Abstract
Background:
The purpose of this study was to determine the effect of fibrinogen concentration on cell viability and migration in a tissue culture tendon healing model.
Methods:
Forty-eight canine flexor digitorum profundus tendons were randomly divided into three groups. In each group the tendons were lacerated and repaired augmented with a canine bone marrow stromal cell seeded fibrin interposition patch using either 5 mg/ml fibrinogen and 25 U/ml thrombin (physiological as a control40 m), g/ml fibrinogen and 250 U/ml thrombin (low adhesive), or 80 mg/ml fibrinogen and 250 U/ml thrombin (high adhesive). The sutured tendons were cultured for two or four weeks.
Results:
Failure load was not significantly different among the groups. Cell-labeling staining showed that the stromal cells migrated across the gap in the control and low adhesive groups, but there was no cell migration in the high adhesive group at two weeks.
Conclusion:
A high fibrinogen concentration in a fibrin patch or glue may impede early cell migration.
Level of evidence:
Not applicable because this study was a laboratory study.
1. Introduction
Tendon repair in zone II is a common and clinically complex problem faced by hand surgeons [1]. While improvements in suture materials [2], suture techniques [3] and protocols for postoperative rehabilitation [4] have generally resulted in improved clinical outcomes, many complications, such as rupture and restrictive adhesions, still occur [5].
A significant and common factor in tendon healing is related to tendon cellularity. Tendon healing commonly includes contributions from intrinsic (i.e., tenocytes) and extrinsic (i.e., migrating fibroblasts) cells [6]. While extrinsic cells provide a good source of cells that aid healing, these migrating cells also result in adhesions between the tendon and surrounding tissue that restrict tendon motion and impair hand function. Although intrinsic cells are better than extrinsic cells for tendon healing with less adhesion, the low intrinsic cell population limits a robust intrinsic tendon healing response. To address this problem, tissue engineering techniques have been adopted to deliver cells to the repair site during surgery, with encouraging preliminary results [7–10]. In this setting, a delivery vehicle such as a suture [11], collagen gel [12], platelet rich plasma clot [13], or fibrin gel [14] is needed. Previous reports showed cell-seed patch group had better healing capacity than no patch group [9,10,15].
Although many hydrogel-based cell delivery vehicles have been investigated, the ideal system remains to be determined. Some studies have investigated the effect of fibrin scaffolds seeded with stem cells with and without growth factor augmentation [13,16–18]. Fibrin has adhesive capability to serve as a repair glue, as well as the ability to act as a matrix for cell seeding. Fibrin matrix can deliver bone marrow stromal cells (BMSCs) and enhance tendon healing in an in vivo tendon repair model [15,19]. However, results have not been consistent, with some studies reporting that fibrin scaffolds increased cell viability, proliferation, or differentiation [16,18] while others have reported that fibrin may adversely affect these parameters [13,16,18]. One study noted that fibrin gel enhanced tendon healing mechanically and histologically compared to a collagen gel using an in vitro tendon repair model [20]. The concentration of fibrinogen and thrombin in that study was based on levels found physiologically, namely 5 mg/ml fibrinogen and 25 U/ml thrombin. Fibrin is also used as tissue repair glue, and the degree of adhesion of fibrin depends on the concentrations of fibrinogen and thrombin [21–24]. In commercially available fibrin glues these range typically from 60 to 110 mg/ml fibrinogen and 500–600 U/ml thrombin. A comparison of these fibrin gel “glues” with physiological fibrin gels showed that the failure load and tensile stiffness of canine tendons repaired with gels containing 80 mg/ml fibrinogen were significantly stronger than those of the physiologic gels. However, in a cell culture scratch migration model, the cells in the 80 mg/ml fibrinogen group were rounded and did not migrate across the scratch, suggesting that the denser fibrin matrix impeded cell migration [25]. That study, however, reported time zero mechanical properties in free tissue, and cell viability and migration in a cell culture model within seven days. Validation of the these results are needed in tendon healing model.
