-2011-000439. http://europepmc.org/abstract/MED/22101970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Khokhar B, Quan H, Kaplan GG, Butalia S, Rabi D. Exploring novel diabetes surveillance methods: a comparison of administrative, laboratory and pharmacy data case definitions using THIN. J Public Health (Oxf) 2018 Sep 01;40(3):652–658. doi: 10.1093/pubmed/fdx096. [DOI] [PubMed] [Google Scholar]
- 203.Klompas M, Eggleston E, McVetta J, Lazarus R, Li L, Platt R. Automated detection and classification of type 1 versus type 2 diabetes using electronic health record data. Diabetes Care. 2013 Apr;36(4):914–21. doi: 10.2337/dc12-0964. http://europepmc.org/abstract/MED/23193215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kosowan L, Wicklow B, Queenan J, Yeung R, Amed S, Singer A. Enhancing Health Surveillance: Validation of a Novel Electronic Medical Records-Based Definition of Cases of Pediatric Type 1 and Type 2 Diabetes Mellitus. Can J Diabetes. 2019 Aug;43(6):392–398. doi: 10.1016/j.jcjd.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 205.Kudyakov R, Bowen J, Ewen E, West SL, Daoud Y, Fleming N, Masica A. Electronic health record use to classify patients with newly diagnosed versus preexisting type 2 diabetes: infrastructure for comparative effectiveness research and population health management. Popul Health Manag. 2012 Mar;15(1):3–11. doi: 10.1089/pop.2010.0084. [DOI] [PubMed] [Google Scholar]
- 206.Lawrence JM, Black MH, Zhang JL, Slezak JM, Takhar HS, Koebnick C, Mayer-Davis EJ, Zhong VW, Dabelea D, Hamman RF, Reynolds K. Validation of pediatric diabetes case identification approaches for diagnosed cases by using information in the electronic health records of a large integrated managed health care organization. Am J Epidemiol. 2014 Jan 01;179(1):27–38. doi: 10.1093/aje/kwt230. [DOI] [PubMed] [Google Scholar]
- 207.Lipscombe LL, Hwee J, Webster L, Shah BR, Booth GL, Tu K. Identifying diabetes cases from administrative data: a population-based validation study. BMC Health Serv Res. 2018 May 02;18(1):316. doi: 10.1186/s12913-018-3148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Makam AN, Nguyen OK, Moore B, Ma Y, Amarasingham R. Identifying patients with diabetes and the earliest date of diagnosis in real time: an electronic health record case-finding algorithm. BMC Med Inform Decis Mak. 2013 Aug 01;13:81. doi: 10.1186/1472-6947-13-81. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/1472-6947-13-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Moreno-Iribas C, Sayon-Orea C, Delfrade J, Ardanaz E, Gorricho J, Burgui R, Nuin M, Guevara M. Validity of type 2 diabetes diagnosis in a population-based electronic health record database. BMC Med Inform Decis Mak. 2017 Apr 08;17(1):34. doi: 10.1186/s12911-017-0439-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nichols GA, Desai J, Elston LJ, Lawrence JM, O'Connor PJ, Pathak RD, Raebel MA, Reid RJ, Selby JV, Silverman BG, Steiner JF, Stewart WF, Vupputuri S, Waitzfelder B, SUPREME- DG. Construction of a multisite DataLink using electronic health records for the identification, surveillance, prevention, and management of diabetes mellitus: the SUPREME-DM project. Prev Chronic Dis. 2012;9:E110. doi: 10.5888/pcd9.110311. http://www.cdc.gov/pcd/issues/2012/11_0311.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Nichols GA, Schroeder EB, Karter AJ, Gregg EW, Desai J, Lawrence JM, O'Connor PJ, Xu S, Newton KM, Raebel MA, Pathak RD, Waitzfelder B, Segal J, Lafata JE, Butler MG, Kirchner HL, Thomas A, Steiner JF, SUPREME- DG. Trends in diabetes incidence among 7 million insured adults, 2006-2011: the SUPREME-DM project. Am J Epidemiol. 2015 Jan 1;181(1):32–9. doi: 10.1093/aje/kwu255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Young JB, Gauthier-Loiselle M, Bailey RA, Manceur AM, Lefebvre P, Greenberg M, Lafeuille M, Duh MS, Bookhart B, Wysham CH. Development of predictive risk models for major adverse cardiovascular events among patients with type 2 diabetes mellitus using health insurance claims data. Cardiovasc Diabetol. 2018 Aug 24;17(1):118. doi: 10.1186/s12933-018-0759-z. https://cardiab.biomedcentral.com/articles/10.1186/s12933-018-0759-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Pacheco JA, Thompson W, Kho A. Automatically detecting problem list omissions of type 2 diabetes cases using electronic medical records. AMIA Annu Symp Proc. 2011;2011:1062–9. http://europepmc.org/abstract/MED/22195167. [PMC free article] [PubMed] [Google Scholar]
