Abstract
COVID-19 is a serious disease that has infected more than 40 million people. Beside significant mortality, the SARS-CoV-2 infection causes considerable and sustained morbidity, dubbed long COVID. This paper argues that some of this morbidity may be due to a persistent systemic infection. Persistent infection is indicated by continued virus RNA shedding. The virus’ superantigen could overstimulate anti-virus immune responses, and thereby induce negative feedback loops, that paradoxically allow the virus to persist. The superantigen would induce strong immune response to any residual infection. This hypothesis suggests that clearing the virus infection completely would be an appropriate intervention against long COVID.
Introduction
In less than one year, COVID-19 caused by SARS-CoV-2 has emerged to a worldwide major health threat [1], with 40 million documented cases and over 1.1 million documented fatal cases [2]. Currently, about 300,000 and rising new cases are identified every day, while the daily deaths are stabilized at about 6000. The estimated infection fatality rate (IFR) is 0.68% (0.53–0.82%) for the overall population, and below 0.2% for those under 60 years old [3].
While mortality after SARS-CoV-2 infection is low, 87% of hospitalized patients had prolonged symptoms two months after the disease [4]. The prolonged symptoms of Long COVID [5], are not only seen in people with severe disease, but also in patient with mild disease [6], [7]. Delayed recovery after disease is not unique to COVID-19, but seen after several other virus infections [8]. Complications of COVID-19 can affect a multiple physiological systems, i.e. immune system, neurologic, cardiologic, respiratory, cutaneous, hepatic, renal and pancreatic (diabetes) function [9], [10], [11], [12].
Three different mechanisms could be hypothesized to play a role in long COVID-19: (i) post-viral fatigue syndromes [13], [14], [15], (ii) autoimmunity [16], [17], and (iii) persistent virus infection (this paper). The symptoms are more diverse and intense than those reported in general post-viral fatigue syndromes. Autoimmunity requires cross-reactive antigens, and some putative candidates have been identified [18], [19]. Further research will identify these antigens. Cross-reacting antigens is not sufficient for the development of autoimmunity, the immune system should signal danger as well. Cell damage or direct activation could induce maturation of dendritic cells [20]. A persistent virus infection could induce both cell damage and direct activation of dendritic cells, which could lead to autoimmunity. But from an immunologic perspective persistent infection and autoimmunity are two clearly distinct mechanisms that could be caused by SARS-CoV-2 [21].
In brief, a persistent infection could explain the symptoms of long COVID. This paper investigates how SARS-CoV-2 could cause a persistent infection.
Outline of the hypothesis
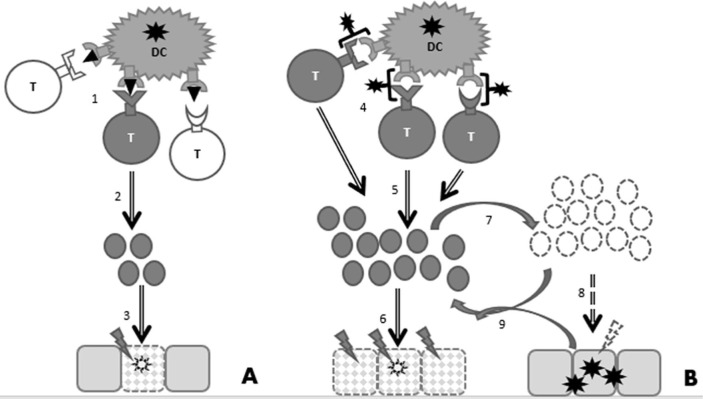
Superantigens are described for SARS-CoV-2 [22], [23], and superantigens are known to cause cytokine storm, by a polyclonal T cell activation [24]. SARS-CoV-2 induces a very strong immune response [25], which could be due to its superantigen(s). Down regulation of the immune system by corticosteroid treatment reduces mortality in severe COVID-19 [26], indicating the need for immune suppression. In most patients down-regulation of superantigen responses occurs naturally, and therefore superantigen-induced immune responses are all but efficient in generating immune response [27]. The negative immunological feedback loop could allow the virus to reproduce in the body, especially where the immune response is relatively weak. Fig. 1 shows the delicate balance between immune activation and suppression in case of a superantigen infection.
