Abstract
Background and Objectives:
Arcobacter species are food-borne and zoonotic enteropathogens. Defined breakpoints for the investigation of antimicrobial resistance of Arcobacter are missing.
Materials and Methods:
The study was performed to investigate the incidence and antimicrobial resistance of Arcobacter species in animals and poultry meat samples procured from slaughterhouses in Iran. To investigate the prevalence of antimicrobial resistance, samples were collected from cattle (n=100), sheep (n=100), goat (n=100), broiler chicken (n=100), turkey (n=100) and quail (n=100). Arcobacter isolates of meat samples were isolated, investigated by PCR method and antibiotic resistance was also investigated. The susceptibility was assessed by Kirby-Bauer disc diffusion.
Results:
The results showed that 52 samples (8.66%) were positive for Arcobacter spp. The most prevalence were observed in broiler chickens (26%, n=26 samples), quail (13%, n=13 samples), turkey (8%, n=8), cattle (3%, n=3), sheep (1%, n=1) and goat (1%, n=1). Arcobacter butzleri had highest prevalence among Arcobacter species. All the isolates showed sensitivity to gentamicin, streptomycin and tetracycline.
Conclusion:
Poultry meat is a potential source of infection with Arcobacter that must be considered in slaughterhouses in Iran. Arcobacter species showed sensitivity for a broad spectrum of antibiotics that can be used during infection with Arcobacter species.
Keywords: Arcobacter, Broiler chicks, Iran, Prevalence, Antimicrobial susceptibility
INTRODUCTION
Arcobacters are foodborne and zoonotic enteropathogens and known as food-borne enteropathogens (1). Arcobacter species are commonly isolated from animal source foods (2). They cause bacteraemia, endocarditis, peritonitis, gastroenteritis in human, and diarrhea in both animals and humans (3). Arcobacter genus belongs to Campylobacteraceae family, the class Epsilonproteobacteria of the phylum Proteobacteria (4). Arcobacter species of A. butzleri, A. cryaerophilus, A. thereius, and A. skirrowii infect hosts and habitats and are mainly transmitted by water routes (5), such as rivers and lakes (6), drinking water (7), groundwater and recreational water (8). Infections induced by A. butzleri are mainly transmitted by water routes (9).
Slaughterhouses are sources for spreading disease (5). Arcobacters are commonly isolated from healthy cattle, sheep and pigs (3). Infections induced by Arcobacter species commonly occur during slaughter process (10). Increased trade of meat products between developing and industrialized countries increases the risk of the animal-associated pathogens in all over world (11). It is a major challenge in countries without surveillance systems and/or sites that pathogens can cause contamination.
Identification of Arcobacter by different biochemical tests is difficult as these organisms are metabolically inert (8). Culture media was previously used for diagnosis of Arcobacter. Cultural differentiation is difficult between Arcobacter and Campylobacter due to their phenotypic similarity. Molecular methods are useful for diagnosis of Arcobacter. PCR is a common and rapid technique to investigate Arcobacter in meat samples. Several antibacterial drugs are used for the treatment of infections with Arcobacter, such as erythromycin, fluoroquinolones and ciprofloxacin (13). Utilization of antibiotics has faced with forbidden, because they cause antimicrobial resistance. It was reported A. butzleri as more resistant compared to A. cryophilus and A. skirrowi (14, 15).
Iranian people raise animals and poultry for meat consumption. Shirzad Aski et al. (16) showed the prevalence of the Arcobacter spp. was 9% for slaughterhouse samples. With regards to Arcobacter species, their contamination rate in animals meat, and providing an effective strategies for alleviation of the both the infections induced by Arcobacter species in humans and animals, this study investigates the incidence and antimicrobial resistance of Arcobacter species in animal and poultry meat samples at slaughterhouses in Iran.
MATERIALS AND METHODS
Samples.
Six-hundred samples were collected from cattle (n=100), sheep (n=100), goat (n=100), broiler chicken (n=100), turkey (n=100) and quail (n=100) during 2019 year. The samples were immediately transferred to laboratory. The samples in weight of 50 g were collected from different parts of animal meat at slaughterhouse and five samples were collected for each animal.