- 214.Pantalone KM, Misra-Hebert AD, Hobbs TM, Wells BJ, Kong SX, Chagin K, Dey T, Milinovich A, Weng W, Bauman JM, Burguera B, Zimmerman RS, Kattan MW. Effect of glycemic control on the Diabetes Complications Severity Index score and development of complications in people with newly diagnosed type 2 diabetes. J Diabetes. 2018 Mar;10(3):192–199. doi: 10.1111/1753-0407.12613. [DOI] [PubMed] [Google Scholar]
- 215.Paul SK, Shaw JE, Montvida O, Klein K. Weight gain in insulin-treated patients by body mass index category at treatment initiation: new evidence from real-world data in patients with type 2 diabetes. Diabetes Obes Metab. 2016 Dec;18(12):1244–1252. doi: 10.1111/dom.12761. [DOI] [PubMed] [Google Scholar]
- 216.Richesson RL, Rusincovitch SA, Wixted D, Batch BC, Feinglos MN, Miranda ML, Hammond WE, Califf RM, Spratt SE. A comparison of phenotype definitions for diabetes mellitus. J Am Med Inform Assoc. 2013 Dec;20(e2):e319–26. doi: 10.1136/amiajnl-2013-001952. http://europepmc.org/abstract/MED/24026307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ritchie MD, Denny JC, Crawford DC, Ramirez AH, Weiner JB, Pulley JM, Basford MA, Brown-Gentry K, Balser JR, Masys DR, Haines JL, Roden DM. Robust replication of genotype-phenotype associations across multiple diseases in an electronic medical record. Am J Hum Genet. 2010 Apr 09;86(4):560–72. doi: 10.1016/j.ajhg.2010.03.003. http://linkinghub.elsevier.com/retrieve/pii/S0002-9297(10)00146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rodgers LR, Weedon MN, Henley WE, Hattersley AT, Shields BM. Cohort profile for the MASTERMIND study: using the Clinical Practice Research Datalink (CPRD) to investigate stratification of response to treatment in patients with type 2 diabetes. BMJ Open. 2017 Oct 12;7(10):e017989. doi: 10.1136/bmjopen-2017-017989. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=29025846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Schroeder EB, Donahoo WT, Goodrich GK, Raebel MA. Validation of an algorithm for identifying type 1 diabetes in adults based on electronic health record data. Pharmacoepidemiol Drug Saf. 2018 Oct;27(10):1053–1059. doi: 10.1002/pds.4377. http://europepmc.org/abstract/MED/29292555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sharma M, Petersen I, Nazareth I, Coton SJ. An algorithm for identification and classification of individuals with type 1 and type 2 diabetes mellitus in a large primary care database. Clin Epidemiol. 2016;8:373–380. doi: 10.2147/CLEP.S113415. doi: 10.2147/CLEP.S113415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Spratt SE, Pereira K, Granger BB, Batch BC, Phelan M, Pencina M, Miranda ML, Boulware E, Lucas JE, Nelson CL, Neely B, Goldstein BA, Barth P, Richesson RL, Riley IL, Corsino L, McPeek Hinz ER, Rusincovitch S, Green J, Barton AB, Phenotype Group DDC, Kelley C, Hyland K, Tang M, Elliott A, Ruel E, Clark A, Mabrey M, Morrissey KL, Rao J, Hong B, Pierre-Louis M, Kelly K, Jelesoff N. Assessing electronic health record phenotypes against gold-standard diagnostic criteria for diabetes mellitus. J Am Med Inform Assoc. 2017 May 01;24(e1):e121–e128. doi: 10.1093/jamia/ocw123. http://europepmc.org/abstract/MED/27616701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Teltsch DY, Fazeli Farsani S, Swain RS, Kaspers S, Huse S, Cristaldi C, Nordstrom BL, Brodovicz KG. Development and validation of algorithms to identify newly diagnosed type 1 and type 2 diabetes in pediatric population using electronic medical records and claims data. Pharmacoepidemiol Drug Saf. 2019 Feb;28(2):234–243. doi: 10.1002/pds.4728. [DOI] [PubMed] [Google Scholar]
- 223.Tu K, Manuel D, Lam K, Kavanagh D, Mitiku TF, Guo H. Diabetics can be identified in an electronic medical record using laboratory tests and prescriptions. J Clin Epidemiol. 2011 May;64(4):431–5. doi: 10.1016/j.jclinepi.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 224.Upadhyaya SG, Murphree DH, Ngufor CG, Knight AM, Cronk DJ, Cima RR, Curry TB, Pathak J, Carter RE, Kor DJ. Automated Diabetes Case Identification Using Electronic Health Record Data at a Tertiary Care Facility. Mayo Clin Proc Innov Qual Outcomes. 2017 Jul;1(1):100–110. doi: 10.1016/j.mayocpiqo.2017.04.005. https://linkinghub.elsevier.com/retrieve/pii/S2542-4548(17)30008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wei W, Leibson CL, Ransom JE, Kho AN, Caraballo PJ, Chai HS, Yawn BP, Pacheco JA, Chute CG. Impact of data fragmentation across healthcare centers on the accuracy of a high-throughput clinical phenotyping algorithm for specifying subjects with type 2 diabetes mellitus. J Am Med Inform Assoc. 