Abstract
The optimal timing of an intervention to support health-related behavior after transient ischemic attack (TIA) or ischemic stroke is unknown. We aimed to assess determinants of patients’ health-related intention to change over time. We prospectively studied 100 patients with TIA or minor ischemic stroke. Patients completed questionnaires on fear, response-efficacy (belief that lifestyle change reduces risk of recurrent stroke), and self-efficacy (patients’ confidence to carry out lifestyle behavior) for behavior change, at baseline, 6 weeks and at 3 months after their TIA or ischemic stroke. We studied differences between these determinants at each visit by means of Wilcoxon signed-rank tests. Median self-efficacy score at baseline was 4.3 [interquartile range (IQ) 3.9–4.7], median fear 16 (IQ 7–21), and response-efficacy 10 (9–12). Fear was significantly higher at baseline than at 3 months (mean difference 2.0; 95% confidence interval: 0.78–3.9) and started to decrease after 6 weeks. No change in self-efficacy or response-efficacy was found. Since fear significantly decreased over time after TIA or ischemic stroke and self-efficacy and response-efficacy scores remained high, the optimal moment to start an intervention to support patients in health-related behavior change after TIA or ischemic stroke seems directly after the stroke or TIA.
Keywords: health-related behavior, intervention, stroke, transient ischemic attack
Introduction
Modification of health behavior after transient ischemic attack (TIA) or ischemic stroke including smoking cessation, healthy diet, and increased physical activity is considered important and strongly recommended in many guidelines. However, at present little is known about effective interventions to support patients in this health behavior change [1–9]. Insight in determinants of lifestyle behavior change and optimal timing of the intervention is essential to develop a successful intervention to support health-related behavior change [10–13].
The protection motivation theory to examine determinants of lifestyle behavior change after TIA or ischemic stroke has shown to be a useful model for predicting health-protective intentions and behavior changes in other conditions, such as diabetes, coronary heart disease, and breast cancer [14]. We earlier found that response-efficacy (the belief that lifestyle change can reduce the risk of recurrent stroke), self-efficacy (patients confidence to carry out behavior necessary to reach a desired goal), and fear were determinants of intention to change health-related behavior after TIA or ischemic stroke [15]. Self-efficacy was the strongest determinant of intention to stop smoking, increase physical activity, and improve healthy diet [15]. Both response-efficacy and self-efficacy were associated with intention to change health behavior in other cardiovascular studies [16–23]. At present, there are no studies focusing on change of these determinants over time after TIA or ischemic stroke. As these determinants probably vary over time, there may be a window of opportunity to start a health-related behavior supporting intervention [24].
To get insight in the timing to start an intervention supporting health-related behavior change after TIA or ischemic stroke, we aimed to assess the determinants of intention to change over time (fear, response-efficacy, and self-efficacy) in patients with recent TIA or ischemic stroke.
Methods
All patients included in the present study participated in the DECIDE study. This study was approved by national and local institutional review boards (MEC-2011-356, NL36454.078.11) and written informed consent was obtained from all patients. The investigation conforms with the principles outlined in the Declaration of Helsinki. Detailed methods of the DECIDE study have been described earlier [15]. DECIDE was a prospective study on determinants of intention to change health-related behavior and actual change in patients with TIA or ischemic stroke. Patients of 18 years or older with a clinical diagnosis of TIA, including amaurosis fugax, or minor ischemic stroke with a modified Rankin Scale (mRS) score of 2 or less were included during admission on the stroke unit or outpatient clinic. The mRS is a commonly used scale for measuring the degree of disability or dependence in the daily activities of people who have suffered a stroke. Scores on the mRS range from 0 (no symptoms at all) to 5 (severe disability); for statistical purposes, death has a score of 6 [25].
Patients were excluded if they were discharged to a nursing home, were not Dutch-speaking, or had severe aphasia. Patients were recruited in the first week after admission to the stroke unit or TIA outpatient clinic. All patients received routine general lifestyle advice including regular physical exercise, healthy diet, and advice against smoking as part of standard care at baseline.
At baseline, we recorded data on clinical features of TIA or ischemic stroke, quantification of stroke severity according to the National Institutes of Health stroke scale [26], stroke etiology according to the Classification of subtype of acute ischemic stroke developed for the Trial of Org 10172 in Acute Stroke Treatment (TOAST) [27], demographic data, vascular risk factors and history, and use of medication. All patients underwent a cognitive assessment including the Montreal Cognitive Assessment (MoCA), a rapid screening instrument for cognitive impairment, particularly in stroke patients [28], and depression was measured with the Center for Epidemiologic Studies Depression Scale (CES-D) for both depression and anxiety [29,30]. Higher scores indicate more depressive symptoms.
