Abstract
Supplemental Digital Content is available in the text.
Keywords: angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, blood pressure, diuretics, hypertension, renin
The 2018 European Society of Cardiology/European Society of Hypertension1 and the 2020 International Society of Hypertension2 guidelines for the management of hypertension proposed that initial combination therapy with 2 antihypertensive agents in a single-pill combination (SPC) is preferred in most patients in need of blood pressure (BP) lowering treatment and should replace the long-standing concept of starting treatment with a single agent, rotating through antihypertensive drug classes, and next moving towards combining drug classes. By moving SPCs forward as the initial BP-lowering strategy, the European1 and International2 Societies of Hypertension Guideline Committees overlooked several principles in hypertension management: (1) understanding the pathophysiology of hypertension; (2) prioritizing evidence from randomized clinical trials above observational studies and expert opinion; and (3) giving consideration to the cost-effectiveness of antihypertensive drug treatment and the sustainability of health care. This article addresses these points. Sources of information included (1) guidelines issued by European,1,3,4 American,5–7 International,2,8,9 and British10–12 Expert Committees, published between 19998 and 2020,2 summarized in Table S1 in the Data Supplement; (2) a PubMed search ran on May 5, 2020, without limitations with as search terms in the abstract or title “hypertension” combined with “fixed combination” OR “hypertension” combined with “single” and “costs”; (3) the placebo-controlled trials of antihypertensive drug treatment, as identified from the reference lists of 5 systematic literature reviews,13–17 of which 2 were published by the Blood Pressure Lowering Trialists’ Collaboration14,16; (4) 3 randomized controlled trials of usual versus intensive BP control18–20; and (5) the retail costs of antihypertensive drugs on the Belgian market (https://www.bcfi.be).
Tailoring Antihypertensive Treatment
In the early 1970s, Laragh’s group coined the terms low-renin, normal-renin, and high-renin hypertension by relating plasma renin activity to the daily sodium excretion.21 Under normal conditions, plasma renin activity increases with sodium restriction but decreases with higher BP.21 Although an imperfect generalization, low-renin hypertension is characterized by volume expansion and its high-renin counterpart by increased peripheral resistance,22,23 and are indications to start BP-lowering treatment with a diuretic as opposed to an inhibitor of the renin-angiotensin system or vasodilator.24 The activity of the renin system decreases with advancing age22 and is lower in Blacks compared with Whites.25–27 These pathophysiological principles explain why guidelines, with the exception of the 2018 European1 and the 2020 International2 guidelines recommend to start antihypertensive drug treatment with ACE (angiotensin-converting enzyme) inhibitors or ARBs below age 55 and with thiazide diuretics (TDs) or dihydropyridine CCBs (calcium-channel blockers) in older patients and in Blacks across the adult age range. Isolated systolic hypertension, which in its initial course is not associated with increased peripheral resistance, but is caused by stiffening of the large arteries28 is an indication for TDs29 or CCBs.30,31 The 2020 International Society of Hypertension guideline2 supported the use of thiazide-like diuretics, that is, indapamide and chlorthalidone, rather than regular TDs (chlorothiazide and hydrochlorothiazide), based on a systematic review of 19 randomized clinical trials involving 112 113 patients.32 The observed benefits were mainly confined to thiazide-like diuretics rather than TDs with reductions in the risk of cardiac events (odds ratio, 0.78; P<0.001), heart failure (odds ratio, 0.57; P<0.001), and stroke (odds ratio, 0.82; P=0.016).32
ACE inhibitors not only inhibit the generation of active angiotensin II, but also the inactivation of the vasodilator bradykinin, explaining their higher potency compared with ARBs and direct renin inhibitors and the recommendation to prescribe ARBs only in ACE inhibitor-intolerant patients.33,34 The involvement of sympathetic drive and the renin-angiotensin system in the cardiovascular and renal complications of hypertension and its comorbidities clarifies why guidelines1–12 unanimously recommend the use ACE inhibitors and ARBs in patients with diabetes or chronic kidney disease, and βBs (beta-blockers),35,36 ACE inhibitors, and ARBs in secondary prevention.
Control Rates on Monotherapy Versus Combination Therapy
With as objective to estimate the proportion of patients with hypertension who can be controlled on monotherapy, we reviewed the placebo-controlled randomized clinical trials listed in systematic reviews of BP-lowering therapies13–17 as well as the trials of intensive versus usual BP control.18–20 We extracted control rates on monotherapy from the trial reports.
Placebo-Controlled Trials
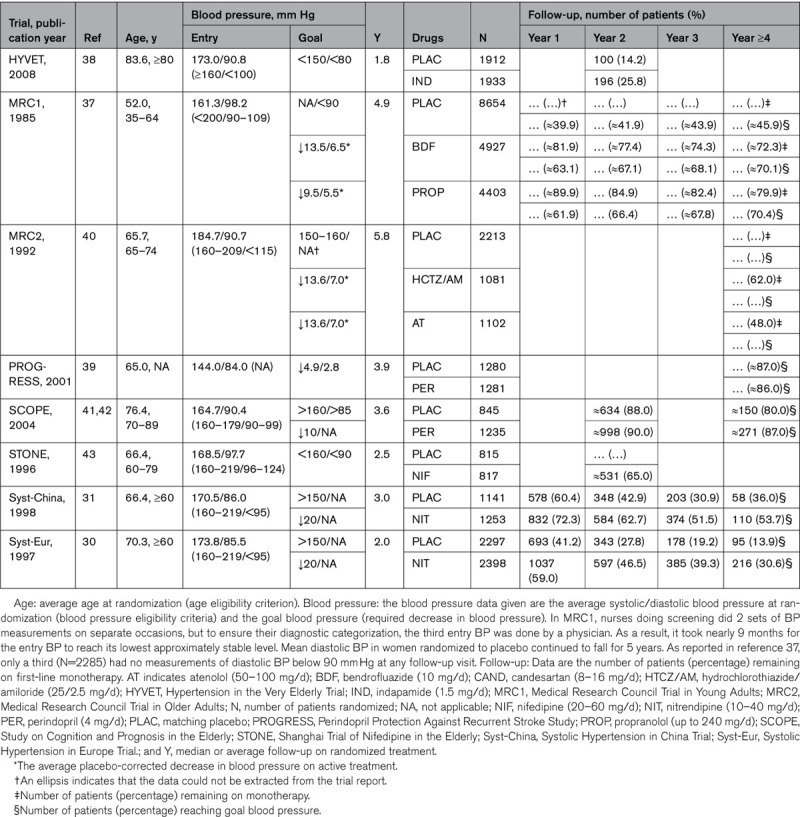
Table 1 lists the placebo-controlled trials from which the proportion of patients remaining on monotherapy could be extracted. These trials were published from 198537 until 2008.38 The first Medical Research Council Trial (age range, 35–64 years)37 and the Perindopril Protection Against Recurrent Stroke Study (age range, not reported)39 enrolled adults, but all other recruited older patients,30,31,38,40–43 including exclusively38 or a substantial proportion of octogenarians.41,42 Considering the patients randomized to active treatment, at 2 years, from 25.8%38 to 90.0%41,42 remained on a single drug and at 4 years from 48.0%40 to 87.0%.41,42 In the Hypertension in the Very Elderly Trial38 and in the Systolic Hypertension in Europe Trial,30 the study coordinating office emailed or faxed recommendations for intensification of treatment to the local investigators, whenever at a visit a patient was not at goal BP, resulting in a substantially smaller proportion of patients remaining on monotherapy in the placebo compared with the active treatment group (Table 1). In the first Medical Research Council Trial,37 at 4 years of follow-up, 70.0% of patients had attained the target BP, defined as a diastolic BP of <90 mm Hg. Thus, a substantial proportion of patients remained on monotherapy or reached goal BP on a single drug in the placebo-controlled trials listed in Table 1.
Table 1.
