Abstract
Background and Objectives:
The aim of present study was to evaluate the prevalence of Listeria monocytogenes and Escherichia coli, characterization and antimicrobial resistance of their serotypes and genotyping profiles in fresh beef and poultry meats marketed in Zanjan, Iran.
Materials and Methods:
A total of 90 (45 chicken and 45 beef) samples were collected from January to June 2018 focusing on retail meat stores of Zanjan city, Iran. Foodborne pathogen detection and antimicrobial resistance of isolates performed by PCR and disc diffusion methods, respectively. Simplex PCR method was used for screening hly and uidA genes in L. monocytogenes and E. coli isolates, respectively.
Results:
Findings revealed high contamination in beef and chicken meats with E. coli (68.89% and 88.89%, respectively) and L. monocytogenes (53.33% and 46.67%, respectively). The most likelihood of E. coli isolates belonged to E. coli 13479 serotype. All L. monocytogenes isolates from beef and chicken meat samples had high similarity with serotypes L. monocytogenes strain NCTC 10357 and strain MF 4545, respectively. Multi drug resistance (MDR) was seen in both L. monocytogenes and E. coli isolates.
Conclusion:
This study shows an insight of the current status of beef and chicken meat contamination maketed in Zanjan, Iran with E. coli and L. monocytogenes isolates (high contamination rate), their genotypic profile, epidemiological relationship and antimicrobial resistance (AMR) that should be considered as a significant public health concern in Zanjan, Iran.
Keywords: Antibiotic resistance, Escherichia coli, Genotyping, Listeria monocytogenes, Meat hygiene
INTRODUCTION
Escherichia coli as a commensal bacterium of intestinal tract in human and animals and Listeria monocytogenes as an ubiquitous bacterium are of major concern to human health and the most commonly studied foodborne pathogens (1). Both L. monocytogenes and E. coli are common in food and clinical samples due to their high prevalence and ability to grow in different conditions such as low temperature (2). Direct contact with the reservoir animals such as ruminants, contaminated unprocessed and processed foods such as meat products, dairy products, ready to eat (RTE) foods, fruits and vegetables are sources to transmission of foodborne pathogen bacteria to human including L. monocytogenes and E. coli (1, 2). High rate of meat consumption (beef, chicken) and high contamination probability of them increase the risk of foodborne pathogens to public health associated with morbidity and mortality (3). Antibiotic therapy is necessary in some cases but it can be complicated by antimicrobial resistance (AMR) of pathogens. Antibiotics have been used successfully in livestock breeding for growth promotion and therapeutics use. In this respect, chicken and cattle can harbor and transmit antimicrobial-resistant strains to human (4). It is demonstrated that antimicrobial resistance has closely related with presence of virulence factors in pathogens which connected to public health and risk of pathogens in food chain (1).
Standard conventional methods for detection of pathogens in food products is time consuming and require skilled staff. Therefore, using rapid and precise methods to detect foodborne pathogens are preferred in food industry (5). PCR has been established as one of the noteworthy and alternative method to the traditional procedures due to its rapidity, simplicity, sensitivity and cost-effectiveness and provides possibility to detect specific genes related to pathogen virulence (5–7). It has been shown that some molecular markers are more specific to detect pathogens. The uidA and hly genes have been shown to be very specific to E. coli and L. monocytogenes, respectively (8, 9). The uidA gene encodes the beta-glucuronidase enzyme and routinely used to assay β-D-glucuronidase activity and unequivocally identify E. coli (10). hly gene encoded Listeriolysin O, a pore-forming toxin protein considered as a virulence factor that is crucial for the virulence of L. monocytogenes (9). In this study, 16S-rRNA sequencing was performed to detect and classification of bacteria.
Several studies have been implemented on different food such as chicken meat and beef to determine molecular characterization and antibiotic resistance of pathogens throughout the world including Iran (11–13). Existent information about the distribution, variety of serotypes, AMR and genetically diversity of foodborne pathogens in contaminated foods including meat are rare in Iran. In this regard, this study aimed to evaluate occurrence of E. coli and L. monocytogenes contaminations and determination of their antimicrobial resistance (AMR) and genotyping profiles in fresh beef and chicken meats marketed in Zanjan city, Iran.
MATERIALS AND METHODS
Sample collection.
In this cross-sectional study, a total of 90 (45 chicken and 45 beef) fresh non-branded samples were collected according to randomized cluster sampling plan from January to June 2018 on retail meat stores of Zanjan city, Iran, and based on the number of the retail meat stores including butcher shops and supermarkets in each cluster in compare with previous studies. 500 g fresh meat sample was collected from each retail store and transported in a sterile plastic bag to the laboratory maintaining cold chain. All samples were processed as soon as possible otherwise preserved at 4°C. Normal physical attributes are mentioned during sampling including odor, color and density.
