Abstract
Aim
We aimed to address the potential impact of COVID-19 on glycemic patterns in a small pilot study.
Method
13 patients with mild COVID-19 who were confirmed without diabetes and another group of 18 healthy individuals with available CGM data were well matched and enrolled into the final analysis.
Results
We noticed significantly higher TARs of >140 mg/dL (median 13.9% vs. 2.3%, P = 0.006), >160 mg/dL (median 4.7% vs. 0.0%, P = 0.011) and >180 mg/dL (median 1.9% vs. 0.0%, P = 0.007) among non-diabetic patients with COVID-19 than those among healthy individuals. There was no significant difference in TBR of <70 mg/dL or <54 mg/dL (all P > 0.1). Consequently, the TIR of 70 mg/dL to 140 mg/dL was significantly lower in non-diabetic patients with COVID-19 than that in healthy individuals (median 80.1% vs. 93.1%, P = 0.001). Significant postprandial glycemic fluctuations were observed among patients with COVID-19. There was a remarkable difference in CV in non-diabetic patients with COVID-19 compared to healthy individuals (median 25.6% vs. 15.7%, P < 0.001).
Conclusion
Significant higher glycemic fluctuation and exposure to hyperglycemia was associated with COVID-19 among previously normoglycemic individuals, characterized with potentially impaired glucose tolerance.
Keywords: COVID-19, Glycemic fluctuation, Glucose tolerance
More and more evidences support an active interaction between coronavirus disease 2019 (COVID-19) and diabetes (Shrestha et al., 2020). Recent researchers revealed that pancreatic β cells are highly permissive to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection with receptor angiotensin-converting enzyme 2 (ACE2) as its entry (Yang et al., 2020; Al-Benna, 2020). Therefore, hyperglycemia and glycemic fluctuations may be caused by the inflammatory cascade of the attack of SARS-CoV-2 to pancreas and the potentially impaired β-cell function (Hoffmann et al., 2020). In accord, an international group of leading diabetes researchers have recently raised their concerns on a new-onset of diabetes potentially precipitated by COVID-19 5.
In order to address the potential impact of COVID-19 on glycemic patterns, we did a small observational pilot study. 13 patients with mild COVID-19 who were confirmed without diabetes and were admitted to Leishenshan Hospital in Wuhan city, China were invited to receive continuous glucose monitoring (CGM) for a median period of 6 days. Meanwhile, another group of 18 healthy individuals with available CGM data for a median period of 3 days from volunteers recruited in Shanghai Sixth People's Hospital were well matched and balanced by age, sex, body mass index, fasting glucose and HbA1c levels (Zhou et al., 2009), resulting in a total of 31 individuals enrolled into the final analysis. All these participants received a standardized meal regimen. All patients in this study were confirmed without use of glucocorticoids. All patients with COVID-19 received arbidol hydrochloride capsules plus traditional Chinese herbs during hospitalization. These participants also took 1-h mild to moderate physical exercises daily as instructed at bedside. Percentage times of sensor glucose levels above (TAR), below (TBR) and in certain ranges (TIR) were calculated. Glycemic fluctuation was assessed by calculation of the coefficient of variation (CV) of the sensor glucose levels. All participants were confirmed non-diabetic by fasting plasma glucose levels <100 mg/dL and HbA1c levels <6.5% (48 mmol/mol). These glycemic metrics were further compared between non-diabetic patients with COVID-19 and healthy individuals using Kruskal-Wallis test. The study protocol was approved by Institutional Review Boards of both Shanghai Jiaotong University Affiliated Sixth People's Hospital and Zhongnan Hospital of Wuhan University, which was subsequently registered in Chinese Clinical Trial Registry (ChiCTR2000030436). We had obtained informed consent from all patients involved in this study.