In this study, we sought to assess the effect over time of fibrin gels of varying concentration on the ability of cells to migrate out of the gel and into a tendon in a tendon repair tissue culture model for two and four weeks. We hypothesized that a gel with a lower concentration of fibrinogen would allow increased cellular migration into the healing tendon ends compared to gels with higher fibrinogen content.
2. Methods
2.1. Study design
Tissue was obtained from six mixed-breed dogs euthanized for other Institutional Animal Care and Use Committee (IACUC) approved studies. A total of 48 flexor digitorum profundus (FDP) tendons from the second to fifth forepaw digits were harvested under sterile conditions. The tendons were divided into three groups to be repaired with cell patches of one of these three fibrin formulations, based on formulations that had been studied in a previous report [25]: 1) 5 mg/mL fibrinogen with 25 U/mL thrombin (physiologic concentration/control group); 2) low adhesive concentration fibrinogen (40 mg/mL) with adhesive concentration thrombin (250 U/mL); and 3) high adhesive concentration fibrinogen (80 mg/mL) with adhesive concentration thrombin (250 U/mL) interposition (Table 1). Tendons were further divided into Zone II (distal) and Zone III (proximal) segments; these segments were then divided and repaired with cell-seeded fibrin gels. After two or four weeks of tissue culturing, the tendons were assessed for mechanical strength and histology. Segments from zone II were used for biomechanical testing, and segments from zone III were used for histological testing.
Table 1.
Experimental design.
| Groups Description | Culture 2 week | Culture 4 week | Total number |
|||
|---|---|---|---|---|---|---|
| MT | HIS | MT | HIS | |||
| Zone II | Zone III | Zone II | Zone III | |||
| 1 | Hemostatic concentration | 8 | 3 | 8 | 3 | 22 |
| 2 | Low fibrinogen | 8 | 3 | 8 | 3 | 22 |
| 3 | High fibrinogen | 8 | 3 | 8 | 3 | 22 |
| Total number | 24 | 9 | 24 | 9 | 66 | |
MT; mechanical testing, HIS; histology.
2.2. BMSCs harvest and suspension
Bone marrow was harvested from one mixed-breed dog euthanized for another IACUC approved study. Bone marrow was aspirated aseptically from each tibia, using a 15 mL syringe containing 2.0 mL heparin solution. The heparin was removed with centrifugation at 1500 rpm for 5 min at room temperature, and the bone marrow pellet was resuspended in cell culture medium and equally divided into three 100 mm culture dishes with 10 mL of standard medium. The medium consisted of minimal essential medium (MEM) with Earle’s salts (GIBCO, Grand Island, NY, USA), 10% fetal bovine serum and 1% antibiotics (Antibiotic-Antimycotic, GIBCO, Grand Island, NY, USA). Then, the bone marrow cells were incubated at 37°C with 5% CO2 and 95% air at 100% humidity. After three days, the non-adherent cells were removed, and the medium was added to dishes with the remaining adherent cells. These adherent cells were defined as BMSCs. The medium was changed every three days. When cells reached 80% confluence, the BMSCs were harvested with 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA) and subcultured. Cells between passage two and four were used for testing.
2.3. Stromal cell tracking and cell identification
BMSCs were labeled with cell labeling solution (VybrantR DiI cell labeling solution, Molecular Probes, Eugene, Oregon) in accordance with the manufacturer’s instructions, before preparing the gel patch.
2.4. Cell-seeded fibrin gel patch preparation
The cultured and passaged BMSCs were washed with phosphate buffered saline (PBS) (GIBCO, Grand Island, NY, USA) and then trypsinized. Cells were counted and 2.0 × 105 cells added to each fibrin gel patch. A 10 μL aliquot of bovine fibrinogen (5 mg/mL, 40 mg/mL, or 80 mg/mL for control, low and high groups, respectively) (Sigma–Aldrich, St. Louis, MO, USA) was mixed with the pelleted BMSCs supplemented with 1 μL of rhGDF-5 (Abcam, Cambridge, MA, USA) (10 ng/μL). A 3 μL bovine thrombin solution (25 U/mL for the control group, 250 U/mL for low and high groups) (Sigma) was mixed with the fibrinogen solution to convert the fibrinogen to fibrin. This cell, fibrinogen and thrombin mixture was then incubated at 37°C for 0.5 h. The BMSCs-seeded patch was then immediately used for the tendon repair as described below.
