Abstract
Objectives
We investigated the impact of COVID-19 and national pandemic response on primary care antibiotic prescribing in London.
Methods
Individual prescribing records between 2015 and 2020 for 2 million residents in north west London were analysed. Prescribing records were linked to SARS-CoV-2 test results. Prescribing volumes, in total, and stratified by patient characteristics, antibiotic class and AWaRe classification, were investigated. Interrupted time series analysis was performed to detect measurable change in the trend of prescribing volume since the national lockdown in March 2020, immediately before the first COVID-19 peak in London.
Results
Records covering 366 059 patients, 730 001 antibiotic items and 848 201 SARS-CoV-2 tests between January and November 2020 were analysed. Before March 2020, there was a background downward trend (decreasing by 584 items/month) in primary care antibiotic prescribing. This reduction rate accelerated to 3504 items/month from March 2020. This rate of decrease was sustained beyond the initial peak, continuing into winter and the second peak. Despite an overall reduction in prescribing volume, co-amoxiclav, a broad-spectrum “Access” antibiotic, prescribing rose by 70.1% in patients aged 50 and older from February to April. Commonly prescribed antibiotics within 14 days of a positive SARS-CoV-2 test were amoxicillin (863/2474, 34.9%) and doxycycline (678/2474, 27.4%). This aligned with national guidelines on management of community pneumonia of unclear cause. The proportion of “Watch” antibiotics used decreased during the peak in COVID-19.
Discussion
A sustained reduction in community antibiotic prescribing has been observed since the first lockdown. Investigation of community-onset infectious diseases and potential unintended consequences of reduced prescribing is urgently needed.
Keywords: Antibiotics, Antimicrobial resistance, Antimicrobial stewardship, Primary care, SARS-CoV-2
Introduction
The coronavirus disease 2019 (COVID-19) pandemic is having significant global impact on healthcare delivery. The influence of COVID-19 on antibiotic use across healthcare and on antimicrobial resistance (AMR) remains unclear.
Antibiotics have been used in hospitals to empirically treat patients with suspected COVID-19 due to the overlapping clinical and radiological features with bacterial respiratory tract infection [1,2]. Initial data from hospitals with a high burden of COVID-19 indicate high rates of antibiotic prescribing despite relatively low detected rates of bacterial co-infections in COVID-19 [3,4].
Although there are increasing data regarding the impact of COVID-19 on antibiotic use in acute care, there are very limited data on the impact of COVID-19 on antibiotic use in community settings [2]. Prescribing in primary care accounts for 81% of total antibiotic prescribing in England [5], and is mainly empirical, based on clinical signs and symptoms rather than a precise diagnosis [6]. Measures implemented in the UK to contain the transmission of COVID-19, such as social distancing, quarantine and travel restrictions, reduced population mobility and drove a shift from face-to-face appointments to telephone and video consultations. This shift in practice has influenced the process of how patients are assessed [7], and how antibiotics are prescribed in primary care [8]. Avoiding visits to primary care facilities, to prevent infections and ‘protect the National Health Service’, has led to delays in care-seeking. In the 15-week lockdown period between 23 March 2020 and 5 July 2020 in England, face-to-face consultation with primary care physicians, referred to as General Practitioners (GPs), accounted for 24% of all GP contacts with patients, compared with over 70% in the previous year [9].
AMR is also a pandemic in nature, but a gradual yet implacable process. To minimize the negative impact on human health during this era of the double pandemic of COVID-19 and AMR, data are urgently needed to assess antibiotic prescribing, examine clinical outcomes of infection, evaluate prescribing guidelines for community care and support design of clinical decision support technologies [10].
This study aimed to describe antibiotic use in primary care during and following the initial surge in COVID-19 cases in north west London (NWL).
Materials and methods
Ethics
The Whole Systems Integrated Care (WSIC) system provides linked health records from primary, secondary, community, mental health and social care in NWL, which have been de-identified and made available for approved research. This study was approved by the Imperial Academic Health Science Centre (AHSC) COVID Research Committee, the COVID-19 NWL Data Prioritisation Group, and the Discover Research Advisory Group (DRAG), which jointly provides a governance mechanism.
