Supplemental Digital Content is available in the text.
Keywords: angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, COVID-19, hospitalization, mortality, pharmacoepidemiology
Abstract
After initially hypothesizing a positive relationship between use of renin-angiotensin-aldosterone system inhibitors and risk of coronavirus disease 2019 (COVID-19), more recent evidence suggests negative associations. We examined whether COVID-19 risk differs according to antihypertensive drug class in patients treated by ACE (angiotensin-converting enzyme) inhibitors and angiotensin receptor blockers (ARBs) compared with calcium channel blockers (CCBs). Three exclusive cohorts of prevalent ACE inhibitors, ARB and CCB users, aged 18 to 80 years, from the French National Health Insurance databases were followed from February 15, 2020 to June 7, 2020. We excluded patients with a history of diabetes, known cardiovascular disease, chronic renal failure, or chronic respiratory disease during the previous 5 years, to only consider patients treated for uncomplicated hypertension and to limit indication bias. The primary end point was time to hospitalization for COVID-19. The secondary end point was time to intubation/death during a hospital stay for COVID-19. In a population of almost 2 million hypertensive patients (ACE inhibitors: 566 023; ARB: 958 227; CCB: 358 306) followed for 16 weeks, 2338 were hospitalized and 526 died or were intubated for COVID-19. ACE inhibitors and ARBs were associated with a lower risk of COVID-19 hospitalization compared with CCBs (hazard ratio, 0.74 [95% CI, 0.65–0.83] and 0.84 [0.76–0.93], respectively) and a lower risk of intubation/death. Risks were slightly lower for ACE inhibitor users than for ARB users. This large observational study may suggest a lower COVID-19 risk in hypertensive patients treated over a long period with ACE inhibitors or ARBs compared with CCBs. These results, if confirmed, tend to contradict previous hypotheses and raise new hypotheses.
Severe acute respiratory coronavirus 2 infection has become a worldwide pandemic. The virus has spread rapidly with >17 million people infected (and 680 000 deaths) as of July 30, 2020 worldwide, 185 000 positive patients, and 30 000 deaths in France (19 500 in-hospital deaths and 10 500 in retirement homes and long-term care facilities).
See Editorial, pp 843–845
Rapidly during the pandemic, it was noted that patients with comorbidities were at higher risk of being hospitalized or dying from coronavirus disease 2019 (COVID-19),1–3 including hypertensive patients. Furthermore, as the mechanism of severe acute respiratory coronavirus 2 infection was shown to involve the ACE2 (angiotensin-converting enzyme 2) the expression of which was thought to be increased by renin-angiotensin-aldosterone system inhibitors, that is, ACE inhibitors and angiotensin receptor blockers (ARBs) drugs, it was hypothesized that these drugs could increase the risk of infection.
Some authors suggested switching from ACE inhibitors or ARBs with calcium channel blockers (CCBs),4 although at the present time, the scientific community and French authorities recommend that patients continue their antihypertensive drug treatment unless they received contrary medical advice.5
Indeed, most studies have concluded on the absence of association or a small negative trend between ACE inhibitors or ARB drugs and the risk of COVID-19 hospitalization, severity, or mortality.6–13 The most recent meta-analysis showed that ACE inhibitors use may not increase the susceptibility to severe acute respiratory coronavirus 2 infection, disease severity, and mortality.14 Most studies were carried on patients who were hospitalized, and not on the overall hypertensive population, with a risk of collider bias.6
We used the SNDS (Système National des Données de Santé) database to study a large population of patients treated for hypertension. While ensuring a sufficient number of events to provide good statistical power, we wanted to consider patients treated for hypertension only, with no known severe cardiometabolic diseases, to limit indication bias.
We examined whether the COVID-19 risk differs according to the pharmacological class of antihypertensive drugs in patients treated by ACE inhibitors or ARBs compared with CCBs.
Methods
According to data protection and the French regulation, the authors cannot publicly release the data from the French national health data system (SNDS). However, any person or structure, public or private, for-profit or nonprofit, is able to access SNDS data upon authorization from the French Data Protection Office (CNIL Commission Nationale de l’Informatique et des Libertés) to carry out a study, a research, or an evaluation of public interest (https://www.snds.gouv.fr/SNDS/Processus-d-acces-aux-donnees and https://www.indsante.fr/).
Setting and Data Sources
We conducted a retrospective cohort study comparing 3 classes of antihypertensive drugs using data from the SNDS, which covers the entire French population, that is, 67 million inhabitants. Since 2006, an anonymous unique individual identifier links information from two main data sources: DCIR (Données de Consommation Inter-Régimes, national health insurance claims database) and PMSI (Programme de Médicalisation des Systèmes d’Information, national hospital and discharge database).