2012;19(2):219–24. doi: 10.1136/amiajnl-2011-000597. http://europepmc.org/abstract/MED/22249968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wiese AD, Roumie CL, Buse JB, Guzman H, Bradford R, Zalimeni E, Knoepp P, Morris HL, Donahoo WT, Fanous N, Epstein BF, Katalenich BL, Ayala SG, Cook MM, Worley KJ, Bachmann KN, Grijalva CG, Rothman RL, Chakkalakal RJ. Performance of a computable phenotype for identification of patients with diabetes within PCORnet: The Patient-Centered Clinical Research Network. Pharmacoepidemiol Drug Saf. 2019 May;28(5):632–639. doi: 10.1002/pds.4718. http://europepmc.org/abstract/MED/30680840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Williams BA, Geba D, Cordova JM, Shetty SS. A risk prediction model for heart failure hospitalization in type 2 diabetes mellitus. Clin Cardiol. 2020 Mar;43(3):275–283. doi: 10.1002/clc.23298. doi: 10.1002/clc.23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wysham CH, Lefebvre P, Pilon D, Lafeuille M, Emond B, Kamstra R, Pfeifer M, Duh MS, Ingham M. An investigation into the durability of glycemic control in patients with type II diabetes initiated on canagliflozin or sitagliptin: A real-world analysis of electronic medical records. J Diabetes Complications. 2019 Feb;33(2):140–147. doi: 10.1016/j.jdiacomp.2018.10.016. https://linkinghub.elsevier.com/retrieve/pii/S1056-8727(18)30329-5. [DOI] [PubMed] [Google Scholar]
- 229.Yang F, Ma Q, Liu J, Ma B, Guo M, Liu F, Li J, Wang Z, Liu M. Prevalence and major risk factors of type 2 diabetes mellitus among adult psychiatric inpatients from 2005 to 2018 in Beijing, China: a longitudinal observational study. BMJ Open Diabetes Res Care. 2020 Mar;8(1) doi: 10.1136/bmjdrc-2019-000996. https://drc.bmj.com/lookup/pmidlookup?view=long&pmid=32139600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yue X, Wu J, Ruan Z, Wolden ML, Li L, Lin Y. The Burden of Hypoglycemia in Patients With Insulin-Treated Diabetes Mellitus in China: Analysis of Electronic Medical Records From 4 Tertiary Hospitals. Value Health Reg Issues. 2020 May;21:17–21. doi: 10.1016/j.vhri.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 231.Zheng L, Wang Y, Hao S, Shin AY, Jin B, Ngo AD, Jackson-Browne MS, Feller DJ, Fu T, Zhang K, Zhou X, Zhu C, Dai D, Yu Y, Zheng G, Li Y, McElhinney DB, Culver DS, Alfreds ST, Stearns F, Sylvester KG, Widen E, Ling XB. Web-based Real-Time Case Finding for the Population Health Management of Patients With Diabetes Mellitus: A Prospective Validation of the Natural Language Processing-Based Algorithm With Statewide Electronic Medical Records. JMIR Med Inform. 2016 Nov 11;4(4):e37. doi: 10.2196/medinform.6328. http://medinform.jmir.org/2016/4/e37/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zheng T, Xie W, Xu L, He X, Zhang Y, You M, Yang G, Chen Y. A machine learning-based framework to identify type 2 diabetes through electronic health records. Int J Med Inform. 2017 Dec;97:120–127. doi: 10.1016/j.ijmedinf.2016.09.014. http://europepmc.org/abstract/MED/27919371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhong VW, Obeid JS, Craig JB, Pfaff ER, Thomas J, Jaacks LM, Beavers DP, Carey TS, Lawrence JM, Dabelea D, Hamman RF, Bowlby DA, Pihoker C, Saydah SH, Mayer-Davis EJ. An efficient approach for surveillance of childhood diabetes by type derived from electronic health record data: the SEARCH for Diabetes in Youth Study. J Am Med Inform Assoc. 2016 Nov;23(6):1060–1067. doi: 10.1093/jamia/ocv207. http://europepmc.org/abstract/MED/27107449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhong VW, Pfaff ER, Beavers DP, Thomas J, Jaacks LM, Bowlby DA, Carey TS, Lawrence JM, Dabelea D, Hamman RF, Pihoker C, Saydah SH, Mayer-Davis EJ, Search for Diabetes in Youth Study Group Use of administrative and electronic health record data for development of automated algorithms for childhood diabetes case ascertainment and type classification: the SEARCH for Diabetes in Youth Study. Pediatr Diabetes. 2014 Dec;15(8):573–84. doi: 10.1111/pedi.12152. http://europepmc.org/abstract/MED/24913103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Agrawal S, Kremsdorf R, Uysal S, Fredette ME, Topor LS. Nephrolithiasis: A complication of pediatric diabetic ketoacidosis. Pediatr Diabetes. 2018 Mar;19(2):329–332. doi: 10.1111/pedi.12559. [DOI] [PubMed] [Google Scholar]