Isolation and identification of bacteria.
For E. coli, 10 grams of each sample was aseptically added to 90 ml of 0.1% buffered peptone water (BPW) (Merck, Darmstadt, Germany) and homogenized at room temperature for 2 min, at 200 rpm speed in a Stomacher blender (Bagmixer, Interscience, France) and serial dilution was prepared. 1 ml of each dilution was inoculated into MacConkey agar. After incubation at 37°C for 24 hr, the Red/pink colonies were considered as suspicious ones and transported to Eosin methylene blue agar (EMB) and incubated at 35°C for 24 hr (14). EMB is a selective and differential medium used to isolate coliforms like E. coli (15). The resulting colonies that produced the metallic green sheen isolated and stored in Brain Heart Infusion (BHI) broth which were incubated at 37°C for 24 hr. PCR was used for confirmation.
Listeria monocytogenes.
Preparation of originally homogenized suspension and serial dilutions performed such as E. coli. 1 ml of each dilution was inoculated into Listeria CHROM agar (DRG International, Inc., Springfield, USA) at 37°C for 24 hr. Then, Blue colonies with halos were selected and characterized by the Gram staining. After the confirmation, the confirmed colonies were transported to Brain Heart Infusion (BHI) broth tubes, which were incubated at 37°C for 24 hr (7). PCR assay used for confirmation.
Polymerase chain reaction assays.
Identification and verification of isolated strains of L. monocytogenes and E. coli by classic methods were performed using PCR and genotyping methods. Simplex PCR method was used for screening hly and uidA genes in L. monocytogenes and E. coli isolates, respectively according to described method of Karimiazar et al. 2019 with some modification (16). YTA DNA extraction kit (Yekta Tajhiz Azma, Tehran, Iran) was used for extraction of DNA from overnight culture of isolates based on kit protocol. Previously published studies were used for selection of primers listed in Table 1 (17, 18). Purified DNA from reference strains of L. monocytogenes (ATCC13932) and E. coli (ATCC 25922) was used as a template in PCR to ensure its specificity and testing primers. PCR mixture for identification of L. monocytogenes and E. coli isolates, based on hly and uidA, is showed in Table 1. PCR cycling conditions for both L. monocytogenes and E. coli isolates is showed in Table 2. PCR conditions for amplification of selected 16S-rRNA genes are summarized in Table 2.
Table 1.
Primers, PCR mixture for detection L. monocytogenes and E. coli isolates based on hly, uidA and 16S-rRNA genes
| Molecular marker | Sequence of nucleotides | Primers Size (bp) | Gene size (bp) |
|---|---|---|---|
| hly gene (L. monocytogenes) | F: GCAGTTGCAAGCGCTTGGAGTGAA | 24 | 495 |
| R: GCAACGTATCCTCCAGAGTGATCG | 24 | ||
| uidA gene (E. coli) | F: GCGAAAACTGTGGAATTGAT | 20 | 250 |
| R: GCGAAAACTGTGGAATTGGG | 20 | ||
| 16S-rRNA | F: AGAGTTTGATCCTGGCTCAG | 20 | 1450 |
| R: ACGGTACCTTGTTACGACTT | 20 |
| Composition | Stock | Content in final volume (20 µl) | Final concentration in a reaction |
|---|---|---|---|
| Master Mix | × 20 | 10 µL | 1× |
| Forward primer | 10 µM | µl 1 | 0.5 µM |
| Reverse primer | 10 µM | µl 1 | 0.5 µM |
| DDW* | - | µl 7 | - |
| DNA | - | µl 1 | - |
Deionized Distilled Water
Table 2.
The PCR cycling conditions for detection of E. coli and L. monocytogenes isolates based on uidA and hly genes, respectively and to detect both pathogens based on 16S-rRNA genes in this study
| The PCR cycling conditions for detection of E. coli and L. monocytogenes isolates based on uidA and hly genes | ||||||
|---|---|---|---|---|---|---|
| Steps | Temperature (ºC) | Time (Sec) | Cycles | |||
| E. coli | L. monocytogenes | E. coli | L. monocytogenes | E. coli | L. monocytogenes | |
| Denaturation | 95 | 94 | 30 | 60 | 34 | 35 |
| Annealing | 65 | 60 | 30 | 120 | ||
| Extension | 72 | 72 | 30 | 60 | ||
| Final extension | 72 | 72 | 300 | 420 | 1 | 1 |
| The PCR cycling conditions based on 16S-rRNA genes in this study to detect both pathogens | |||
|---|---|---|---|
| Stage | Temperature (ºC) | Time (s) Cycle | Cycle |
| Initial denaturation | 95 | 900 | 1 |
| Denaturation | 95 | 30 | 35 |
| Annealing | 66 | 30 | |
| Extension | 72 | 60 | |
| Final extension | 72 | 420 | 1 |
PCR products were detectable by electrophoresis on 1.5% agarose gel. To determine the size of PCR products, a 100 bp Ladder was used. Sequencing of 16S-rRNA gene is known as a basic step to identify and classification of bacteria (20). DNA fragments was verified at 1450 bp were considered for sequencing based on 16S-rRNA gene. The PCR mixture was same for amplification of all genes (Table 1).