2.5. Tendon repair and tissue culture
The zone II and zone III [26] tendon segments were cut to a standardized length of 30 mm. The repair site was made at the midpoint of each segment. The fibrin patch was interposed between lacerated tendon ends. A simple loop suture of 6–0 Prolene (Ethicon, Somerville, NJ) was applied to maintain the fibrin patch between the transected tendon ends (Fig. 1).
Fig. 1.
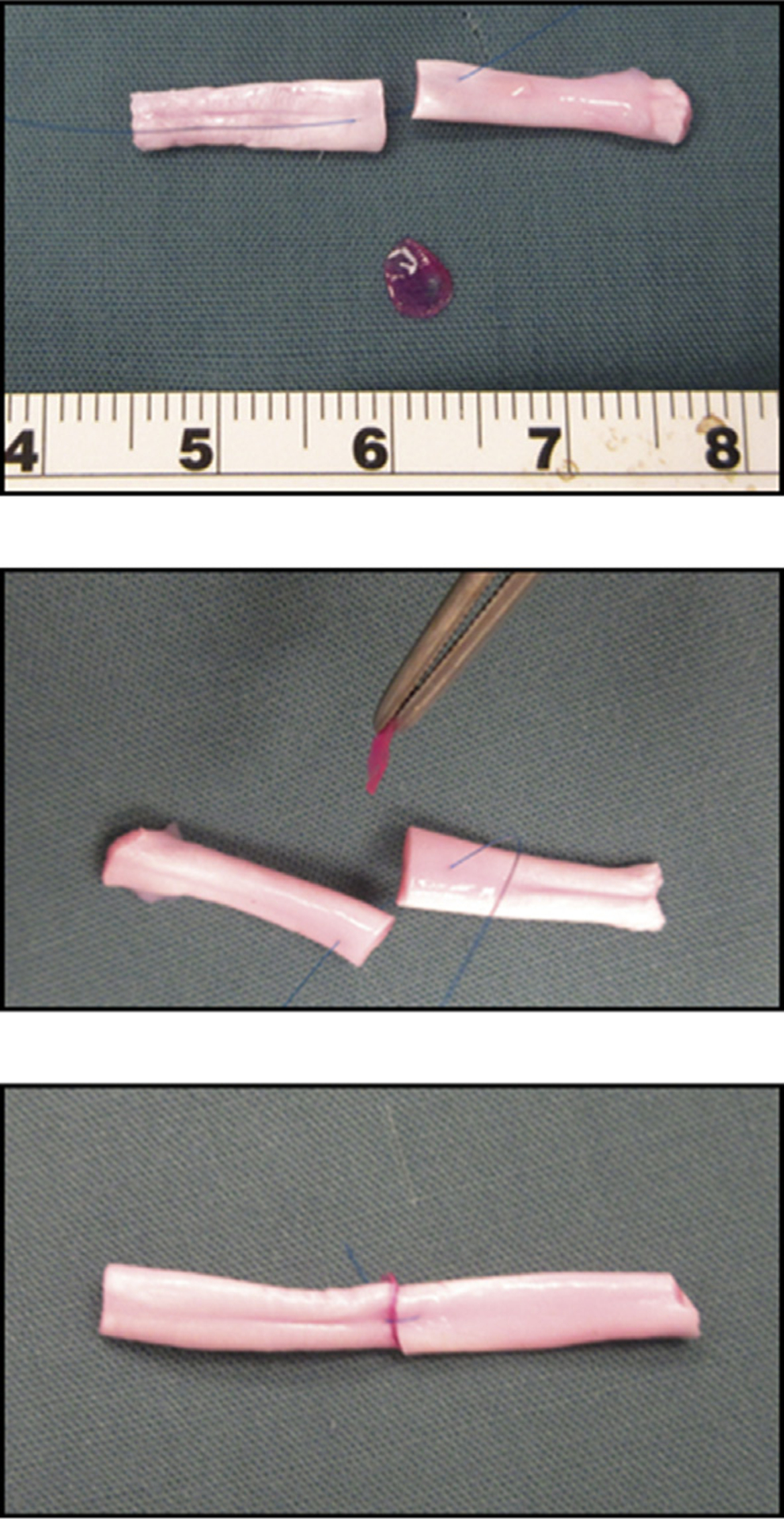
The repair site was made at the midpoint of each segment. The fibrin patch was interposed between lacerated tendon ends. A simple loop suture was applied to maintain the fibrin patch between the transected tendon ends.
