Abstract
Background and Objectives:
Essential oils (EOs) with different biological activities, such as antibacterial properties, are a valuable resource for developing new drugs.
Materials and Methods:
Ingredients of six medicinally important EOs, including Artemisia dracunculus, Anethum graveolens, Citrus limon, Citrus sinensis, Cinnamomum zeylanicum and Zingiber officinale, were identified using GC-MS analysis. Moreover, their five major compounds were also listed. Furthermore, the half-maximal inhibitory concentration (IC50) against four important human bacteria was also investigated using the 96-well plate microdilution.
Results:
C. sinensis EO with IC50 of 1.0 and 4.7 mg.mL–1 have the most effect on the growth of S. aureus and P. aeruginosa. Moreover, EOs of Cinnamomum zeylanicum (IC50: 1.0 mg. Ml–1) and Artemisia dracunculus (IC50: 1.3 mg.mL–1) significantly showed better inhibitory effect on E. coli and K. pneumoniae.
Conclusion:
These EOs could be used for developing inexpensive, potent, and green antibacterial agents.
Keywords: Essential oil, Antibacterial activity, Pathogens, Microdilution
INTRODUCTION
Essential oils (EOs) are a concentrated mixture of hydrophobic compounds in the oil phase, characterized by a strong odor (1). They are secreted as secondary metabolites from different parts of aromatic plants, such as flowers, fruits, seeds, stems, and roots (2). Hydrodistillation using the Clevenger type apparatus is the most common approach for the extraction of EOs (3). Recently, a growing number of studies on different medical properties of EOs have been being performed (4). For example, as flavorings in the food (5), larvicidal activity (6), anticancer drug discovery (7), antioxidant properties (8), and antifungal bioassays (9). In addition to such uses, EOs possess antibacterial effects against human pathogens such as Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Klebsiella pneumoniae (10, 11).
In the past, antibacterial properties were mainly reported by minimum inhibitory concentration (MIC) which is described as the lowest concentration of an agent to prevent bacterial visible growth (12). For instance, the MIC of Artemisia dracunculus EO on S. aureus was 62.4 mg.mL–1 (13). Besides, Anethum graveolens EO showed a good antibacterial effect on E. coli with MIC of 2.5 mg.mL–1 (14). However, by developing optical density (OD) dependent technics, the growth of microorganisms was observed as turbidity, determined by analytical instruments (15). By investigating the antibacterial activity of active agents at various concentrations and using software such as CalcuSyn, half-maximal inhibitory concentration (IC50) is measurable. This value is defined as observing a 50% decrease in bacterial growth in the treated sample compared to the control group. It is a reliable and quantitative unit with upper and lower confidence limits (16).
In this study, ingredient and antibacterial activities of six EOs, including Artemisia dracunculus (ADEO), Anethum graveolens (AGEO), Citrus limon (CLEO), Citrus sinensis (CSEO), Cinnamomum zeylanicum (CZEO), and Zingiber officinale (ZOEO) were investigated. Then for the first time, their IC50s were calculated.
MATERIALS AND METHODS
Materials.
Standard species of bacteria, including S. aureus (ATCC 25923), E. coli (ATCC 25922), P. aeruginosa (ATCC 27853), and K. pneumoniae (ATCC 13883) were provided by the laboratory of microbiology, Fasa University of Medical Sciences (FUMS). ADEO was bought from Zardband Pharmaceutical co, Iran. Barij Essence Pharmaceutical Co, Iran, provided AGEO and CLEO. Moreover, Green Plants of Life Co. Ltd, Iran, supplied CSEO, CZEO, and ZOEO. Muller Hinton Broth (Bacterial culture media) was purchased from Merck Chemicals, Germany.
The procedure of GC-MS analysis.
For the identification of ingredients of the EOs, GC-MS analysis was used. Briefly, The GC-MS analyses were performed using a 7890A Network GC system coupled with 5975C VL MSD with Triple-Axis, mass selective detector (Agilent Technologies, Santa Clara, CA, USA). The separation of the components of the EOs was carried out on HP-5MS silica fused columns (30 m length; 0.25 mm i.d.; and 25 µM film thickness). The GC-MS column temp was programmed as follows: the initial temp was set at 40°C and fixed for 1 min, then increased with the rate of 3°C.min–1 to the final temperature of 250°C and held for 20 min. Temperature of the injection port and detector fixed at 250 and 230°C, respectively. Other instrument parameters were set as split flow: 100 mL.min–1 and column flow rate: 1 mL.min–1. Helium gas with a purity of 99.99% was used as the carrier gas. The EOs components were identified using the method described in our previous report (17).