To sequence the amplified genome of identified isolates, 20 μl of purified PCR products of 16S-rRNA gene and primers of both hly and uidA genes were sent to Macrogen inc. (South Korea). Received profiles of sequencing were analyzed by Mega 6.0 software and blasted in NCBI database to determine and comparing similarity with reported and existing strains in NCBI gene bank.
Antimicrobial susceptibility testing.
Disc diffusion method was used to determine AMR of the isolates using commercial antimicrobial susceptibility discs (Hi-Media, Mumbai, India) on Mueller Hinton Agar (Merck, Darmstadt, Germany) based on the guidelines of the Clinical and Laboratory Standards Institute (CLSI 2018-M100-S28). The used antibiotic discs were ciprofloxacin, crimethoprim/sulfame-thoxazole, tetracycline, streptomycin, vancomycin, carbenicillin, erythromycin, penicillin, cephalexin, cephalothin, gentamycin, chloramphenicol. The growth inhibition zone (GIZ) for each disk was measured after incubation at 37°C for 24 hr and evaluated according to the CLSI guidelines. The reference strains, L. monocytogenes (ATCC13932) and E. coli (ATCC 25922) were included as a quality controls.
Statistical analysis.
Microsoft Excel software was used to capture and sorting of obtained data and a simple descriptive statistic was performed to determine frequency and percentage using this software.
RESULTS
Prevalence in chicken and beef.
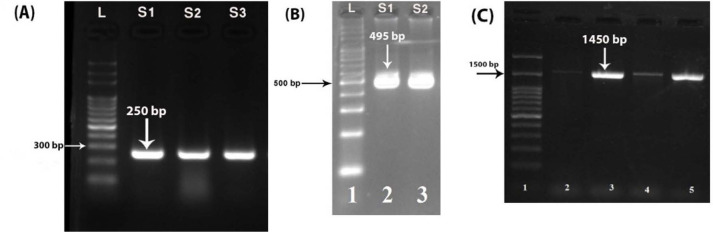
The prevalence of L. monocytogenes and E. coli isolated from examined samples was showed in Table 3. A single band at the expected size generated for each of the pathogen-specific primer sets (Fig. 1). According to Iran national standard, maximum allowed limit of E. coli in beef and chicken meat is 0 and 50 CFU/gr respectively. This limit for L. monocytogenes is 102 CFU/gr of beef and chicken meat samples (15, 19).
Table 3.
Prevalence of bacterial pathogens in meat samples
| Bacteria | Beef (%) | Chicken meat (%) | Total (%) |
|---|---|---|---|
| E. coli | 31 (68.89) | 40 (88.89) | 71 (78.94) |
| L. monocytogenes | 21 (46.67) | 24 (53.33) | 45 (50.20) |
Fig. 1.
Analysis of the PCR products in a 1.5% agarose gel. PCR products of target gene in: (A) E. coli, S1–S3 are positive samples. (B) Listeria monocytogenes; S1–S2 are positive samples. (C) PCR product of 16S-rRNA amplification. L: molecular mass marker (100 bp ladder)
Genotyping.