Repaired tendons were placed in wire meshes with longitudinal grooves designed to maintain the tendons in a straight position. The meshes with repaired tendons were set into a 100 mm Petri dish with MEM with Earle’s salts (GIBCO, Grand Island, NY, USA), 10% fetal bovine serum and 1% antibiotics (Antibiotic-Antimycotic, GIBCO, Grand Island, NY, USA) and incubated at 37°C in a 5% CO2 humidified incubator for 2 or 4 weeks [9,27]. Culture medium was changed every three days.
2.6. Biomechanical testing
After incubating for two or four weeks, repaired tendons were removed from the culture dish. A single loop suture of 6–0 poly-propylene (Prolene, Ethicon, Somerville, NJ, USA) 5 mm in length was placed at each end of the test specimen to connect the repaired tendon construct to a custom-designed micro tester (Fig. 2). The testing device included a load transducer (GSO-50, Transducer Techniques Inc., Temecula, CA, USA), a linear potentiometer (TR-50, Novotechnik, Southborough, MA, USA), and a stepper-motor-driven stage with hooks to capture the suture loops. Before testing, the suture at the repair site was cut, without disrupting the repair site, so that the testing would assess only the strength of the cell-laden fibrin interposition/tendon composite without suture holding strength.
Fig. 2.

The scheme of the custom-designed micro tester. The tendon was placed on a flat, plastic platform moistened with physiological saline. Then, the specimen was distracted at a rate of 0.1 mm/s until the repair site was completely separated.
The tendon was placed on a flat, plastic platform moistened with physiological saline. Then, the specimen was distracted at a rate of 0.1 mm/s until the repair site was completely separated. The displacement and load to failure were measured by the transducer at a sample rate of 100 Hz. Failure load was normalized by each tendon cross-sectional area. Tensile stiffness was defined by the slope of the linear region of the force/displacement curve.
2.7. Cell viability assessment and histology
Each group and time course had three specimens for each of two histological studies: fluorescent antibody staining (DiI and DAPI) and hematoxylin and eosin (H&E). DAPI and DiI staining were observed with confocal microscopy (X 400, LSM780; Zeiss, Germany) and H&E staining was evaluated with light microscopy (X 40) within 5 mm of the tendon laceration. The tendon specimen was set in optimal cutting temperature (OCT) compound (Tissue-Tek, Sakura Finetek, Japan) and 7 mm sections were prepared with a cryostat (Leica CM 1850, Wetzlar, Germany). Cell density was obtained and assessed for the DiI labeled bone marrow stromal cells and total cells in the repair site (unlabeled tenocytes and DiI labeled cells). Specimens stained with DAPI were also stained with hematoxylin and eosin (H&E) to evaluate gross morphology. The assessments were performed without blinding.
2.8. Statistical analysis
For the mechanical study, previous data showed that the average failure loads of a canine FDP tendon after repair using a hemostatic concentration of fibrin gel in a tendon tissue culture model of tendon healing at 2 weeks and 4 weeks were 12.4 mN and 29.3 mN, respectively (standard deviations 7.8 mN and 15.9 mN, respectively) [20]. Using these values, a sample size of 8 provided 80% power at a significance level of p < 0.05 to detect a difference in failure load of 9.46 mN.
Failure load and tensile stiffness were analyzed by one-way factorial analysis of variance (ANOVA). The Tukey–Kramer post hoc test for each pairwise comparison was done if a significant difference is observed. Statistical analysis for histological study was evaluated by Fisher’s exact test. All measurements were expressed as mean values and standard deviations. The significance level was set to p < 0.05 in all cases.