Evaluation of the antibacterial activity of EOs.
96-well plate microdilution method was used for determining the growth inhibitory effect of EOs against target bacteria with slight modification (15, 18). New cultured bacterial colonies (overnight culture) were suspended in Muller Hinton broth to reach 1.5 × 108 CFU/mL to reach the level of 0.5 McFarland turbidity. Then 20 µL of the bacterial suspension was added to each well using an 8-channel pipette.
A serial dilution of each EO was prepared by dissolving in Muller Hinton Broth (containing 0.5% DMSO) in a concentration range of 10.00–0.39 mg.mL–1. By the addition of 80 µL form serial dilutions to each well, the concentration of EOs eventually fixed at 8.00, 4.00, 2.00, 1.00, 0.50, 0.25, 0.13, 0.06, and 0.03 mg.mL–1. Three control wells were considered in each plate, filled with 20 and 80 µL of the bacteria suspension and the Muller Hinton Broth (containing DMSO 0.5%). Treated plates were then incubated at 37ºC for 24 h. The turbidity of each well was read at 630 nm by a plate reader (Synergy HTX Multi-Mode Reader, USA), and the growth of bacteria was calculated using Equation 1.
| Equation 1 |
Statistical methods.
Antibacterial tests were performed in triplicates. For calculation of means, standard deviations, and drawing charts, Excel software (Version 2010, Microsoft Corporation, USA) was used. IC50 of the EOs was calculated using CalcuSyn software (Free version, BIOSOFT, UK). For comparing determined IC50 of the EOs together, independent sample t-test and one-way ANOVA using SPSS software (Version 22, SPSS Inc, USA) were performed. In this study, a confidence interval of 95% (CI 95%) was considered.
RESULTS
GC-MS analysis.
The five major constituents of each EO with their retention times and retention indices are listed in Table 1. The most abundant components for EOs were as follow; ADEO: p-allylanisole (67.62%), AGEO: p-cymene (20.81%) and α.phellandrene (20.75%), CLEO: limonene (61.83%), CSEO: limonene (71.26%), CZEO: cinnamaldehyde (62.04%), and ZOEO: zingiberene (30.28%).
Table 1.
Identified components in the EOs using GC-MS analysis
| EOs | Major components | aRT | bRI | % |
|---|---|---|---|---|
| ADEO | Limonene | 10.73 | 673.23 | 4.34 |
| cis-Ocimene | 11.32 | 696.48 | 8.69 | |
| β-Ocimene Y | 11.90 | 712.26 | 7.58 | |
| p-Allylanisole | 19.18 | 876.22 | 67.62 | |
| 3-Methoxycinnamaldehyde | 34.25 | 1166.13 | 1.49 | |
| AGEO | α-Phellandrene | 9.73 | 634.08 | 20.75 |
| p-Cymene | 10.80 | 675.94 | 20.81 | |
| Dill ether | 17.38 | 839.99 | 9.88 | |
| cis-Sabinol | 18.21 | 856.67 | 3.61 | |
| Carvone | 20.25 | 897.85 | 10.97 | |
| CLEO | α-Pinene | 9.45 | 643.87 | 3.46 |
| Sabinene | 11.35 | 800.60 | 16.99 | |
| Limonene | 13.98 | 764.62 | 61.83 | |
| Limonene oxide, cis- | 18.57 | 864.00 | 2.27 | |
| Limonene oxide, trans- | 18.80 | 868.71 | 3.08 | |
| CSEO | Limonene | 13.97 | 764.32 | 71.26 |
| trans-p-2,8-Menthadien-1-ol | 18.60 | 864.66 | 4.96 | |
| Limonene oxide, cis- | 18.77 | 868.04 | 2.59 | |
| Limonene oxide, trans- | 18.82 | 869.09 | 2.29 | |
| trans-Carveol | 22.69 | 943.77 | 2.91 | |
| CZEO | Linalool | 17.23 | 837.05 | 6.96 |
| Cinnamaldehyde | 25.76 | 1001.60 | 62.04 | |
| trans-Caryophyllene | 31.36 | 1108.55 | 6.60 | |
| transS-Cinnamyl acetate | 32.57 | 1132.76 | 4.30 | |
| Benzyl Benzoate | 44.52 | 1383.62 | 3.33 | |
| ZOEO | Camphene | 10.11 | 1625.67 | 6.73 |
| α-Curcumene | 34.00 | 1161.19 | 11.61 | |
| Zingiberene | 34.70 | 1175.25 | 30.28 | |
| β-Bisabolene | 35.07 | 1182.57 | 10.69 | |
| β-Sesquiphellandrene | 35.73 | 1195.68 | 12.37 |
Retention Time
Retention index
Effect of the EOs on the growth of bacteria.