- 236.Cahn A, Altaras T, Agami T, Liran O, Touaty CE, Drahy M, Pollack R, Raz I, Chodick G, Zucker I. Validity of diagnostic codes and estimation of prevalence of diabetic foot ulcers using a large electronic medical record database. Diabetes Metab Res Rev. 2019 Feb;35(2):e3094. doi: 10.1002/dmrr.3094. [DOI] [PubMed] [Google Scholar]
- 237.Dong Y, Gao W, Zhang L, Wei J, Hammar N, Cabrera CS, Wu X, Qiao Q. Patient characteristics related to metabolic disorders and chronic complications in type 2 diabetes mellitus patients hospitalized at the Qingdao Endocrine and Diabetes Hospital from 2006 to 2012 in China. Diab Vasc Dis Res. 2017 Jan;14(1):24–32. doi: 10.1177/1479164116675489. https://journals.sagepub.com/doi/10.1177/1479164116675489?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed. [DOI] [PubMed] [Google Scholar]
- 238.DuBrava S, Mardekian J, Sadosky A, Bienen EJ, Parsons B, Hopps M, Markman J. Using Random Forest Models to Identify Correlates of a Diabetic Peripheral Neuropathy Diagnosis from Electronic Health Record Data. Pain Med. 2017 Dec 01;18(1):107–115. doi: 10.1093/pm/pnw096. [DOI] [PubMed] [Google Scholar]
- 239.Lee CS, Lee AY, Baughman D, Sim D, Akelere T, Brand C, Crabb DP, Denniston AK, Downey L, Fitt A, Khan R, Mahmood S, Mandal K, Mckibbin M, Menon G, Lobo A, Kumar BV, Natha S, Varma A, Wilkinson E, Mitry D, Bailey C, Chakravarthy U, Tufail A, Egan C, UK DR EMR Users Group The United Kingdom Diabetic Retinopathy Electronic Medical Record Users Group: Report 3: Baseline Retinopathy and Clinical Features Predict Progression of Diabetic Retinopathy. Am J Ophthalmol. 2017 Aug;180:64–71. doi: 10.1016/j.ajo.2017.05.020. http://europepmc.org/abstract/MED/28572062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Martín-Merino E, Fortuny J, Rivero-Ferrer E, García-Rodríguez LA. Incidence of retinal complications in a cohort of newly diagnosed diabetic patients. PLoS One. 2014;9(6):e100283. doi: 10.1371/journal.pone.0100283. https://dx.plos.org/10.1371/journal.pone.0100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Song X, Waitman LR, Hu Y, Yu ASL, Robins D, Liu M. Robust clinical marker identification for diabetic kidney disease with ensemble feature selection. J Am Med Inform Assoc. 2019 Mar 01;26(3):242–253. doi: 10.1093/jamia/ocy165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.VanderWeele J, Pollack T, Oakes DJ, Smyrniotis C, Illuri V, Vellanki P, O'Leary K, Holl J, Aleppo G, Molitch ME, Wallia A. Validation of data from electronic data warehouse in diabetic ketoacidosis: Caution is needed. J Diabetes Complications. 2018 Jul;32(7):650–654. doi: 10.1016/j.jdiacomp.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 243.Abhyankar S, Demner-Fushman D, Callaghan FM, McDonald CJ. Combining structured and unstructured data to identify a cohort of ICU patients who received dialysis. J Am Med Inform Assoc. 2014;21(5):801–7. doi: 10.1136/amiajnl-2013-001915. http://europepmc.org/abstract/MED/24384230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Afzal Z, Schuemie MJ, van Blijderveen JC, Sen EF, Sturkenboom MCJM, Kors JA. Improving sensitivity of machine learning methods for automated case identification from free-text electronic medical records. BMC Med Inform Decis Mak. 2013 Mar 02;13:30. doi: 10.1186/1472-6947-13-30. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/1472-6947-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Alcober-Morte L, Barrio-Ruiz C, Parellada-Esquius N, Subirana I, Comín-Colet J, Grau M, Dégano IR, Cainzos-Achirica M, Cunillera-Puértolas O, Cobo-Guerrero S, Mestre-Ferrer J, Pascual-Benito L, Cerain-Herrero MJ, Gil-Terrón N, Rodríguez-Latre L, Tamayo-Ojeda C, Salvador-González B. Heart failure admission across glomerular filtration rate categories in a community cohort of 125,053 individuals over 60 years of age. Hypertens Res. 2019 Dec;42(12):2013–2020. doi: 10.1038/s41440-019-0315-6. [DOI] [PubMed] [Google Scholar]
- 246.Broder A, Mowrey WB, Izmirly P, Costenbader KH. Validation of Systemic Lupus Erythematosus Diagnosis as the Primary Cause of Renal Failure in the US Renal Data System. Arthritis Care Res (Hoboken) 2017 Apr;69(4):599–604. doi: 10.1002/acr.22972. http://europepmc.org/abstract/MED/27390299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Crawford DC, Bailey JNC, Miskimen K, Miron P, McCauley JL, Sedor JR, ƠToole JF, Bush WS. Somatic T-cell Receptor Diversity in a Chronic Kidney Disease PatientPopulation Linked to Electronic Health Records. AMIA Jt Summits Transl Sci Proc. 2018;2017:63–71. http://europepmc.org/abstract/MED/29888042. [PMC free article] [PubMed] [Google Scholar]