3. Results
3.1. Mechanical testing
Failure load was not significantly different among the groups at 2 weeks (p = 0.06) or 4 weeks (p = 0.22) (Fig. 3). Stiffness at 4 weeks was significantly greater in the low fibrinogen group as compared to the high fibrinogen group (p 0.02), but was not significantly different at 2 weeks among any of the groups (p = 0.37) (Fig. 3).
Fig. 3.

Failure load and stiffness at two and four weeks. Error bar represents standard deviation.
3.2. Histology
H&E staining showed partial connection of collagen bundles between the opposed tendon ends as follows: at two weeks, two of three specimens in the control group, three of three specimens in the low group, and one of three specimens in the high group; at four weeks, one of three specimens in the control group, three of three specimens in the low group, and one of three specimens in the high group (Fig. 4).
Fig. 4.

H&E staining show partial repair in each group. The dotted boxes show partial repair sites. Scale bar indicates 500 μm.
Qualitative observation by confocal microscopy revealed that labeled, viable BMSCs were present within the repair site in all groups after both two and four weeks of tissue culture (Fig. 5). We did not quantify the number of cells in each section, but we did note a suggestion of more cells in the low fibrinogen group at 4 weeks as compared to the other groups. Vybrant DiI staining indicated that bone marrow stromal cells migrated to the transected tendon ends and across the gap in the control and low group (three of three specimens) at two weeks and all groups (three of three specimens) at four weeks. In the high group, there was no cell migration observed in the two-week group (none of three specimens).
Fig. 5.

Vybrant DiI staining indicated bone marrow stromal cells migrated to the site of transected tendon ends in the control and low group at two weeks. The dotted boxes show cell migration sites. The dotted yellow line show the contour of the stamps. Scale bar indicates 300 μm (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article).
4. Discussion
Fibrin hydrogels have been widely used as scaffolds for the reconstruction of various tissue and have been used as a biological adhesive for various procedures, such as abdominal, vascular oral, thoracic, and endoscopic surgeries [28]. In the tendon repair setting, while many materials have been used as scaffolds for seeding cells and delivering growth factors, fibrin has been suggested to be an appropriate candidate for tendon tissue engineering, because of its adherent ability, biocompatibility, malleability, and ease of use and availability [29]. There is controversy, though regarding optimal concentrations of fibrinogen and thrombin for a fibrin gel used for tendon repair. The concentration of thrombin plays an important role in forming the fibrin fibril network; an increased thrombin concentration was found to be associated with shorter gelation time, the formation of more fiber bundles with thinner fibers size, and with lower porosity [28]. Fibrinogen concentration is important for both adhesive strength and cell viability [21,25]. The results of this study support the notion that concentrations of fibrinogen higher than those associated with its physiological function in hemostasis but lower than those used in the current commercially available fibrin glues should be used to compose a scaffold if the goal is to provide both some adhesive strength and cell delivery for tendon repair [25].
In our study, the low fibrinogen group showed higher stiffness after four weeks in tissue culture than the high fibrinogen groups, while both the control and low fibrinogen group showed better migration ability than the high group at two weeks. DiI stain results at two weeks were consistent with a previous report of migration assay in cell culture [25]. Previously it was reported that the concentration of fibrinogen in the gel affected cell viability, shape, and motility, which might have important implications for the use of fibrin gels as cell carriers for the purpose of tissue engineering. In that study, the low-fibrinogen gels allowed for cell migration, whereas the high-fibrinogen gels did not [25]. Kim et al. similarly reported that mesenchymal stem cells maintained a rounded shape in fibrin gels with a higher, nonphysiological concentration of fibrinogen (127–256 mg/mL) [30]. The currrent study demonstrated that the high fibrinogen concentration reduced cell migration at two weeks, but not at four weeks. Thus, our data show that eventually cells do migrate out of the higher fibrinogen gel, but in a delayed fashion compared to the lower fibrinogen formulation. While of course no tissue culture system can truly mimic in vivo conditions, the model system that we use has been employed to study cell migration and healing in other publications [9,27]. In the normal process of tendon healing, fibroblastic stage at 2 weeks and remodeling stage at 4 weeks [31]. During fibroblastic stage, extra-cellular matrix is deposited and becomes hyper-cellular condition. Initially, collagen type III deposition occurs in a disorganized fashion, then the structure is reorganized into longitudinal pattern. The collagen type III is subsequently replaced with collagen type I during remodeling phase. Finally, longitudinal reorientation of the collagen fibers are occurred with the tendon tissue maturation and prevailing tension forces [31]. Collagen bridging in these stages may be crucial for the final outcomes. Our study did not have a “no patch group” because previous publications have demonstrated that a no patch group had lower healing potential than inserting a cell-laden patch between cut tendons in a tissue culture model [27,29].