Data
We used the de-identified individual-level primary care prescribing data from WSIC, covering more than 2 million patients across 400 GP practices in NWL [11]. Medication order records were extracted by identifying all antibiotic prescriptions using BNF codes.
In 2020, Imperial NIHR Biomedical Research Centre (BRC) developed the secure Clinical Analytics, Research and Evaluation (iCARE) high-performance analytics environment [12], which hosts WSIC data and secondary care relevant to COVID-19 from Imperial College NHS Healthcare Trust (ICNHT). COVID-19 pathology data are a direct feed from COVID-19-designated laboratories processing SARS-CoV-2 nasopharyngeal swab samples in NWL. The SARS-CoV-2 test results from these sites were deterministically linked to community prescribing records using a pseudonymized patient identifier and age. We used the NHS Digital GP consultation data of the 8 Clinical Commissioning Groups (CCGs) in NWL, equivalent to the population covered by WSIC [13].
Definitions
Patient characteristics
Gender, age, ethnicity and deprivation level were defined using primary care data at the time when the primary care prescribing event took place. Deprivation level was determined using the 2011 Lower Layer Super Output Area (LSOA) code of a patient's current address and the 2019 Index of Multiple Deprivation (IMD) [14]. COVID-19 infection status was defined as a positive SARS-CoV-2 test result between 01 January 2020 and 30 November 2020. Negative, indeterminate, error, or no test result reported for SARS-CoV-2 was coded as negative. A patient is classified as positive from the date of his/her first positive test if multiple SARS-CoV-2 tests were performed.
Antibiotic prescriptions
We analysed antibacterial agents listed in British National Formulary (BNF) sub-chapter 5.1 (Antibacterial drugs), excluding anti-mycobacterial drugs (BNF sub-chapter 5.1.9, 5.1.10) [15]. The consumption volume was calculated in the number of items, and Defined Daily Dose (DDD) values using the World Health Organization (WHO) Anatomical Therapeutic Chemical (ATC) index 2019 [16].
GP consultations
We counted the number of attended GP consultations delivered face to face (on NHS premise or via home visit) and remotely (using telephone or video device). The consultation appointments coded as ‘patient did not attend’ or ‘status unknown’ were excluded from analysis.
Descriptive analysis
We analysed GP prescribing records of antibiotics from 01 January 2015 to 30 November 2020, and reported number of antibiotic items prescribed in 2020, stratified by gender (female, male), age group (children: under 18 years old, adults: 18–64 years old, and elderly people: above 64 years old), ethnic group (Black, Asian and minority ethnic (BAME) and mixed background, white and unknown), deprivation level (divided into quintiles: 1 = most deprived; 5 = least deprived). We also reported the proportion of DDDs of antibiotic prescribed using the WHO Access, Watch and Reserve (AWaRe) classification [17]. In addition, we reported the number of attended GP appointments and whether these were conducted face to face or virtually via telephone or video, to provide the context of prescribing.
Statistical analysis
Interrupted time series analysis (ITSA) is a quasi-experimental research design for evaluation of longitudinal effects of interventions or incidents. It is particularly appropriate for the assessment of interventions or incidents that impact population-level health outcomes [18].
We conducted ITSA to determine whether there was a measurable change in the underlying trend of antibiotic prescribing volume in primary care associated with COVID-19 and the national pandemic response. We used the date when the first national lockdown was imposed in England as a clear ‘interruption’ date, around which the data trends would be analysed [19]. This date was just 1 week before the peak of the first wave of COVID-19. The impact of the pandemic disease itself cannot be separated from the societal responses. We constructed a monthly time series of the number of antibiotic items prescribed from January 2015 to November 2020, and set the lockdown date as March 2020 [19]. We adjusted for seasonality by including each calendar month as an independent variable. We performed ITSA to produce Newey–West standard errors for coefficients estimated by ordinary least squared (OLS) regression, which assumes the errors to follow a first-order autoregressive process [20]. We plotted the gradients of the resulting regression line as monthly changes in number of antibiotic items and demonstrated the impact of COVID-19 by showing the observed trend line and the continuation of the pre-COVID trend that would have been expected in the absence of the COVID-19 pandemic. The seasonal variation was smoothed using locally weighted scatterplot smoothing (LOWESS) regression. We performed the same analysis using a weekly time series from 5 January 2015 to 30 November 2020 and introduced the lockdown in the week commencing 23 March 2020 to predict weekly prescribing volume during 2020 to allow zoomed-in visualization.