Health-related behavior including smoking cessation, healthy diet, and increased physical activity was assessed:
Physical activity was measured with the International Physical Activity Questionnaire short (IPAQ-S) questionnaire. Patients were asked to report activities performed for at least 10 min during the last 7 days, and time spent in physical activity performed across leisure time, work, domestic activities, and transport at each of the three intensities: walking, moderate, and vigorous [31]. We used reported minutes of moderate and vigorous physical activity to calculate a total physical activity score of minutes a day.
Dietary behavior was evaluated with the short Food Frequency Questionnaire (FFF). This 14-item scale assesses the intake of saturated fatty acids, unsaturated fatty acids, and fruits and vegetables over the week before the visit. An overall cardiovascular dietary score was calculated, ranging from –17 to +19, the higher the score, the more favorable the dietary pattern [32].
Actual smoking status was assessed with questions on current smoking status, how many years they have smoked, and how much cigarettes a patient smokes a day. Smoking was defined as current smoking.
Patients were assessed at baseline (directly after inclusion), at 6 weeks and 3 months after inclusion. The initial assessment included self-reported questionnaires on all sociocognitive determinants of the protection motivation theory including severity, susceptibility, fear, response-efficacy, and self-efficacy. In this study, we used self-efficacy, response-efficacy, and fear as we previously identified them as determinants of intention to change health-related behavior after a TIA or stroke [15]. Patients completed the following questionnaires:
Self-efficacy was measured with the self-efficacy scale, a 9-item scale with scores that range from 1 to 5 [33]. Higher values indicate more confidence to carry out the behavior necessary to reach the desired goal. Cronbach’s α of the self-efficacy questionnaire was 0.75 [15]. This scale has been used successfully before in vascular patients [16,20,34,35].
Fear was assessed with 8 questions. Patients were asked on a scale of 1–5 how nervous they are when thinking of getting another stroke, how upset they get, depressed or jittery, if their heart beats faster, an if they feel uneasy or anxious [36].
Response-efficacy, assessed with the following statement: ‘For me, regular physical activity will reduce my chances of getting another stroke’ (1 = strongly disagree; 5 = strongly agree). Similar questions were asked for dietary change and smoking cessation [31,36].
Statistical analysis
Statistical analysis was performed with STATA 12.1 statistical package (Statacorp, College Station, Texas). Continuous variables were expressed as means and SD, scores were expressed as medians and interquartile ranges (IQs), and counts in patient numbers (n) and percentage (%). Fear, self-efficacy, and response-efficacy at baseline, after 6 weeks and 3 months were described and differences were tested with Wilcoxon signed-rank tests. Since the questionnaires for self-efficacy and response-efficacy included an additional question for smokers, data were presented and analyzed separately for smokers and nonsmokers. We studied the relation between determinants of health-related behavior change at baseline and after 3 months with univariable and multivariable linear regression. Adjustments were made for age, sex, baseline scores, and other determinants. For instance, in analyzing self-efficacy, adjustments were made for age, sex, baseline self-efficacy scores, and response-efficacy and fear.
Results
We included 100 patients between February and October 2012. Follow up was completed in 92 patients: one patient was lost to follow-up, one patient was excluded because of severe other comorbidity, one because of intracerebral hematoma during follow-up, two patients because of misdiagnosis, and three patients were discharged to another hospital (Fig. 1). No significant differences in baseline characteristics were found between included patients and excluded patients (data not shown). Mean age was 64 years (SD 12), 60% of the patients were male, and 53% had a TIA (Table 1). Median self-efficacy score at baseline was 4.3 (IQ 3.9–4.7), median fear 16 (IQ 7–21), and response-efficacy 10 (IQ 9–12). There were no differences between smokers and nonsmokers. Fear was significantly higher at baseline than at 3 months [mean difference 2.0; 95% confidence interval (CI): 0.78–3.9] (Table 2). This significance remained after adjustment for age, sex, baseline self-efficacy, and response-efficacy (0.37; 95% CI 0.11–0.64, Table 3). Fear started to decrease after 6 weeks (median fear at 6 weeks 16, at three months 11; P = 0.02). No change in self-efficacy or response-efficacy was found.