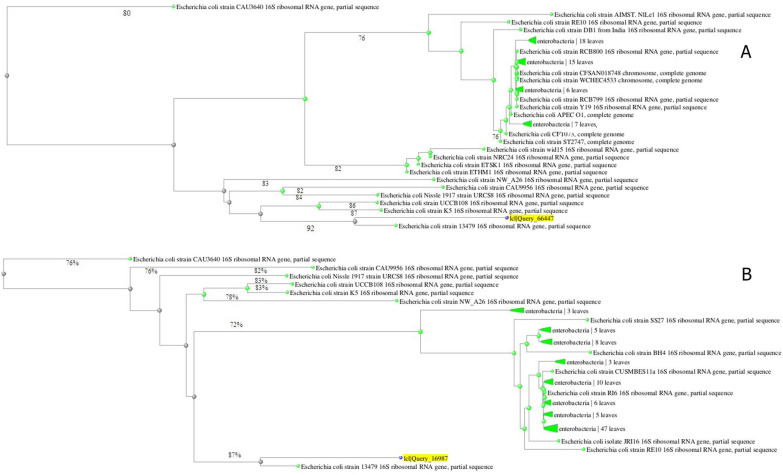
The PCR technique was able to verify and identification L. monocytogenes and E. coli isolates by amplify 250 bp and 495 bp fragment of the targeted gene (hly and uidA for L. monocytogenes and E. coli, respectively) from their genomic DNA, successfully. Screening of isolated bacteria was conducted by genus specific PCR (16S rRNA gene of L. monocytogenes and E. coli) and amplification of 1450 bp fragments of hly and uidA genes (Fig. 1). In general, sequencing of 16S-rRNA PCR products showed that all sequencing profiles in beef and chicken meat samples samples for both E. coli and L. monocytogenes isolates were similar. Neighbor-joining method and 16S rRNA sequence analyzing of E. coli in beef samples showed two main clusters in which clusters 1 and 2 were composed of one and 21 isolates, respectively (Fig. 2B). This phylogenetic relationship was in two clusters for thirteen isolates in chicken meat samples (Fig. 2A). The most likelihood of E. coli isolates in both beef and chiken meat belonged to E. coli 13479 serotype (Fig. 2).
Fig. 2.
Maximum likelihood phylogenetic tree of E. coli isolated from (A) Chicken meat and (B) Beef
16S rRNA sequence analyzing using neighbour-joining method for L. monocytogenes in beef and chicken meat samples showed two and one clusters with phylogenetic relationship of 3 and 21 isolates in beef and chicken meat samples, respectively (Fig. 3). All isolates of L. monocytogenes from beef showed high similarity with serotype L. monocytogenes strain NCTC10357 (Fig. 3B), whereas all isolated L. monocytogenes from chicken meat had high similarity with serotype L. monocytogenes strain MF4545 (Fig. 3A).
Fig. 3.
Maximum likelihood phylogenetic tree of L. monocytogenes isolated from (A) chicken meat and (B) beef
Antimicrobial resistance.
Antimicrobial resistance profile of E. coli and L. monocytogenes isolates are shown in Table 4. All isolates of E. coli were resistant to vancomycin and penicillin. In addition, most of them (96.6% and 87.5% of isolated strains from meat and chicken samples, respectively) were semi-sensitive to streptomycin and susceptible to other antibiotics. All of the isolated E. coli strains from meat and chicken had same antibiotic resistance profile (Table 4). All isolates of L. monocytogenes were resistant to trimethoprim/sulfamethoxazole, tetracycline, penicillin and gentamycin. In addition, some of these isolates were semi-sensitive to carbenicillin (71.4% and 91.7% of isolated strains from meat and chicken samples, respectively), erythromycin (33.3% and 37.5% of isolated strains from meat and chicken samples, respectively) and susceptible to other antibiotics (Table 4). All L. monocytogenes isolates from meat and chicken showed similar antibiotic resistance profile.
Table 4.
Antimicrobial resistance pattern of isolated E. coli and L. monocytogenes from beef /chicken meat samples
| No | Antibiotics | Concentration and diameter of inhibition zone | Isolates |
Sensitive No. (%) of isolates |
Intermediate No. (%) of isolates |
Resistance No. (%) of isolates |
|||
|---|---|---|---|---|---|---|---|---|---|
| beef | chicken | beef | chicken | beef | chicken | ||||
| 1 | Ciprofloxacin | 5 μg, ≥21 mm | E. coli | 28 (90.3) | 40 (100) | 3 (9.7) | 0 | 0 | 0 |
| L. monocytogenes | 19 (90.4) | 18 (75) | 2 (9.6) | 6 (25) | 0 | 0 | |||
| 2 | Trimethoprim/sulfamethoxazole | 1.25/23.75 µg, ≥16 mm | E. coli | 26 (83.6) | 35 (87.5) | 5 (16.4) | 5 (22.5) | 0 | 0 |
| L. monocytogenes | 0 | 0 | 0 | 2 (8.3) | 21 (100) | 22 (91.7) | |||
| 3 | Tetracycline | 30 μg≥15 mm | E. coli | 24 (77.4) | 30 (75) | 7 (23.6) | 10 (25) | 0 | 0 |