Fig. 1.
Antiviral immune responses by antigens and superantigens. Simplified representation with T lymphocytes as representatives of the orchestration of antigen-specific immune responses. A. Normal viral antigens are presented by Dendritic cells (DCs) to T lymphocytes. (1) Only T cells with an antigen-specific T-cell receptor (TCR) matching the peptide in MHC context are activated, and (2) have clonal expansion. The (3) expanded antigen-specific T-cell population results in effective clearance of the virus. B. Superantigens are presented by DCs to T lymphocytes. (4) A large subgroup of T lymphocytes is activated, e.g. through their common Vβ Receptor. Due to the binding to the outside of the TCR, this binding is independent of the specificity of the TCR. (5) A large group of activated T cells have clonal expansion and is capable of (6) immune overreaction, i.e. sepsis. Due to the self-destructing nature of sepsis, (7) the immune response is downregulated by negative feedback loops, e.g. by CD4+CD25+ Treg lymphocytes and interleukine-10 (IL-10). (8) The result is an partial effective immune response that allows virus persistence. (9) The persistent virus infection reactivates the immune system, resulting in an ineffective, but tissue-damaging virus-immune reaction loop.
Immune suppression, as induced by its superantigens, might be crucial for SARS-CoV-2 to establish persistent infections. Many RNA viruses can cause persistent infections [28]. Routine clinical practice, only tests for SARS-CoV-2 in samples from the (upper) respiratory tract. It should be noted that SARS-CoV-2, like many other viruses, can cause a dispersed infection [29], possibly by antibody-mediated infection [30]. The virus superantigen would cause a strong immune response to anywhere in the body, where it would be produced.
Evaluation of the hypothesis
Evidence for persistent infection is shown by prolonged viral shedding in feces of several groups of patients [31], [32], [33], [34]. Irrespective of their mechanisms, persistent infections of SARS-CoV-2 are dispersed into various organs [35], [36]. These papers often show that persistent virus infection might be missed by regular RT-PCR diagnosis based on sampling in the nasopharyngeal and/or oropharyngeal cavity. Persistent infections have also been described for a murine coronavirus, mouse hepatitis virus (MHV) [37], [38], [39]. Just like, SARS-CoV-2, MHV contains superantigen activity [40].
The SARS-CoV-2 virus has a long-term coexistence with immune response in COVID-19 patients, indicating that immunity, in spite of its severity, is not very effective [41]. A strong immune response by superantigens could lead to incomplete immunity, as has been shown for other respiratory viruses [42].
A persistent infection is a strong indication for incomplete immunity. This is in accordance with the short-lasting protective immunity against seasonal coronaviruses [43]. Several papers report early reinfection by SARS-CoV-2, sometimes even more severe [44], [45], suggesting that if some immunity remains after an earlier infection, it is not of great benefit to the host. This contra intuitive immunological finding fits nicely with the characteristics of superantigens.
Consequences of the hypothesis
A persistent virus infection would imply persistent superantigen exposure in the host. Chronic superantigen exposure induces systemic inflammation, and has a possible role in the development diabetes [46]. Indeed the risk of developing diabetes after COVID-19 has been described [12]. Also the development of cardiovascular disease could be a morbidity due to COVID-19 [10], [11]. Incomplete and/or short-lived immunity also plays a crucial role in reinfection by SARS-CoV-2, which could be more severe [44]. Diabetes and cardiovascular comorbidities cold worsen the outcome a reinfection [47], and also the continued immunological exacerbation due to superantigen re-exposure could contribute to a more severe outcome after reinfection.
Both a residual inflammation or an autoimmune reaction are undesired immune reactions. Incomplete immunity could lead to a persistent infection, with ongoing inflammation and the putative development of autoimmune reaction as a result. For clinical-decision making, it is invaluable to know whether symptoms are caused by a too strong immune reaction or due to a persistent infection. In the first case, interventions should aim at suppressing the immune responses, e.g. by corticosteroids. In the later, it is suggested to boost anti-viral immunity with type I Interferons, Interleukin-2 (IL-2), IL-7 or antivirals [48], [49]. The argument of this paper is that fighting the persistent virus infection would be the appropriate intervention in at least some cases. The virus and/or immune status of the patient should be decisive for the choice of intervention. The status of virus persistent may be hard to detect, as currently there is not generic methodology for sampling from systemic SARS-CoV-2 infections, that are absent from the nasopharyngeal and oropharyngeal cavity. Immune-based clinical-decision making is not new to immunologists [50], and formed the basis for personalized medicine [51]. A similar immunology-based clinical-decision system might be crucial to select the right treatment of long COVID patients.