Fig. 1.
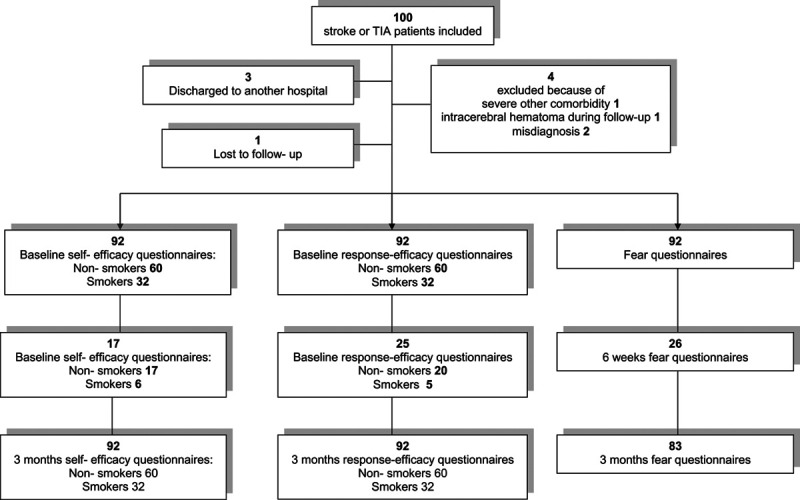
Flow chart of inclusion and follow up of patients.
Table 1.
Baseline characteristics (N = 100)
| Sex (male), n (%) | 60 (60) |
| Age (years), mean (SD) | 64 (12) |
| Event characteristics | |
| Event type (TIA), n (%) | 53 (53) |
| Stroke etiology (TOAST)a, n (%) | |
| Large vessel disease | 13 (13) |
| Cardiac embolism | 15 (15) |
| Small vessel disease | 19 (19) |
| Other | 0 |
| Undetermined | 53 (53) |
| NIHSS scoreb, median (IQ) | 3 (1–5) |
| Vascular history, n (%) | |
| TIA | 18 (18) |
| Ischemic stroke | 15 (15) |
| Ischemic heart disease | 36 (36) |
| Atrial fibrillation | 11 (11) |
| Peripheral arterial disease | 8 (8) |
| No vascular history | 49 (49) |
| Cognition and depression | |
| Score on MoCAc, median (IQ) scores from 0 to 30 | 24 (21–26) |
| Score on CES-Dd, median (IQ) scores from 0 to 30 | 7 (5–13) |
| Vascular risk factors | |
| Hypertension, n (%) | 65 (65) |
| Systolic blood pressure (mmHg), mean (SD) | 135 (22) |
| Diastolic blood pressure (mmHg), mean (SD) | 78 (13) |
| Hypercholesterolemia, n (%) | 79 (79) |
| LDL level (mmol/l), mean (SD) | 3.17 (1.0) |
| Blood glucose level (mmol/l), mean (SD) | 5.9 (1.4) |
| Diabetes mellitus, n (%) | 30 (30) |
| Health-related behavior | |
| Smoking, n (%) | 36 (36) |
| Alcohol abuse, n (%) | 5 (5.2) |
| Physical exercisee (min/day), median (IQ) | 129.6 (60–218.6) |
| Physical exercise >30 min a day n (%) | 75 (75) |
| Overall dietscoref, median (IQ) scores from –17 to +19 | 1.0 (–2 to 2.5) |
| BMI (kg/m2), mean (SD) | 26.5 (3.6) |
| Overweight (BMI > 25), n (%) | 64 (64) |
CES-D, Center for Epidemiologic Studies Depression Scale; IQ, interquartile range; MoCA, Montreal Cognitive Assessment; NIHSS, National Institutes of Health stroke scale; TIA, transient ischemic attack; TOAST, Trial of Org 10172 in Acute Stroke Treatment.
aClassification of subtype of acute ischemic stroke developed for the Trial of Org 10172 in Acute Stroke Treatment.
bQuantification of stroke severity according to the National Institutes of Health stroke scale), a 15-item scale with scores that range from 0 to 42 and higher values indicating greater severity.
cAssessed with the Montreal Cognitive assessment.
dScored with the Centre for Epidemiologic Studies Depression Scale.
eMeasured with the International Physical Activity Questionnaire short questionnaire.
fEvaluated with the short Food Frequency Questionnaire. The higher the score, the more favorable the dietary pattern.