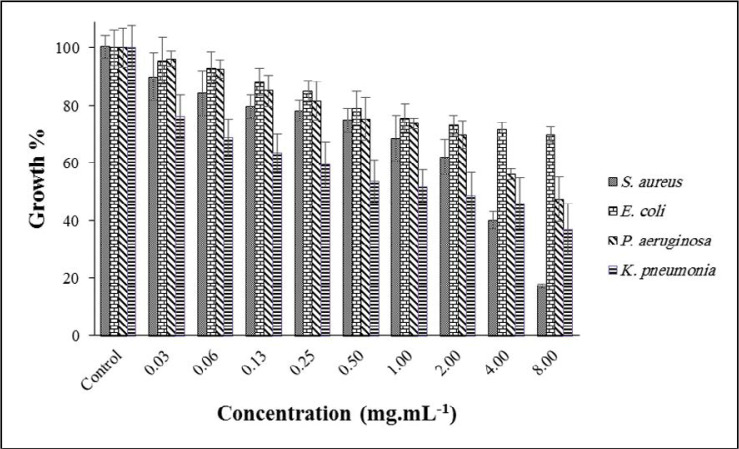
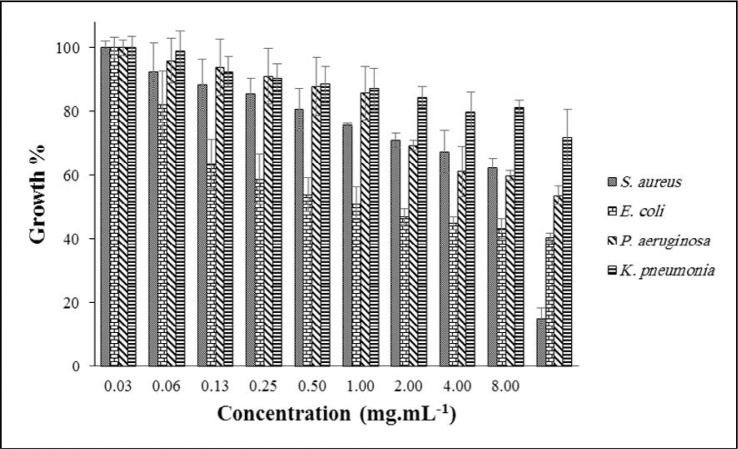
The effect of ADEO at different concentrations (0.03–8.00 mg.mL–1) on the targeted bacterial growth is depicted in Fig. 1. The best result was observed at a concentration of 8.00 mg.mL–1 against S. aureus; the growth was reduced to ~ 17%, while K. pneumoniae, P. aeruginosa and E. coli were decreased to 36, 47 and 69%, respectively. From the literature, MIC of ADEO on S. aureus and E. coli were reported as 1.25 and 2.50 mg.mL–1 (19). Moreover, its zone of inhabitation in the disk diffusion approach was reported as 8 mm for E. coli and 10 mm for S. aureus (8).
Fig. 1.