Results
Patient characteristics
Patients from 351 GP practices who had at least one antibiotic prescription between 1 January 2020 and 30 November 2020 were identified. Most patients were female (224 853, 62.1%) and adults (231 138, 63.8%) (Table 1 ).
Table 1.
Patient characteristics
| Patient characteristics | n (N = 366 059) | % | |
|---|---|---|---|
| Gender | Female | 224 853 | 62.1 |
| Male | 141 190 | 39.0 | |
| Unknown | 16 | 0.0 | |
| Age group, years | Children (<18) | 59 333 | 16.2 |
| Adult (18-64) | 231 138 | 63.1 | |
| Elderly (>64) | 75 588 | 20.6 | |
| Ethnicity | BAME and mixed background | 153 681 | 42.0 |
| White | 120 763 | 33.3 | |
| Unknown | 91 615 | 25.3 | |
| Deprivation level, Index of Multiple Deprivation | 1 (most) | 68 601 | 33.0 |
| 2 | 123 104 | 33.6 | |
| 3 | 84 447 | 23.1 | |
| 4 | 45 929 | 13.0 | |
| 5 (least) | 21 503 | 5.9 | |
| Unknown | 22 475 | 6.1 | |
| COVID-19 status | Had SARS-CoV-2 test positive | 6158 | 1.7 |
| Had SARS-CoV-2 test negative | 59 577 | 16.3 | |
| Had no SARS-CoV-2 test | 300 324 | 82.0 | |
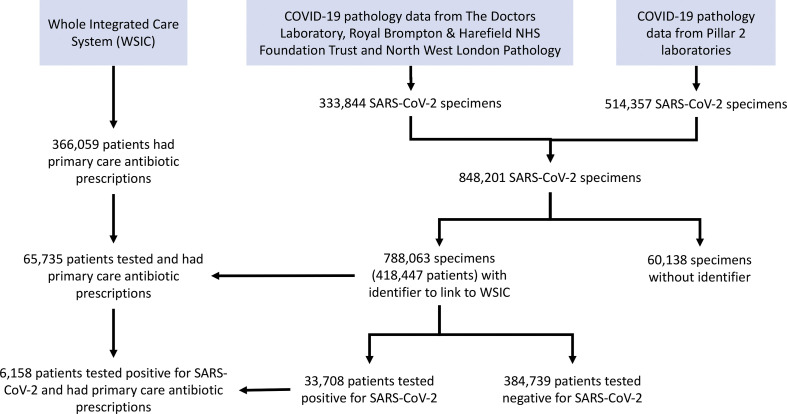
Pathology data were available for 848 201 SARS-CoV-2 test samples processed between 1 January 2020 and 30 November 2020. Test results of 788 063 SARS-CoV-2 samples (92.9%) were linked to WSIC primary care records of 418 447 patients, of which, 33 708 patients were tested positive for SARS-CoV-2.6158 patients who tested positive for SARS-CoV-2 had at least one primary care antibiotic prescription during the 11-month study period (Table 1, Supplementary material 1, Figure S1).
Antibiotic use during the COVID-19 pandemic
During the 11-month study period, 730 001 GP antibiotic prescriptions were identified, after excluding prescriptions of anti-mycobacterial drugs. We observed a decreasing trend of prescribing volume following the introduction of the first national lockdown in March 2020 (Fig. 1 ). The predicted weekly prescribing volume is presented in Fig. 1 to illustrate the observed variation from the historical trend before 2020. The long-term trend of antibiotic prescribing volume across NWL has been constantly decreasing by 38 items per week before March 2020 (gradient –38.1, 95% CI –43.0 to –33.2, p 0.000). After introducing the lockdown in the week commencing 23 March 2020, there was a downward change in gradient from –38.1 to –187.1 (95% CI –233.1 to –141.1, p 0.000).