- 248.Ernecoff NC, Wessell KL, Hanson LC, Lee AM, Shea CM, Dusetzina SB, Weinberger M, Bennett AV. Electronic Health Record Phenotypes for Identifying Patients with Late-Stage Disease: a Method for Research and Clinical Application. J Gen Intern Med. 2019 Dec;34(12):2818–2823. doi: 10.1007/s11606-019-05219-9. http://europepmc.org/abstract/MED/31396813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Fraccaro P, van der Veer S, Brown B, Prosperi M, O'Donoghue D, Collins GS, Buchan I, Peek N. An external validation of models to predict the onset of chronic kidney disease using population-based electronic health records from Salford, UK. BMC Med. 2016 Jul 12;14:104. doi: 10.1186/s12916-016-0650-2. https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-016-0650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hao S, Fu T, Wu Q, Jin B, Zhu C, Hu Z, Guo Y, Zhang Y, Yu Y, Fouts T, Ng P, Culver DS, Alfreds ST, Stearns F, Sylvester KG, Widen E, McElhinney DB, Ling XB. Estimating One-Year Risk of Incident Chronic Kidney Disease: Retrospective Development and Validation Study Using Electronic Medical Record Data From the State of Maine. JMIR Med Inform. 2017 Jul 26;5(3):e21. doi: 10.2196/medinform.7954. http://medinform.jmir.org/2017/3/e21/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kitsos A, Peterson GM, Jose MD, Khanam MA, Castelino RL, Radford JC. Variation in Documenting Diagnosable Chronic Kidney Disease in General Medical Practice: Implications for Quality Improvement and Research. J Prim Care Community Health. 2019;10:2150132719833298. doi: 10.1177/2150132719833298. https://journals.sagepub.com/doi/10.1177/2150132719833298?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Koyner JL, Adhikari R, Edelson DP, Churpek MM. Development of a Multicenter Ward-Based AKI Prediction Model. Clin J Am Soc Nephrol. 2016 Nov 07;11(11):1935–1943. doi: 10.2215/CJN.00280116. https://cjasn.asnjournals.org/cgi/pmidlookup?view=long&pmid=27633727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Magvanjav O, Cooper-DeHoff RM, McDonough CW, Gong Y, Segal MS, Hogan WR, Johnson JA. Antihypertensive therapy prescribing patterns and correlates of blood pressure control among hypertensive patients with chronic kidney disease. J Clin Hypertens (Greenwich) 2019 Jan;21(1):91–101. doi: 10.1111/jch.13429. doi: 10.1111/jch.13429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Malas MS, Wish J, Moorthi R, Grannis S, Dexter P, Duke J, Moe S. A comparison between physicians and computer algorithms for form CMS-2728 data reporting. Hemodial Int. 2017 Jan;21(1):117–124. doi: 10.1111/hdi.12445. [DOI] [PubMed] [Google Scholar]
- 255.Malas MS, Wish J, Moorthi R, Grannis S, Dexter P, Duke J, Moe S. A comparison between physicians and computer algorithms for form CMS-2728 data reporting. Hemodial Int. 2017 Jan;21(1):117–124. doi: 10.1111/hdi.12445. [DOI] [PubMed] [Google Scholar]
- 256.Meyers JL, Candrilli SD, Kovacs B. Type 2 diabetes mellitus and renal impairment in a large outpatient electronic medical records database: rates of diagnosis and antihyperglycemic medication dose adjustment. Postgrad Med. 2011 May;123(3):133–43. doi: 10.3810/pgm.2011.05.2291. [DOI] [PubMed] [Google Scholar]
- 257.Nadkarni GN, Gottesman O, Linneman JG, Chase H, Berg RL, Farouk S, Nadukuru R, Lotay V, Ellis S, Hripcsak G, Peissig P, Weng C, Bottinger EP. Development and validation of an electronic phenotyping algorithm for chronic kidney disease. AMIA Annu Symp Proc. 2014;2014:907–16. http://europepmc.org/abstract/MED/25954398. [PMC free article] [PubMed] [Google Scholar]
- 258.Robertson LM, Denadai L, Black C, Fluck N, Prescott G, Simpson W, Wilde K, Marks A. Is routine hospital episode data sufficient for identifying individuals with chronic kidney disease? A comparison study with laboratory data. Health Informatics J. 2016 Jun;22(2):383–96. doi: 10.1177/1460458214562286. https://journals.sagepub.com/doi/10.1177/1460458214562286?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed. [DOI] [PubMed] [Google Scholar]
- 259.Salvador-González B, Rodríguez-Latre LM, Güell-Miró R, Álvarez-Funes V, Sanz-Ródenas H, Tovillas-Morán FJ. Estimation of glomerular filtration rate by MDRD-4 IDMS and CKD-EPI in individuals of 60 years of age or older in primary care. Nefrologia. 2013;33(4):552–63. doi: 10.3265/Nefrologia.pre2013.Apr.11929. http://www.revistanefrologia.com/es/linksolver/ft/ivp/0211-6995/33/552. [DOI] [PubMed] [Google Scholar]