Effect of ADEO on the growth of targeted bacteria
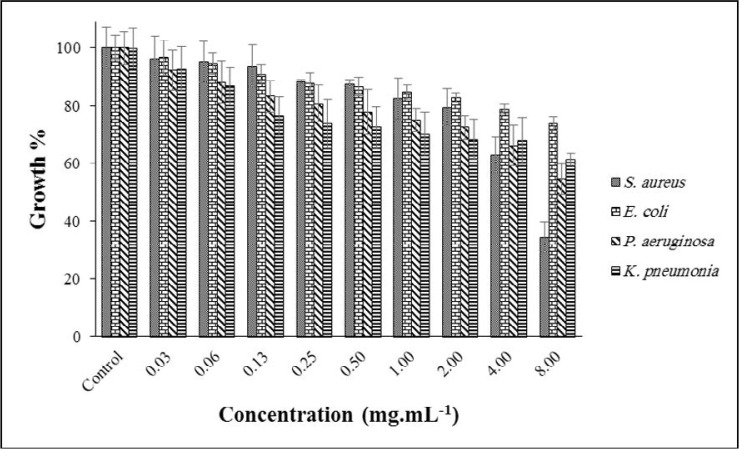
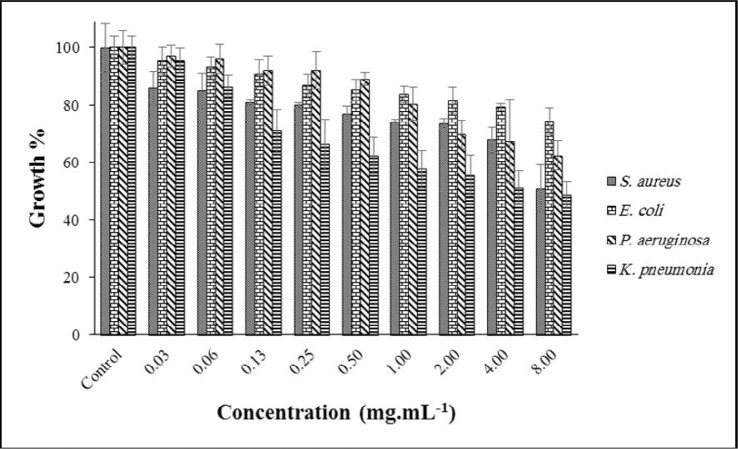
Fig. 2 shows the antibacterial activity of AGEO at various concentrations. The highest antibacterial activity was achieved at 8.00 against S. aureus, with inhibition in 34% growth. However, other bacterial growth was 54, 61, 73% for P. aeruginosa, K. pneumoniae and E. coli, respectively. Some reports on the MIC of ADEO against many bacteria have been found; For example, E. coli 1.25 mg.mL–1, P. aeruginosa 1.5 mg.mL–1, and S. aureus 0.62 mg.mL–1 (20). In another study, the MIC of AGEO on K. pneumoniae was reported as >10 mg.mL–1 (21).
Fig. 2.
Effect of AGEO on the growth of targeted bacteria
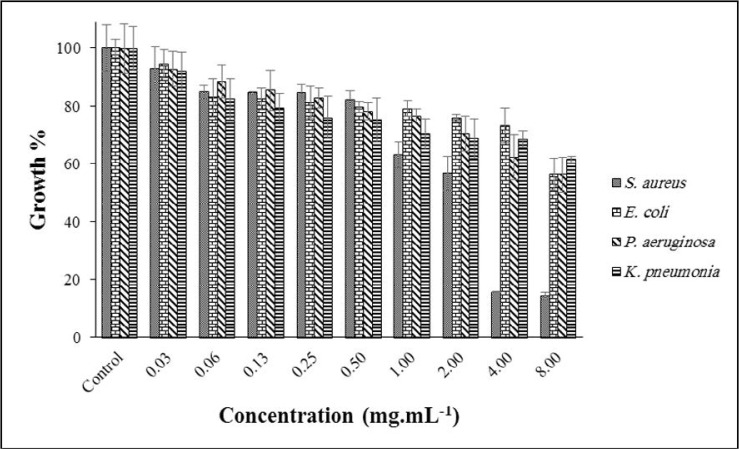
Results of the growth inhibitory effect of CLEO on some bacteria are demonstrated in Fig. 3. With the maximum growth of 14%, S. aureus was more affected after 24 h exposure with CLEO at a concentration of 8.00 mg. mL–1; observed growth for three other bacteria was ~ 60%. Antibacterial effect (MIC) of CLEO on E. coli, K. pneumoniae, P. aeruginosa and S. aureus was reported previously. These values were 6.4, 12.8, 12.8 and 12.8 mg.mL–1, respectively (22).
Fig. 3.