Patients Remaining on First-Line Drug Treatment in Placebo-Controlled Randomized Clinical Trials
Intensive Versus Usual BP Control
Of the 3 trials18–20 comparing intensive with usual BP control, 219,20 reported on treatment status by randomization group. In the ACCORD Trial (Action to Control Cardiovascular Risk in Diabetes)19 and in SPRINT (Systolic Blood Pressure Intervention Trial),20 patients with a systolic BP of 130 mm Hg or higher and an increased cardiovascular risk were randomly assigned to a systolic BP target of <120 mm Hg (intensive treatment) or a systolic target of <140 mm Hg (usual treatment). In the type-2 diabetic patients randomized to intensive (N=2174) and usual (N=2208) BP control in ACCORD,19 after 1 year, the achieved systolic BP averaged 119.3 mm Hg on intensive treatment and 133.5 mm Hg in the control group; in SPRINT,20 these levels were 121.4 mm Hg (N=4683) and 136.2 mm Hg (N=4683), respectively. In ACCORD (median follow-up, 4.7 years),19 at 1 year, 174 (8.0%) and 265 (28.0%) of patients randomized to intensive and standard treatment were on monotherapy and at the last visit 184 (8.0%) and 553 (24.0%); in SPRINT (median follow-up, 3.3 years),20 these numbers at last follow-up were 493 (10.5%) and 1455 (31.1%), respectively.
Evidence Supporting SPCs
The literature on SPCs focuses on efficiency, adherence (also known as compliance),44 persistence, and safety. Over time, these notions permeated to several,1–9 but not all,10–12 guidelines. What is the evidence?
Randomized Clinical Trials
Our extensive literature review revealed only one randomized clinical trial comparing the efficacy and safety of a SPC with its components.45,46 The COACH Study (Combination of Olmesartan Medoxomil and Amlodipine Besylate in Controlling High Blood Pressure) was a double-blind trial, conducted at 172 clinical sites in the United States.45,46 Patients aged 18 years or older with a diastolic BP ranging from 95 to 120 mm Hg were randomized in equal proportions to combination therapy with olmesartan/amlodipine (daily doses, 10/5, 20/5, 40/5, 10/10, 20/10, or 40/10 mg) or monotherapy with olmesartan (10, 20, or 40 mg) or amlodipine (5 or 10 mg). Of 4234 patients, who entered the 2-week washout phase, 1940 (45.8%) were randomized (women, 45.7%; mean age, 54.0 years; mean entry BP, 164/102 mm Hg) and 1689 (87.1%) completed the 8-week trial. Predictably, each treatment modality, compared with placebo, produced dose-dependent decreases in systolic and diastolic BP and at each dose, combination therapy reduced BP more and achieved BP control more frequently (<140/<90 and <130/<90 mm Hg in diabetic patients) than the equivalent dose of the single-component drug. Limitations of the COACH trial were selection of patients (45.8% of those screened were randomized), the short washout (2 weeks) and follow-up (8 weeks), the highly predictable BP results,47 and the post hoc analysis of patients with isolated systolic hypertension.46
The Simplified Treatment Intervention to Control Hypertension Study was a cluster-randomized trial, involving 45 family practices in Ontario, Canada and compared control rates of hypertension as achieved by a simplified treatment algorithm (experimental group) or following the Canadian Hypertension Program guideline (control group).48 The systolic/diastolic target BP was <140/<90 mm Hg and <130/<90 mm Hg in diabetic patients. The simplified treatment algorithm consisted of the following: (1) initial therapy with a low-dose ACE inhibitor/TD or ARB/TD SPC; (2) uptitration of the combination therapy to the highest dose; (3) addition and subsequent uptitration of a CCB; and (4) addition of a βB, α-blocker, or spironolactone. The proportion of patients achieving target BP at 6 months was higher in the experimental (N=802) than the control (N=1246) group (64.7% versus 52.7%; P=0.026). At 6 months, 82.8% of patients in the experimental group were on SPCs and 16.4% in the control group. However, no information on BP control beyond 6 months was provided.48
A third randomized double-blind study evaluated the efficacy and safety of triple therapy with amlodipine/valsartan/hydrochlorothiazide for moderate or severe hypertension (systolic/diastolic BP, 145/100 mm Hg or higher).49 After a 1-week single-blind placebo run-in, patients were randomly assigned to valsartan/amlodipine/hydrochlorothiazide 320/10/25 mg, valsartan/hydrochlorothiazide 320/25 mg, valsartan/amlodipine 320/10 mg, or amlodipine/hydrochlorothiazide 10/25 mg with uptitration of these once daily SPCs from week 1 to week 3. Of the 4285 patients screened, 2271 (53.0%) were randomized (women, 44.7%; mean age, 53.2 years; mean entry BP, 169.9/106.5 mm Hg) and 2060 (90.7%) completed the 8-week trial. Triple therapy was significantly superior to all of the dual therapies in reducing BP (P<0.0001).49 Results were similar across sex, age, and ethnicity strata. The limitations of this study were like those of the COACH trial.45,46
In the double-blind PATHWAY-1 study (Prevention and Treatment of Hypertension With Algorithm-Based Therapy Trial),24 of 796 screened patients, 605 (76.0%) were randomized and 432 (71.4%) completed the 1-year follow-up period. Eligible patients were untreated, aged 18 to 79 years, and had a self-measured home systolic/diastolic BP of ≥150/≥95 mm Hg. They were randomized to initial monotherapy with losartan 50 to 100 mg/d (N=151) or hydrochlorothiazide 12.5 to 25 mg/d (N=150), crossing over at 8 weeks (switching to the alternative monotherapy), or initial combination treatment with losartan 50 to 100 mg/d plus hydrochlorothiazide 12.5 to 25 mg/d (N=304). In phase 2 (weeks 17–32), all patients received losartan 100 mg and hydrochlorothiazide 12.5 to 25 mg. In phase 3 (weeks 33–52), amlodipine with or without doxazosin could be added to achieve target BP. The primary end point was the change in the systolic home BP (target systolic/diastolic home BP >140/>90 mm Hg). The original protocol prespecified the time of the primary end point at the end of phase 2, namely, 32 weeks after randomization, at which time all patients were receiving the same therapy. The statistical analysis plan, published before the data lock and unblinding, introduced 2 hierarchical co-primary end points.50 The first was the reduction in the systolic home BP averaged over phases 1 and 2, testing for the superiority of initial combination therapy over monotherapy. The co-primary end point, to be tested only if the first hypothesis was confirmed, was the reduction in systolic home BP at week 32, a time point, when all participants were receiving the same treatment. Comparing initial monotherapy with initial combination therapy (Figure 1), the systolic/diastolic reductions in the home BP were 13.3/6.5 versus 21.9/12.1 mm Hg (end of phase 1), 20.1/10.7 versus 19.5/10.6 mm Hg at week 24 (midpoint of phase 2), 23.6/12.7 versus 22.0/11.9 mm Hg at week 32 (end of phase 2), and 24.5/13.9 versus 23.6/13.4 mm Hg at week 52 (end of study). By the end of phase 3, over 75% of participants in the initial monotherapy and combination therapy groups had attained the target home BP with no difference between groups at the end of either phase 2 or 3.24 Based on the redefinition of the primary end points,50 the PATHWAY-1 researchers reported the average BP results combining phases 2 and 3 and all study periods.24 They concluded that initial combination therapy achieved target BP in twice as many participants as initial monotherapy,24 whereas in fact starting from week 24 (Figure 1), home BP was similar irrespective of whether antihypertensive treatment was started with SPC or free SD combination therapy. In the context of the current debate, a relevant finding of the PATHWAY-1 trial was that the BP reductions induced by losartan and hydrochlorothiazide were greatest in the top and bottom plasma renin activity tertiles, respectively,24 an argument supporting an insightful rather than a simplistic initiation of antihypertensive drug therapy.
Figure 1.