Fig. 1.
Weekly GP antibiotic prescriptions and positive SARS-CoV-2 cases, January 2020 – November 2020, north west London.
A similar trend was seen in the monthly time series analysis. Antibiotic prescribing volume decreased at a rate of 584 items per month (gradient –584.3, 95% CI –761.3 to –407.4, p 0.000) before March 2020. After March 2020 the rate of decrease was 3504 items per month (gradient –3504.4, 95% CI –4885.9 to –2123.0, p 0.000). The regression line (indicating the long-term trend without considering seasonality) and the predicted monthly prescribing volume are presented in Fig. 2 with the observed prescribing volume. While antibiotic prescribing remained low, GP consultation rates began to recover in NWL. Between September and November 2020, the total number of attended GP appointments were higher than the previous year, though 46.3% were remote appointments, compared to 17.2% in 2019 (Fig. 2).
Fig. 2.
Monthly GP antibiotic prescriptions and consultations with historical trend, January 2019 – November 2020, north west London.
The observed decline in prescribing volume following the first national lockdown and the first peak of COVID-19 occurred consistently across all gender, age, ethnic and socioeconomic groups (Supplementary material 1, Figure S2). However, such a decline was not sustained in BAME population since August. The potential relationships between antibiotic prescribing and ethnicity in the context of COVID-19 is being explored further.
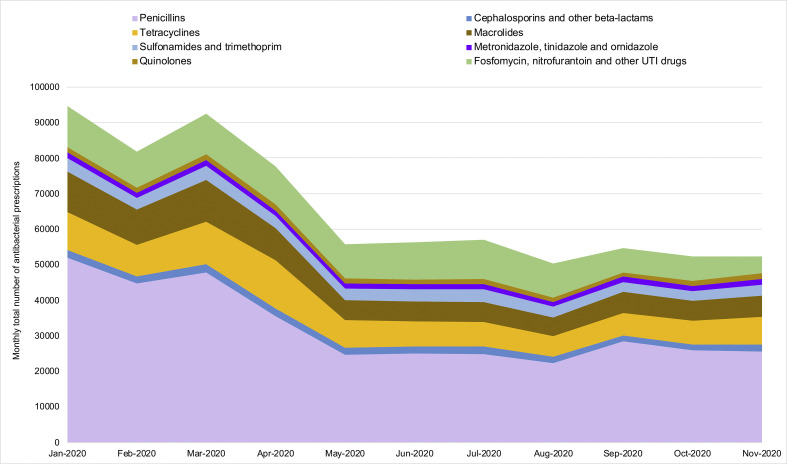
Antibiotic prescribing volume reached its lowest in August 2020. From January to August, prescribing of penicillins decreased most, by 57.2%, followed by macrolides (54.6%) and tetracyclines (45.0%). The reduction in prescribing of cephalosporins (14.1%), and nitrofurantoin and urinary tract infection (UTI) drugs (16.9%) was smaller (Fig. 3 ).
Fig. 3.
Monthly GP antibiotic prescriptions by antibiotic class, January 2020 – November 2020, north west London.
The volume of the agents commonly prescribed in primary care, including amoxicillin, cefalexin, trimethoprim and nitrofurantoin, increased from February to March before decreasing in April. The use of co-amoxiclav, in number of items, rose by 70.1% from February to April 2020 among patients above 50 years old, while declining in younger age groups during April.
Antibiotic use among SARS-CoV-2 positive patients
We identified 6158 patients who tested positive for SARS-COV-2 and had at least one GP prescription of antibiotics between January and August 2020. Of these patients, 1940 were prescribed at least one antibiotic within 14 days of a positive SARS-CoV-2 result. The documented indications for these antibiotic prescriptions were not available. The most prescribed antibiotics within the time window of 14 days before or after a positive SARS-CoV-2 test were amoxicillin (863/2474, 34.9%), doxycycline (678/2474, 27.4%), clarithromycin (229/2474, 9.3%), phenoxymethylpenicillin (140/2474, 5.7%) and co-amoxiclav (112/2474, 4.5%).