Effect of CLEO on the growth of targeted bacteria
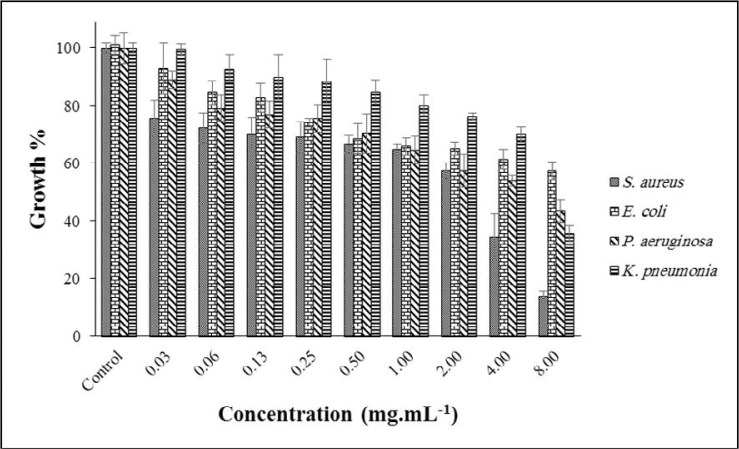
The antibacterial effect of CSEO is shown in Fig. 4. Totally, by increasing the concentration of EO, the growth of bacteria was reduced. At the highest level (8.00 mg.mL–1), the growth of S. aureus, E. coli, P. aeruginosa and K. pneumoniae decreased to 13%, 57%, 43% and 35%, respectively. Like the previously mentioned EOs, S. aureus was more susceptible than other examined bacteria. Reviewing the literature, MIC of CSEO against S. aureus, K. pneumoniae and E. coli was reported as 0.062, 0.25, and 0.12 mg.mL–1 (23). The related value for P. aeruginosa was 0.75 mg.mL–1 (24).
Fig. 4.
Effect of CSEO on the growth of targeted bacteria
After 24 h exposure with CZEO (8.00 mg.mL–1), the growth of bacteria had a substantial difference from each other (see Fig. 5). For instance, the observed growth for S. aureus was around 15%, while this amount for K. pneumoniae was 71%. This value for the other bacteria falls between those values (P. aeruginosa: 53% and E. coli: 40%). Antibacterial effect (MIC mg.mL–1) of CZEO on such bacteria, i.e., E. coli (1.6), K. pneumoniae (3.2), P. aeruginosa (0.8), and S. aureus (3.2) was reported previously (22).
Fig. 5.
Effect of CZEO on the growth of targeted bacteria
As shown in Fig. 6, only the growth of K. pneumoniae decreased to <50% after treatment with ZOEO. E. coli, with a growth of 74%, was more resistant than others. In previously published papers, MIC of ZOEO on targeted bacteria, including P. aeruginosa 31.25, S. aureus 7.81, E. coli 62.5 (25), and K. pneumoniae 20 (26) were reported.
Fig. 6.
Effect of ZOEO on the growth of targeted bacteria
In Table 2, IC50s (with lower and upper confidence limits: LCL and UCL) of the EOs against four human pathogens are summarized.
Table 2.
| Bacteria | ADEO | AGEO | ClEO | CSEO | CZEO | ZOEO |
|---|---|---|---|---|---|---|
| S. aureus | 1.9 (1.1–3.6) | 8.0 (4.1–15.6) | 1.3 (0.7–2.3) | 1.0 (0.4–2.5) | 2.9 (1.2–7.1) | 37.3 (11.2–124.2) |
| E. coli | 29.8 (10.5–85.1) | 101.9 (33.3–311.4) | 41.7 (5.7–303.9) | 10.0 (3.8–26.1) | 1.0 (0.5–2.0) | 189.8 (75.0–480.8) |
| P. aeruginosa | 6.1 (3.6–10.3) | 19.1 (10.5–35.0) | 16.2 (11.1–23.7) | 4.7 (3.1–7.3) | 7.2 (4.7–10.9) | 14.0 (8.9–22.0) |
| K. pneumoniae | 1.3 (1.0–1.8) | 22.2 (4.5–108.9) | 33.1 (7.3–150.0) | 5.8 (2.0–16.9) | 42.9 (5.4–343.2) | 3.0 (1.1–8.2) |
The half-maximal inhibitory concentration
Lower Confidence Limit
Upper Confidence Limit
Values are presented in mg.mL–1
DISCUSSION
IC50 of four EOs on S. aureus was around 2 mg.mL–1, CSEO (1.0), CLEO (1.3), ADEO (1.9), and CZEO (2.9). Their IC50 is not significantly different from each other (one-way ANOVA, sig > 0.05), but substantially better than AGEO and ZOEO (one-way ANOVA, sig < 0.05). S. aureus is Gram-positive cocci, which is usually found in the nasal cavity and on the skin. Although most S. aureus strains often act as normal flora of the human microbiota, it can become an opportunistic pathogen, a common cause of various infections, such as skin infections and food poisoning. S. aureus is one of the most common reasons for hospital-acquired infections and is usually the cause of wound infections following surgery (27, 28).