With regard to mean failure load, in vitro a higher fibrinogen concentration makes a gel with greater adhesive strength [25]. In our ex vivo study, however, there was no such effect, suggesting that, over time and in the presence of living cells, the effect of fibrinogen concentration is less important, and the effect of cellular migration and healing begin to dominate between time zero and 2 weeks after repair. Moreover, the mechanical results show that even the strongest gels fail to approach the strength of a suture repair. The magnitude of the strength with fibrin gel alone (without suture repair) was far less than that of a repaired tendon. However, in our opinion the best use of fibrin in the context of tendon healing is not to improve initial strength but rather to serve as a vehicle to deliver therapeutic agents such as stem cells. In this study, we have shown that the specific proportions of fibrinogen and thrombin are very important in determining the permeability of the resulting gel to cell diffusion. Specifically, a lower concentration of fibrinogen seems to be more conducive to cell migration. As for the control group, the lower viscosity of the control gel allowed the patch to spread out more and became thinner, resulting in difficulty handling it, as previous manuscripts have reported [13,25]. Gels with higher fibrinogen concentrations impede cell migration, while gels at physiological concentration are soft and difficult to manipulate. Unfortunately, gels with a fibrinogen concentration of 40 mg/ml and 250 U/ml thrombin are not currently available commercially. We believe, though, that such a gel would provide an important improvement for cell-based therapy for tissue engineering applications.
The main strength of this study is that it isolated a factor, fibrinogen concentration, in order to assess its effect on cell migration. The main limitations of this study include the fact that it was done in tissue culture, and thus could not capture all of the interactions that would be presented in vivo. Loading of the tendons may have altered the healing response; we did not load the tendons because we did not wish to risk disrupting the repair site. We did not quantitatively assess the cell migration. The study was performed on canine tendons, and the results in humans may be different. Confirmation of the beneficial effects of low fibrinogen gels in a more complex model in vivo will provide important information on potential clinical efficacy.
In conclusion, lower fibrinogen concentrations result in improved cell migration from a fibrin gel in our canine tendon tissue culture model. This finding suggests that a lower concentration of fibrinogen should be used if a fibrin gel is used to deliver cells for tissue repair.
Funding statement
This work was supported by a grant from National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases [R01AR044391].
Footnotes
Ethical approval details
In this study, tendons and bone marrow stromal cells were harvested from mixed-breed dogs which had been euthanized for other Institutional Animal Care and Use Committee (IACUC) approved studies. Because we used specimens from dogs that had been euthanized for other studies this study did not require IACUC approval.
Declaration of competing interest
None of the authors has a financial conflict with any commercial entity relating to the subject of this work.