Antibiotic use and AWaRe classification
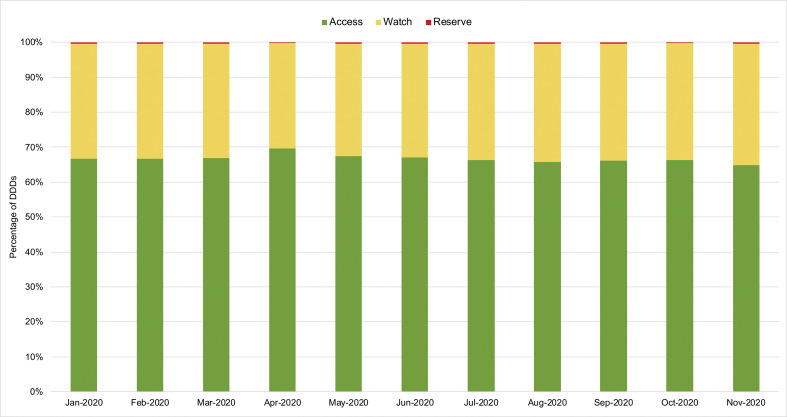
We compared the proportion of DDDs prescribed in each AWaRe antibiotic category to examine if there was any change in use of “Watch” or “Reserve” antibiotics. Our results suggested no overall change in proportion of AWaRe antibiotic use in NWL between since the national lockdown was introduced. While COVID-19 cases peaked in April, the proportion of “Watch” antibiotics use decreased (Fig. 4 ).
Fig. 4.
Monthly proportion of AWaRe antibiotic use, January 2020 – November 2020, north west London.
Discussion
We report an overall decrease in antibiotic prescribing volume in primary care from the date when the national lockdown was introduced in March 2020, just before COVID-19 cases first peaked in NWL. This reduction in primary care prescribing has been sustained for a further eight months, extending 6 months beyond the initial peak of the pandemic, and into the winter months and second wave. The reduction was sustained while GP consultation rates are seen to be returning back to normal. Despite an overall reduction in antibiotic prescribing during this period, the prescribing volume of co-amoxiclav, a broad-spectrum β-lactam–β-lactamase inhibitor combination, has increased, especially in older patients. This may be because co-amoxiclav was prescribed to cover a range of potential infections by primary care clinicians facing less certainty about diagnosis, when not being able to assess their patients face to face or perform basic tests, such as urinalysis and chest auscultation.
Amoxicillin and doxycycline were the most prescribed antibiotics within 14 days of a positive test for SARS-CoV-2, which aligned with the guideline published by the National Institute for Health and Care Excellence (NICE). These guidelines recommended the use of amoxicillin and doxycycline to manage suspected pneumonia in the community when the cause was unclear [21].
Our results suggested no overall change of AWaRe antibiotic use in NWL since the introduction of national lockdown in March 2020. ‘Access’ antibiotics were used to manage non-hospitalized patients, as per recommendations in the WHO Interim guidance for suspected or confirmed COVID-19 [22,23]. The WHO AWaRe classification has been adopted by secondary care in England to estimate the relative use of narrow-spectrum antibiotics, and aid evaluation of progress in optimizing antibiotic use and limiting AMR. This strategy has not been previously explored in primary care before this research.
Our analysis of prescribing volume considered the existing national initiatives to optimize antibiotic use in England, with focus on UTI management and reduction in co-amoxiclav and quinolones. Prescribing volume had decreased since 2015 following the introduction of the Quality Premiums to financially reward local commissioners of healthcare in England for targeted reductions in primary care antibiotic prescribing [24,25].