Effect of CZEO on E. coli was significantly better than the other examined EO (one-way ANOVA, sig < 0.05); IC50 (LCL-UCL): 1.0 (0.5–2.0) mg.mL–1. However, the calculated IC50 for ZOEO (189.8) and AGEO (101.9) differ substantially against this bacterium, but they were also larger than the total IC50s calculated in this study. E. coli is a Gram-negative, facultative anaerobe rod and a genus of Enterobacteriaceae. Most strains of E. coli are harmless and are part of the normal microbiota of the gut. Still, some strains (pathotypes) can cause severe infections in humans, usually through food contamination. E. coli is one of the most important bacteria in a hospital and community-acquired infections in humans. Fecal–oral transmission is the usual route through which patho-types of the E. coli cause disease (29, 30).
CSEO has the lowest IC50 (4.7 mg.mL–1) against P. aeruginosa, this amount significantly better than AGEO (19.1), CLEO (16.2), and ZOEO (14.0) (oneway ANOVA, sig < 0.05). Furthermore, ADEO and CZEO with IC50 of 6.1 and 7.2 mg. mL–1, respectively, showed good antibacterial activity, and their IC50 were not significantly different from CSEO (one-way ANOVA, sig > 0.05). P. aeruginosa is a Gram-negative rod found in soil, water, and skin flora. An opportunistic microorganism in which severe infection often occurs during existing diseases or conditions, such as damaged tissues, cystic fibrosis, and wound burns, is common in acute illness, especially hospital-acquired infections. Treatment of P. aeruginosa infections can be difficult due to its natural resistance to antibiotics (multidrug-resistant pathogen) (31, 32).
The lowest observed IC50 (LCL-UCL) against K. pneumoniae was related to ADEO: 1.3 (1.0–1.8) mg.mL–1. ZOEO, CSEO, AGEO, CLEO and CZEO with IC50 of 3.0, 5.8, 22.2, 33.1 and 42.9 mg.mL–1 were situated in other ranks. K. pneumoniae is a Gram-negative rod, facultatively anaerobic, found in the intestine normal flora. K. pneumoniae can cause destructive changes to the human lungs if aspirated, resulting in bloody sputum. In recent years, Klebsiella species have become important pathogens in hospital-acquired infections (33, 34).
In other researches, MIC of ADEO on S. aureus and E. coli were reported as 1.25 and 2.50 mg.mL–1 (19). MIC of CLEO on E. coli, K. pneumoniae, P. aeruginosa and S. aureus was 6.4, 12.8, 12.8 and 12.8 mg.mL–1, respectively (22). MIC of CSEO against S. aureus, K. pneumoniae and E. coli was reported as 0.062, 0.25 and 0.12 mg.mL–1 (23). The related value for P. aeruginosa was 0.75 mg.mL–1 (24). MIC of ZOEO on targeted bacteria, including P. aeruginosa 31.25, S. aureus 7.81 E. coli 62.5 (25), and K. pneumoniae 20 (26) were reported.
CONClUSION
Antibacterial activity of six EOs was investigated in a quantitative approach on four important human pathogens. CSEO (IC50: 1.0 mg.mL–1), CZEO (IC50: 1.0 mg.mL–1), CSEO (4.7 mg.mL–1), and ADEO (IC50: 1.3 mg.mL–1) were the most effective against S. aureus, E. coli, P. aeruginosa and K. pneumoniae, respectively. These EOs could be used for developing inexpensive, potent, and green antibacterial agents.