Declaration of Competing Interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Zhou P., Yang X.-L., Wang X.-G., et al. A pneumonia outbreak associated with a new coronavirus of probably bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. https://www.nature.com/articles/s41586-020-2012-7.pdf CrossRefView. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymus COVID-19 coronavirus pandemic. Assessed 18-10-2020. https://www.worldometers.info/coronavirus/ CrossRefView N.A.
- 3.Meyerowitz-Katz G, Merone L. A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates medRxiv (2020) preprint https://www.medrxiv.org/content/10.1101/2020.05.03.20089854v4.full.pdf CrossRefView. https://doi.org/10.1101/2020.05.03.20089854. [DOI] [PMC free article] [PubMed]
- 4.Carfi A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA 324; 2020: 603–605. https://jamanetwork.com/journals/jama/fullarticle/2768351 CrossRefView. https://doi.org/10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed]
- 5.Mahase E. Covid-19: what do we know about “long covid”? BMJ 370; 2020: m2815. https://www.bmj.com/content/370/bmj.m2815 CrossRefView. http://dx.doi.org/10.1136/bmj.m2815. [DOI] [PubMed]
- 6.Goërtz YMG, Van Herck M, Delbressine JM, et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res 2020 [in press]. https://openres.ersjournals.com/content/erjor/early/2020/09/01/23120541.00542-2020.full.pdf CrossRefView. https://doi.org/10.1183/23120541.00542-2020. [DOI] [PMC free article] [PubMed]
- 7.Dennis A, Wamil M, Kapur S, et al. Multi-organ impairment in low-risk individuals with long COVID Submitted https://www.medrxiv.org/content/10.1101/2020.10.14.20212555v1.full.pdf CrossRefView. https://doi.org/10.1101/2020.10.14.20212555.
- 8.Nabavi N. Long covid: how to define it and how to manage it. BMJ 370; 2020: m3489. https://www.bmj.com/content/bmj/370/bmj.m3489.full.pdf CrossRefView. http://dx.doi.org/10.1136/bmj.m3489. [DOI] [PubMed]
- 9.Bridwell R, Long B, Gottlieb M. Neurologic complications of COVID-19. Am J Emerg Med 38; 2020: 1549.e3–1549.e7. https://linkinghub.elsevier.com/retrieve/pii/S0735675720303648 CrossRefView. https://doi.org/10.1016\j.ajem.2020.05.024. [DOI] [PMC free article] [PubMed]
- 10.Dasgupta A, Kalhan A, Kalra S.. Long term complications and rehabilitation of COVID-19 patients. J Pak Med Assoc 70; 2020: S131–S13. https://www.ejmanager.com/mnstemps/33/33-1589041918.pdf?t=1601748998 CrossRefView. https://doi.org/10.5455/JPMA.32. [DOI] [PubMed]
- 11.Leung T, Chan A, Chan EW, et al. Short- and potential long-term adverse health outcomes of COVID-19: a rapid review. Emerg Microbes Infect 17; 2020: 1–19. https://doi.org/10.1080/22221751.2020.1825914. [DOI] [PMC free article] [PubMed]
- 12.Mongioì LM, Barbagallo F, Condorelli RA, et al. Possible long-term endocrine-metabolic complications in COVID-19: lesson from the SARS model. Endocrine 68; 2020: 467–470. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7266418/pdf/12020_2020_Article_2349.pdf CrossRefView. https://doi.org/10.1007/s12020-020-02349-7. [DOI] [PMC free article] [PubMed]
- 13.Wilson C. Concern coronavirus may trigger post-viral fatigue syndromes. New Sci 246; 2020: 10–11. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7194715/pdf/main.pdf CrossRefView. https://dx.doi.org/10.1016%2FS0262-4079(20)30746-6. [DOI] [PMC free article] [PubMed]
- 14.Bannister BA. Post-infectious disease syndrome. Postgrad Med J 64; 1988: 559–567. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2428896/pdf/postmedj00187-0079.pdf CrossRefView. https://dx.doi.org/10.1136%2Fpgmj.64.753.559. [DOI] [PMC free article] [PubMed]
- 15.Ho-Yen DO. The epidemiology of post viral fatigue syndrome scott. Med J 33; 1988: 368–9. https://journals.sagepub.com/doi/10.1177/003693308803300607?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed CrossRefView. https://doi.org/10.1177%2F003693308803300607. [DOI] [PubMed]