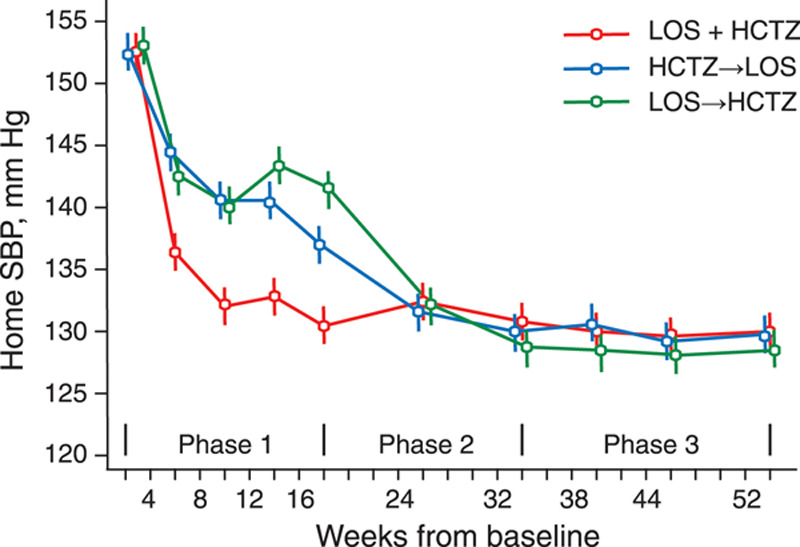
Systolic home blood pressure (BP) by randomization group and follow-up duration. Data points are means. Vertical bars indicate 95% CI. Patients were randomized to initial monotherapy with losartan (LOS) 50–100 mg (N=151) or hydrochlorothiazide (HCTZ) 12.5–25 mg (N=150), crossing over at 8 weeks (switching to the alternative monotherapy), or initial combination treatment with losartan 50–100 mg plus hydrochlorothiazide 12.5–25 mg (N=304). In phase 2 (weeks 17–32), all patients received losartan 100 mg and hydrochlorothiazide 12.5 to 25 mg. In phase 3 (weeks 33–52), amlodipine with or without doxazosin could be added to achieve target BP. SBP indicates systolic BP. Reproduced from MacDonald et al24 with permission. Copyright ©2017, Wiley.
Observational Studies
A common denominator of all observational studies was that they had a retrospective design. A meta-analysis published in 201151 summarized 12 studies published from 200052 until 2010.53 It compared health care costs, adherence, and persistence between groups of patients taking antihypertensive agents as SPCs versus free-equivalent SDs. The mean difference in the annual all-cause and hypertension-related health care costs was $1357 (CI, $778–$1935) lower in favor of SPCs than free SD combinations. Adherence, measured as the mean difference in medication possession ratio, was 8% higher in patients naive to prior antihypertensive drugs and 14% higher in non-naive SPC patients compared with their counterparts on free SD combinations. Persistence in the SPC groups was twice as likely as in the free SD combination groups (pooled risk ratio, 2.1 [CI, 1.1–4.1]). The authors hypothesized that improved adherence and persistence likely contributed to the lower health care costs in the SPCs groups via improved clinical outcomes. Of the 12 studies included in the meta-analysis,52–63 2 did not include a conflict of interest statement,54,56 10 were directly funded by the pharmaceutical industry,52,53,55,57–63 and 752,56–59,61,63 had one or more co-authors employed by drug companies having a commercial interest in SPCs.
The early literature was almost unanimous in stating that SPCs, in comparison with SDs or free combinations of SDs, were more efficacious in lowering BP, increasing adherence and persistence, and lowering health care costs. In view of this exceptional consistency, we searched PubMed for publications with discordant results. We identified 10 studies,51,64–72 published between 201064 until 2020,73 of which the principal outcome measures, data sources consulted, the methods applied, and principal limitations are summarized in Table S2. Of the 10 studies,51,64–72 751,64–67,69,70 were directly supported by SPCs producers, 551,64–67 involved a subcontractor to these manufacturers, and 551,64,65,67,69 were co-authored by one or more industry employees. The study by Hong et al68 stands out, because it was a publication not supported by industry, in which none of the authors reported a conflict of interest. In this article, free SD combinations had average monthly drug costs similar to the respective SPCs, when SPCs were not generically available.68 However, free SD combinations were more expensive compared with generic SPCs.68
A study published in 2020 without industry support,72 applied the 2014 to 2015 Medical Expenditure Panel Survey data, to assess the uses and expenses of antihypertensive drugs among American men and nonpregnant women, aged 18 or older, who had a diagnosis of hypertension. Multiple medications users were patients who used 2 or more antihypertensive medications each year, including SPCs or multiple free SD combinations, or who switched BP-lowering agents within or between classes. Among 10 971 hypertensive adults, 4759 (44.1%) were SD users and 6212 (55.9%) were multiple medication users. The average annual total cost for antihypertensive medications was $336 per person: $199 for SD users and $436 for multiple medication users. The average annual costs for each medication class were estimated at $438 for ARBs and $49 for TDs. Thus, users of multiple medications, including SPCs, incurred more than twice the expense than single medication users.72 When comparing classes of medications, the costs for ARBs were highest, whereas those for TDs were lowest (Figure 2), a trend still visible in the 2020 retail prizes of antihypertensive drugs on the Belgian market (Table S3).72
Figure 2.
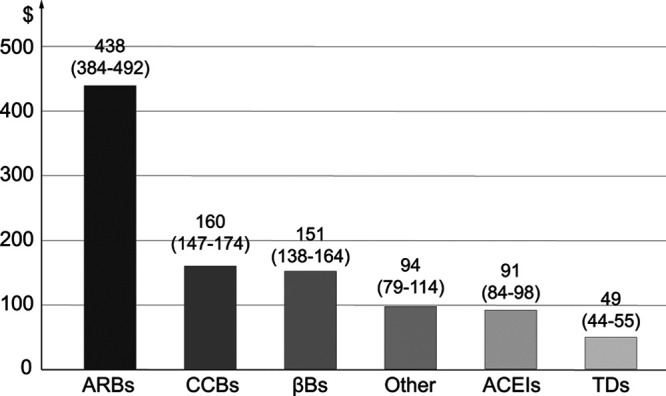
Estimated average annual per capita expenses of each medication class (95% CI), expressed in US dollars based on the 2014–2015 Medical Expenditure Panel Survey Notes. ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium-channel blocker; and TD, thiazide diuretics. Reproduced from Park et al72 with permission. Copyright ©2020, Elsevier.