Isolation and identification of Arcobacter species.
Arcobacter species were isolated as reported by Maruyama et al. (17). To identify and differentiate of Arcobacter species, DNA was extracted from meat samples using DNA kit as reported by Cinnagen Company (Tehran-Iran). The used primers were as follows;
(Arcobacter): TTCGCTTGCGCTGCATCAT
(A. butzleri): AGCGTTCTATTCAGCGTAGAAGATGT
(A. cryaerophilus): ACCGAAGCTTTAGATTCGAATTTATTCA
(A. skirrowii): CGAGGTCACGGATGGAAGTG
Amplification was conducted in a thermal cycler (Master Cycle Gradient, Eppendorf, Germany) as follows: initial denaturation and denaturation at 94°C, and annealing at 64°C. The final elongation was conducted at 72°C for 7 min. The quality and quantity of extracted DNA was determined by measurement of the concentration. Test method and thermal program were as reported by Son et al. (18). Purity was investigated by a UV spectrophotometer (NanoDrop™ 1000). Absorption was investigated at 260 nm (A260) and 280 nm (A280). DNA purities were calculated through calculating the A260/A280 ratios. Samples containing A260/A280 ratios of 1.7–2.0 were considered as pure samples, free from protein and other impurities (19). The samples were held at 4°C until the PCR products were analyzed. Agarose gel 1.5% was used to trace the products. The amplified DNA products were electrophoresed on 1.5% agarose gels at 90 V for 6 h using 1× TBE (0.89 M Tris borate, 0.02 M EDTA, pH 8.3) as the running buffer, and then stained with ethidium bromide. Gels were visualized using a UV gel documentation system. A set of molecular weight standards was included on each gel. DNA samples from reference strains were considered as positive controls. Negative controls in that DNA was replaced with sterile distilled water were included in all assays. Negative controls were prepared from American Type Culture Collection. Species were identified by 16SrRNA using specific described primers. Product size was 298 bp, 214 bp, and 421 bp for A. butzleri, A. cryaerophilus and A. skirrowii, respectively.
Antibiotic resistance.
Kirby–Bauer disc methods was used for assessing antimicrobial susceptibility testing in Mueller-Hinton agar (HiMedia Laboratories, Mumbai, India, MV1084) enriched with 5% defibrinated sheep blood as reported by Rahimi (19). Arcobacter isolates of meat samples were investigated for antibiotic resistance for chloramphenicol (30 µg), erythromycin (15 µg), gentamicin (10 µg), penicillin (10 µg), streptomycin (30 µg), tetracycline (15 µg), azithromycin (10 µg), and nalidixic Acid (30 µg) by disc method as reported by rahimi (19).
Statistical analysis.
The data were analyzed by Chi-square and Fisher tests using SPSS software. The data were reported as mean and frequency (%).
RESULTS
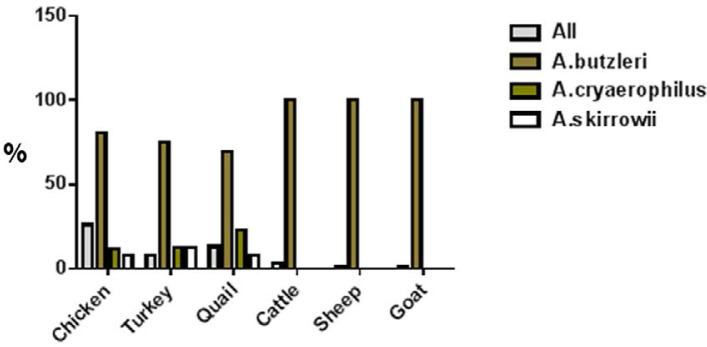
The results for prevalence of Arcobacter species in animals and poultry are shown in Table 1. The results showed that 52 samples (8.66%) were positive for Arcobacter spp. The most prevalence were observed in broiler chickens (26%, n=26), quail (13%, n=13), turkey (8%, n=8), cattle (3%, n=3), sheep (1%, n=1) and goat (1%, n=1). In all positive samples, A. butzleri species were positive (Fig. 1). Fig. 2 shows electrophoresis gel for the samples.