Table 2.
Determinants of health-related behavior change after 6 weeks and 3 months
| Baseline | 6 weeks | P-value | 3 months | P-value | |
|---|---|---|---|---|---|
| Self-efficacy total, median (IQ) | 4.3 (3.9–4.7) | 4.4 (4–4.7) | 0.13 | 4.5 (4–4.8) | 0.28 |
| Self-efficacy smokers total, median (IQ) | 4.3 (3.9–4.7) | 4.2 (3.6–4.3) | 0.83 | 4.4 (3.7–4.9) | 0.97 |
| Self-efficacy nonsmokers, median (IQ) | 4.3 (3.8–4.8) | 4.5 (4.3–4.8) | 0.24 | 4.5 (4.2–4.7) | 0.46 |
| Response-efficacy smokers, median (IQ) | 10 (9–12) | 12 (12–12) | 0.09 | 10 (8–11) | 0.73 |
| Response-efficacy nonsmokers (IQ) | 8 (6–8) | 8 (6–8) | 1.00 | 8 (6–8) | 0.81 |
| Fear, median (IQ) | 16 (7–21) | 16 (7–23) | 0.83 | 11 (7–18) | 0.02 |
IQ, interquartile range.
Table 3.
Univariable and multivariable relations between determinants of health-related behavior change at baseline and after 3 months
| Beta | P-value | aBeta | P-value | |
|---|---|---|---|---|
| Self-efficacy total, median (IQ) | 0.55 (0.32–0.78) | 0.00 | 0.64 (0.40–0.88)a | 0.00 |
| Self-efficacy smokers total, median | 0.53 (0.21–0.85) | 0.53 | 0.76 (0.47–1.06)a | 0.00 |
| Self-efficacy nonsmokers, median | 0.57 (0.25–0.90) | 0.00 | 0.63 (0.28–0.99)a | 0.00 |
| Response-efficacy smokers | 0.08 (–0.23 to 0.39) | 0.61 | 0.06 (–0.26 to 0.39)b | 0.69 |
| Response-efficacy nonsmokers | 0.35 (0.00–0.69) | 0.05 | 0.34 (-0.01–0.71)b | 0.06 |
| Fear, median (IQ) scores | 0.42 (0.25–0.60) | 0.00 | 0.37 (0.11–0.64)c | 0.01 |
IQ, interquartile range.
aAdjusted for age sex, baseline response-efficacy, and baseline fear.
bAdjusted for age, sex baseline self-efficacy, and baseline fear.
cAdjusted for age, sex baseline self-efficacy, and baseline response-efficacy.
Discussion
Fear significantly decreased in 3 months after TIA or ischemic stroke. Patients with TIA or ischemic stroke (both smokers and nonsmokers) have high self-efficacy and response-efficacy scores for health-related behavior change and these do not vary over time. This suggests that confidence in changing health behavior capacities and the belief that this change can prevent a new stroke are still high at 3 months. However, fear decreased after 3 months suggesting that the best time to start the intervention may be directly after the stroke or TIA or at least within 3 months.
To the best of our knowledge, this is the first study that focusses on changes in determinants of health-related behavior change after TIA or ischemic stroke. Self-efficacy has been studied before in patients with vascular disease (coronary heart disease, cerebrovascular disease, or peripheral artery disease) [20,35]. In those studies, comparably high self-efficacy levels were found, but the self-efficacy was not monitored over time. Response-efficacy for behavior change has not been described in patients with TIA or ischemic stroke before. Fear seems moderate (with a median of 16 on a scale of 32) and decreased strongly within 3 months after stroke or TIA. Fear started to decrease after 6 weeks. During this period, patients often undergo additional examinations to assess the underlying etiology at different medical specialists. This might lead to uncertainty and fear. Possibly patients also adapt to the uncertainty, which lowers the fear. We could not compare our findings with those of others as fear in relation to behavior change has not been studied quantitatively or in patients with TIA or stroke before. Fear of a recurrent stroke has been found in several other studies [37–41] and can possibly be used as an opportunity to motivate patients to change their health-related behavior in order to reduce risk of recurrence [24]. In two small studies with stroke patients, fear was mentioned by patients as a motivating factor to change health behavior [37,42]. Previous studies in patients with coronary artery syndrome have shown that the majority of patients who quit smoking successfully stopped immediately after the event [43]. Perceived feeling of a life-threatening disease seems to play a role in this process [44]. The European Society of Cardiology (ESC) guidelines recommend to seize this opportunity by addressing the issue of smoking before discharge [45]. These guidelines also recommend that support for cessation of smoking is initiated for all smokers during hospital admission and is continued for a prolonged period after discharge [45,46]. Although there is no evidence for this approach in stroke patients, it seems reasonable to assume that this advice can also be effective after ischemic stroke or TIA.