This study has provided an insight into the impact of COVID-19 and the national response in the UK on antibiotic prescribing in primary care. There are a number of explanations for the reduction in antibiotic prescribing volume. Firstly, interventions introduced to mitigate COVID-19 transmission such as social distancing, increased home working and decreased use of public transport will affect other infectious diseases. Data from the Royal College of General Practitioners did show a large decline in consultation rates from many infectious diseases [26]. Secondly, GP consultation rates and the use of other parts of the NHS (e.g. accident and emergency departments) were reduced in the early lockdown period. However, our analysis revealed that while consultation rates were returning to normal levels, schools were re-opened and social distancing measures were relaxed, the decline in antibiotic prescribing sustained. Further analysis is required to assess whether the reduced community prescribing will have a continuing effect on community infection rates and AMR. We will also need to evaluate the longer-term impact of a shift to remote consultations in primary care on antibiotic prescribing and clinical outcomes such as delayed treatment of bacterial infections, increase in hospital admissions and deaths [27].
Our study also has several limitations that provide scope for future research. First, the analysis of prescribing for SARS-CoV-2 positive patients was limited due to the lack of documented indication for the antibiotic. The testing capacity and specificity further restricted the comparison between COVID-19 and non-COVID-19 patients. We aim to link primary care and hospital records for SARS-CoV-2 positive patients to assess whether they required hospital admission and the outcomes of treatment. Second, we did not assess how the shift from face to face to remote GP consultations might have influenced antibiotic prescribing and dispensing. Remote delivery of primary care service grew dramatically in the pandemic, formal evaluation is urgently required to re-design antimicrobial stewardship interventions, maintain quality of antibiotic prescribing, and avoid unintended consequences [28]. Third, GP-led ‘hot hubs’ were providing face to face consultations and issuing pre-packed antibiotics with pneumonia symptoms in NWL [29]. Antibiotics dispensed in this route outside standard opening hours was not captured in our data; however, this was estimated to account for less than 0.5% of GP prescribing in NWL [30].
Conclusion
Primary care accounts for over 81% of antibiotic consumption in the UK. The reduction in community prescribing associated with COVID-19 and the national pandemic response could potentially have a favourable impact on antimicrobial resistance. Our results need to be seen in the context of all antibiotic prescribing, and further monitoring is required to see whether this reduction persists and the impact that it will have. Prospectively investigating the impact this will have on AMR is critically important [31]. However, surveillance and a rapid understanding of potential unintended consequences of a reduction in prescribing, such as an increase in more severe cases of bacterial infections is essential.
Transparency declaration
The authors have nothing to disclose. Funding: This research was funded by (1) the National Institute for Health Research (NIHR) Health Protection Research Unit (HPRU) in Healthcare Associated Infections and Antimicrobial Resistance at Imperial College London in partnership with Public Health England (PHE), in collaboration with Imperial College Health Partners, University of Cambridge and University of Warwick, and (2) the Department for Health and Social Care, who funded Centre for Antimicrobial Optimisation (CAMO) at Imperial College London, and (3) the NIHR Imperial Biomedical Research Centre (BRC), who developed and funded the iCARE high performance analytics environment to provide data used in this research. AH is a NIHR Senior Investigator. PA and AM are supported by the NIHR Applied Research Collaboration Northwest London. The views expressed in this publication are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Author contributions
N.Z., P.A. and A.H. developed the concept and methodology for this research. N.Z. and P.A. undertook data extraction and analysis. N.Z. drafted the initial manuscript. T.M.R., M.G., A.M. and A.H. contributed significantly to data interpretation, revision of the manuscript and finalization for submission.
Acknowledgements
The authors thank Elizabeth Beech for the valuable discussion. The authors thank Isa Ahmad for the support in consultation data. The authors thank the colleagues, Dimitri Papadimitriou from the Imperial Colleague Healthcare NHS Trust, Owain Griffiths and Eamon O'Doherty from the North West London Collaboration of Clinical Commissioning Groups, for their support in data.