Several studies addressed the health-economic aspects of the use of SPCs versus SDs or free combinations of SDs,65–67,70,73 or triple versus dual SPCs.69 Data from the MarketScan Database 2006 to 2008 in the United States showed that SPC patients (N=382 476) fared better over a 6-month period than their counterparts on free SD combinations (N=197 375).73 The analyses were adjusted for the baseline characteristics of the selected patients, a reason why this article73 was excluded from the 2011 meta-analysis.51 SPC patients had higher medication possession rates (+11.6%), fewer all-cause hospitalizations (−23.0%), and emergency room visits (−13.0%). SPC patients showed greater reductions in post-therapy initiation in all-cause medical costs ($208 [CI, −$302 to −$114]), but larger increases in hypertension-related prescription costs (+$53 [CI, +$51 to +$55]).73 Similarly, in a study conducted in UK general-practice, hospitalization costs validated up to 2011 were lower in SPC patients compared with free SD users (N=9929 versus 18 665; £62 versus £112; P<0.001), whereas drug costs were higher (£126 versus £78; P<0.001), resulting in similar mean annual management costs in the 2 groups (£192 versus £192).66
All observational reviewed above (Table S2) had a retrospective design and were, therefore, vulnerable to overt and hidden sources of bias, for which analyses did not account. Particularly, most studies had no information on the severity of hypertension at the time of initiation or adjustment of BP-lowering treatment, higher BP being an indication for SPCs or multiple drugs, or on the patients’ health insurance status as determinant of the out-of-pocket costs and adherence (Table S2). Data on health behaviors, patients’ lifestyle, and use of over-the-counter drugs were unavailable. In several analyses, there was a remarkable imbalance between SPC and free SD combination users,65–67,73 indicating selection bias in the patients being prescribed SPCs versus free SD combinations or in data extraction from the claims databases by researchers. Medication possession rate, although an objective measure, but only in settings with a closed pharmacy system,44 is an ambiguous concept. Although there is moderate association between claims for filled prescriptions and measured drug levels44 or prevention of adverse health outcomes,74 claims databases do not ensure that the medication was taken as prescribed. Moreover, information from claims databases disfavors free SD drug combinations, because in their publications investigators selected the SD with the worse adherence,73 or when 2 or more SDs were prescribed, probabilities of nonadherence were multiplicative, not additive. Furthermore, the claims data used for the health-economic analyses were collected for payment purposes rather than for research. A diagnostic code on a medical claim is no proof for the presence of disease, because diagnoses might be incorrectly coded or included as a rule-out criterion rather than as an actual disease. All reviewed health-economic studies only accounted for direct health care costs, disregarding patient values,75 and out-of-pocket costs.71 A follow-up duration ranging from 6 months64 to 5 years66 is not representative of the life course of hypertension. No study measured adverse health outcomes in a prospective manner (Table S2). Transitions between health states applied in Markov modeling were not directly measured, but extrapolated,69,70 introducing arbitrariness in selecting data sources best fitting the hypothesis to be proven.
Narrative Reviews
Of 7 reviews on the use of SPCs,76–82 published from 200976 until 2019,82 6 were written with direct financial support from SPC manufacturers,77–82 3 included co-authors employed by these manufacturers,77,79,82 2 involved a for-profit company running the literature search77 or providing assistance in writing the text,78 and 1 article’s co-author received research support from a company marketing SPCs.76
Take Home Messages
Table 2 lists the major limitations of the recommended policy to initiate antihypertensive treatment using SPCs in most patients.1,2
Table 2.
Take Home Messages
Weaknesses of Current Guidelines
Lengthy guidelines comprehensible only by hypertension specialists, lead to therapeutic inertia in primary care and fall short of their very reason of existence. The 98-page 2018 European recommendations1 go as far as stating that initial combination therapy is invariably more effective in lowering BP than monotherapy and is, therefore, indicated in most patients. The reference cited to substantiate this claim was a meta-analysis, not of SPCs versus SD free combinations, but comparing treatment strategies consisting of increasing the dose of the first-line antihypertensive agent or adding a second drug class.47 Two of its authors held patents for a combination pill (polypill) for the prevention of cardiovascular disease.47 To permeate clinical practice, recommendations must excel in simplicity, allowing summarizing key issues in a simple mnemonic rule, such as the AB/CD algorithm in the 2006 British guideline (Figure 3).10 Admittedly, the position of βBs as first-line treatment remains a matter of debate, albeit not in the last author’s interpretation of the literature.36,83 One might argue that SPCs combining a βB and an ACE inhibitor, as for instance marketed in Belgium (www.bcfi.be) might allow initiating treatment in high-renin hypertensive patients in line with pathophysiologic insights, but in line with the older literature84 no guideline1–12 supports this combination for BP lowering. Nevertheless, guidelines do support such combination in secondary prevention.
Figure 3.
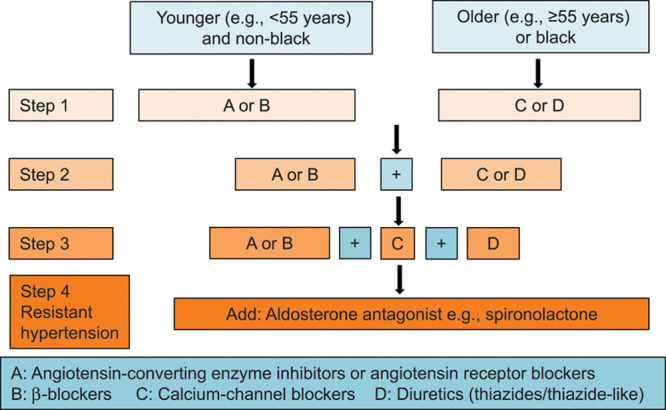
Recommendation for combining blood pressure-lowering drugs. First-line drugs with different modes of action should be combined according to the AB/CD rule. Reproduced from British Cardiac Society, British Hypertension Society, Diabetes UK, HEART UK, Primary Care Cardiovascular Society, The Stroke Association10 with permission. Copyright ©2020, BMJ Publishing Group Ltd.
The pharmaceutical industry is an important motor in creating therapeutic innovation. To remain profitable, there is nothing wrong in SPC manufacturers highlighting the potential benefits of their products. However, a problem arises when retrospective observational studies52–72 (Table S2) or systematic51 or narrative76–82 reviews of such studies become the source of information in evidence-based recommendations. Guidelines should be incremental over time, meaning that evidence published between successive versions should lead to removing or adjusting previous recommendation or introducing new ones. The British guidelines10–12 are exemplary in this respect, giving great weight to new evidence as justification for any change in treatment advice (Table S1). The 2019 National Institute for Health and Care Excellence recommendation11 stated that there was some limited evidence from a single study85 that initial dual therapy, compared with placebo, might reduce cardiovascular complications in people with hypertension and type-2 diabetes, but the Committee Members were disappointed that more comprehensive data were unavailable.11 The Committee discussed the benefits of optimizing treatment for hypertension early and agreed that this could substantially improve quality of life. However, they found that there was not enough evidence to determine confidently the benefits or harms of starting antihypertensive treatment with dual therapy.11
The 2018 European guideline1 went on proposing that the combination of medications targeting multiple mechanisms, such as blocking the renin-angiotensin system and inducing vasodilatation and diuresis, reduces the heterogeneity of the BP responses to initial treatment and provides a steeper dose response than is observed with escalating doses of monotherapy.1 Whereas this might be true during first 6 months after starting BP-lowering treatment,50,86 this certainly does not apply to the long-term life course treatment of hypertension (Figure 1). A post hoc analysis of the Valsartan Antihypertensive Long-Term Use Evaluation Trial did not confirm the widely promoted notion in SPC publications that earlier short-term differences in BP lowering over the long run would reduce cardiovascular end points.87 Furthermore, the European1 and International2 Societies of Hypertension instructions ignored that the association of multiple drugs in a single pharmaceutical formulation may have effects on the pharmacokinetic and pharmacodynamic properties of each and every individual component and may lead to undesired interactions between components.88,89 The trials45,46,48–50 and observational studies51–72 reviewed in this debate article were generally not powered or had a duration not long enough to highlight serious adverse effects. As demonstrated by an observational study of patients aged 50 years or more and reflecting a real-world setting, use of SPCs was associated with a greater risk of hypotension than titrated SD free combinations.90 Moreover, abstraction made of commonly attributable adverse effects, for example, leg edema or cough respectively on treatment with CCBs or ACE inhibitors, many drug-induced complaints are vaguer and more difficult to be picked up, such as for instance fatigue or dizziness and in theory require rechallenge with the SD components of an SPC to identify the culprit drug. Fewer pills to be taken daily is a central concept in the promotion of SPCs,91 but a literature review with as search terms “preference” AND “patient” AND “pills” or “SPC”, ran on October 20, 2020, with no limitations, did not yield any article among the 46 hits that directly translated patient convenience into preference for SPCs in primary or secondary cardiovascular prevention. As a corollary, treatment-experienced persons living with HIV valued minimizing side-effects and long-term toxicities over dosing and administration characteristics.92 Preferences varied widely,92 highlighting the need to elicit individual patient preferences, when decisions about dosing schemes of medications are made, certainly in the light of the potential adverse events of SPCs as mentioned before. Finally, the advice to initiate antihypertensive drug therapy with SPCs also goes against pathophysiological principles supporting the use of TDs in low-renin hypertension, Blacks and older patients and the use vasodilators (ACE inhibitors, ARBs, or CCBs) in high-renin patients or younger individuals (Figure 3).