- 260.Schroeder EB, Powers JD, O'Connor PJ, Nichols GA, Xu S, Desai JR, Karter AJ, Morales LS, Newton KM, Pathak RD, Vazquez-Benitez G, Raebel MA, Butler MG, Lafata JE, Reynolds K, Thomas A, Waitzfelder BE, Steiner JF, SUPREME-DM Study Group Prevalence of chronic kidney disease among individuals with diabetes in the SUPREME-DM Project, 2005-2011. J Diabetes Complications. 2015 Jul;29(5):637–43. doi: 10.1016/j.jdiacomp.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 261.Semler MW, Rice TW, Shaw AD, Siew ED, Self WH, Kumar AB, Byrne DW, Ehrenfeld JM, Wanderer JP. Identification of Major Adverse Kidney Events Within the Electronic Health Record. J Med Syst. 2016 Jul;40(7):167. doi: 10.1007/s10916-016-0528-z. http://europepmc.org/abstract/MED/27234478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Sun AZ, Shu Y, Harrison TN, Hever A, Jacobsen SJ, O'Shaughnessy MM, Sim JJ. Identifying Patients with Rare Disease Using Electronic Health Record Data: The Kaiser Permanente Southern California Membranous Nephropathy Cohort. Perm J. 2020;24 doi: 10.7812/TPP/19.126. http://europepmc.org/abstract/MED/32069207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Anand V, Hyun C, Khan QM, Hall C, Hessefort N, Sonnenberg A, Fimmel CJ. Identification and Fibrosis Staging of Hepatitis C Patients Using the Electronic Medical Record System. J Clin Gastroenterol. 2016 Sep;50(8):664–9. doi: 10.1097/MCG.0000000000000519. [DOI] [PubMed] [Google Scholar]
- 264.Atiemo K, Skaro A, Maddur H, Zhao L, Montag S, VanWagner L, Goel S, Kho A, Ho B, Kang R, Holl JL, Abecassis MM, Levitsky J, Ladner DP. Mortality Risk Factors Among Patients With Cirrhosis and a Low Model for End-Stage Liver Disease Sodium Score (≤15): An Analysis of Liver Transplant Allocation Policy Using Aggregated Electronic Health Record Data. Am J Transplant. 2017 Oct;17(9):2410–2419. doi: 10.1111/ajt.14239. doi: 10.1111/ajt.14239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Bateman-Steel CR, Smedley EJ, Kong M, Ferson MJ. Hepatitis C enhanced surveillance: results from a southeastern Sydney pilot program. Public Health Res Pract. 2015 Mar 30;25(2):e2521520. doi: 10.17061/phrp2521520. doi: 10.17061/phrp2521520. [DOI] [PubMed] [Google Scholar]
- 266.Corey KE, Kartoun U, Zheng H, Shaw SY. Development and Validation of an Algorithm to Identify Nonalcoholic Fatty Liver Disease in the Electronic Medical Record. Dig Dis Sci. 2016 Mar;61(3):913–9. doi: 10.1007/s10620-015-3952-x. http://europepmc.org/abstract/MED/26537487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Cuthbert JA, Arslanlar S, Yepuri J, Montrose M, Ahn CW, Shah JP. Predicting short-term mortality and long-term survival for hospitalized US patients with alcoholic hepatitis. Dig Dis Sci. 2014 Jul;59(7):1594–602. doi: 10.1007/s10620-013-3020-3. http://europepmc.org/abstract/MED/24445730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Fialoke S, Malarstig A, Miller MR, Dumitriu A. Application of Machine Learning Methods to Predict Non-Alcoholic Steatohepatitis (NASH) in Non-Alcoholic Fatty Liver (NAFL) Patients. AMIA Annu Symp Proc. 2018;2018:430–439. http://europepmc.org/abstract/MED/30815083. [PMC free article] [PubMed] [Google Scholar]
- 269.Kaplan DE, Dai F, Aytaman A, Baytarian M, Fox R, Hunt K, Knott A, Pedrosa M, Pocha C, Mehta R, Duggal M, Skanderson M, Valderrama A, Taddei TH, VOCAL Study Group Development and Performance of an Algorithm to Estimate the Child-Turcotte-Pugh Score From a National Electronic Healthcare Database. Clin Gastroenterol Hepatol. 2015 Dec;13(13):2333–41.e1. doi: 10.1016/j.cgh.2015.07.010. http://europepmc.org/abstract/MED/26188137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kartoun U, Corey KE, Simon TG, Zheng H, Aggarwal R, Ng K, Shaw SY. The MELD-Plus: A generalizable prediction risk score in cirrhosis. PLoS One. 2017;12(10):e0186301. doi: 10.1371/journal.pone.0186301. https://dx.plos.org/10.1371/journal.pone.0186301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Lai JC, Wong GL, Yip TC, Tse Y, Lam KL, Lui GC, Chan HL, Wong VW. Chronic Hepatitis B Increases Liver-Related Mortality of Patients With Acute Hepatitis E: A Territorywide Cohort Study From 2000 to 2016. Clin Infect Dis. 2018 Sep 28;67(8):1278–1284. doi: 10.1093/cid/ciy234. [DOI] [PubMed] [Google Scholar]