- 16.Galeotti C, Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol 4; 2020: 1–2. https://www.nature.com/articles/s41584-020-0448-7 CrossRefView. https://dx.doi.org/10.1038%2Fs41584-020-0448-7. [DOI] [PMC free article] [PubMed]
- 17.Rodríguez Y, Novelli L, Rojas M, et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J Autoimmun 16; 2020: 102506. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7296326/pdf/main.pdf CrossRefView. https://dx.doi.org/10.1016%2Fj.jaut.2020.102506. [DOI] [PMC free article] [PubMed]
- 18.Angileri F, Legare S, Marino Gammazza A, Conway de Macario E, Macario AJL, Cappello F. Molecular mimicry may explain multi-organ damage in COVID-19. Autoimmun Rev 19; 2020: 102591. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7289093/pdf/main.pdf CrossRefView. https://dx.doi.org/10.1016%2Fj.autrev.2020.102591. [DOI] [PMC free article] [PubMed]
- 19.McMillan P, Uhal BD. COVID-19-A theory of autoimmunity to ACE-2. MOJ Immunol 7; 2020: 17–19. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7351250/pdf/nihms-1601975.pdf CrossRefView. https://doi.org/10.15406/moji.2020.07.00257. [PMC free article] [PubMed]
- 20.Jacobs JJL, Lehë CL, Hasegawa H, Elliott GR, Das PK. Skin irritants and contact sensitizers induce Langerhans cell migration and maturation at irritant concentration. Exp Dermatol 15; 2006: 432–40. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.0906-6705.2006.00420.x CrossRefView. https://doi.org/10.1111/j.0906-6705.2006.00420.x. [DOI] [PubMed]
- 21.Gousseff M, Penot P, Gallay L, et al. Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect 2020: S0163-4453(20)30454-0. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7326402/pdf/main.pdf. https://doi.org/10.1016/j.jinf.2020.06.073. [DOI] [PMC free article] [PubMed]
- 22.Cheng MH, Zhang S, Porritt RA, et al. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation. Proc Nat Acad Sci 2020 [in press]. https://www.pnas.org/content/pnas/early/2020/09/25/2010722117.full.pdf CrossRefView https://doi.org/10.1073/pnas.2010722117. [DOI] [PMC free article] [PubMed]
- 23.Lu Y, Luo C, Li W. Immunity and virulence of SARS coronavirus. Viral Immunol 17; 2004: 528–34. https://www.liebertpub.com/doi/10.1089/vim.2004.17.528?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed CrossRefView. https://doi.org/10.1089/vim.2004.17.528. [DOI] [PubMed]
- 24.Scaglioni V, Soriano ER. Are superantigens the cause of cytokine storm and viral sepsis in severe COVID-19? Observations and hypothesis Scand J Immunol 2020: e12944. https://doi.org/10.1111/sji.12944. [DOI] [PMC free article] [PubMed]
- 25.Yazdanpanah F, Hamblin MR, Rezaei N. The immune system and COVID-19: friend or foe? Life Sci 256; 2020: 117900. https://reader.elsevier.com/reader/sd/pii/S0024320520306500?token=56891288C7D70B0EB0692A90F3A030F47FA11015D1CC6FE05CCEEB33233DE6063C2D0BC4DFD76B7A43DD5EB033F82DC9 CrossRefView. https://dx.doi.org/10.1016%2Fj.lfs.2020.117900. [DOI] [PMC free article] [PubMed]
- 26.Bartoletti M, Marconi L, Scudeller L, et al. Efficacy of corticosteroid treatment for hospitalized patients with severe COVID-19: a multicentre study. Clin Microbiol Infect 2020: S1198-743X(20)30563-2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7506332/pdf/main.pdf CrossRefView. https://doi.org/10.1016/j.cmi.2020.09.014. [DOI] [PMC free article] [PubMed]
- 27.Huber BT, Hsu P-N, Sutkowski N. Virus-encoded superantigens. Microbiol Rev 60; 1996: 473–482. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC239452/pdf/600473.pdf CrossRefView N.A. [DOI] [PMC free article] [PubMed]
- 28.Randall RE, Griffin DE. Within host RNA virus persistence: mechanisms and consequences. Curr Op Virol 23; 2017: 35–42. https://www.sciencedirect.com/science/article/pii/S1879625716301559?via%3Dihub CrossRefView. https://doi.org/10.1016/j.coviro.2017.03.001. [DOI] [PMC free article] [PubMed]
- 29.Feng Z, Diao B, Wang R, et al. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes. medRxiv 2020. Preprint. https://www.medrxiv.org/content/10.1101/2020.03.27.20045427v1.full.pdf CrossRefView. https://doi.org/10.1101/2020.03.27.20045427.