In an era of epidemiological transition,93 payers, doctors, and patients should join forces to keep health care sustainable in aging populations. In the placebo-controlled outcome trials (Table 1),30,31,37–43 a substantial proportion of hypertensive patients could be controlled on a single drug. Arguably, the entry and target BPs in these trials were higher than those currently proposed. However, mutatis mutandis, lower BP levels, at which antihypertensive drug treatment should be initiated,7 would increase the control rates on monotherapy. BP lowering to <140/90 mm Hg was achieved by monotherapy in about one-third of patients randomized to standard treatment in ACCORD19 and SPRINT.20 In needy patients, out-of-pocket costs are a major hurdle in long-term adherence.71 In a Canadian cluster-randomized trial involving 76 primary care practices,71 3592 patients with uncomplicated hypertension were followed up for 5 years. Physicians were randomized to an out-of-pocket expenditure software module that provided alerts for out-of-reimbursement costs and recommended TDs as first-line therapy and control. In the intervention group, there was a significant increase in the prescription of TDs in newly treated patients (26.6% versus 19.8%). For patients already treated, older patients were less likely to be switched to a TD. Translating these findings to the Belgian context (Table S3), starting a patient on monotherapy with low-dose treatment with chlorthalidone, bisoprolol, amlodipine, perindopril, valsartan, or olmesartan entails an annual cost of €19, €38, €44, €72, €87, and €107, respectively, if the drug with the lowest retail prize would be prescribed; the corresponding annual expense for the lowest-cost SPC with valsartan/hydrochlorothiazide, olmesartan/hydrochlorothiazide, valsartan/amlodipine, and olmesartan/amlodipine amounts to €85, €107, €128, and €141. Giving that one-third of the patients started on antihypertensive therapy can be controlled by a SD, the potential savings for the Belgian health insurance are huge, if patients would no longer be started on dual SPCs.
Rational Use of SPCs
We proposed that starting antihypertensive therapy in treatment-naive hypertensive patients might be based on a few simple principles. First, use antihypertensive drugs with different modes of action in line with the AB/CD algorithm (Figure 3). Second, use antihypertensive agents with a long duration of action based on their molecular structure, so-called forgiving drugs, rather than extended-release dosage formulations.94,95 Third, titrate each drug to the highest dose that does not produce adverse effects. Fourth, include a thiazide in the drug combination. Finally, once the right combination has been found by rotating through and combining drug classes as well as the timing of dosing, stimulate adherence by reducing the pill load by prescribing SPCs including 2 or 3 antihypertensive agents in adjustable doses. Initiating antihypertensive drug treatment with SDs overcomes the inflexibility of SPCs in titrating the doses of the SD components and the timing of their administration, for instance to prevent nocturnal diuresis or hypotension. In line with the above proposal, in Japan, only one triple-drug SPC is being marketed (telmisartan/amlodipine/hydrochlorothiazide 80/5/12.5 mg). It can only be prescribed after 8 weeks of successful treatment with its components given as dual SPCs plus one SD or as free 3-drug SD combinations
None of the trials of SPCs had a cardiovascular end point. In line with the 2019 National Institute for Health and Care Excellence guideline,11 an important research issue is to mount outcome-driven randomized clinical trials to delineate particular subgroups of hypertensive patients who might benefit from starting dual therapy. Furthermore, compared with hydrochlorothiazide, chlorthalidone is 1.5 to 2.0 × more potent, has a substantially longer duration of action (plasma half-life, 8 versus >24 hours), and is not metabolized but excreted unchanged in urine, thereby preventing drug-drug interactions.32,95 Unfortunately, the diuretic in SPCs currently marketed is overwhelmingly hydrochlorothiazide, an issue to be addressed by manufacturers (Table 2). Finally, payers should better inform physicians and patients on the costs of antihypertensive drugs to reduce health care costs, decrease out-of-pocket costs as a factor limiting adherence,71 and to support the sustainability of health care by lower drug costs and better prevention of the cardiovascular-renal hypertension-associated complications.
Acknowledgment
We acknowledge the clerical assistance of Vera De Leebeeck and Renilde Wolfs, Research Unit Hypertension and Cardiovascular Epidemiology, Department of Cardiovascular Sciences, University of Leuven, Leuven, Belgium.
Source of Funding
The Research Institute Alliance for the Promotion of Preventive Medicine received a nonbinding grant from OMRON Healthcare Co Ltd, Kyoto, Japan.
Disclosures
None.
Supplementary Material
Footnotes
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
This article was sent to Morris J. Brown, Guest Editor, for review by expert referees, editorial decision, and final disposition.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.120.12858.
For Sources of Funding and Disclosures, see page 796.
Contributor Information
Zhen-Yu Zhang, Email: zhenyu.zhang@kuleuven.be.
Yu-Ling Yu, Email: yyl0603@sina.cn.
Kei Asayama, Email: kei@asayama.org.
Tine W. Hansen, Email: tine.willum.hansen@regionh.dk.
Gladys E. Maestre, Email: gladysmaestre@gmail.com.
Response to Starting Antihypertensive Drug Treatment With Combination Therapy: Controversies in Hypertension - Con Side of the Argument
Alexandre Persu, Marilucy Lopez-Sublet, Engi Abd El-Hady Algharably, Reinhold Kreutz
Jan Staessen is an internationally recognized expert known for his independent and integer positions. He and his coauthors have produced a highly informative, original document against the extensive use of first-line single-pill combinations.
Still, we feel that it does not detract from our plea in favor of the use of combination therapy as first-line treatment in most patients with hypertension. Although studies supporting the benefit of this compared to other strategies have limitations, this is also the case for other treatment strategies used in daily practice. For example, the arguments in favor of the National Institute for Health and Care Excellence algorithm supported by Staessen and colleagues rest more on clinical expertise and general principles of pharmacology than on rigorously designed randomized controlled trials.
The recommendation to use dual antihypertensive therapy in most patients with hypertension is more a public health than a trialist’s perspective.
As indicated by Prof. Staessen, two-thirds of patients with hypertension eventually need ≥2 antihypertensive drugs to achieve blood pressure control.
We simply think that using single-pill combinations as first-line therapy in those patients is the most effective way to overcome poor drug adherence and inertia, currently the main barriers to improve blood pressure control worldwide.
Admittedly, this recommendation may benefit the pharma industry. However, it is the responsibility of public health authorities to negotiate properly the price of single-pill combinations while supporting less expensive, generic alternatives.
Finally, the gap between Prof. Staessen’s and our conception is less wide than it may appear.
While he focuses on cases in which monotherapy is the preferred approach—basically the same as us, patients with mild hypertension, particularly older patients with isolated systolic hypertension—we emphasize the big picture in favor of first-line dual antihypertensive therapy in most patients with hypertension, while mentioning the exceptions.