The DCIR includes individual information on outpatient medical care, laboratory tests, and reimbursed drugs coded according to the Anatomic Therapeutic Chemical classification. The health expenditure of patients with serious and costly long-term diseases, such as cancer, or diabetes, is fully reimbursed, and their diagnosis is recorded according to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. The DCIR also collects a few patient data.15,16
The PMSI includes dates of admission and discharge to all public and private hospitals in France, discharge diagnoses coded according to International Statistical Classification of Diseases and Related Health Problems, Tenth Revision and type of medical or surgical procedures coded according to the French common classification of medical procedures (Classification Commune des Actes Médicaux). Disease and expenditure mapping is a tool developed from the DCIR and PMSI databases by using 58 medical algorithms identifying diseases.17
The SNDS has been extensively used in France to conduct real-life studies and pharmaco-epidemiological studies.15,16,18–20
Three Cohorts of Antihypertensive Drugs
We defined 3 mutually exclusive cohorts of prevalent ACE inhibitors, ARB, and CCB users (Table S1 in the Data Supplement). Patients who concomitantly received another class of antihypertensive drug (eg, diuretics, beta-blockers) were considered to receive a combination therapy for hypertension (ie, antihypertensive polymedication) but were not excluded. We included adults aged from 18 to 80 years, who received their last drug reimbursement between November 15, 2019, and February 15, 2020, and to whom antihypertensive drugs had been dispensed at least 3× during the previous 12 months with no dispensing of either of the other 2 drug classes. We excluded individuals who were identified, in the 5 years before study entry, with diabetes, cardiovascular disease (myocardial infarction, stroke, heart failure or severe arrhythmia), chronic renal failure, chronic respiratory diseases to only consider patients known to be currently treated for uncomplicated hypertension, to limit indication bias (eg, ACE inhibitors are more likely to be used in patients with heart failure and CCBs are more likely to be used for coronary heart disease), and to exclude already described risk factors for COVID-19,21 which could be the cause of or share a common cause with hypertension (Table S2), and therefore constitute potential confounding factors.
We considered age, sex, and region of residence as demographic variables. Age was used as a categorical variable in 10-year age-groups except for the first category due to the small number of cases: 18 to 50, 51 to 60, 61 to 70, and 71 to 80 years. Quintiles of the social deprivation index were used as a measure of socioeconomic status based on the area’s median household income, percentage of high school graduates in the population aged 15 years and older, percentage of manual workers in the active population, and unemployment in individual’s city of residence.
Smoking was defined by the presence of specific hospitalization diagnoses, nicotine replacement therapy, or annual lump sum payment for smoking cessation. Patients who did not take another class of antihypertensive drug (eg, beta-blockers, diuretics) were classified as patients on monotherapy. History of known comorbidities was assessed using both the disease and expenditure mapping medical algorithms and additional data from the DCIR and PMSI databases. Hospitalization for comorbidities was identified in the 5 years before the last antihypertensive drug dispensing; drugs other than the 3 classes of antihypertensive drugs considered were identified in the 12 months before the last antihypertensive drug dispensing. Use of NSAID, statins, aspirin, anticoagulants, antiplatelet drugs, heparin, anxiolytics, antidepressants, and antipsychotics was each defined by at least two reimbursements during the 1-year period. For further details about variable definitions, see Table S3.
Hospitalization With COVID-19
Information on all hospital stays is integrated into the SNDS (usually in July of the following year). In April 2020, the French government encouraged hospitals to report COVID hospital stays according to an exceptional, fast-track modality (once a week or once a fortnight; called fast-track PMSI). Our study was based on hospital discharge data available on June 7, 2020. At this date, 86 623 patients hospitalized with COVID-19 were reported and identified, including 83 445 (96%) for whom hospital discharge data could be linked to reimbursement data (ie, with no error concerning the anonymous identifier). Of these 83 445 patients, 14 566 died in hospital. At this date, the number of new cases of COVID-19 had decreased and lockdown in France had been partly withdrawn for about one month.
End Points
The primary end point was time from February 15, 2020, the end of the inclusion period, to hospitalization for COVID-19. The secondary end point was time from February 15, 2020, to intubation or death during a hospital stay for COVID-19. Note that all patients who were intubated or who died constituted a subset of all hospitalized patients. During the hospital stay, the date of intubation was not available, and we used the date of hospitalization for intubated patients. For patients who died after intubation, the secondary end point was defined as the date of death.
Statistical Analysis
Crude associations between treatment group or outcomes and patient characteristics were described.
Cox proportional hazards models were used to estimate the association between groups of selected antihypertensive drugs and hospitalization for COVID-19. We reported the results of crude associations and multivariable Cox model including all the variables listed above in Table 1 with an additional interaction term between age and gender. We used propensity score methods to take into account nonrandomized antihypertensive drugs. The individual propensities for exposure to one of the 3 antihypertensive classes were estimated by means of a multinomial multivariable logistic regression model.22 The predicted probabilities from the propensity score model were used to calculate the stabilized inverse probability of treatment weighting. We assessed the balance of individual covariates before and after inverse probability of treatment weighting. For each combination of 2 treatment groups, standardized differences were calculated as the differences in means or proportions divided by the pooled SD. For variables with >2 categories, calculations were based on the Mahalanobis distance.23 The negligible difference was defined as an absolute standardized difference <0.1. We then used different strategies to consider propensity scores: inverse probability of treatment weighting with (as main analysis) or without additional adjustment for the same variables, patient matching based on propensity scores, and use of propensity score as an adjustment variable. For the last 2 strategies, the propensity score was calculated from several binomial logistic models.