- 30.Jacobs JJL. Neutralizing antibodies mediate virus-immune pathology of COVID-19. Med Hypotheses 143; 2020: 109884. https://www.sciencedirect.com/science/article/pii/S0306987720311270 CrossRefView. https://doi.org/10.1016/j.mehy.2020.109884. [DOI] [PMC free article] [PubMed]
- 31.Xing Y-H, Ni W, Wu Q, et al. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol Immunol Infect 53; 2020: 473–480. https://www.sciencedirect.com/science/article/pii/S1684118220300815?via%3Dihub CrossRefView. https://doi.org/10.1016/j.jmii.2020.03.021. [DOI] [PMC free article] [PubMed]
- 32.Agarwal V, Venkatakrishnan AJ, Puranik A, et al. Quantifying the prevalence of SARS-CoV-2 long-term shedding among non-hospitalized COVID-19 patients. medRxiv 2020: 2020.06.02.20120774 (preprint). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7302207/pdf/nihpp-2020.06.02.20120774.pdf CrossRefView. https://doi.org/10.1101/2020.06.02.20120774.
- 33.Sethuraman M, Stanleyraj Jeremiah S, Ryo A. Interpreting diagnostic tests for SARS-CoV-2 JAMA.323 2020: 2249–2251. https://jamanetwork.com/journals/jama/fullarticle/2765837 CrossRefView. https://doi.org/10.1001/jama.2020.8259. [DOI] [PubMed]
- 34.Widders A, Broom A, Broom J. SARS-CoV-2: the viral shedding vs infectivity dilemma. Infect Dis Health 25; 2020: 210–215. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7237903/pdf/main.pdf CrossRefView. https://doi.org/10.1016/j.idh.2020.05.002. [DOI] [PMC free article] [PubMed]
- 35.Kalkeri R., Goebel S., Sharma G.D. SARS-CoV-2 shedding from asymptomatic patients: contribution of potential extrapulmonary tissue reservoirs. Am J Trop Med Hyg. 2020;103:18–21. doi: 10.4269/ajtmh.20-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoang VT, Dao TL, Gautret P. Recurrence of positive SARS-CoV-2 in patients recovered from COVID-19. J Med Virol 2020. 10.1002/jmv.26056. [DOI] [PMC free article] [PubMed]
- 37.Bergmann CC, Lane TE, Stohlman SA. Coronavirus infection of the central nervous system: host–virus stand-off. Nat Rev Microbiol 4; 2006: 121–132. https://www.nature.com/articles/nrmicro1343 CrossRefView. https://doi.org/10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed]
- 38.Skinner D, Marro BS, Lane TE. Chemokine CXCL10 and coronavirus-induced neurologic disease. Viral Immunol 32; 2019: 25–37. https://doi.org/10.1089/vim.2018.0073. [DOI] [PMC free article] [PubMed]
- 39.Gaebler Christian, Wang Zijun, Lorenzi Julio, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021 doi: 10.1038/s41586-021-03207-w. https://pubmed.ncbi.nlm.nih.gov/33461210/ In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gagne S, Thibodeau L, Lamontagne L. Clonal deletion of some V beta+ T cells in peripheral lymphocytes from C57BL/6 mice infected with MHV3. In: Enjuanes L., Siddell S.G., Spaan W. (eds) Coronaviruses and Arteriviruses. Advances in Experimental Medicine and Biology, vol. 440. Springer: Boston, MA; 1998. pp. 485-489. https://doi.org/10.1007/978-1-4615-5331-1_62. [DOI] [PubMed]