Editor: A. Kalil
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.02.007.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
References
- 1.Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71:2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nieuwlaat R., Mbuagbaw L., Mertz D., Burrows L., Bowdish D.M.E., Moja L. COVID-19 and antimicrobial resistance: parallel and interacting health emergencies. Clin Infect Dis. 2020:ciaa773. doi: 10.1093/cid/ciaa773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaughn V.M., Gandhi T., Petty L.A., Patel P.K., Prescott H.C., Malani A.N. Empiric antibacterial therapy and community-onset bacterial co-infection in patients hospitalized with COVID-19: a multi-hospital cohort study. Clin Infect Dis. 2020:ciaa1239. doi: 10.1093/cid/ciaa1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens R.W., Jensen K., O’Horo J.C., Shah A. Antimicrobial prescribing practices at a tertiary care center in patients diagnosed with COVID-19 across the continuum of care. Infect Control Hosp Epidemiol. 2020;42:89–92. doi: 10.1017/ice.2020.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Public Health England . 2019. English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) report 2019 [Internet]https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/936199/ESPAUR_Report_2019-20.pdf [cited 2020 Oct 20]. Available from: [Google Scholar]
- 6.Cooke J., Llor C., Hopstaken R., Dryden M., Butler C. Respiratory tract infections (RTIs) in primary care: narrative review of C reactive protein (CRP) point-of-care testing (POCT) and antibacterial use in patients who present with symptoms of RTI. BMJ Open Respir Res. 2020;7 doi: 10.1136/bmjresp-2020-000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majeed A., Maile E.J., Bindman A.B. The primary care response to COVID-19 in England’s National Health Service. J R Soc Med. 2020;113:208–210. doi: 10.1177/0141076820931452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rawson T.M., Moore L.S.P., Castro-Sanchez E., Charani E., Davies F., Satta G. COVID-19 and the potential long-term impact on antimicrobial resistance. J Antimicrob Chemother. 2020;75:1681–1684. doi: 10.1093/jac/dkaa194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Royal College of General Practitioners . 2020. RCGP survey provides snapshot of how GP care is accessed in latest stages of pandemic [Internet]https://www.rcgp.org.uk/about-us/news/2020/july/rcgp-survey-provides-snapshot-of-how-gp-care-is-accessed-in-latest-stages-of-pandemic.aspx [cited 2021 Jan 20]. Available from: [Google Scholar]
- 10.Rawson T.M., Ming D., Ahmad R., Moore L.S.P., Holmes A.H. Antimicrobial use, drug-resistant infections and COVID-19. Nat Rev Microbiol. 2020;18:409–410. doi: 10.1038/s41579-020-0395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bottle A., Cohen C., Lucas A., Saravanakumar K., Ul-Haq Z., Smith W. How an electronic health record became a real-world research resource: comparison between London’s Whole systems integrated care database and the clinical practice research datalink. BMC Med Inform Decis Mak. 2020;20:71. doi: 10.1186/s12911-020-1082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NIHR Imperial Biomedical Research Centre . 2020. Imperial’s Clinical Analytics, Research and Evaluation (iCARE) project started in Autumn 2019 as a proof-of-concept high performance analytics environment, funded by NIHR Imperial BRC [Internet]https://imperialbrc.nihr.ac.uk/facilities/icare/ Available from: [Google Scholar]
- 13.NHS Digital . 2020. Appointments in General Practice [Internet]. Appointments in General Practice.https://digital.nhs.uk/data-and-information/publications/statistical/appointments-in-general-practice [cited 2020 Dec 22]. Available from: [Google Scholar]
- 14.Ministry of Housing C & LG . National Statistics; 2019. English indices of deprivation 2019 [Internet]https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019 [cited 2020 Sep 20]. Available from: [Google Scholar]
- 15.BMJ Publishing Group Ltd and Royal Pharmaceutical Society . 2015. British National Formulary (BNF) 70. Sept 2015. [Google Scholar]