Table 1.
Prevalence of Arcobacter species in animal and poultry meat samples
| Arcobacter species | |||||
|---|---|---|---|---|---|
| Samples | Number | Positive samples | A. butzleri | A. cryaerophilus | A. skirrowii |
| Broiler | 100 | 26 (26.00%) | 21 (80.76%) | 3 (11.53%) | 2 (7.69%) |
| Turkey | 100 | 8 (8.00%) | 6 (75.00%) | 1 (12.50%) | 1 (12.50%) |
| Quail | 100 | 13 (13.00%) | 9 (69.23%) | 3 (23.07%) | 1 (7.69%) |
| Cattle | 100 | 3 (3.00%) | 3 (100%) | - | - |
| Sheep | 100 | 1 (1.00%) | 1 (100%) | - | - |
| Goat | 100 | 1 (1.00%) | 1 (100%) | - | - |
Fig. 1.
Arcobacter species in animal and poultry meat samples
Fig. 2.
Agarose gel electrophoresis of A. butzleri (A), A. cryaerophilus (B) and A. skirrowii (C). Lane M: Trackit 100 bp ladder
The results for antimicrobial resistance are shown in Table 2. The results showed that all the species were sensitive to gentamicin, streptomycin and tetracycline. Higher resistance rate were observed for chloramphenicol (41/52=78.84%), azithromycin (41/52=78.84%) and clindamycin (36/52=69.23%). Among species, A. butzleri showed high resistance to chloramphenicol (36/41=87.80%) and azithromycin (32/41=78.04%). A. cryaerophilus showed highest resistance to azithromycin (6/7=85.71%), clindamycin (5/7=71.42%) and erythromycin (5/7=71.42%). However, A. skirrowii showed the highest resistance to Azithromycin (3/4=75.00%).
Table 2.
Antibiotic resistance of Arcobacter species isolated from birds
| Antibiotics | Arcobacter (n=52) | A. butzleri (n=41) | A. cryaerophilus (n=7) | A. skirrowii (n=4) |
|---|---|---|---|---|
| Gentamicin | - | - | - | - |
| Chloramphenicol | 41 (78.84%) | 36 (87.80%) | 3 (42.85%) | 2 (50.00%) |
| Clindamycin | 36 (69.23%) | 29 (70.73%) | 5 (71.42%) | 2 (50.00%) |
| Erythromycin | 25 (48.07%) | 20 (48.78%) | 5 (71.42%) | - |
| Streptomycin | - | - | - | - |
| Tetracycline | - | - | - | - |
| Azithromycin | 41 (78.84%) | 32 (78.04%) | 6 (85.71%) | 3 (75.00%) |
| Nalidixic Acid | 30 (57.69%) | 26 (63.41%) | 2 (28.57%) | 2 (50.00%) |
DISCUSSION
Arcobacter prevalence is increasing in food and most species show antimicrobial resistance. Increased in the prevalence and resistance pushed us to conduct this study. This study investigated the incidence and antimicrobial resistance of Arcobacter species in animal and poultry meat samples at slaughterhouses in Iran. The results showed that 52 samples (8.66%) were positive for Arcobacter spp. The most prevalence were observed in broiler chickens (26%, n=26 samples), quail (13%, n=13 samples), turkey (8%, n=8), cattle (3%, n=3), sheep (1%, n=1) and goat (1%, n=1). In contrast to our findings, Shirzad Aski et al. (16) investigated the incidence of Arcobacter spp. in animal meat samples at slaughterhouses in Southern Iran and reported 9% and 14% positive samples for cattle and sheep, respectively. The most prevalence was observed for broiler chicks meat samples. Similar to our findings, Atabay et al. (20) reported a significant contamination (65.3%) for broiler chickens. These findings confirm that broiler chicks are sensitive to Arcobacter species. The results showed 36.25% contamination in samples procured from turkey meat. Aydin et al. (21) reported rates of 68% and 4% for contamination of chicken and turkey meat samples, respectively. Bogantes et al. (22) reported prevalence rate to be 36% in duck meat sample which is lower than the present study (54%). In the current study, in the animals, prevalence rate was very low that may be attributed to geographical condition and animal types that influence bacterial growths. Merga et al. (23, 24) reported