References
- 1.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. ; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 2.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, Ramirez A, Schlaich M, Stergiou GS, Tomaszewski M, et al. 2020 International Society of hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026 [DOI] [PubMed] [Google Scholar]
- 3.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, et al. 2007 Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236 [DOI] [PubMed] [Google Scholar]
- 4.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151 [DOI] [PubMed] [Google Scholar]
- 5.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, et al. ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2 [DOI] [PubMed] [Google Scholar]
- 6.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). J Am Med Assoc. 2014;311:507–520. doi: 10.1001/jama.2013.284427 [DOI] [PubMed] [Google Scholar]
- 7.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 8.Guidelines Subcommittee. 1999 World Health Organization-International Society of Hypertension guidelines for the management of hypertension. J Hypertens. 1999;17:151–183. [PubMed] [Google Scholar]
- 9.Whitworth JA; World Health Organization, International Society of Hypertension Writing Group. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–1992. doi: 10.1097/00004872-200311000-00002 [DOI] [PubMed] [Google Scholar]
- 10.British Cardiac Society, British Hypertension Society, Diabetes UK, HEART UK, Primary Care Cardiovascular Society, The Stroke Association. JBS 2: Joint British Societies’ guidelines on prevention of cardiovascular diseases in clinical practice. Heart. 2005Suppl 5v1–52. doi: 10.1136/hrt.2005.079988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institute for Health and Clinical Excellence (NICE). Hypertension in Adults: Diagosis and Management. NICE Guideline [NG136]. 2019. https://www.nice.org.uk/guidance/ng136. Accessed January 23, 2021
- 12.National Institute for Health and Clinical Excellence (NICE). The Clinical Management of Primary Hypertension in Adults. Clinical Guideline 127. Methods, Evidence and Recommendations. 2011. http://www.nice.org.uk/guidance/CG127. Accessed January 23, 2021
- 13.Staessen JA, Wang JG, Thijs L. Cardiovascular protection and blood pressure reduction: a meta-analysis. Lancet. 2001;358:1305–1315. doi: 10.1016/S0140-6736(01)06411-X [DOI] [PubMed] [Google Scholar]
- 14.Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1535. doi: 10.1097/HJH.0b013e3280bad9b4 [DOI] [PubMed] [Google Scholar]
- 15.Verdecchia P, Reboldi G, Angeli F, Gattobigio R, Bentivoglio M, Thijs L, Staessen JA, Porcellati C. Angiotensin-converting enzyme inhibitors and calcium channel blockers for coronary heart disease and stroke prevention. Hypertension. 2005;46:386–392. doi: 10.1161/01.HYP.0000174591.42889.a2 [DOI] [PubMed] [Google Scholar]
- 16.Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure dependent and independent effects of agents that inhibit the renin-angiotensin system. J Hypertens. 2007;25:951–958. doi: 10.1097/HJH.0b013e3280bad9b4 [DOI] [PubMed] [Google Scholar]
- 17.Verdecchia P, Angeli F, Cavallini C, Gattobigio R, Gentile G, Staessen JA, Reboldi G. Blood pressure reduction and renin-angiotensin system inhibition for prevention of congestive heart failure: a meta-analysis. Eur Heart J. 2009;30:679–688. doi: 10.1093/eurheartj/ehn575 [DOI] [PubMed] [Google Scholar]
- 18.Verdecchia P, Staessen JA, Angeli F, de Simone G, Achilli A, Ganau A, Mureddu G, Pede S, Maggioni AP, Lucci D, et al. ; Cardio-Sis investigators. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009;374:525–533. doi: 10.1016/S0140-6736(09)61340-4 [DOI] [PubMed] [Google Scholar]
- 19.The ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, et al. ; The SPRINT Research Group. A randomized trial of intensive versus standard blood pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunner HR, Laragh JH, Baer L, Newton MA, Goodwin FT, Krakoff LR, Bard RH, Bühler FR. Essential hypertension: renin and aldosterone, heart attack and stroke. N Engl J Med. 1972;286:441–449. doi: 10.1056/NEJM197203022860901 [DOI] [PubMed] [Google Scholar]
- 22.Dunn MJ, Tannen RL. Low-renin hypertension. Kidney Int. 1974;5:317–325. doi: 10.1038/ki.1974.47 [DOI] [PubMed] [Google Scholar]
- 23.Julius S. Interaction between renin and the autonomic nervous system in hypertension. Am Heart J. 1988;1162 pt 2611–616. doi: 10.1016/0002-8703(88)90559-5 [DOI] [PubMed] [Google Scholar]
- 24.MacDonald TM, Williams B, Webb DJ, Morant S, Caulfield M, Cruickshank JK, Ford I, Sever P, Mackenzie IS, Padmanabhan S, et al. ; British Hypertension Society Programme of Prevention and Treatment of Hypertension with Algorithm-based Therapy (PATHWAY). Combination therapy is superior to sequential monotherapy for the initial treatment of hypertension: a double-blind randomized controlled trial. J Am Heart Assoc. 2017;6:e006986. doi: 10.1161/JAHA.117.006986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helmer OM, Judson WE. Metabolic studies on hypertensive patients with suppressed plasma renin activity not due to hyperaldosternosm. Circulation. 1968;38:965–976. doi: 10.1161/01.cir.38.5.965 [DOI] [PubMed] [Google Scholar]
- 26.Channick BJ, Adlin EV, Marks AD. Suppressed plasma renin activity in hypertension. Arch Intern Med. 1969;123:131–140. doi:10.1001/archinte.1969.00300120019003 [PubMed] [Google Scholar]
- 27.Mroczek WJ, Finnerty FA, Catt KJ. Lack of association between plasma-renin and history of heart-attack or stroke in patients with essential hypertension. Lancet. 1973;2:464–468. doi: 10.1016/s0140-6736(73)92069-2 [DOI] [PubMed] [Google Scholar]
- 28.Staessen J, Amery A, Fagard R. Editorial review. Isolated systolic hypertension. J Hypertens. 1990;8:393–405. [DOI] [PubMed] [Google Scholar]
- 29.SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). J Am Med Ass. 1991;265:3255–3264. doi:10.1001/jama.1991.03460240051027 [PubMed] [Google Scholar]
- 30.Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhäger WH, Bulpitt CJ, de Leeuw PW, Dollery CT, Fletcher AE, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350:757–764. doi: 10.1016/s0140-6736(97)05381-6 [DOI] [PubMed] [Google Scholar]
- 31.Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. J Hypertens. 1998;1612 pt 11823–1829. doi: 10.1097/00004872-199816120-00016 [DOI] [PubMed] [Google Scholar]
- 32.Chen P, Chaugai S, Zhao F, Wang DW. Cardioprotective effect of thiazide-like diuretics: a meta-analysis. Am J Hypertens. 2015;28:1453–1463. doi: 10.1093/ajh/hpv050 [DOI] [PubMed] [Google Scholar]
- 33.Staessen JA, Wang J, Bianchi G, Birkenhäger WH. Essential hypertension. Lancet. 2003;361:1629–1641. doi: 10.1016/S0140-6736(03)13302-8 [DOI] [PubMed] [Google Scholar]
- 34.Staessen JA, Li Y, Richart T. Oral renin inhibitors. Lancet. 2006;368:1449–1456. doi: 10.1016/S0140-6736(06)69442-7 [DOI] [PubMed] [Google Scholar]
- 35.Staessen J, Bulpitt C, Cattaert A, Fagard R, Vanhees L, Amery A. Secondary prevention with beta-adrenoceptor blockers in post-myocardial infarction patients. Am Heart J. 1982;104:1395–1399. doi: 10.1016/0002-8703(82)90184-3 [DOI] [PubMed] [Google Scholar]
- 36.Staessen JA, Wang JG, Birkenhäger WH, Fagard R. Treatment with beta-blockers for the primary prevention of the cardiovascular complications of hypertension. Eur Heart J. 1999;20:11–24. doi: 10.1053/euhj.1998.1121 [DOI] [PubMed] [Google Scholar]