Stratified analyses were performed by sex, age, region, and antihypertensive polymedication status. Competitive risk models were also tested considering all-cause deaths.
All calculations were performed using SAS, version 9.4 software (SAS Institute, Inc).
Results
We identified 3 617 556 patients between the ages of 18 and 80 years with exclusive exposure to 1 of the 3 drug classes of interest (patients could concomitantly receive another class of antihypertensive drug; they were identified as antihypertensive polymedicated): 1 326 476 patients in the ACE inhibitors cohort, 1 661 197 patients in the ARB cohort, and 629 883 patients in the CCB cohort. After exclusion of patients with known comorbidities, 566 023 individuals remained in the ACE inhibitors cohort, 958 227 individuals in the ARB cohort, and 358 306 individuals in the CCB cohort (Figure 1).
Figure 1.
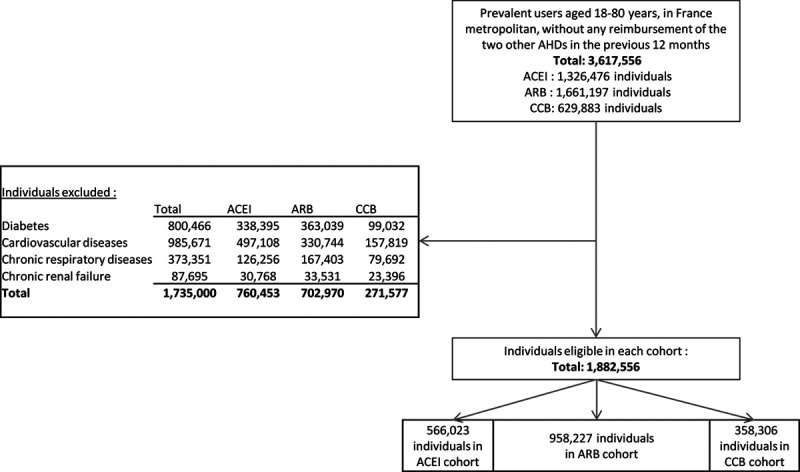
Flowchart. ACE indicates angiotensin-converting enzyme and ACEI, ACE inhibitors; ARB, angiotensin receptor blockers; and CCB, calcium channel blockers.
Baseline characteristics according to antihypertensive drugs are shown in Table 1. The 3 cohorts were relatively well balanced in terms of sociodemographic characteristics and other medications, but with several differences: ACE inhibitors prevalent users were more likely to be men compared with ARB or CCB prevalent users (46% versus 41% and 40%, respectively), less likely to be in the older age-group (28% versus 32% and 31%, respectively) and less likely to live in more deprived cities than ARB and CCB users. Fewer ACE inhibitor prevalent users were identified as living in the Ile-de-France region (which includes Paris; 12% versus 17% and 21%, respectively).
Table 1.
Baseline Characteristics of the 3 Cohorts of Antihypertensive Drug Prevalent Users (CCB, ACE Inhibitors, ARB)
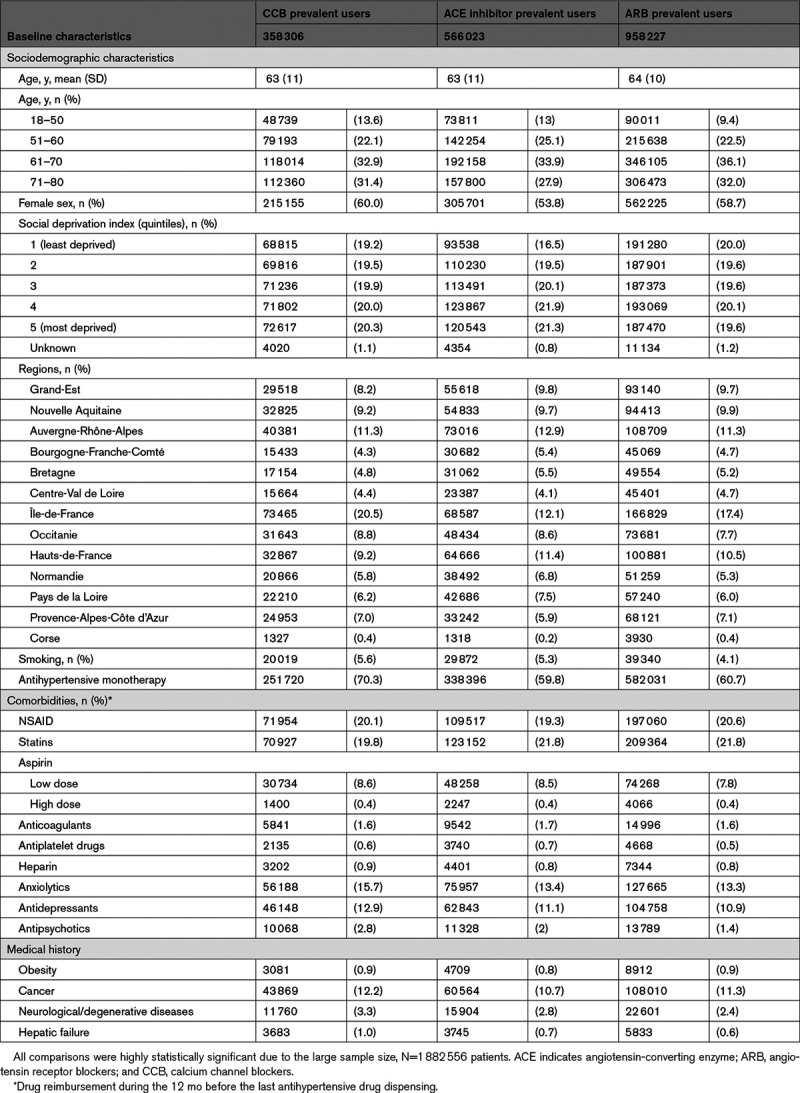
The main most recently dispensed molecules before the index date in each antihypertensive drug category were perindopril (34%) and ramipril (32%) in the ACE inhibitors cohort; irbesartan (43%) and candesartan (28%) in the ARB cohort; and lercanidipine (37%) and amlodipine (34%) in the CCB cohort. Other molecules each represented <10% of most recently dispensed drugs (Table S1). Among ACE inhibitors and ARB patients, 60% did not take any other antihypertensive drug, compared with 70% of CCB patients. The other drugs most commonly used were diuretics in the ACE inhibitors (81%) and ARB (83%) cohorts, compared with 47% in the CCB cohort, in which the other class most commonly used was beta-blockers (74%).