the incidence of 43% and 40% from feces of cattle and sheep in the United Kingdom. A difference between our findings and Merga et al. findings could be attributed to sampling types (meat vs feces). The results also showed that in all the positive samples, A. butzleri species were positive. Similar to our findings previous studies also have reported A. butzleri as most prevalent species (7, 26, 27). A. butzleri is a prevalent organism in dairy farms compared with other Arcobacter species, because it survives in different environmental conditions (27). Badilla-Ramırez et al. (28) reported that A. butzleri could grow at 4°C and 10°C. It was reported that A. butzleri species could survive in different storage temperatures, and they contaminate poultry meat samples (29). Our findings for contamination with A. butzleri, A. skirrowii and A. cryaerophilus that are parallel with the results reported by Verma et al. (30). The results showed that Arcobacter species were sensitive to gentamicin, streptomycin and tetracycline. The data for antimicrobial resistance are important, because the agents can be used as first-line drugs for the treatment of infection induced by Arcobacter (31). The results showed that Arcobacter species showed sensitivity to tetracycline. Yesilmen et al. (26) showed that the acquired resistance for Arcobacter species against tetracycline and ampicillin antibiotics. Several studies have reported high susceptibility of Arcobacter species to tetracycline (14, 20, 32). Shah et al. (33) reported that Arcobacter species are susceptible to gentamicin. Unver et al. (34) showed that A. skirrowii and most of the A. cryaerophilus isolates were resistant to chloramphenicol, erythromycin, and amoxicillin. Our results for the effect of tetracycline on Arcobacter species are consistent with previous results that showed Arcobacter species are susceptible to tetracycline (19). In sum, the results suggest that gentamicin, streptomycin and tetracycline are efficient agents for the treatment of infections induced by Arcobacter spp. It means that some antibiotics do not kill them, due to bacterial structure.
In conclusion, poultry meat was more sensitive to Arcobacter species that might be attributed to nutrient nature of poultry meat samples. In all the positive samples, A. butzleri species were positive. Arcobacter species were sensitive to tetracycline. Since high levels of contamination with Arcobacter spp. can occur in poultry slaughterhouses, considering hygiene in slaughterhouses is an essential principle for alleviating the risk of contamination and the use of tetracycline as an efficient agent for the treatment of contamination with Arcobacter species is suggested.
ACKNOWLEDGEMENTS
This study was conducted in the form of an internal study opportunity at Islamic Azad University Sanandaj Branch (Kurdistan, Iran). The authors thank the Dr. Ali Olfati for all efforts to improve the written language of the paper.
REFERENCES
- 1.Çelik C, Ikiz S. The investigation of the presence and antimicrobial profiles of Arcobacter species in sheep carcasses and feces. Acta Vet Eurasia 2019; 45:42–49. [Google Scholar]
- 2.Aydin F, Yağiz A, Abay S. Prevalence of Arcobacter and Campylobacter in beef meat samples and characterization of the recovered isolates. J Consum Prot Food Saf 2020; 15:15–25. [Google Scholar]
- 3.Ferreira S, Queiroz JA, Oleastro M, Domingues F. Insights in the pathogenesis and resistance of Arcobacter: a review. Crit Rev Microbiol 2016; 42:364–383. [DOI] [PubMed] [Google Scholar]