Strengths of our study are that this is the first study that focuses on determinants of health behavior change after TIA or ischemic stroke over time. Also we collected detailed information about potential determinants such as difference between smokers and nonsmokers. This study has also some limitations. First, we studied patients for a relatively short period of time. Patients are often rehabilitating longer than 3 months, which can cause further changes over time. Second, not all patients completed questionnaires at 6 weeks (n = 17–25, Fig. 1), which may have affected the results. Baseline and 3-month follow-up questionnaires were completed during an outpatient clinic visit. However, not all patients visited the outpatient clinic after 6 weeks and many patients did not return the submitted questionnaires by mail. We mainly drew conclusions based on the analyses of the difference between baseline and 3 months follow-up. Self-efficacy and response-efficacy scores did not change during all measurements. And most patients had completed all questionnaires after 3 months. Furthermore social desirability bias during questionnaire completion may also have played a role as self-efficacy is high in these patients where our earlier study showed that most patients do not actually change their behavior, due to the intention-behavior gap [47,48].
In summary, at present it is unclear how and at which moment patients can be best supported in health-related behavior change after TIA or ischemic stroke. Fear, self-efficacy, and response-efficacy play a role in this behavior change process. We found that response-efficacy and self-efficacy remain high after 3 months, and fear decreased significantly after 6 weeks. Therefore, the optimal timing of supporting patients in health-related behavior change after TIA or ischemic stroke seems to be directly after the stroke or TIA.
Acknowledgements
The corresponding author also completed the statistical analysis. This study is not industry-sponsored.
Conflicts of interest
There are no conflicts of interest.
References
- 1.European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008; 25:457–507. [DOI] [PubMed] [Google Scholar]
- 2.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. ; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014; 45:2160–2236. [DOI] [PubMed] [Google Scholar]
- 3.Rudd AG, Bowen A, Young GR, James MA. The latest national clinical guideline for stroke. Clin Med (Lond). 2017; 17:154–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawrence M, Kerr S, Watson H, Paton G, Ellis G. An exploration of lifestyle beliefs and lifestyle behaviour following stroke: findings from a focus group study of patients and family members. BMC Fam Pract. 2010; 11:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lennon O, Galvin R, Smith K, Doody C, Blake C. Lifestyle interventions for secondary disease prevention in stroke and transient ischaemic attack: a systematic review. Eur J Prev Cardiol. 2014; 21:1026–1039. [DOI] [PubMed] [Google Scholar]
- 6.Rodgers H, Atkinson C, Bond S, Suddes M, Dobson R, Curless R. Randomized controlled trial of a comprehensive stroke education program for patients and caregivers. Stroke. 1999; 30:2585–2591. [DOI] [PubMed] [Google Scholar]
- 7.Ellis G, Rodger J, McAlpine C, Langhorne P. The impact of stroke nurse specialist input on risk factor modification: a randomised controlled trial. Age Ageing. 2005; 34:389–392. [DOI] [PubMed] [Google Scholar]
- 8.Maasland E, Koudstaal PJ, Habbema JD, Dippel DW. Effects of an individualized multimedia computer program for health education in patients with a recent minor stroke or transient ischemic attack - a randomized controlled trial. Acta Neurol Scand. 2007; 115:41–48. [DOI] [PubMed] [Google Scholar]
- 9.Sit JW, Yip VY, Ko SK, Gun AP, Lee JS. A quasi-experimental study on a community-based stroke prevention programme for clients with minor stroke. J Clin Nurs. 2007; 16:272–281. [DOI] [PubMed] [Google Scholar]