| L. monocytogenes | 0 | 0 | 0 | 2 (8.3) | 21 (100) | 22 (91.7) | |||
| 4 | Streptomycin | 10 μg, ≥15 mm | E. coli | 2 (3.4) | 5 (2.5) | 29 (96.6) | 35 (87.5) | 0 | 0 |
| L. monocytogenes | 18 (85.7) | 24 (100) | 3 (14.3) | 0 | 0 | 0 | |||
| 5 | Vancomycin | 30 μg, ≥14 mm | E. coli | 0 | 0 | 0 | 0 | 31 (100) | 40 (100) |
| L. monocytogenes | 21 (100) | 24 (100) | 0 | 0 | 0 | 0 | |||
| 6 | Carbenicillin | 100 μg, ≥17 mm | E. coli | 31 (100) | 40 (100) | 0 | 0 | 0 | 0 |
| L. monocytogenes | 0 | 0 | 15 (71.4) | 22 (91.7) | 6 (28.6) | 2 (8.3) | |||
| 7 | Erythromycin | 30 μg, ≥23 mm | E. coli | 21 (67.7) | 35 (87.5) | 10 (32.3) | 5 (22.5) | 0 | 0 |
| L. monocytogenes | 14 (66.7) | 15 (62.5) | 7 (33.3) | 9 (37.5) | 0 | 0 | |||
| 8 | Penicillin | 10 U, ≥21 mm | E. coli | 0 | 0 | 0 | 0 | 31 (100) | 40 (100) |
| L. monocytogenes | 0 | 0 | 0 | 0 | 21 (100) | 24 (100) | |||
| 9 | Cephalexin | 30 μg, ≥15 mm | E. coli | 30 (96.8) | 40 (100) | 1 (3.2) | 0 | 0 | 0 |
| L. monocytogenes | 21 (100) | 24 (100) | 0 | 0 | 0 | 0 | |||
| 10 | Cephalothin | 30 μg, ≥23 mm | E. coli | 28 (90.3) | 40 (100) | 3 (9.7) | 0 | 0 | 0 |
| L. monocytogenes | 21 (100) | 24 (100) | 0 | 0 | 0 | 0 | |||
| 11 | Gentamycin | 10 μg, ≥15 mm | E. coli | 26 (83.9) | 40 (100) | 5 (16.1) | 0 | 0 | 0 |
| L. monocytogenes | 0 | 0 | 0 | 0 | 21 (100) | 24 (100) | |||
| 12 | Chloramphenicol | 30 μg, ≥18 mm | E. coli | 31 (100) | 40 (100) | - | 0 | 0 | 0 |
| L. monocytogenes | 21 (100) | 24 (100) | 0 | 0 | 0 | 0 | |||
DISCUSSION
Escherichia coli.
According to this study, prevalence of E. coli was very high. Out of 90 samples about 79% were found to be positive for E. coli. The occurrence of E. coli in chicken (88.89%) was higher than in beef (68.89%). This prevalence in beef and chicken meat is very higher than reported occurrence in previous studies in Iran conducted by Momtaz and Jamshidi (2013) (34.59% in chicken meat) (20), Jafareyan-Sedigh and Doosti, 2011 (29.1% in beef) (8), Momtaz et al. (2013) (29.02% in ruminant’s meat) (21). Occurrence rate of E. coli strains in this study was inconsistent with that reported in other countries. In the United States E. coli was isolated from 38.7% of chicken meat, 19.0% of beef meat, and 16.3% of pork meat samples (7). Another study in Egypt showed 6.67 and 16.67% contamination rate of beef and chicken meat samples, respectively (22).
The antimicrobial susceptibility of E. coli isolates was demonstrated in Table 4. Antibiotic resistance in Gram-negative bacteria is on the rise in Iran, particularly in E. coli. Different patterns of antibiotic resistance were seen in various regions of Iran. More than 90% of E. coli isolates were resistant to penicillin (ampicillin or amoxicillin) in Tehran (capital) (23).
Generally, the Enterobacteriaceae are resistant to erythromycin, penicillin, ampicillin, streptomycin, tetracycline and kanamycin. AMR were lowest to chloramphenicol and gentamycin (31). E. coli is the most common producers of Extended-Spectrum Beta-Lactamases (ESBLs) (24). Presence of ESBLs enzymes compromises the efficacy of all β-lactams, except cephamycins and carbapenems, by hydrolysis of the β-lactam ring and play a major role in the inhibition of penicillin-binding protein targets (25). In this study, high contamination rate in beef and chicken meat samples indicates the low attention to health regulations during the processing, packaging, storage and distribution of meat products. On the other hand, the staff hands of the distribution and sales centers, as well as the water used to wash the slaughtered carcasses, may have been contaminated by E. coli.
Listeria monocytogenes.