The mean number of dispensations was 6 (SD, 2.5) over a 12-month period. As a result of large pack sizes (for 3 months), the mean number of months covered by at least one reimbursement in each cohort was 11 (SD, 3) over the same period. From February 16 to May 31, 2020, >90% of individuals continued to receive at least one dispensing of a drug from the same class and <2% switched to a drug from another class.
Hazard ratios (HR) and 95% CIs for mutually adjusted associations between outcomes and all variables, including exposure to antihypertensive drugs, are shown in Tables S4 and S5. After inverse probability of treatment weighting, baseline characteristics of individuals according to antihypertensive drug were well balanced (Figure S1).
From February 15, 2020 to June 7, 2020, the primary end point occurred in 2338 patients (585 in the ACE inhibitors group, 1194 in the ARB group, and 559 in the CCB group); a total of 526 patients were intubated or died (125 in the ACE inhibitors group, 267 in the ARB group and 134 in the CCB group). Kaplan-Meier survival curves of time to hospitalization for COVID-19 or to death/intubation stratified by exposure group are shown in Figures 2 and 3, respectively. In crude analyses, patients exposed to ACE inhibitors or ARB drugs were less likely to have experienced a primary end point event compared with individuals exposed to CCBs (HR, 0.66 [95% CI, 0.59–0.74] for ACE inhibitors; 0.80 [0.72–0.88] for ARB; Table 2).
Figure 2.
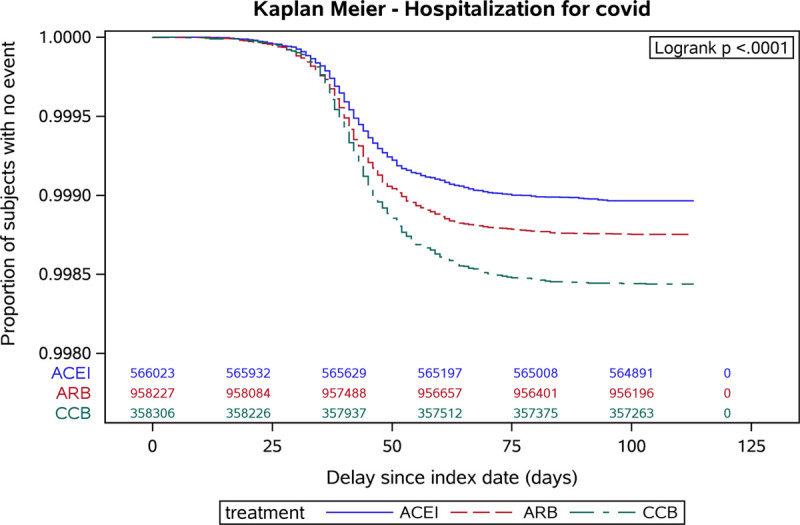
Kaplan-Meier survival curves of time to hospitalization for coronavirus disease 2019 (COVID-19) stratified by treatment group and showing the proportion of subjects with no event from the index date (February 15, 2020) until the end of follow-up. y axis adjusted to account for rare events. ACE indicates angiotensin-converting enzyme and ACEI, ACE inhibitors; ARB, angiotensin receptor blockers; and CCB, calcium channel blockers.
Figure 3.