- 10.Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ. 2015; 350:h1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartholomew Eldredge LK, Markham CM, Ruiter RAC, Fernández ME, Kok G, Parcel GS. Planninghealth Promotion Programs: An Intervention Mapping Approach. San Fransisco: Jossey- Bass Inc; 4th ed 2016. [Google Scholar]
- 12.Wight D, Wimbush E, Jepson R, Doi L. Six steps in quality intervention development (6SQuID). J Epidemiol Community Health. 2016; 70:520–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Araújo-Soares V, Hankonen N, Presseau J, Rodrigues A, Sniehotta F. Developing Behavior Change Interventions for Self-Management in Chronic Illness. 2018. pp. 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Floyd DL, Prentice-Dunn S, Rogers RW. A meta-analysis of research on protection motivation theory. J Appl Soc Psychol. 2000; 30:407–429. [Google Scholar]
- 15.Brouwer-Goossensen D, Genugten LV, Lingsma H, Dippel D, Koudstaal P, Hertog HD. Determinants of intention to change health-related behavior and actual change in patients with TIA or minor ischemic stroke. Patient Educ Couns. 2016; 99:644–650. [DOI] [PubMed] [Google Scholar]
- 16.Sol BG, van der Bijl JJ, Banga JD, Visseren FL. Vascular risk management through nurse-led self-management programs. J Vasc Nurs. 2005; 23:20–24. [DOI] [PubMed] [Google Scholar]
- 17.Sniehotta FF, Scholz U, Schwarzer R. Bridging the intention-behaviour gap: planning, self-efficacy, and action control in the adoption and maintenance of physical exercise. Psychol Health. 2005; 20:143–160. [Google Scholar]
- 18.Garcia K, Mann T. From ‘I Wish’ to ‘I Will’: social-cognitive predictors of behavioral intentions. J Health Psychol. 2003; 8:347–360. [DOI] [PubMed] [Google Scholar]
- 19.Vries Hd, Dijkstra M, Kuhlman P. Self- efficacy: the third factor besides attitude and subjective norm as a predictor of behavioural intentions. Health Educ Res. 1988; 3:272–282. [Google Scholar]
- 20.Sol BG, van der Graaf Y, van der Bijl JJ, Goessens NB, Visseren FL. Self-efficacy in patients with clinical manifestations of vascular diseases. Patient Educ Couns. 2006; 61:443–448. [DOI] [PubMed] [Google Scholar]
- 21.Tulloch H, Reida R, D’Angeloa MS, Plotnikoff RC, Morrina L, Beatona L, et al. Predicting short and long-term exercise intentions and behaviour in patients with coronary artery disease: a test of protection motivation theory. Psychol Health. 2009; 24:255–269. [DOI] [PubMed] [Google Scholar]
- 22.Blanchard CM, Reid RD, Morrin LI, McDonnell L, McGannon K, Rhodes RE, et al. Does protection motivation theory explain exercise intentions and behavior during home-based cardiac rehabilitation? J Cardiopulm Rehabil Prev. 2009; 29:188–192. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan KA, White KM, Young RM, Scott CJ. Predictors of intention to exercise to reduce stroke risk among people at risk of stroke: an application of an extended Health Belief Model. Rehabil Psychol. 2008; 53:505–512. [Google Scholar]
- 24.White CL, Barrientos R, Dunn K. Dimensions of uncertainty after stroke: perspectives of the stroke survivor and family caregiver. J Neurosci Nurs. 2014; 46:233–240. [DOI] [PubMed] [Google Scholar]
- 25.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988; 19:604–607. [DOI] [PubMed] [Google Scholar]
- 26.Schlegel D, Kolb SJ, Luciano JM, Tovar JM, Cucchiara BL, Liebeskind DS, Kasner SE. Utility of the NIH Stroke Scale as a predictor of hospital disposition. Stroke. 2003; 34:134–137. [DOI] [PubMed] [Google Scholar]
- 27.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24:35–41. [DOI] [PubMed] [Google Scholar]
- 28.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005; 53:695–699. [DOI] [PubMed] [Google Scholar]
- 29.Beekman AT, van Limbeek J, Deeg DJ, Wouters L, van Tilburg W. [A screening tool for depression in the elderly in the general population: the usefulness of Center for Epidemiological Studies Depression Scale (CES-D)]. Tijdschr Gerontol Geriatr. 1994; 25:95–103. [PubMed] [Google Scholar]
- 30.Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychol Med. 1997; 27:231–235. [DOI] [PubMed] [Google Scholar]