Reports from Iran show until 2018 prevalence of L. monocytogenes in Iranian food samples was estimated to range from 0–50% (26). In this study, the overall prevalence of L. monocytogenes in chicken meat (53.33%) was higher than in beef (46.67%). Reported prevalence of Listeria in poultry products was lower in Iran (33.4%) (27). Prevalence of L. monocytogenes in raw meat samples were reported 41.9%, 8.5%, 11% and 8.5–19.8% from turkey, China, Bangladesh and Iran, respectively that is lower than obtained results in this study (28–31). The most prevalent serotypes of L. monocytogenes isolated from beef and chicken samples were 1/2a, 1/2b and 4b which is in accordance with reported document of Ranjbar and Halaji (2018) (26). Contamination rate depends on respecting to hygienic measures in processing by food handlers and the extent of cross-contamination (32). Several parameters allow this pathogen to reach to hazardous levels prior to consumption including growth ability at cold temperature, and desirable conditions at the retail level, during transport, and home storage (2).
Antimicrobial resistance pattern of L. monocytogenes isolates is shown in Table 4. According to our results, some of isolate was multidrug-resistant (≥3 antibiotics). These results are in agreement with previous studies (32, 33). Horizontal gene transfer between L. monocytogenes and commensal microorganisms found in food and obtaining various antibiotic resistance genes can be the main reason of increasing number and type of new antibiotic resistant strains of this bacterium reported worldwide (16). On the other hand, genetic mutation in the new strains is possible, too. AMR is a global concern of public health. Therefore, continuous surveillance and taking some preventive control measures about resistant bacteria are necessary.
Inappropriate, excessive dose, and extensive use of antibiotics in human and veterinary medicine are known as the main causes of antibiotic resistance. Existence of genetic elements such as integrons and transposons are often the main reason of multi-resistant phenotypes among bacteria (22). Conjugative transposons and mobile plasmids can be the reasons to multi drug resistance with the ability to identify and capturing carriers of related genes (mobile gene cassettes) (16).
Genotyping.
Rapid and cost effective methods play a prominent role to detect foodborne pathogens, particularly in the foods, immediately and are able to reduce human errors in their detection. However, rapid methods have some advantages and limitations. To accurate molecular detection of pathogens, choosing the specific gene amplicons is critical (10). In this study, hly and uidA genes were used to characterize L. monocytogenes and E. coli isolates but identification of species was based on sequencing of 16SrRNA gene. This method depends on matching level of the primers in different species which can lead to bias in the detection of species (34). The main limitation for application of 16S rRNA genes is alteration in gene sequence within a genome or closely related taxa and undetermined copy numbers in bacterial genomes. In present study, due to high diversity of closely related taxa and use of universal PCR primers, determination of precise identity is impossible and unexpected results can be occurred. Direct sequencing of 16S rRNA is the best way to overcome on this diversity (16, 34).
To better assessment of the prevalence and antibiotic resistance and decline potential hazards to public health of E. coli and L. monocytogenes, continuous and constant monitoring programs and comprehensive studies are needed. There are many issues have made it difficult to risk assessments of foodborne diseases in most countries including Iran such as the rare available epidemiological and quantitative data on occurrence of foodborne diseases and pathogens in the food chain. High rate contamination of meat and meat products, particularly chicken meat with foodborne pathogens remains a significant public health concern. Several issues such as geographic and regional features of animal feeding systems, various meat processing environments, sampling method, frequency and quantity of sampling, seasonal variation and etc. can impress on contamination rate (27). In conclusion, the results of this study revealed a high contamination rate with L. monocytogenes and E. coli in chicken and beef meat in Zanjan, Iran. Most of the isolates were antibiotic-resistant which can threaten public health in this area by consuming these products. Neighbor-joining method and 16S rRNA sequence analyzing of E. coli in beef and chicken meat samples showed that the most likelihood of E. coli isolates belonged to E. coli 13479 serotype. These methods for L. monocytogenes that all beef isolates had high similarity with serotype L.monocytogenes strain NCTC10357, whereas all isolated L. monocytogenes from chicken meat had high similarity with serotype L. monocytogenes strain MF4545.
ACKNOWLEDGEMENTS
Authors would like to thank Zanjan University of Medical Sciences for the financial support of this study (grant number A-11-940-13).