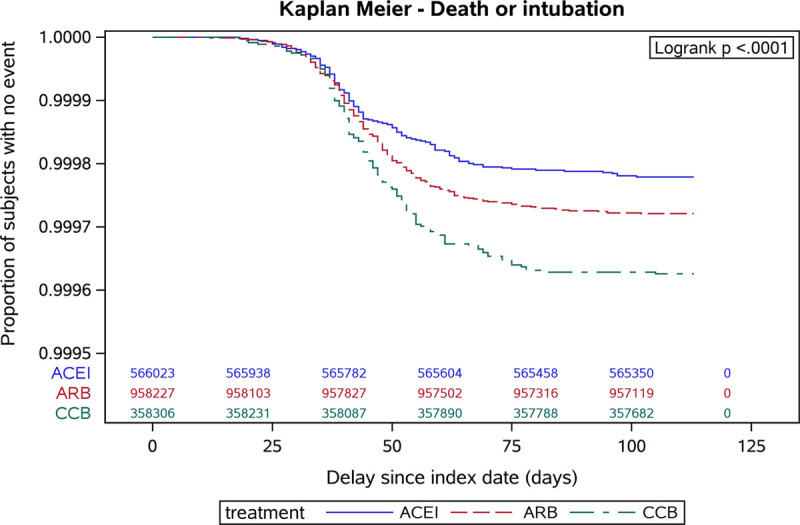
Kaplan-Meier survival curves of time to hospitalization for coronavirus disease 2019 (COVID-19) death or intubation stratified by treatment group and showing the proportion of subjects with no event from the index date (February 15, 2020) until the end of follow-up. y axis adjusted to account for rare events. ACE indicates angiotensin-converting enzyme and ACEI, ACE inhibitors; ARB, angiotensin receptor blockers; and CCB, calcium channel blockers.
Table 2.
Association Between Exposure to Antihypertensive Drugs (CCB, ACE inhibitors, ARB) and the Risk of COVID-19 Outcomes (Hospitalization and Death or Intubation for COVID-19)
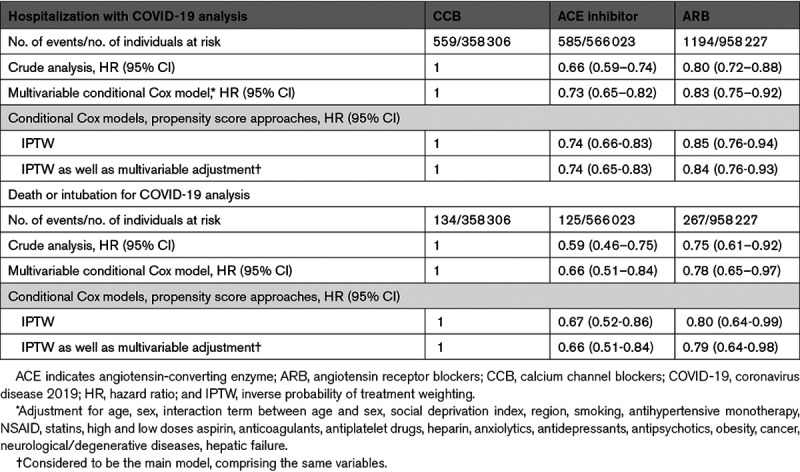
In the main analysis with inverse probability of treatment weighting, ACE inhibitors, and ARB exposures were associated with a decreased risk of hospitalization with COVID-19 compared with exposure to CCB (HR, 0.74 [95% CI, 0.65–0.83] 0.84 [95% CI, 0.76–0.93], respectively). ACE inhibitors and ARB exposures were also associated with a decreased risk of intubation or death in the main analysis compared with CCB exposure (HR, 0.66 [95% CI, 0.51–0.84]; 0.79 [95% CI, 0.64–0.98], respectively). We observed a lower risk for hospitalization (HR, 0.87 [95% CI, 0.79–0.96]) and death or intubation (HR, 0.83 [95% CI, 0.67–1.03]) in patients in the ACE inhibitors group compared with patients in the ARB group (data not shown).
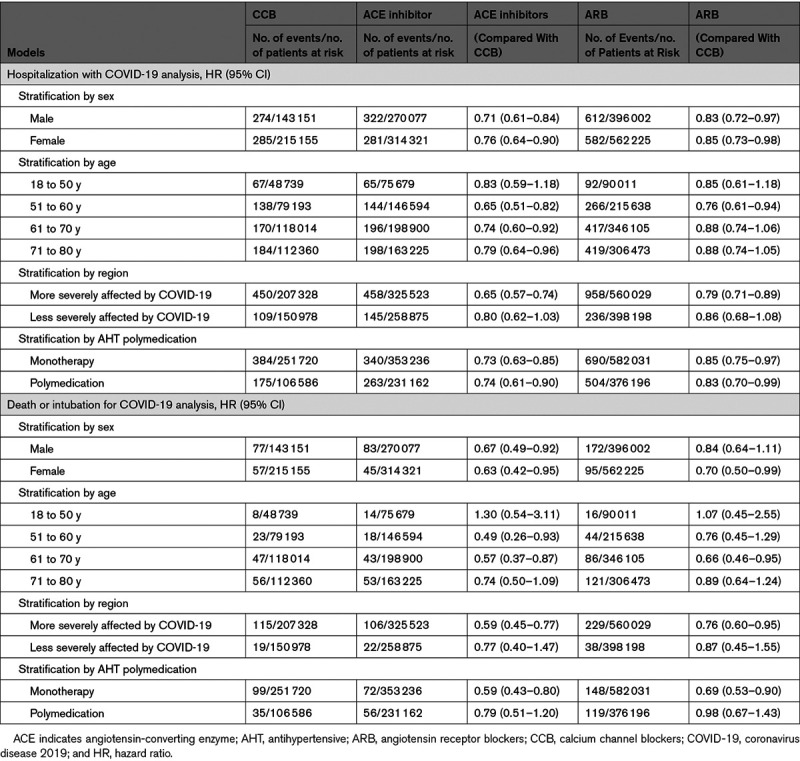
Additional multivariable analyses yielded similar results. The negative associations between ARB and ACE inhibitors exposures and hospitalization for COVID-19 compared with exposure to CCB were observed in almost all subgroups according to sex, age, whether the region was among the regions most severely affected by COVID-19, or whether individuals were reimbursed for other antihypertensive drugs (Table 3). The association tended to be more pronounced in individuals aged 51 to 60 years (HR, 0.65 [95% CI, 0.51–0.82] for ACE inhibitors versus CCB; HR, 0.76 [95% CI, 0.61–0.94] for ARB versus CCB), in the regions most severely affected by COVID-19 (HR, 0.65 [95% CI, 0.57–0.74] and 0.79 [0.71–0.89]) and for death/intubation outcomes only, when patients did not take any other antihypertensive drug (HR, 0.59 [95% CI, 0.43–0.80] and 0.69 [0.53–0.89], respectively). Results were still consistent after exclusion of patients (Table S6) with cancer, after excluding patients who switched treatment to one of the other 2 drug classes or who stopped treatment during the 3 months following the index date, and after excluding patients reimbursed for anticoagulants, heparin, or antiplatelet drugs (n=17 080 [3%], 26 100 [3%], 10 779 [3%] in the ACE inhibitors, ARB, and CCB cohorts, respectively). In the later analysis, HRs of ACE inhibitors and ARB exposures for the risk of hospitalization with COVID-19 compared with CCB were 0.74 (95% CI, 0.65–0.83) and 0.84 (95% CI, 0.76–0.93), respectively.
Table 3.
Association Between Exposure to Antihypertensive Drugs (CCB, ACE Inhibitors, ARB) and Risk of 2 COVID-19 Outcomes (Hospitalization and Death or Intubation for COVID-19) in a Multivariable Cox Model With Inverse Probability of Treatment Weighting by Sex, Age Range, Region and AHT Polymedication
Matching or adjustment for propensity score or competitive risk model taking into account all-cause of deaths showed very consistent results (Table S7, S8; Figure S2).