References
- [1].Tang JB. Clinical outcomes associated with flexor tendon repair. Hand Clin 2005;21(2):199–210. [DOI] [PubMed] [Google Scholar]
- [2].McDonald E, Gordon JA, Buckley JM, Gordon L. Comparison of a new multifilament stainless steel suture with frequently used sutures for flexor tendon repair. J Hand Surg Am 2011;36(6):1028–34. [DOI] [PubMed] [Google Scholar]
- [3].Peltz TS, Haddad R, Scougall PJ, Nicklin S, Gianoutsos MP, Walsh WR. Influence of locking stitch size in a four-strand cross-locked cruciate flexor tendon repair. J Hand Surg Am 2011;36(3):450–5. [DOI] [PubMed] [Google Scholar]
- [4].Trumble TE, Vedder NB, Seiler 3rd JG, Hanel DP, Diao E, Pettrone S. Zone-II flexor tendon repair: a randomized prospective trial of active place-and-hold therapy compared with passive motion therapy. J Bone Joint Surg Am 2010;92(6):1381–9. [DOI] [PubMed] [Google Scholar]
- [5].Dy CJ, Hernandez-Soria A, Ma Y, Roberts TR, Daluiski A. Complications after flexor tendon repair: a systematic review and meta-analysis. J Hand Surg Am 2012;37(3):543–551 e1. [DOI] [PubMed] [Google Scholar]
- [6].Galvez MG, Crowe C, Farnebo S, Chang J. Tissue engineering in flexor tendon surgery: current state and future advances. J Hand Surg Eur Vol 2014;39(1): 71–8. [DOI] [PubMed] [Google Scholar]
- [7].Juncosa-Melvin N, Boivin GP, Gooch C, Galloway MT, West JR, Dunn MG, Butler DL. The effect of autologous mesenchymal stem cells on the biomechanics and histology of gel-collagen sponge constructs used for rabbit patellar tendon repair. Tissue Eng 2006;12(2):369–79. [DOI] [PubMed] [Google Scholar]
- [8].Martinello T, Bronzini I, Perazzi A, Testoni S, De Benedictis GM, Negro A, Caporale G, Mascarello F, Iacopetti I, Patruno M. Effects of in vivo applications of peripheral blood-derived mesenchymal stromal cells (PB-MSCs) and platlet-rich plasma (PRP) on experimentally injured deep digital flexor tendons of sheep. J Orthop Res 2013;31(2):306–14. [DOI] [PubMed] [Google Scholar]
- [9].Morizaki Y, Zhao C, An KN, Amadio PC. The effects of platelet-rich plasma on bone marrow stromal cell transplants for tendon healing in vitro. J Hand Surg Am 2010;35(11):1833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhao C, Chieh HF, Bakri K, Ikeda J, Sun YL, Moran SL, An KN, Amadio PC. The effects of bone marrow stromal cell transplants on tendon healing in vitro. Med Eng Phys 2009;31(10):1271–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Guyette JP, Fakharzadeh M, Burford EJ, Tao ZW, Pins GD, Rolle MW, Gaudette GR. A novel suture-based method for efficient transplantation of stem cells. J Biomed Mater Res A 2013;101(3):809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Young RG, Butler DL, Weber W, Caplan AI, Gordon SL, Fink DJ. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res 1998;16(4):406–13. [DOI] [PubMed] [Google Scholar]
- [13].Zurita M, Otero L, Aguayo C, Bonilla C, Ferreira E, Parajon A, Vaquero J. Cell therapy for spinal cord repair: optimization of biologic scaffolds for survival and neural differentiation of human bone marrow stromal cells. Cytotherapy 2010;12(4):522–37. [DOI] [PubMed] [Google Scholar]
- [14].Breen A, O’Brien T, Pandit A. Fibrin as a delivery system for therapeutic drugs and biomolecules. Tissue Eng Part B Rev 2009;15(2):201–14. [DOI] [PubMed] [Google Scholar]
- [15].Sato D, Takahara M, Narita A, Yamakawa J, Hashimoto J, Ishikawa H, Ogino T. Effect of platelet-rich plasma with fibrin matrix on healing of intrasynovial flexor tendons. J Hand Surg Am 2012;37(7):1356–63. [DOI] [PubMed] [Google Scholar]