- 4.Fanelli F, Pinto AD, Mottola A, Mule G, Chieffi D, Baruzzi F, et al. Genomic characterization of Arcobacter butzleri isolated from shellfish: novel insight into antibiotic resistance and virulence determinants. Front Microbiol 2019; 10: 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collado L, Figueras MJ. Taxonomy, epidemiology, and clinical relevance of the genus Arcobacter. Clin Microbiol Rev 2011; 24:174–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park S, Jung YT, Kim S, Yoon JH. Arcobacter acticola sp. Nov isolated from seawater on the east sea in south Korea. J Microbiol 2016; 54:655–659. [DOI] [PubMed] [Google Scholar]
- 7.Chieffi D, Fanelli F, Fusco V. Arcobacter butzleri: Up-to-date taxonomy, ecology, and pathogenicity of an emerging pathogen. Compr Rev Food Sci Food Saf 2020; 19:2071–2109. [DOI] [PubMed] [Google Scholar]
- 8.Collado L, Levican A, Perez J, Figueras MJ. Arcobacter defluvii sp. nov., isolated from sewage samples. Int J Syst Evol Microbiol 2011; 61:2155–2161. [DOI] [PubMed] [Google Scholar]
- 9.Lee C, Agidi S, Marion JW, Lee J. Arcobacter in Lake Erie beach waters: an emerging gastrointestinal pathogen linked with human-associated fecal contamination. Appl Environ Microbiol 2012; 78:5511–5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Smet S, De Zutter L, Van Hende J, Houf K. Arcobacter contamination on pre- and post-chilled bovine carcasses and in minced beef at retail. J Appl Microbiol 2010; 108:299–305. [DOI] [PubMed] [Google Scholar]
- 11.Van Driessche E, Houf K, Van Hoof J, De Zutter L, Vandamme P. Isolation of Arcobacter species from animal feces. FEMS Microbiol Lett 2003; 229:243–248. [DOI] [PubMed] [Google Scholar]
- 12.Dekker DD, Eibach KG, Boahen C, Wiafe Akenten Y, Pfeifer AE, Zautner E, et al. Fluoroquinolone-resistant Salmonella enterica, Campylobacter spp., and Arcobacter butzleri from local and imported poultry meat in Kumasi, Ghana. Foodborne Pathog Dis 2019; 16: 352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skirrow MB. (2000). Clinical aspects of Campylobacter infection, in: Nachamkin I., Blaser M.J. (Eds.), Campylobacter, second ed., ASM Press, Washington, DC: 69–88. [Google Scholar]
- 14.Kabeya H, Maruyama S, Morita Y, Ohsuga T, Ozawa S, Kobayashi Y, et al. Prevalence of Arcobacter species in retail meats and antimicrobial susceptibility of the isolates in Japan. Int J Food Microbiol 2004; 90:303–308. [DOI] [PubMed] [Google Scholar]
- 15.Abay S, Kayman T, Hizlisoy H, Aydin F. In vitro anti-bacterial susceptibility of Arcobacter butzleri isolated from different sources. J Vet Med Sci 2012; 74:613–616. [DOI] [PubMed] [Google Scholar]
- 16.Shirzad Aski H, Tabatabaei M, Khoshbakht R, Raeisi M. Occurrence and antimicrobial resistance of emergent Arcobacter spp. isolated from cattle and sheep in Iran. Comp Immunol Microbiol Infect Dis 2016; 44:37–40. [DOI] [PubMed] [Google Scholar]
- 17.Kabeya H, Maruyama S, Morita Y, Ohsuga T, Ozawa S, Kobayashi Y, et al. Prevalence of Arcobacter species in retail meats and antimicrobial susceptibility of the isolates in Japan. Int J Food Microbiol 2004;90:303–308. [DOI] [PubMed] [Google Scholar]
- 18.Son I, Englen MD, Berrang ME, Fedorka-Cray PJ, Harrison MA. Prevalence of Arcobacter and Campylobacter on broiler carcasses during processing. Int J Food Microbiol 2007; 113:16–22. [DOI] [PubMed] [Google Scholar]
- 19.Rahimi E. Prevalence and antimicrobial resistance of Arcobacter species isolated from poultry meat in Iran. Br Poult Sci 2014; 55:174–180. [DOI] [PubMed] [Google Scholar]
- 20.Atabay HI, Aydin F. Susceptibility of Arcobacter butzleri isolates to 23 antimicrobial agents. Lett Appl Microbiol 2001; 33:430–433. [DOI] [PubMed] [Google Scholar]