- 31.Plotnikoff RC, Higginbotham N. Protection Motivation theory and the prediction of exercise and low- fat diet behaviors among Australian cardiac patients. Psychol Health. 1998; 13:411–429. [Google Scholar]
- 32.Mahe G, Ronziere T, Laviolle B, Golfier V, Cochery T, De Bray JM, Paillard F. An unfavorable dietary pattern is associated with symptomatic ischemic stroke and carotid atherosclerosis. J Vasc Surg. 2010; 52:62–68. [DOI] [PubMed] [Google Scholar]
- 33.Bijl JV, Poelgeest-Eeltink AV, Shortridge-Baggett L. The psychometric properties of the diabetes management self-efficacy scale for patients with type 2 diabetes mellitus. J Adv Nurs. 1999; 30:352–359. [DOI] [PubMed] [Google Scholar]
- 34.Sol BG, van der Graaf Y, van der Bijl JJ, Goessens BM, Visseren FL. The role of self-efficacy in vascular risk factor management: a randomized controlled trial. Patient Educ Couns. 2008; 71:191–197. [DOI] [PubMed] [Google Scholar]
- 35.Sol BG, van der Graaf Y, van Petersen R, Visseren FL. The effect of self-efficacy on cardiovascular lifestyle. Eur J Cardiovasc Nurs. 2011; 10:180–186. [DOI] [PubMed] [Google Scholar]
- 36.Champion VL, Skinner CS, Menon U, Rawl S, Giesler RB, Monahan P, Daggy J. A breast cancer fear scale: psychometric development. J Health Psychol. 2004; 9:753–762. [DOI] [PubMed] [Google Scholar]
- 37.Carlsson GE, Möller A, Blomstrand C. Managing an everyday life of uncertainty–a qualitative study of coping in persons with mild stroke. Disabil Rehabil. 2009; 31:773–782. [DOI] [PubMed] [Google Scholar]
- 38.Martin BJ, Yip B, Hearty M, Marletta S, Hill R. Outcome, functional recovery and unmet needs following acute stroke. Experience of patient follow up at 6 to 9 months in a newly established stroke service. Scott Med J. 2002; 47:136–137. [DOI] [PubMed] [Google Scholar]
- 39.Townend E, Tinson D, Kwan J, Sharpe M. Fear of recurrence and beliefs about preventing recurrence in persons who have suffered a stroke. J Psychosom Res. 2006; 61:747–755. [DOI] [PubMed] [Google Scholar]
- 40.Horne J, Lincoln NB, Preston J, Logan P. What does confidence mean to people who have had a stroke? A qualitative interview study. Clin Rehabil. 2014; 28:1125–1135. [DOI] [PubMed] [Google Scholar]
- 41.Nordin Å, Sunnerhagen KS, Axelsson ÅB. Patients’ expectations of coming home with Very Early Supported Discharge and home rehabilitation after stroke - an interview study. BMC Neurol. 2015; 15:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuki T, Kudo M. Factors related to continuation of health behaviours among stroke survivors. J Jpn Phys Ther Assoc. 2011; 14:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snaterse M, Scholte Op Reimer WJ, Dobber J, Minneboo M, Ter Riet G, Jorstad HT, et al. Smoking cessation after an acute coronary syndrome: immediate quitters are successful quitters. Neth Heart J. 2015; 23:600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scholte op Reimer W, de Swart E, De Bacquer D, Pyorala K, Keil U, Heidrich J, et al. Smoking behaviour in European patients with established coronary heart disease. Eur Heart J. 2006; 27:35–41. [DOI] [PubMed] [Google Scholar]
- 45.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren WM, et al. ; European Association for Cardiovascular Prevention & Rehabilitation (EACPR). European guidelines on cardiovascular disease prevention in clinical practice (version 2012): the fifth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Int J Behav Med. 2012; 19:403–488. [DOI] [PubMed] [Google Scholar]
- 46.Rigotti NA, Clair C, Munafo MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2012; 5:CD001837. doi: 10.1002/14651858.CD001837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brouwer-Goossensen D, Genugten LV, Lingsma H, Dippel D, Koudstaal P, Hertog HD. Determinants of intention to change health-related behavior and actual change in patients with TIA or minor ischemic stroke. Patient Educ Couns. 2016; 99:644–650. [DOI] [PubMed] [Google Scholar]
- 48.Sheeran P. Intention- behavior relations: a conceptual and empirical review. Eur Rev Soc Psychol. 2002; 12:1–36. [Google Scholar]