Discussion
In this large nationwide retrospective cohort study, we observed differences in the risk of hospitalization with COVID-19 according to antihypertensive drug class in a population free of any known cardiovascular or respiratory diseases. Compared with chronic CCB users, patients with long-term use of ACE inhibitors or ARBs up until the start of the epidemic were less likely to be hospitalized with COVID-19 (HR, 0.74 [95% CI, 0.65–0.83] and 0.84 [95% CI, 0.76–0.93], respectively) and less likely to die or be intubated.
Several studies have investigated the risk difference according to antihypertensive drug classes. To our knowledge, apart from one study which reported a positive association,24 most studies, including several meta-analyses, concluded on either no association or a negative trend with COVID-19 risk or severity.6–13,25–29
These studies can have a risk of confounding bias or collider bias.30,31 To limit confounding, we restricted the study population to a more homogeneous population of treated hypertensive patients with no known cardiovascular comorbidity and no major comorbidity suspected to increase the risk of COVID-19.32 We selected patients younger than 80 years treated over a long period of time with drugs with similar indications in 3 mutually exclusive classes of antihypertensive drugs to limit indication bias and confounding. Thereafter, adjustments had little impact on the main associations. Our population was also not restricted to hospitalized or tested-positive patients to limit collider bias.31
If the presence of cough is a specific side effect of this ACE inhibitors users, selecting patients with long-term use should limit this bias, as such patients would be more likely to tolerate their treatment. Moreover, such a bias would not explain the results observed with ARBs. Although we cannot exclude residual confounding or selection bias, we do not think these biases could entirely explain the negative associations observed in this study.
Although this database does not guarantee that the subject actually took the antihypertensive drug dispensed, selecting patients to whom the drug was dispensed at least 3× should reduce this drawback. The mean number of dispensations was 6 over a 12-month period and the mean number of months covered by at least one reimbursement in each cohort was 11 which suggests that the majority of the patients probably presented a good adherence to treatment. Finally, little information is available on lifestyle, and variables, such as obesity or smoking, are underestimated.
Santé publique France, the French center comparable to US Centers for Disease Control and Prevention, estimated that between March 1, 2020 and June 9, 2020, 102 863 patients were hospitalized with COVID-19 in France, including 18 912 patients who died in hospitals. Before March 1, 2020, there were fewer than 400 hospitalizations with COVID-19 in France (2 in our study). Of these 102 863 patients, 11 961 were still hospitalized in France on June 9, 2020, including 955 patients in intensive care (with or without intubation). Our study, therefore, included ≈80% of all patients (83 455/102 863) hospitalized with COVID-19 in France, and >90% of these patients after excluding those patients who were still hospitalized.