- 37.Medical Research Council Working Party. MRC trial of treatment of mild hypertension: principal results. Br Med J. 1985;291:97–104. doi: 10.1136/bmj.291.6488.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, et al. ; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369 [DOI] [PubMed] [Google Scholar]
- 39.PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood pressure lowering regimen among 6105 individuals with prior stroke or transient ischaemic attack. Lancet. 2001;358:1033–1041. doi: 10.1016/S0140-6736(01)06178-5 [DOI] [PubMed] [Google Scholar]
- 40.MRC Working Party. Medical research council trial of treatment of hypertension in older adults: principal results. Br Med J. 1992;304:405–412. doi: 10.1136/bmj.304.6824.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, Trenkwalder P, Zanchetti A; SCOPE Study Group. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens. 2003;21:875–886. doi: 10.1097/00004872-200305000-00011 [DOI] [PubMed] [Google Scholar]
- 42.Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, Trenkwalder P, Zanchetti A; SCOPE Study Group. The Study on COgnition and Prognosis in the Elderly (SCOPE); outcomes in patients not receiving add-on therapy after randomization. J Hypertens. 2004;22:1605–1612. doi: 10.1097/01.hjh.0000133730.47372.4c [DOI] [PubMed] [Google Scholar]
- 43.Gong L, Zhang W, Zhu Y, Zhu J, Kong D, Pagé V, Ghadirian P, LeLorier J, Hamet P. Shanghai trial of nifedipine in the elderly (STONE). J Hypertens. 1996;14:1237–1245. doi: 10.1097/00004872-199610000-00013 [DOI] [PubMed] [Google Scholar]
- 44.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100 [DOI] [PubMed] [Google Scholar]
- 45.Chrysant SG, Melino M, Karki S, Lee J, Heyrman R. The combination of olmesartan medoxomil and amlodipine besylate in controlling high blood pressure: COACH, a randomized, double-blind, placebo-controlled, 8-week factorial efficacy and safety study. Clin Ther. 2008;30:587–604. doi: 10.1016/j.clinthera.2008.04.002 [DOI] [PubMed] [Google Scholar]
- 46.Mourad JJ, Le Jeune S. Effective systolic blood pressure reduction with olmesartan medoxomil/amlodipine combination therapy. Post hoc analysis of data from a randomized, double-blind, parallel-group, multicentre study. Clin Drug Invest. 2009;29:419–425. doi: 10.2165/00044011-200929060-00005 [DOI] [PubMed] [Google Scholar]
- 47.Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290–300. doi: 10.1016/j.amjmed.2008.09.038 [DOI] [PubMed] [Google Scholar]
- 48.Dasgupta K, Quinn RR, Zarnke KB, Rabi DM, Ravani P, Daskalopoulou SS, Rabkin SW, Trudeau L, Feldman RD, Cloutier L, et al. ; Canadian Hypertension Education Program. The 2014 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2014;30:485–501. doi: 10.1016/j.cjca.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 49.Calhoun DA, Lacourcière Y, Chiang YT, Glazer RD. Triple antihypertensive therapy with amlodipine, valsartan, and hydrochlorothiazide: a randomized clinical trial. Hypertension. 2009;54:32–39. doi: 10.1161/HYPERTENSIONAHA.109.131300 [DOI] [PubMed] [Google Scholar]
- 50.MacDonald TM, Williams B, Caulfield M, Cruickshank JK, McInnes G, Sever P, Webb DJ, Mackenzie IS, Salsbury J, Morant S, et al. Monotherapy versus dual therapy for the initial treatment of hypertension (PATHWAY-1): a randomised double-blind controlled trial. BMJ Open. 2015;5:e007645. doi: 10.1136/bmjopen-2015-007645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. J Clin Hypertens (Greenwich). 2011;13:898–909. doi: 10.1111/j.1751-7176.2011.00550.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dezii CM. A retrospective study of persistence with single-pill combination therapy vs. concurrent two-pill therapy in patients with hypertension. Manag Care. 2000;10:6–10. [PubMed] [Google Scholar]
- 53.Malesker MA, Hilleman DE. Comparison of amlodipine/valsartan fixed-dose combination therapy and conventional therapy. Manag Care. 2010;19:36–42. [PubMed] [Google Scholar]
- 54.Taylor AA, Shoheiber O. Adherence to antihypertensive therapy with fixed-dose amlodipine besylate/benazepril HCl versus comparable component-based therapy. Congest Heart Fail. 2003;9:324–332. doi: 10.1111/j.1527-5299.2003.03269.x [DOI] [PubMed] [Google Scholar]
- 55.Gerbino PP, Shoheiber O. Adherence patterns among patients treated with fixed-dose combination versus separate antihypertensive agents. Am J Health Syst Pharm. 2007;64:1279–1283. doi: 10.2146/ajhp060434 [DOI] [PubMed] [Google Scholar]
- 56.Hasford J, Schröder-Bernhardi D, Rottenkolber M, Kostev K, Dietlein G. Persistence with antihypertensive treatments: results of a 3-year follow-up cohort study. Eur J Clin Pharmacol. 2007;63:1055–1061. doi: 10.1007/s00228-007-0340-2 [DOI] [PubMed] [Google Scholar]
- 57.Schweizer J, Hilsmann U, Neumann G, Handrock R, Klebs S. Efficacy and safety of valsartan 160/HCTZ 25 mg in fixed combination in hypertensive patients not controlled by candesartan 32 mg plus HCTZ 25 mg in free combination. Curr Med Res Opin. 2007;23:2877–2885. doi: 10.1185/030079907x242539 [DOI] [PubMed] [Google Scholar]
- 58.Barron JJ, Daniel G, Makin C, Preblick R. Treatment modifications and resource use for fixed-dose vs separate-agent antihypertensive regimens. Drug Benefit Trends. 2008;20:226–247. [Google Scholar]
- 59.Brixner DI, Jackson KC, 2nd, Sheng X, Nelson RE, Keskinaslan A. Assessment of adherence, persistence, and costs among valsartan and hydrochlorothiazide retrospective cohorts in free-and fixed-dose combinations. Curr Med Res Opin. 2008;24:2597–2607. doi: 10.1185/03007990802319364 [DOI] [PubMed] [Google Scholar]
- 60.Dickson M, Plauschinat CA. Compliance with antihypertensive therapy in the elderly: a comparison of fixed-dose combination amlodipine/benazepril versus component-based free-combination therapy. Am J Cardiovasc Drugs. 2008;8:45–50. doi: 10.2165/00129784-200808010-00006 [DOI] [PubMed] [Google Scholar]
- 61.Hess G, Hill J, Lau H, Dastani H, Chaudhari P. Medication utilization patterns and hypertension-related expenditures among patients who were switched from fixed-dose to free-combination antihypertensive therapy. P T. 2008;33:652–666. [PMC free article] [PubMed] [Google Scholar]
- 62.Jackson KC, 2nd, Sheng X, Nelson RE, Keskinaslan A, Brixner DI. Adherence with multiple-combination antihypertensive pharmacotherapies in a US managed care database. Clin Ther. 2008;30:1558–1563. doi: 10.1016/j.clinthera.2008.08.010 [DOI] [PubMed] [Google Scholar]
- 63.Shaya FT, Du D, Gbarayor CM, Frech-Tamas F, Lau H, Weir MR. Predictors of compliance with antihypertensive therapy in a high-risk medicaid population. J Natl Med Assoc. 2009;101:34–39. doi: 10.1016/s0027-9684(15)30808-7 [DOI] [PubMed] [Google Scholar]
- 64.Yang W, Kahler KH, Fellers T, Orloff J, Chang J, Bensimon AG, Wu EQ, Fan CP, Yu AP. Copayment level, treatment persistence, and healthcare utilization in hypertension patients treated with single-pill combination therapy. J Med Econ. 2011;14:267–278. doi: 10.3111/13696998.2011.570401 [DOI] [PubMed] [Google Scholar]
- 65.Baser O, Andrews LM, Wang L, Xie L. Comparison of real-world adherence, healthcare resource utilization and costs for newly initiated valsartan/amlodipine single-pill combination versus angiotensin receptor blocker/calcium channel blocker free-combination therapy. J Med Econ. 2011;14:576–583. doi: 10.3111/13696998.2011.596873 [DOI] [PubMed] [Google Scholar]