REFERENCES
- 1.El Garch F, De Jong A, Bertrand X, Hocquet D, Sauget M. mcr-1-like detection in commensal Escherichia coli and Salmonella spp. from food-producing animals at slaughter in Europe. Vet Microbiol 2018;213:42–46. [DOI] [PubMed] [Google Scholar]
- 2.Şanlıbaba P, Tezel BU, Çakmak GA. Prevalence and antibiotic resistance of Listeria monocytogenes isolated from ready-to-eat foods in Turkey. J Food Qual 2018;2018: 7693782. [Google Scholar]
- 3.Mitchell NM, Johnson JR, Johnston B, Curtiss R, Mellata M. Zoonotic potential of Escherichia coli isolates from retail chicken meat products and eggs. Appl Environ Microbiol 2015;81:1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dallal MMS, Doyle MP, Rezadehbashi M, Dabiri H, Sanaei M, Modarresi S, et al. Prevalence and antimicrobial resistance profiles of Salmonella serotypes, Campylobacter and Yersinia spp. isolated from retail chicken and beef, Tehran, Iran. Food Control 2010;21:388–392. [Google Scholar]
- 5.Sonnier JL, Karns JS, Lombard JE, Kopral CA, Haley BJ, Kim S-W, et al. Prevalence of Salmonella enterica, Listeria monocytogenes, and pathogenic Escherichia coli in bulk tank milk and milk filters from US dairy operations in the National Animal Health Monitoring System Dairy 2014 study. J Dairy Sci 2018;101:1943–1956. [DOI] [PubMed] [Google Scholar]
- 6.Campion A, Morrissey R, Field D, Cotter PD, Hill C, Ross RP. Use of enhanced nisin derivatives in combination with food-grade oils or citric acid to control Cronobacter sakazakii and Escherichia coli O157: H7. Food Microbiol 2017;65:254–263. [DOI] [PubMed] [Google Scholar]
- 7.Zhao C, Ge B, De Villena J, Sudler R, Yeh E, Zhao S, et al. Prevalence of Campylobacter spp., Escherichia coli, and Salmonella serovars in retail chicken, turkey, pork, and beef from the Greater Washington, DC, area. Appl Environ Microbiol 2001;67:5431–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godambe LP, Bandekar J, Shashidhar R. Species specific PCR based detection of Escherichia coli from Indian foods. 3 Biotech 2017; 7:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poimenidou SV, Dalmasso M, Papadimitriou K, Fox EM, Skandamis PN, Jordan K. Virulence gene sequencing highlights similarities and differences in sequences in Listeria monocytogenes serotype 1/2a and 4b strains of clinical and food origin from 3 different geographic locations. Front Microbiol 2018; 9: 1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molina F, López-Acedo E, Tabla R, Roa I, Gómez A, Rebollo JE. Improved detection of Escherichia coli and coliform bacteria by multiplex PCR. BMC Biotechnol 2015; 15:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kačániová M, Kluz M, Petrová J, Mellen M, Kunová S. Incidence of Listeria monocytogenes in meat product samples by real time PCR. Mod Chem Appl 2015;3:2. [Google Scholar]
- 12.Köppel R, Tolido I, Marti G, Peier M. Detection of DNA from Escherichia coli, Clostridium perfringens, Staphylococcus aureus and Bacillus cereus after simplified enrichment using a novel multiplex real-time PCR system. Eur Food Res Technol 2017;243:521–530. [Google Scholar]
- 13.Tao T, Chen Q, Bie X, Lu F, Lu Z. Investigation on prevalence of Listeria spp. and Listeria monocytogenes in animal-derived foods by multiplex PCR assay targeting novel genes. Food Control 2017;73:704–711. [Google Scholar]
- 14.ISIRI (Institute of Standards & Industrial Research of Iran) 2946, Microbiology of food and animal feeding stuffs-Detection and enumeration of presumptive Escherichia coli-Most probable number technique. 2011.