ACKNOWLEDGEMENTS
The authors appreciate Fasa University of Medical Sciences for financial support of this work (Grant No. 97090). Also, this research has been ethically approved (IR.FUMS.REC.1397.152).
REFERENCES
- 1.Pandey AK, Kumar P, Singh P, Tripathi NN, Bajpai VK. Essential oils: sources of antimicrobials and food preservatives. Front Microbiol 2017;7:2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Firmino DF, Cavalcante TTA, Gomes GA, Firmino NCS, Rosa LD, de Carvalho MG, et al. Antibacterial and antibiofilm activities of Cinnamomum sp. essential oil and Cinnamaldehyde: antimicrobial activities. ScientificWorldJournal 2018;2018:7405736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tongnuanchan P, Benjakul S. Essential oils: extraction, bioactivities, and their uses for food preservation. J Food Sci 2014;79:R1231–1249. [DOI] [PubMed] [Google Scholar]
- 4.Maekawa LE, Valera MC, Oliveira LD, Carvalho CA, Camargo CH, Jorge AO. Effect of Zingiber officinale and propolis on microorganisms and endotoxins in root canals. J Appl Oral Sci 2013;21:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguefack J, Budde BB, Jakobsen M. Five essential oils from aromatic plants of Cameroon: their antibacterial activity and ability to permeabilize the cytoplasmic membrane of Listeria innocua examined by flow cytometry. Lett Appl Microbiol 2004;39:395–400. [DOI] [PubMed] [Google Scholar]
- 6.Osanloo M, Sedaghat MM, Esmaeili F, Amani A. Larvicidal activity of essential oil of Syzygium aromaticum (Clove) in comparison with its major constituent, eugenol, against Anopheles stephensi. J Arthropod Borne Dis 2018;12:361–369. [PMC free article] [PubMed] [Google Scholar]
- 7.Husain I, Ahmad R, Chandra A, Raza ST, Shukla Y, Mahdi F. Phytochemical characterization and biological activity evaluation of ethanolic extract of Cinnamomum zeylanicum. J Ethnopharmacol 2018;219:110–116. [DOI] [PubMed] [Google Scholar]
- 8.Lopes-Lutz D, Alviano DS, Alviano CS, Kolodziejczyk PP. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry 2008;69: 1732–1738. [DOI] [PubMed] [Google Scholar]
- 9.Brochot A, Guilbot A, Haddioui L, Roques C. Antibacterial, antifungal, and antiviral effects of three essential oil blends. Microbiologyopen 2017;6(4):e00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Man A, Santacroce L, Jacob R, Mare A, Man L. Antimicrobial activity of six essential oils against a group of human pathogens: A comparative study. Pathogens 2019;8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saeb S, Amin M, Gooybari RS, Aghel N. Evaluation of antibacterial activities of Citrus limon, Citrus reticulata, and Citrus grandis against pathogenic bacteria. Int J Enteric Pathog 2016;4(4): e37103. [Google Scholar]
- 12.Lambert R, Skandamis PN, Coote PJ, Nychas GJ. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol 2001;91:453–462. [DOI] [PubMed] [Google Scholar]
- 13.Chaleshtori RS, Rokni N, Razavilar V, Kopaei MR. The evaluation of the antibacterial and antioxidant activity of Tarragon (Artemisia dracunculus L.) essential oil and its chemical composition. Jundishapur J Microbiol 2013;6 (9); e7877. [Google Scholar]
- 14.Said-Al Ahl HA, Sarhan AM, Dahab ADMA, Abou-Zeid E-SN, Ali MS, Naguib NY, et al. Essential oils of Anethum graveolens L.: chemical composition and their antimicrobial activities at vegetative, flowering and fruiting stages of development. Int J Plant Sci 2015;1:98–102. [Google Scholar]
- 15.Kuglerova M, Tesarova H, Grade JT, Halamova K, Wanyana-Maganyi O, Van Damme P, et al. Antimicrobial and antioxidative effects of Ugandan medicinal barks. Afr J Biotechnol 2011;10:3628–3632. [Google Scholar]