- 66.Belsey JD. Optimizing adherence in hypertension: a comparison of outcomes and costs using single tablet regimens vs individual component regimens. J Med Econ. 2012;15:897–905. doi: 10.3111/13696998.2012.689792 [DOI] [PubMed] [Google Scholar]
- 67.Breitscheidel L, Ehlken B, Kostev K, Oberdiek MS, Sandberg A, Schmieder RE. Real-life treatment patterns, compliance, persistence, and medication costs in patients with hypertension in Germany. J Med Econ. 2012;15:155–165. doi: 10.3111/13696998.2011.635229 [DOI] [PubMed] [Google Scholar]
- 68.Hong SH, Wang J, Tang J. Dynamic view on affordability of fixed-dose combination antihypertensive drug therapy. Am J Hypertens. 2013;26:879–887. doi: 10.1093/ajh/hpt035 [DOI] [PubMed] [Google Scholar]
- 69.Stafylas P, Kourlaba G, Hatzikou M, Georgiopoulos D, Sarafidis P, Maniadakis N. Economic evaluation of a single-pill triple antihypertensive therapy with valsartan, amlodipine, and hydrochlorothiazide against its dual components. Cost Eff Resour Alloc. 2015;13:10. doi: 10.1186/s12962-015-0036-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ren M, Xuan D, Lu Y, Fu Y, Xuan J. Economic evaluation of olmesartan/amlodipine fixed-dose combination for hypertension treatment in China. J Med Econ. 2020;23:394–400. doi: 10.1080/13696998.2019.1699799 [DOI] [PubMed] [Google Scholar]
- 71.Tamblyn R, Winslade N, Qian CJ, Moraga T, Huang A. What is in your wallet? A cluster randomized trial of the effects of showing comparative patient out-of-pocket costs on primary care prescribing for uncomplicated hypertension. Implement Sci. 2018;13:7. doi: 10.1186/s13012-017-0701-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park C, Wang G, Ng BP, Fang J, Durthaler JM, Ayala C. The uses and expenses of antihypertensive medications among hypertensive adults. Res Social Adm Pharm. 2020;16:183–189. doi: 10.1016/j.sapharm.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 73.Yang W, Chang J, Kahler KH, Fellers T, Orloff J, Wu EQ, Bensimon AG. Evaluation of compliance and health care utilization in patients treated with single pill vs. free combination antihypertensives. Curr Med Res Opin. 2010;26:2065–2076. doi: 10.1185/03007995.2010.494462 [DOI] [PubMed] [Google Scholar]
- 74.Rasmussen JN, Chong A, Alter DA. Relationship between adherence and evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. J Am Med Assoc. 2007;297:177–186. [DOI] [PubMed] [Google Scholar]
- 75.Marzorati C, Pravettoni G. Value as the key concept in the health care system: how it has influenced medical practice and clinical decision-making processes. J Multidiscip Healthc. 2017;10:101–106. doi: 10.2147/JMDH.S122383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cowart JB, Taylor AA. Should two-drug initial therapy for hypertension be recommended for all patients? Curr Hypertens Rep. 2012;14:324–332. doi: 10.1007/s11906-012-0280-9 [DOI] [PubMed] [Google Scholar]
- 77.Burnier M, Brown RE, Ong SH, Keskinaslan A, Khan ZM. Issues in blood pressure control and the potential role of single-pill combination therapies. Int J Clin Pract. 2009;63:790–798. doi: 10.1111/j.1742-1241.2009.01999.x [DOI] [PubMed] [Google Scholar]
- 78.Ram CV. Fixed-dose triple-combination treatments in the management of hypertension. Manag Care. 2013;22:45–55. [PubMed] [Google Scholar]
- 79.Schäfer HH, Scheunert U. Costs of current antihypertensive therapy in Switzerland: an economic evaluation of 3,489 patients in primary care. Swiss Med Wkly. 2013;143:w13854. doi: 10.4414/smw.2013.13854 [DOI] [PubMed] [Google Scholar]
- 80.Hilleman DE. Adherence and health care costs with single-pill fixed-dose combinations in hypertension management. J Manag Care Pharm. 2014;20:93–100. doi: 10.18553/jmcp.2014.20.1.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Costa FV. Improving adherence to treatment and reducing economic costs of hypertension: the role of olmesartan-based treatment. High Blood Press Cardiovasc Prev. 2017;24:265–274. doi: 10.1007/s40292-017-0221-4 [DOI] [PubMed] [Google Scholar]
- 82.Poulter NR, Borghi C, Parati G, Pathak A, Toli D, Williams B, Schmieder RE. Medication adherence in hypertension. J Hypertens. 2020;38:579–587. doi: 10.1097/HJH.0000000000002294 [DOI] [PubMed] [Google Scholar]
- 83.Wei FF, Zhang ZY, Huang QF, Staessen JA. Diagnosis and management of resistant hypertension: state of the art. Nat Rev Nephrol. 2018;14:428–441. doi: 10.1038/s41581-018-0006-6 [DOI] [PubMed] [Google Scholar]
- 84.Staessen J, Fagard R, Lijnen P, Verschueren LJ, Amery A. Double-blind comparison between propranolol and bendroflumethiazide in captopril-treated resistant hypertensive patients. Am Heart J. 1983;106:321–328. doi: 10.1016/0002-8703(83)90199-0 [DOI] [PubMed] [Google Scholar]
- 85.ADVANCE Collaborative Group. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–840. doi: 10.1016/S0140-6736(07)61303-8 [DOI] [PubMed] [Google Scholar]
- 86.Feldman RD, Zou GY, Vandervoort MK, Wong CJ, Nelson SA, Feagan BG. A simplified approach to the treatment of uncomplicated hypertension: a cluster randomized, controlled trial. Hypertension. 2009;53:646–653. doi: 10.1161/HYPERTENSIONAHA.108.123455 [DOI] [PubMed] [Google Scholar]
- 87.Julius S, Weber MA, Kjeldsen SE, McInnes GT, Zanchetti A, Brunner HR, Laragh J, Schork MA, Hua TA, Amerena J, et al. The Valsartan Antihypertensive Long-Term Use Evaluation (VALUE) trial: outcomes in patients receiving monotherapy. Hypertension. 2006;48:385–391. doi: 10.1161/01.HYP.0000236119.96301.f2 [DOI] [PubMed] [Google Scholar]
- 88.García-Donaire JA, Ruilope LM. Multiple action fixed combination. Present or future? Fund Clin Pharmacol. 2010;24:37–42. doi: 10.1111/j.1472-8206.2009.00799.x [DOI] [PubMed] [Google Scholar]
- 89.Guglietta A, Guerrero M. Issues to consider in the pharmaceutical development of a cardiovascular polypill. Nat Clin Pract Cardiovasc Med. 2009;6:112–119. doi: 10.1038/ncpcardio1424 [DOI] [PubMed] [Google Scholar]
- 90.Nowak E, Happe A, Bouget J, Paillard F, Vigneau C, Scarabin PY, Oger E. Safety of fixed dose of antihypertensive drug combinations compared to (single pill) free-combinations: a nested matched case-control analysis. Medicine (Baltimore). 2015;94:e2229. doi: 10.1097/MD.0000000000002229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aslam F, Haque A, Lee V, Foody J. Patient adherence and preference considerations in managing cardiovascular risk: focus on single pill and amlodipine/atorvastatin fixed combination. Patient Prefer Adherence. 2009;3:61–66. doi: 10.2147/ppa.s4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ostermann J, Mühlbacher A, Brown DS, Regier DA, Hobbie A, Weinhold A, Alshareef N, Derrick C, Thielman NM. Heterogeneous patient preferences for modern antiretroviral therapy: results of a discrete choice experiment. Value Health. 2020;23:851–861. doi: 10.1016/j.jval.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369:448–457. doi: 10.1056/NEJMra1201534 [DOI] [PubMed] [Google Scholar]
- 94.Hernández-Hernández R, Armas de Hernández MJ, Armas-Padilla MC, Carvajal AR, Guerrero-Pajuelo J. The effects of missing a dose of enalapril versus amlodipine on ambulatory blood pressure. Blood Press Monit. 1996;1:121–126. [PubMed] [Google Scholar]
- 95.Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43:4–9. doi: 10.1161/01.HYP.0000103632.19915.0E [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