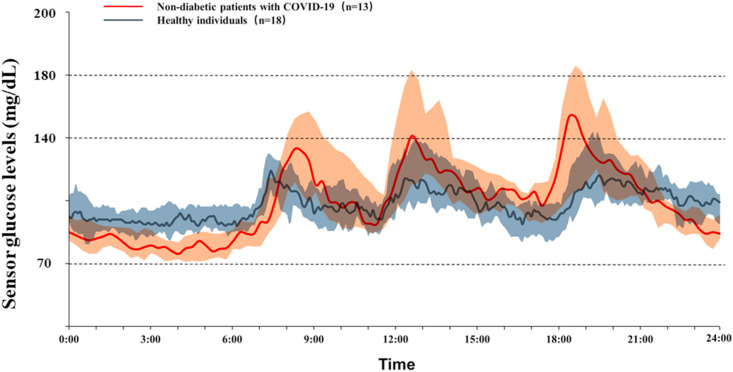
For all glycemic metrics listed in Table 1 , we noticed significantly higher TARs of >140 mg/dL (median 13.9% vs. 2.3%, P = 0.006), >160 mg/dL (median 4.7% vs. 0.0%, P = 0.011) and >180 mg/dL (median 1.9% vs. 0.0%, P = 0.007) among non-diabetic patients with COVID-19 than those among healthy individuals. There was no significant difference in TBR of <70 mg/dL or <54 mg/dL (all P > 0.1). Consequently, the TIR of 70 mg/dL to 140 mg/dL was significantly lower in non-diabetic patients with COVID-19 than that in healthy individuals (median 80.1% vs. 93.1%, P = 0.001). Significant postprandial glycemic fluctuations were observed among patients with COVID-19 (Fig. 1 ). There was a remarkable difference in CV in non-diabetic patients with COVID-19 compared to healthy individuals (median 25.6% vs. 15.7%, P < 0.001).
Table 1.
Summary of glycemic metrics by continuous glucose monitoring in normoglycemic patients with COVID-19 and healthy individuals.
| Percentage of sensor glucose levels, % | Healthy individuals N = 18 |
Normoglycemic patients with COVID-19 N = 13 |
|---|---|---|
| TAR | ||
| >10.0 mmol/L | 0.0 (0.0–0.0) | 1.9 (0.0–8.3)b |
| >8.9 mmol/L | 0.0 (0.0–2.4) | 4.7 (0.7–17.8)a |
| >7.8 mmol/L | 2.3 (0.0–9.8) | 13.9 (4.8–30.9)b |
| TBR | ||
| <3.9 mmol/L | 0.0 (0.0–0.0) | 0.5 (0.0–11.9) |
| <3.0 mmol/L | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| TIR | ||
| 3.9–7.8 mmol/L | 93.1 (86.8–99.5) | 80.1 (68.9–88.2)b |
| 3.9–10.0 mmol/L | 100 (94.5–100) | 92.1 (82.5–97.5) |
Data were presented with median (interquartile range).
P < 0.05.
P < 0.01.
Fig. 1.
Median sensor glucose levels with their 25th and 75th percentiles during a period of 24 h of non-diabetic patients with COVID-19 and healthy individuals. Solid line represents the median sensor glucose levels and the cloud shades present the 25th to 75th percentile range. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In this case series study using CGM, we demonstrated a significantly greater degree of glycemic fluctuation especially postprandial excursions in non-diabetic patients with COVID-19 when compared to healthy individuals. Since normal glycemic profiles suggest very tight physiological control, with only brief postprandial excursions above 140 mg/dl (Zhou et al., 2009; Shah et al., 2019), our findings indicate that the glycemic profiles of the previously normoglycemic individuals (e.g., TAR of >140 mg/dL: 13.9%) could no longer be regarded as “normal” after COVID-19 infection, especially at the postprandial state. Accordingly, the measurement of postprandial glucose is warranted to better address the burden of hyperglycemia in these patients. Regarding the mechanism underlying this observation, a recent study reported that human pancreatic beta cells could be infected by SARS-CoV-2 2, suggesting that impaired glucose tolerance may at least partially account for the glucose dysregulation, although the impact of COVID-19 on insulin resistance could not be ruled out. Based on these preliminary results, we suggest further studies and focus on the interplay between COVID-19 and diabetes. Studies on the exposure to COVID-19 and the subsequent metabolic outcomes with enough sample size will help elucidate the detrimental effect of COVID-19 on diabetes and other metabolic disorders.