- 21.Aydin F, Gümüşsoy KS, Atabay HI, Iça T, Abay S. Prevalence and distribution of Arcobacter species in various sources in Turkey and molecular analysis of isolated strains by ERIC-PCR. J Appl Microbiol 2007; 103:27–35. [DOI] [PubMed] [Google Scholar]
- 22.Bogantes EV, Fallas-Padilla KL, Rodriguez-Rodriguez CE, Jaramillo HF, Echandi MLA. Zoonotic species of the genus Arcobacter in poultry from different regions of Costa Rica. J Food Prot 2015; 78:808–811. [DOI] [PubMed] [Google Scholar]
- 23.Merga JY, Leatherbarrow A, Winstanley C, Bennett M, Hart CA, Miller WG, et al. Comparison of Arcobacter isolation methods, and diversity of Arcobacter spp. in Cheshire, United Kingdom. Appl Environ Microbiol 2011; 77:1646–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merga JY, Williams NJ, William G, Miller WG, Leatherbarrow AJH, Bennett M, et al. Exploring the diversity of Arcobacter butzleri from cattle in the UK using MLST and whole genome sequencing. PLoS One 2013; 8(2): e55240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levican A, Alkeskas A, G€unter C, Forsythe SJ, Figueras MJ. Adherence to and invasion of human intestinal cells by Arcobacter species and their virulence genotypes. Appl Environ Microbiol 2013; 79:4951–4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yesilmen S, Vural A, Erkan ME, Yildirim IH. Prevalence and antimicrobial susceptibility of Arcobacter species in cow milk, water buffalo milk and fresh village cheese. Int J Food Microbiol 2014; 188:11–14. [DOI] [PubMed] [Google Scholar]
- 27.Giacometti F, Lucchi A, Di Francesco A, Delogu M, Grilli E, Guarniero I, et al. Arcobacter butzleri, Arcobacter cryaerophilus and Arcobacter skirrowii circulation in a dairy farm and sources of milk contamination. Appl Environ Microbiol 2015; 81:5055–5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badilla-Ramırez Y, Fallas-Padilla KL, Fernandez-Jaramillo H, Arias-Echandi ML. Survival capacity of Arcobacter butzleri inoculated in poultry meat at two different refrigeration temperatures. Rev Inst Med Trop Sao Paulo 2016; 58:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramees K, Kumaragurubaran K, Ramswaroop S, Ashok K, Mani Sa, Ruchi T, et al. Arcobacter: an emerging food-borne zoonotic pathogen, its public health concerns and advances in diagnosis and control-a comprehensive review. Vet Q 2017; 37:136–161. [DOI] [PubMed] [Google Scholar]
- 30.Verma M, Joshi N, Rathore RS, Mohan HV. Detection of Arcobacter spp. in poultry, pigs, their meat and environmental samples by conventional and PCR assays. Indian J Anim Sci 2015; 85:954–957. [Google Scholar]
- 31.Vandenberg O, Houf K, Douat N, Vlaes L, Retore P, Butzler JP. Antimicrobial susceptibility of clinical isolates of non-jejuni/coli campylobacters and Arcobacters from Belgium. J Antimicrob Chemother 2006; 57:908–913. [DOI] [PubMed] [Google Scholar]
- 32.Zacharow I, Bystron J, Waecka-Zacharska E, Podkowik M, Bania J. Prevalence and antimicrobial resistance of Arcobacter butzleri and Arcobacter cryaerophilus isolates from retail meat in Lower Silesia region. Pol J Vet Sci 2015; 18:63–69. [DOI] [PubMed] [Google Scholar]
- 33.Shah AH, Saleha AA, Zunita Z, Murugaiyah M, Aliyu AB, Jafri N. Prevalence, distribution and antibiotic resistance of emergent Arcobacter spp. from clinically healthy cattle and goats. Transbound Emerg Dis 2013; 60:9–16. [DOI] [PubMed] [Google Scholar]
- 34.Unver A, Atabay HI, Sahin M, Celebi €O. Antimicrobial susceptibilities of various Arcobacter species. Turk J Med Sci 2013; 43:548–552. [Google Scholar]