- 272.Loomis AK, Kabadi S, Preiss D, Hyde C, Bonato V, St Louis M, Desai J, Gill JMR, Welsh P, Waterworth D, Sattar N. Body Mass Index and Risk of Nonalcoholic Fatty Liver Disease: Two Electronic Health Record Prospective Studies. J Clin Endocrinol Metab. 2016 Mar;101(3):945–52. doi: 10.1210/jc.2015-3444. http://europepmc.org/abstract/MED/26672639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lu M, Chacra W, Rabin D, Rupp LB, Trudeau S, Li J, Gordon SC. Validity of an automated algorithm using diagnosis and procedure codes to identify decompensated cirrhosis using electronic health records. Clin Epidemiol. 2017;9:369–376. doi: 10.2147/CLEP.S136134. doi: 10.2147/CLEP.S136134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Nguyen TA, DeShazo JP, Thacker LR, Puri P, Sanyal AJ. The Worsening Profile of Alcoholic Hepatitis in the United States. Alcohol Clin Exp Res. 2016 Jun;40(6):1295–303. doi: 10.1111/acer.13069. http://europepmc.org/abstract/MED/27147285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Singal AG, Rahimi RS, Clark C, Ma Y, Cuthbert JA, Rockey DC, Amarasingham R. An automated model using electronic medical record data identifies patients with cirrhosis at high risk for readmission. Clin Gastroenterol Hepatol. 2013 Oct;11(10):1335–1341.e1. doi: 10.1016/j.cgh.2013.03.022. http://europepmc.org/abstract/MED/23591286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Xu Y, Li N, Lu M, Myers RP, Dixon E, Walker R, Sun L, Zhao X, Quan H. Development and validation of method for defining conditions using Chinese electronic medical record. BMC Med Inform Decis Mak. 2016 Aug 20;16:110. doi: 10.1186/s12911-016-0348-6. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-016-0348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Jamil K, Huang X, Lovelace B, Pham AT, Lodaya K, Wan G. The burden of illness of hepatorenal syndrome (HRS) in the United States: a retrospective analysis of electronic health records. J Med Econ. 2019 May;22(5):421–429. doi: 10.1080/13696998.2019.1580201. [DOI] [PubMed] [Google Scholar]
- 278.Koola JD, Davis SE, Al-Nimri O, Parr SK, Fabbri D, Malin BA, Ho SB, Matheny ME. Development of an automated phenotyping algorithm for hepatorenal syndrome. J Biomed Inform. 2018 Apr;80:87–95. doi: 10.1016/j.jbi.2018.03.001. https://linkinghub.elsevier.com/retrieve/pii/S1532-0464(18)30039-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Lin C, Karlson EW, Dligach D, Ramirez MP, Miller TA, Mo H, Braggs NS, Cagan A, Gainer V, Denny JC, Savova GK. Automatic identification of methotrexate-induced liver toxicity in patients with rheumatoid arthritis from the electronic medical record. J Am Med Inform Assoc. 2015 May;22(e1):e151–61. doi: 10.1136/amiajnl-2014-002642. http://europepmc.org/abstract/MED/25344930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wing K, Bhaskaran K, Smeeth L, van Staa TP, Klungel OH, Reynolds RF, Douglas I. Optimising case detection within UK electronic health records: use of multiple linked databases for detecting liver injury. BMJ Open. 2016 Sep 02;6(9):e012102. doi: 10.1136/bmjopen-2016-012102. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=27591023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Feller DJ, Zucker J, Yin MT, Gordon P, Elhadad N. Using Clinical Notes and Natural Language Processing for Automated HIV Risk Assessment. J Acquir Immune Defic Syndr. 2018 Feb 01;77(2):160–166. doi: 10.1097/QAI.0000000000001580. http://europepmc.org/abstract/MED/29084046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Felsen UR, Bellin EY, Cunningham CO, Zingman BS. Development of an electronic medical record-based algorithm to identify patients with unknown HIV status. AIDS Care. 2014;26(10):1318–25. doi: 10.1080/09540121.2014.911813. http://europepmc.org/abstract/MED/24779521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Goetz MB, Hoang T, Kan VL, Rimland D, Rodriguez-Barradas M. Development and validation of an algorithm to identify patients newly diagnosed with HIV infection from electronic health records. AIDS Res Hum Retroviruses. 2014 Jul;30(7):626–33. doi: 10.1089/AID.2013.0287. [DOI] [PubMed] [Google Scholar]
- 284.McInnes DK, Shimada SL, Midboe AM, Nazi KM, Zhao S, Wu J, Garvey CM, Houston TK. Patient Use of Electronic Prescription Refill and Secure Messaging and Its Association With Undetectable HIV Viral Load: A Retrospective Cohort Study. J Med Internet Res. 2017 Feb 15;19(2):e34. doi: 10.2196/jmir.6932. http://www.jmir.org/2017/2/e34/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Paul DW, Neely NB, Clement M, Riley I, Al-Hegelan M, Phelan M, Kraft M, Murdoch DM, Lucas J, Bartlett J, McKellar M, Que LG. Development and validation of an electronic medical record (EMR)-based computed phenotype of HIV-1 infection. J Am Med Inform Assoc. 2018 Feb 01;25(2):150–157. doi: 10.1093/jamia/ocx061. http://europepmc.org/abstract/MED/28645207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Bodenreider O. The Unified Medical Language System (UMLS): integrating biomedical terminology. Nucleic Acids Res. 2004 Jan 1;32(Database issue):D267–70. doi: 10.1093/nar/gkh061. http://nar.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=14681409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Donnelly K. SNOMED-CT: The advanced terminology and coding system for eHealth. Stud Health Technol Inform. 2006;121:279–90. [PubMed] [Google Scholar]