- 15.Safarpor dehkordi F, Yahaghi E, Darian E. Prevalence of antibiotic resistance in Escherichia coli isolated from poultry meat supply in Isfahan. Iran J Med Microbiol 2014; 8:41–47. [Google Scholar]
- 16.Karimiazar F, Soltanpour MS, Aminzare M, Hassanzadazar H. Prevalence, genotyping, serotyping, and antibiotic resistance of isolated Salmonella strains from industrial and local eggs in Iran. J Food Saf 2019;39(1):e12585. [Google Scholar]
- 17.Paziak-Domańska B, Bogusławska E, Więckowska-Szakiel M, Kotłowski R, Różalska B, Chmiela M, et al. Evaluation of the API test, phosphatidylinositol-specific phospholipase C activity and PCR method in identification of Listeria monocytogenes in meat foods. FEMS Microbiol Lett 1999;171:209–214. [DOI] [PubMed] [Google Scholar]
- 18.Wen Y, Wang L, Xu L, Li L, Ren S, Cao C, et al. Electrochemical detection of PCR amplicons of Escherichia coli genome based on DNA nanostructural probes and polyHRP enzyme. Analyst 2016;141:5304–5310. [DOI] [PubMed] [Google Scholar]
- 19.Solomakos N, Govaris A, Koidis P, Botsoglou N. The antimicrobial effect of thyme essential oil, nisin, and their combination against Listeria monocytogenes in minced beef during refrigerated storage. Food Microbiol 2008; 25:120–127. [DOI] [PubMed] [Google Scholar]
- 20.Momtaz H, Jamshidi A. Shiga toxin-producing Escherichia coli isolated from chicken meat in Iran: Serogroups, virulence factors, and antimicrobial resistance properties. Poult Sci 2013; 92: 1305–1313. [DOI] [PubMed] [Google Scholar]
- 21.Momtaz H, Safarpoor Dehkordi F, Rahimi E, Ezadi H, Arab R. Incidence of Shiga toxin-producing Escherichia coli serogroups in ruminant’s meat. Meat Sci 2013; 95: 381–388. [DOI] [PubMed] [Google Scholar]
- 22.Moawad AA, Hotzel H, Awad O, Tomaso H, Neubauer H, Hafez HM, et al. Occurrence of Salmonella enterica and Escherichia coli in raw chicken and beef meat in northern Egypt and dissemination of their antibiotic resistance markers. Gut Pathog 2017;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soltani J, Poorabbas B, Miri N, Mardaneh J. Health care associated infections, antibiotic resistance and clinical outcome: A surveillance study from Sanandaj, Iran. World J Clin Cases 2016;4:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilonzo-Nthenge A, Rotich E, Nahashon S. Evaluation of drug-resistant Enterobacteriaceae in retail poultry and beef. Poult Sci 2013;92:1098–1107. [DOI] [PubMed] [Google Scholar]
- 25.Tozzoli R, Maugliani A, Michelacci V, Minelli F, Caprioli A, Morabito S. Validation on milk and sprouts of EN ISO 16654: 2001-Microbiology of food and animal feeding stuffs-Horizontal method for the detection of Escherichia coli O157. Int J Food Microbiol 2019;288:53–57. [DOI] [PubMed] [Google Scholar]
- 26.Ranjbar R, Halaji M. Epidemiology of Listeria monocytogenes prevalence in foods, animals and human origin from Iran: a systematic review and meta-analysis. BMC Public Health 2018; 18:1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fallah A, Saei-Dehkordi, Rahnama M, Tahmasby H, Mahzounieh M. Prevalence and antimicrobial resistance patterns of Listeria species isolated from poultry products marketed in Iran. Food Control 2012;28: 372–332. [Google Scholar]
- 28.Arslan S, Baytur S. Prevalence and antimicrobial resistance of Listeria species and subtyping and virulence factors of Listeria monocytogenes from retail meat. J Food Saf 2018; 39(9):e12578. [Google Scholar]
- 29.Liu Y, Sun W, Sun T, Gorris LGM, Wang X, Liu B, et al. The prevalence of Listeria monocytogenes in meat products in China: A systematic literature review and novel meta-analysis approach. Int J Food Microbiol 2020; 312: 108358. [DOI] [PubMed] [Google Scholar]
- 30.Islam MS, Husna AA, Islam MA, Khatun MM. Prevalence of Listeria monocytogenes in Beef, Chevon and Chicken in Bangladesh. Am J Food Sci Health 2016; 2: 39–44. [Google Scholar]
- 31.Hamidiyan N, Salehi-Abargouei A, Rezaei Z, Dehghani Tafti R, Akrami-Mohajeri F. The prevalence of Listeria spp. food contamination in Iran: A systematic review and meta-analysis. Food Res Int 2018; 107:437–450. [DOI] [PubMed] [Google Scholar]
- 32.Maktabi S, Pourmehdi M, Zarei M, Moalemian R. Occurrence and antibiotic resistance of Listeria monocytogenes in retail minced beef distributed in Ahvaz, South-West of Iran. J Food Qual Hazards Control 2015;2:101–106. [Google Scholar]
- 33.Pesavento G, Ducci B, Nieri D, Comodo N, Nostro AL. Prevalence and antibiotic susceptibility of Listeria spp. isolated from raw meat and retail foods. Food Control 2010;21:708–713. [Google Scholar]
- 34.Rosselli R, Romoli O, Vitulo N, Vezzi A, Campanaro S, De Pascale F, et al. Direct 16S rRNA-seq from bacterial communities: a PCR-independent approach to simultaneously assess microbial diversity and functional activity potential of each taxon. Sci Rep 2016;6:32165. [DOI] [PMC free article] [PubMed] [Google Scholar]