Several limitations should be addressed. Although all patients have undergone a standardized diet and physical activity regimen, the quantitative metrics of lifestyle variables were not calculated. In addition, three days of CGM data may be a little bit short to assess TIR. Finally, we failed to compare the CGM data before and after infection with SARS-CoV-2 of the same patients for the accurate evaluation of the influence of COVID-19 on blood glucose levels.
In summary, in this observational pilot study, significant higher glycemic fluctuation and exposure to hyperglycemia was associated with COVID-19 among previously normoglycemic individuals, characterized with potentially impaired glucose tolerance. We recommended regular glucose monitoring on these patients on both fasting and postprandial glucose levels. These findings also underscore the concerns of new-onset diabetes in COVID-19.
Ethics approval and consent to participate
The study and the analysis plan were approved by the Institutional Review Boards (Research Ethics Committees) of Shanghai Sixth People's Hospital. We have obtained informed consent from all participants.
Consent for publication
All authors have read and approved submission of abstract and the abstract has not been published and is not being considered for publication elsewhere in whole or part in any language.
Availability of data and materials
The datasets generated and/or analyzed in the current study are not publicly available but are available from the corresponding author on reasonable request.
Funding
This work was funded by the National Key R&D Program of China (2018YFC2000802), the Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (20161430) and Shanghai Municipal Key Clinical Specialty.
Authors’ contributions
J.Z. conceived and designed the study. Y.S., and L.Z. contributed to data collection, data analysis, and writing the paper. Y.S. and L.Z. contributed to data analysis. X.F. and J.Z. contributed to interpretation of data and revision of the manuscript. All authors revised the manuscript for important intellectual content and have approved the final version.
Declaration of competing interest
The authors really appreciate and respect all the healthcare workers fighting against COVID-19. We also appreciate all the doctors and nurses in the Wuhan team from Shanghai Jiaotong University Affiliated Sixth People's Hospital and Fifth People's Hospital of Shanghai Fudan University, as well as all the patients who participated in this study.
Acknowledgements
The authors really appreciate and respect all the healthcare workers fighting against COVID-19. We also appreciate all the doctors and nurses in the Wuhan team from Shanghai Jiaotong University Affiliated Sixth People's Hospital and Fifth People's Hospital of Shanghai Fudan University, as well as all the patients who participated in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.obmed.2021.100328.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Al-Benna S. Association of high level gene expression of ACE2 in adipose tissue with mortality of COVID-19 infection in obese patients. Obes Med. 2020;19 doi: 10.1016/j.obmed.2020.100283. 100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. Epub 2020 Mar 5. PMID: 32142651; PMCID: PMC7102627, e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V.N., DuBose S.N., Li Z., et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J. Clin. Endocrinol. Metab. 2019;104(10):4356–4364. doi: 10.1210/jc.2018-02763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha E, Charkhviani M, Musurakis C, Kansakar AR, Devkota A, Banjade R, Pudasainee P, Chitrakar S, Sharma A, Sous M, Padhamanbhan S, Friedman HJ, Nava GR, et al. Type 2 diabetes is associated with increased risk of critical respiratory illness in patients COVID-19 in a community hospital. Obes Med. 2020 doi: 10.1016/j.obmed.2020.100316. Epub ahead of print. PMID: 33392411; PMCID: PMC7772088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Han Y, Nilsson-Payant BE, Gupta V, Wang P, Duan X, Tang X, Zhu J, Zhao Z, Jaffré F, Zhang T, Kim TW, Harschnitz O, Redmond D, Houghton S, Liu C, Naji A, Ciceri G, Guttikonda S, Bram Y, Nguyen DT, Cioffi M, Chandar V, Hoagland DA, Huang Y, Xiang J, Wang H, Lyden D, Borczuk A, Chen HJ, Studer L, Pan FC, Ho DD, TenOever BR, Evans T, Schwartz RE, Chen S, et al. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27(1):125–136. doi: 10.1016/j.stem.2020.06.015. Epub 2020 Jun 19. PMID: 32579880; PMCID: PMC7303620, e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Li H., Ran X., et al. Reference values for continuous glucose monitoring in Chinese subjects. Diabetes Care. 2009;32(7):1188–1193. doi: 10.2337/dc09-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed in the current study are not publicly available but are available from the corresponding author on reasonable request.