- 288.Savova GK, Masanz JJ, Ogren PV, Zheng J, Sohn S, Kipper-Schuler KC, Chute CG. Mayo clinical Text Analysis and Knowledge Extraction System (cTAKES): architecture, component evaluation and applications. J Am Med Inform Assoc. 2010;17(5):507–13. doi: 10.1136/jamia.2009.001560. http://jamia.oxfordjournals.org/lookup/pmidlookup?view=long&pmid=20819853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Torii M, Wagholikar K, Liu H. Using machine learning for concept extraction on clinical documents from multiple data sources. J Am Med Inform Assoc. 2011;18(5):580–7. doi: 10.1136/amiajnl-2011-000155. http://europepmc.org/abstract/MED/21709161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Doering TA, Plapp F, Crawford JM. Establishing an evidence base for critical laboratory value thresholds. Am J Clin Pathol. 2014 Dec;142(5):617–28. doi: 10.1309/AJCPDI0FYZ4UNWEQ. [DOI] [PubMed] [Google Scholar]
- 291.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009 Aug;(113):S1–130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 292.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G, National Kidney Foundation National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003 Jul 15;139(2):137–47. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 293.McCarty CA, Chisholm RL, Chute CG, Kullo IJ, Jarvik GP, Larson EB, Li R, Masys DR, Ritchie MD, Roden DM, Struewing JP, Wolf WA, eMERGE Team The eMERGE Network: a consortium of biorepositories linked to electronic medical records data for conducting genomic studies. BMC Med Genomics. 2011 Jan 26;4:13. doi: 10.1186/1755-8794-4-13. https://bmcmedgenomics.biomedcentral.com/articles/10.1186/1755-8794-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.McCue ME, McCoy AM. The Scope of Big Data in One Medicine: Unprecedented Opportunities and Challenges. Front Vet Sci. 2017;4:194. doi: 10.3389/fvets.2017.00194. doi: 10.3389/fvets.2017.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Sweet LE, Moulaison HL. Electronic Health Records Data and Metadata: Challenges for Big Data in the United States. Big Data. 2013 Dec;1(4):245–51. doi: 10.1089/big.2013.0023. [DOI] [PubMed] [Google Scholar]
- 296.Saito T, Rehmsmeier M. The precision-recall plot is more informative than the ROC plot when evaluating binary classifiers on imbalanced datasets. PLoS One. 2015;10(3):e0118432. doi: 10.1371/journal.pone.0118432. http://dx.plos.org/10.1371/journal.pone.0118432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Reimer AP, Milinovich A, Madigan EA. Data quality assessment framework to assess electronic medical record data for use in research. Int J Med Inform. 2016 Jul;90:40–7. doi: 10.1016/j.ijmedinf.2016.03.006. http://europepmc.org/abstract/MED/27103196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Overhage JM, Ryan PB, Reich CG, Hartzema AG, Stang PE. Validation of a common data model for active safety surveillance research. J Am Med Inform Assoc. 2012;19(1):54–60. doi: 10.1136/amiajnl-2011-000376. http://jamia.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=22037893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Denaxas S, Gonzalez-Izquierdo A, Fitzpatrick N, Direk K, Hemingway H. Phenotyping UK Electronic Health Records from 15 Million Individuals for Precision Medicine: The CALIBER Resource. Stud Health Technol Inform. 2019 Jul 04;262:220–223. doi: 10.3233/SHTI190058. [DOI] [PubMed] [Google Scholar]
- 300.Birtwhistle RV. Canadian Primary Care Sentinel Surveillance Network: a developing resource for family medicine and public health. Can Fam Physician. 2011 Oct;57(10):1219–20. http://www.cfp.ca/cgi/pmidlookup?view=long&pmid=21998241. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Developed search terms (Medical Subject Headings) for scoping literature review.
Embase and Medical Literature Analysis and Retrieval System Online search results of Charlson terms.
Preferred Reporting Items for Systematic reviews and Meta-analyses flow diagram.
Summary spreadsheet of identified articles between January 2000 and April 2020.