Perspectives
In our study based on a large population of hypertensive patients, renin-angiotensin-aldosterone system inhibitors use compared with CCB use was negatively associated with the risk of COVID-19, and stronger associations were observed for ACE inhibitors than for ARBs. Although the observational design of this study prevents us from drawing any definitive conclusions about the nature of this association, this study provides additional evidence in support of a potential protective effect of ACE inhibitors or ARBs compared with CCBs in hypertensive patients and, if it is confirmed, raises new questions about the pharmacological mechanisms of this effect.
Sources of Funding
None.
Disclosures
None.
Supplementary Material
Footnotes
Nonstandard Abbreviation and Acronyms
- ACE
- angiotensin-converting enzyme
- ARB
- angiotensin receptor blocker
- CCB
- calcium channel blocker
- COVID-19
- coronavirus disease 2019
- HR
- hazard ratio
These authors contributed equally to this work.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.120.16314.
For Sources of Funding and Disclosures, see page 841.
Contributor Information
Laura Semenzato, Email: laura.semenzato@assurance-maladie.fr.
Jérôme Drouin, Email: jerome.drouin@assurance-maladie.fr.
Bérangère Baricault, Email: berangere.baricault@assurance-maladie.fr.
Clémentine Vabre, Email: Clementine.VABRE@ansm.sante.fr.
François Cuenot, Email: francois.cuenot@ansm.sante.fr.
Laetitia Penso, Email: laetitia.penso@ansm.sante.fr.
Philippe Herlemont, Email: pl.herlemont@lavache.com.
Emilie Sbidian, Email: emilie.sbidian@aphp.fr.
Alain Weill, Email: alain.weill@assurance-maladie.fr.
Rosemary Dray-Spira, Email: rosemary.dray-spira@ansm.sante.fr.
Mahmoud Zureik, Email: mahmoud.zureik@ansm.sante.fr.
Novelty and Significance
What Is New?
Our study included almost 2 million hypertensive patients.
We selected prevalent long-term users of antihypertensive drugs with no known cardiometabolic or respiratory comorbidities.
We paid particular attention to the risk of bias.
What is Relevant?
Three major classes of antihypertensive drugs were compared.
Our study included patients with uncomplicated hypertension.
Summary
Although the observational design prevents us from drawing any conclusions about the nature of this association, this study provides additional evidence in favor of a potentially lower coronavirus disease 2019 (COVID-19) risk in renin-angiotensin-aldosterone system inhibitor users compared with calcium channel blocker users.
References
- 1.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciulla MM. Switching to another antihypertensive effective drug when using ACEIs/ARBs to treat arterial hypertension during COVID-19. Eur Heart J. 2020;41:1856. doi: 10.1093/eurheartj/ehaa331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lévy BI, Fauvel JP; Société Française d’Hypertension Artérielle. Renin-angiotensin system blockers and severe acute respiratory syndrome coronavirus 2. Arch Cardiovasc Dis. 2020;113:572–578. doi: 10.1016/j.acvd.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiaoming G, Yueli Z, Yuan H. Decreased mortality of COVID-19 with renin-angiotensin-aldosterone system inhibitors therapy in patients with hypertension. Hypertension. 2020;76:e13–e14. doi: 10.1161/HYPERTENSIONAHA.120.15572 [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Yu J, Pan LY, Jiang HY. ACEI/ARB use and risk of infection or severity or mortality of COVID-19: a systematic review and meta-analysis. Pharmacol Res. 2020;158:104927. doi: 10.1016/j.phrs.2020.104927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pirola CJ, Sookoian S. Estimation of renin-angiotensin-aldosterone-system (RAAS)-inhibitor effect on COVID-19 outcome: a meta-analysis. J Infect. 2020;81:276–281. doi: 10.1016/j.jinf.2020.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baral R, White M, Vassiliou VS. Effect of renin-angiotensin-aldosterone system inhibitors in patients with COVID-19: a systematic review and meta-analysis of 28,872 patients. Curr Atheroscler Rep. 2020;22:61. doi: 10.1007/s11883-020-00880-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flacco ME, Acuti Martellucci C, Bravi F, Parruti G, Cappadona R, Mascitelli A, Manfredini R, Mantovani LG, Manzoli L. Treatment with ACE inhibitors or ARBs and risk of severe/lethal COVID-19: a meta-analysis. Heart. 2020;106:1519–1524. doi: 10.1136/heartjnl-2020-317336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barochiner J, Martínez R. Use of inhibitors of the renin-angiotensin system in hypertensive patients and COVID-19 severity: a systematic review and meta-analysis. J Clin Pharm Ther. 2020;45:1244–1252. doi: 10.1111/jcpt.13246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Castelnuovo A, Costanzo S, Antinori A, Berselli N, Blandi L, Bonaccio M, Cauda R, Gialluisi A, Guaraldi G, Menicanti L, et al. RAAS inhibitors are not associated with mortality in COVID-19 patients: findings from an observational multicenter study in Italy and a meta-analysis of 19 studies. Vascul Pharmacol. 2020;135:106805. doi: 10.1016/j.vph.2020.106805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneeweiss MC, Leonard S, Weckstein A, Schneeweiss S, Rassen J. Renin-angiotensin-aldosterone-system inhibitor use in patients with COVID-19 infection and prevention of serious events: a cohort study in commercially insured patients in the US. medRxiv. [Google Scholar]