- 16.Osanloo M, Abdollahi A, Valizadeh A, Abedinpour N. Antibacterial potential of essential oils of Zataria multiflora and Mentha piperita, micro- and nano-formulated forms. Iran J Microbiol 2020;12:43–51. [PMC free article] [PubMed] [Google Scholar]
- 17.Osanloo M, Sedaghat MM, Sereshti H, Rahmani M, Saeedi Landi F, Amani A. Chitosan nanocapsules of tarragon essential oil with low cytotoxicity and long-lasting activity as a green nano-larvicide. J Nanostruct 2019;9:723–735. [Google Scholar]
- 18.Valizadeh A, Shirzad M, Esmaeili F, Amani A. Increased antibacterial activity of Cinnamon Oil Microemulsionin Comparison with Cinnamon Oil Bulk and Nanoemulsion.Nanomed Res J 2018;3:37–43. [Google Scholar]
- 19.Raeisi M, Tajik H, Razavi RS, Maham M, Moradi M, Hajimohammadi B, et al. Essential oil of tarragon (Artemisia dracunculus) antibacterial activity on Staphylococcus aureus and Escherichia coli in culture media and Iranian white cheese. Iran J Microbiol 2012;4:30–34. [PMC free article] [PubMed] [Google Scholar]
- 20.Derakhshan S, Navidinia M, Ahmadi A. Antibacterial activity of Dill (Anethum graveolens) essential oil and antibiofilm activity of Cumin (Cuminum cyminum) alcoholic extract. Infect Epidemiol Microbiol 2017;3:122–126. [Google Scholar]
- 21.Ruangamnart A, Buranaphalin S, Temsiririrkkul R, Chuakul W, Pratuangdejkul J. Chemical compositions and antibacterial activity of essential oil from dill fruits (Anethum graveolens L.) cultivated in Thailand. Mahidol Univ J Pharm Sci 2015;42:135–143. [Google Scholar]
- 22.Prabuseenivasan S, Jayakumar M, Ignacimuthu S. In vitro antibacterial activity of some plant essential oils. BMC Complement Altern Med 2006;6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eldahshan OA, Halim AF. Comparison of the composition and antimicrobial activities of the essential oils of green branches and leaves of Egyptian navel orange (Citrus sinensis (L.) Osbeck var. Malesy). Chem Biodivers 2016;13:681–685. [DOI] [PubMed] [Google Scholar]
- 24.Frassinetti S, Caltavuturo L, Cini M, Della Croce C, Maserti B. Antibacterial and antioxidant activity of essential oils from Citrus spp. J Essent Oil Res 2011;23:27–31. [Google Scholar]
- 25.Debbarma J, Kishore P, Nayak BB, Kannuchamy N, Gudipati V. Antibacterial activity of ginger, eucalyptus and sweet orange peel essential oils on fish-borne bacteria. J Food Process Preserv 2013;37:1022–1030. [Google Scholar]
- 26.Abdalla WE, Abdallah EM. Antibacterial activity of ginger (Zingiber Officinale Rosc.) Rhizome: a mini review. Int J Pharmacogn Chinese Med 2018;2:000142. [Google Scholar]
- 27.Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015;28:603–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis 2001;7:178–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol 2004;2:123–140. [DOI] [PubMed] [Google Scholar]
- 30.Jafari A, Aslani M, Bouzari S. Escherichia coli: a brief review of diarrheagenic pathotypes and their role in diarrheal diseases in Iran. Iran J Microbiol 2012;4:102–117. [PMC free article] [PubMed] [Google Scholar]
- 31.Streeter K, Katouli M. Pseudomonas aeruginosa: A review of their pathogenesis and prevalence in clinical settings and the environment. Infect Epidemiol Microbiol 2016;2:25–32. [Google Scholar]
- 32.Breidenstein EB, de la Fuente-Núñez C, Hancock RE. Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol 2011;19:419–426. [DOI] [PubMed] [Google Scholar]
- 33.Piperaki E-T, Syrogiannopoulos GA, Tzouvelekis LS, Daikos GL. Klebsiella pneumoniae: virulence, biofilm and antimicrobial resistance. Pediatr Infect Dis J 2017;36:1002–1005. [DOI] [PubMed] [Google Scholar]
- 34.Bengoechea JA, Sa Pessoa J. Klebsiella pneumoniae infection biology: living to counteract host defences. FEMS Microbiol Rev 2019;43:123–144. [DOI] [PMC free article] [PubMed] [Google Scholar]