- 41.Wang B, Wang L, Kong X, et al. Long-term coexistence of SARS-CoV-2 with antibody response in COVID-19 patients. J Med Virol 92; 2020: 1684–1689. https://doi.org/10.1002/jmv.25946. [DOI] [PMC free article] [PubMed]
- 42.Le Nouën C, Hillyer P, Munir S, et al. Effects of human respiratory syncytial virus, metapneumovirus, parainfluenza virus 3 and influenza virus on CD4+ T cell activation by dendritic cells. PLoS One 5; 2010: e15017. https://doi.org/10.1371/journal.pone.0015017. [DOI] [PMC free article] [PubMed]
- 43.Edridge AWD, Kaczorowska J, Hoste ACR, et al. Seasonal coronavirus protective immunity is short-lasting. Nat Med 2020 [in press]. https://doi.org/10.1038/s41591-020-1083-1. [DOI] [PubMed]
- 44.Lafaie L, Célarier T, Goethals L, et al. Recurrence or relapse of COVID-19 in older patients: a description of three cases. J Am Geriatr Soc 2020. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7361461/pdf/JGS-9999-na.pdf CrossRefView https://dx.doi.org/10.1111%2Fjgs.16728. [DOI] [PMC free article] [PubMed]
- 45.Shastri J, Parikh S, Agrawal S, et al. Whole genome sequencing confirmed SARS-CoV-2 reinfections among healthcare workers in india with increased severity in the second episode. The Lancet 2020. preprint. https://papers.ssrn.com/sol3/Delivery.cfm/17b5cdc9-d7f9-4c47-8700-abfaec970b2e-MECA.pdf?abstractid=3688220&mirid=1 CrossRefView. https://dx.doi.org/10.2139/ssrn.3688220.
- 46.Vu BG, Stach CS, Kulhaankova K, Salgado-Pabón W, Klingelhutz AJ, Schlievert PM. Chronic superantigen exposure induces systemic inflammation, elevated bloodstream endotoxin, and abnormal glucose tolerance in rabbits: possible role in diabetes. mBio 6; 2015: e02554-14. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4358007/pdf/mBio.02554-14.pdf CrossRefView. https://doi.org/10.1128/mBio.02554-14. [DOI] [PMC free article] [PubMed]
- 47.Ghisolfi S, Almås I, Sandefur JC, Von Carnap T, Heitner J, Bold T. Predicted COVID-19 fatality rates based on age, sex, comorbidities and health system capacity. BMJ Global Health 5; 2020: e003094. https://gh.bmj.com/content/bmjgh/5/9/e003094.full.pdf CrossRefView. http://dx.doi.org/10.1136/. [DOI] [PMC free article] [PubMed]
- 48.Jamilloux Y, Henry T, Belot A, et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions Autoimmun Rev 19; 2020: 102567. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7196557/pdf/main.pdf CrossRefView. https://doi.org/10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed]
- 49.Tomova R, Antonov K, Ivanova A, et al. Low-dose IL-2 therapy reduces HCV RNA and HBV DNA: case report. Anticancer Res 12; 2009: 5241–4. http://ar.iiarjournals.org/content/29/12/5241.full.pdf+html CrossRefView N.A. [PubMed]
- 50.Characiejus D, Pasukoniene V, Kazlauskaite N, et al. Predictive value of CD8highCD57+ lymphocyte subset in interferon therapy of patients with renal cell carcinoma. Anticancer Res 22; 2002: 3679–83. https://pubmed.ncbi.nlm.nih.gov/12552976/ CrossRefView N.A. [PubMed]
- 51.Characiejus D., Hodzic J., Jacobs J.J.L. “First do no harm” and the importance of prediction in oncology. EPMA J. 2010;1:369–375. doi: 10.1007/s13167-010-0042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]