- 14.Chan C-K, Huang Y-S, Liao H-W, Tsai I-J, Sun C-Y, Pan H-C, Chueh JS, Wang J-T, Wu V-C, Chu T-S. Renin-angiotensin-aldosterone system inhibitors and risks of SARS-CoV-2 infection: a systematic review and meta-analysis. Hypertension. 2020;76:1563–1571. doi: 10.1161/HYPERTENSIONAHA.120.15989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouillon K, Bertrand M, Bader G, Lucot JP, Dray-Spira R, Zureik M. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018;319:375–387. doi: 10.1001/jama.2017.21269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weill A, Dalichampt M, Raguideau F, Ricordeau P, Blotière PO, Rudant J, Alla F, Zureik M. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ. 2016;353:i2002. doi: 10.1136/bmj.i2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rachas A, Gastaldi-Menager C, Denis P, Lesuffleur T, Nicolas M, Pestel L, Mette C, Drouin J, Riviere S, Tajahmady A, et al. Prevalences and healthcare expenditures related to 58 health conditions from 2012 to 2017 in France: diseases and healthcare expenditure mapping, a national population-based study [Google Scholar]
- 18.Maura G, Blotière PO, Bouillon K, Billionnet C, Ricordeau P, Alla F, Zureik M. Comparison of the short-term risk of bleeding and arterial thromboembolic events in nonvalvular atrial fibrillation patients newly treated with dabigatran or rivaroxaban versus vitamin K antagonists: a French nationwide propensity-matched cohort study. Circulation. 2015;132:1252–1260. doi: 10.1161/CIRCULATIONAHA.115.015710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer A, Rudant J, Drouin J, Weill A, Carbonnel F, Coste J. Effectiveness and safety of reference infliximab and biosimilar in Crohn disease: a French Equivalence Study. Ann Intern Med. 2019;170:99–107. doi: 10.7326/M18-1512 [DOI] [PubMed] [Google Scholar]
- 20.Tubiana S, Blotière PO, Hoen B, Lesclous P, Millot S, Rudant J, Weill A, Coste J, Alla F, Duval X. Dental procedures, antibiotic prophylaxis, and endocarditis among people with prosthetic heart valves: nationwide population based cohort and a case crossover study. BMJ. 2017;358:j3776. doi: 10.1136/bmj.j3776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leslie S, Thiebaud P. Using Propensity Scores to Adjust for Treatment Selection Bias. SAS Glob Forum. published online 2007:4. https://support.sas.com/resources/papers/proceedings/proceedings/forum2007/184-2007.pdf. Accessed May 1, 2020. [Google Scholar]
- 23.Yang D, Dalton J. A Unified Approach to Measuring the Effect Size Between Two Groups Using SAS®. SAS Glob Forum. published online 2012:6. http://support.sas.com/resources/papers/proceedings12/335-2012.pdf. Accessed May 1, 2020. [Google Scholar]
- 24.Liabeuf S, Moragny J, Bennis Y, Batteux B, Brochot E, Schmit JL, Lanoix J-P, Andrejak C, Ganry O, Slama M, et al. Association between renin–angiotensin system inhibitors and COVID-19 complications [published online June 12, 2020]. Eur Hear J Cardiovasc Pharmacother. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Abajo FJ, Rodríguez-Martín S, Lerma V, Mejía-Abril G, Aguilar M, García-Luque A, Laredo L, Laosa O, Centeno-Soto GA, Ángeles Gálvez M, et al. ; MED-ACE2-COVID19 study group. Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet. 2020;395:1705–1714. doi: 10.1016/S0140-6736(20)31030-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, Hausvater A, Newman JD, Berger JS, Bangalore S, et al. Renin-angiotensin-aldosterone system inhibitors and risk of covid-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of covid-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khera R, Clark C, Lu Y, Guo Y, Ren S, Truax B, Spatz ES, Murugiah K, Lin Z, Omer SB, et al. Association of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers with the risk of hospitalization and death in hypertensive patients with coronavirus disease-19 [published online May 19, 2020]. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hippisley-Cox J, Young D, Coupland C, Channon KM, Tan PS, Harrison DA, Rowan K, Aveyard P, Pavord ID, Watkinson PJ. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020;106:1503–1511. doi: 10.1136/heartjnl-2020-317393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lahens A, Mullaert J, Gressens S, Gault N, Flamant M, Deconinck L, Joly V, Yazdanpanah Y, Lescure F-X, Vidal-Petiot E. Association between renin–angiotensin–aldosterone system blockers and outcome in coronavirus disease 2019: analysing in-hospital exposure generates a biased seemingly protective effect of treatment [published online October 5, 2020]. J Hypertens. doi: 10.1097/HJH.0000000000002658 https://journals.lww.com/jhypertension/Fulltext/9000/Association_between_renin_angiotensin_aldosterone.96834.aspx [DOI] [PubMed] [Google Scholar]
- 31.Griffith G, Morris TT, Tudball M, Herbert A, Mancano G, Pike L, Sharp GC, Palmer TM, Davey Smith G, Tilling K, et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun. 2020;11:5749. 10.1038/s41467-020-19478-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLOS ONE. 2020;15:e0238215. 10.1371/journal.pone.0238215 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