- [16].Aoyagi Y, Kuroda M, Asada S, Bujo H, Tanaka S, Konno S, Tanio M, Ishii I, Aso M, Saito Y. Fibrin glue increases the cell survival and the transduced gene product secretion of the ceiling culture-derived adipocytes transplanted in mice. Exp Mol Med 2011;43(3):161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].de la Puente P, Ludena D. Cell culture in autologous fibrin scaffolds for applications in tissue engineering. Exp Cell Res 2014;322(1):1–11. [DOI] [PubMed] [Google Scholar]
- [18].Hankemeier S, van Griensven M, Ezechieli M, Barkhausen T, Austin M, Jagodzinski M, Meller R, Bosch U, Krettek C, Zeichen J. Tissue engineering of tendons and ligaments by human bone marrow stromal cells in a liquid fibrin matrix in immunodeficient rats: results of a histologic study. Arch Orthop Trauma Surg 2007;127(9):815–21. [DOI] [PubMed] [Google Scholar]
- [19].Chong AK, Ang AD, Goh JC, Hui JH, Lim AY, Lee EH, Lim BH. Bone marrow-derived mesenchymal stem cells influence early tendon-healing in a rabbit achilles tendon model. J Bone Joint Surg Am 2007;89(1):74–81. [DOI] [PubMed] [Google Scholar]
- [20].Ozasa Y, Gingery A, Thoreson AR, An KN, Zhao C, Amadio PC. A comparative study of the effects of growth and differentiation factor 5 on muscle-derived stem cells and bone marrow stromal cells in an in vitro tendon healing model. J Hand Surg Am 2014;39(9):1706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Buen EP, Orozco-Mosqueda A, Leal-Cortes C, Vazquez-Camacho G, Fuentes-Orozco C, Alvarez-Villasenor AS, Macias-Amezcua MD, Gonzalez-Ojeda A. Fibrinogen and thrombin concentrations are critical for fibrin glue adherence in rat high-risk colon anastomoses. Clinics 2014;69(4):259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Byrne DJ, Hardy J, Wood RA, McIntosh R, Cuschieri A. Effect of fibrin glues on the mechanical properties of healing wounds. Br J Surg 1991;78(7):841–3. [DOI] [PubMed] [Google Scholar]
- [23].Murakami M, Tono T, Okada K, Yano H, Monden T. Fibrin glue injection method with diluted thrombin for refractory postoperative digestive fistula. Am J Surg 2009;198(5):715–9. [DOI] [PubMed] [Google Scholar]
- [24].Yoshida H, Hirozane K, Kamiya A. Adhesive strength of autologous fibrin glue. Biol Pharm Bull 2000;23(3):313–7. [DOI] [PubMed] [Google Scholar]
- [25].Uehara K, Zhao C, Gingery A, Thoreson AR, An KN, Amadio PC. Effect of fibrin formulation on initial strength of tendon repair and migration of bone marrow stromal cells in vitro. J Bone Joint Surg Am 2015;97(21):1792–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Amadio PC, Berglund LJ, An KN. Biochemically discrete zones of canine flexor tendon: evaluation of properties with a new photographic method. J Orthop Res 1992;10(2):198–204. [DOI] [PubMed] [Google Scholar]
- [27].Hayashi M, Zhao C, An KN, Amadio PC. The effects of growth and differentiation factor 5 on bone marrow stromal cell transplants in an in vitro tendon healing model. J Hand Surg Eur Vol 2011;36(4):271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rajangam T, An SS. Fibrinogen and fibrin based micro and nano scaffolds incorporated with drugs, proteins, cells and genes for therapeutic biomedical applications. Int J Nanomedicine 2013;8:3641e–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ozasa Y, Gingery A, Amadio PC. Muscle-derived stem cell seeded fibrin gel interposition produces greater tendon strength and stiffness than collagen gel in vitro. J Hand Surg Eur Vol 2015;40(7):747–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kim I, Lee SK, Yoon JI, Kim DE, Kim M, Ha H. Fibrin glue improves the therapeutic effect of MSCs by sustaining survival and paracrine function. Tissue Eng Part A 2013;19(21–22):2373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Titan AL, Foster DS, Chang J, Longaker MT. Flexor tendon: development, healing, adhesion formation, and contributing growth factors. Plast Reconstr Surg 2019;144(4):639e–47e. [DOI] [PMC free article] [PubMed] [Google Scholar]