- 16.World Health Organization . WHO Collaborating Centre for Drug Statistics Methodology; 2018. ATC/DDD Index 2019 [Internet]https://www.whocc.no/atc_ddd_index/ [cited 2020 Sep 20]. Available from: [Google Scholar]
- 17.World Health Organization . Essential medicines and health products; 2019. WHO releases the 2019 AWaRe Classification Antibiotics [Internet] [cited 2020 Sep 20]. Available from: WHO releases the 2019 AWaRe Classification Antibiotics. [Google Scholar]
- 18.Bernal J.L., Cummins S., Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46:348–355. doi: 10.1093/ije/dyw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.UK Government . 2020. Prime Minister’s statement on coronavirus (COVID-19): 23 March 2020 [Internet]https://www.gov.uk/government/speeches/pm-address-to-the-nation-on-coronavirus-23-march-2020 [cited 2020 Sep 20]. Available from: [Google Scholar]
- 20.Linden A. Statistical Software Components; 2015. ITSA: Stata module to perform interrupted time series analysis for single and multiple groups [Internet]https://ideas.repec.org/c/boc/bocode/s457793.html [cited 2020 Jan 20]. Available from: [Google Scholar]
- 21.National Institute for Health and Care Excellence . NICE Guidel; 2020. COVID-19 rapid guideline : managing suspected or confirmed pneumonia in adults in the community. [PubMed] [Google Scholar]
- 22.World Health Organization . 2020. Clinical management of COVID-19 Interim Guidance (updated May 2020) [Internet]https://www.who.int/publications-detail/clinical-management-of-covid-19 [cited 2020 Sep 20]. Available from: [Google Scholar]
- 23.Getahun H., Smith I., Trivedi K., Paulin S., Balkhy H.H. Bulletin of the World Health Organization; 2020. Tackling antimicrobial resistance in the COVID-19 pandemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balinskaite V., Johnson A.P., Holmes A., Aylin P. The impact of a national antimicrobial stewardship program on antibiotic prescribing in primary care: an interrupted time series analysis. Clin Infect Dis. 2019;69:227–232. doi: 10.1093/cid/ciy902. [DOI] [PubMed] [Google Scholar]
- 25.Bou-Antoun S., Costelloe C., Honeyford K., Mazidi M., Hayhoe B.W.J., Holmes A. Age-related decline in antibiotic prescribing for uncomplicated respiratory tract infections in primary care in England following the introduction of a national financial incentive (the Quality Premium) for health commissioners to reduce use of antibiotic. J Antimicrob Chemother. 2018;73:2883–2892. doi: 10.1093/jac/dky237. [DOI] [PubMed] [Google Scholar]
- 26.Royal College of General Practitioners . 2020. Communicable and respiratory disease reports [Internet]https://www.rcgp.org.uk/clinical-and-research/our-programmes/research-and-surveillance-centre/public-health-data.aspx [cited 2020 Jan 21]. Available from: [Google Scholar]
- 27.Han S.M., Greenfield G., Majeed A., Hayhoe B. Impact of remote consultations on antibiotic prescribing in primary healthcare: systematic review. J Med Internet Res. 2020;22 doi: 10.2196/23482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balinskaite V., Bou-Antoun S., Johnson A.P., Holmes A., Aylin P. An assessment of potential unintended consequences following a national antimicrobial stewardship program in England: an interrupted time series analysis. Clin Infect Dis. 2019;69:233–242. doi: 10.1093/cid/ciy904. [DOI] [PubMed] [Google Scholar]
- 29.NHS Confederation . Pulse; 2020. GPs set to diagnose Covid-19 face to face in ‘hot hubs’ [Internet]https://www.pulsetoday.co.uk/news/uncategorised/gps-set-to-diagnose-covid-19-face-to-face-in-hot-hubs/ [cited 2020 Sep 20]. Available from: [Google Scholar]
- 30.NHS Business Services Authority . 2020. ePACT2 [Internet]https://www.nhsbsa.nhs.uk/epact2 [cited 2020 Sep 20]. Available from: [Google Scholar]
- 31.Knight G.M., Costelloe C., Deeny S.R., Moore L.S.P., Hopkins S., Johnson A.P. Quantifying where human acquisition of antibiotic resistance occurs: a mathematical modelling study. BMC Med. 2018;16:137. doi: 10.1186/s12916-018-1121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]