Abstract
Objective:
Increasing evidence supports that early initiation of biologics may dramatically improve disease course and reduce glucocorticoid exposure for children with systemic juvenile idiopathic arthritis (JIA). We characterized variation in the use of first-line biologic and glucocorticoid therapy and identified drivers of variation in children hospitalized with new-onset systemic JIA.
Methods:
We conducted a retrospective cohort study of children hospitalized with new-onset systemic JIA from 2008–2019 utilizing a comparative pediatric database from 52 tertiary care children’s hospitals. Subjects and treatment receipt were identified using International Classification of Diseases (ICD)-9 and ICD-10 discharge diagnosis codes, pharmacy billing data and clinical transaction classification codes. Mixed-effects logistic regression was used to identify patient and hospital-level factors associated with receipt of glucocorticoids and biologics.
Results:
534 children with new-onset systemic JIA hospitalized during the study period met inclusion criteria. Twenty-nine percent received biologics and 58% received glucocorticoids. Biologic use increased over time (p < 0.001), methotrexate use decreased (p < 0.01), and glucocorticoid use remained unchanged. Biologics and glucocorticoid use varied significantly between hospitals. High annual hospital volume, intensive care unit stay, and later discharge year were significantly associated with biologic exposure. Medium-high and high annual hospital volume were significantly associated with less glucocorticoid exposure.
Conclusion:
Despite increasing evidence demonstrating improved outcomes with first-line treatment with biologics, we found significant treatment variation across hospitals with many children not receiving biologics and a persistent high rate of glucocorticoid exposure. These results underscore the need for comparative efficacy studies and improved treatment standardization.
Systemic juvenile idiopathic arthritis (JIA) is an autoinflammatory condition with a very distinct clinical phenotype from other subtypes of JIA, characterized by daily high-spiking fevers, evanescent rashes, hepatosplenomegaly, lymphadenopathy, and serositis. These systemic disease manifestations are often more prominent than arthritis, which can present weeks to months later in the disease course. Management approaches for patients with new-onset systemic JIA have historically included the use of glucocorticoids. In 2012, multiple randomized controlled trials demonstrated efficacy of IL-1 and IL-6 inhibitors (biologics) in treating both the systemic and articular manifestations of systemic JIA, leading to more widespread use and improved outcomes (1,2,3). With the availability of more diverse therapeutic options, provider self-reports indicate significant variation in treatment of patients with new-onset systemic JIA (4).
Increasing evidence continues to emerge that early initiation of biologics may dramatically improve disease course and reduce glucocorticoid exposure (5). However, the frequency of first-line biologic use and temporal trends in treatment of patients with new-onset systemic JIA are not known. In order to move towards a more standardized therapeutic approach, it is first necessary to characterize treatment variation in a real-world setting and identify drivers of variation. Patients diagnosed with systemic JIA during inpatient hospitalization represent the highest acuity patient population, for which glucocorticoids and biologics are most commonly used. We leveraged encounter data from the Pediatric Health Information System (PHIS) to describe treatment variation among US children’s hospitals, temporal trends, and patient and hospital-level factors associated with first-line biologic and glucocorticoid use in a large inpatient multi-site cohort of new-onset systemic JIA patients.
PATIENTS & METHODS
This is a retrospective cohort study of children in the United States hospitalized with new-onset systemic JIA. This study was reviewed and determined to be exempt by the Children’s Hospital of Philadelphia (CHOP) Internal Review Board.
Data source
Subjects were obtained from the Pediatric Health Information System (PHIS) from 1/1/2008–1/1/2019. PHIS is a comparative pediatric database that contains inpatient, emergency department, ambulatory surgery, and observation unit information from 52 not-for-profit, tertiary care pediatric hospitals. Data include demographics, dates of service, discharge disposition, and daily inpatient billing data for medications, laboratory tests, imaging, procedures, clinical services, and supplies. Records can be linked longitudinally across admissions based on unique patient identifiers. Data are deidentified at the time of submission and data quality is assured through a joint effort between the Children’s Hospital Association and participating hospitals.
Study population
Children (< 19 years) discharged from one of 52 PHIS hospitals between 1/1/2008 and 3/31/2019 were considered for inclusion if they had at least one hospitalization with an International Classification of Diseases-9 (ICD-9) or ICD-10 discharge code for juvenile rheumatoid arthritis with systemic onset (714.30, M08.2x). The index admission was identified for patients with multiple admissions, which was defined as the first admission during the inclusion period without a prior discharge code of M08.2x or 714.30. Patients admitted to a hospital with ≤ 1 year of participation time in PHIS prior to the index admission were excluded to ensure a prior diagnosis of systemic JIA could be detected. Exclusion criteria included the following: 1) a primary discharge diagnosis of infection or malignancy; 2) patients who did not receive systemic JIA therapy to avoid including other diagnoses coded as 714.30, such as IBD-arthritis; 3) receipt of systemic JIA therapy other than scheduled NSAIDs within the first two hospital days of admission to avoid inclusion of disease flare. The ICD-9 code for systemic JIA (714.30) is less specific than the ICD-10 code (M08.2x), necessitating additional exclusion criteria for patients discharged from 1/1/2008 to 9/30/2015 (last date prior to transition to ICD-10 coding system): 1) discharge diagnostic codes for other rheumatologic conditions, uveitis, inflammatory bowel disease (IBD); 2) absence of laboratory billing code for ferritin during the hospitalization. Supplementary table S1 includes a list of discharge ICD codes and clinical transaction classification (CTC) codes used in exclusion criteria.
Medication exposure
Medication usage was determined using pharmacy billing data and CTC codes. Systemic JIA therapy was defined as methotrexate, glucocorticoids, anakinra, canakinumab, tocilizumab, or scheduled nonsteroidal anti-inflammatory drugs. Glucocorticoid exposure included oral or intravenous administration of dexamethasone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Biologic exposure included anakinra, rilonacept, canakinumab, or tocilizumab. Scheduled nonsteroidal anti-inflammatory drug (NSAID) use was defined as a code for ibuprofen, naproxen, indomethacin, piroxicam, diclofenac, meloxicam, or celecoxib on two or more consecutive hospital days. Oral and subcutaneous methotrexate and intravenous and subcutaneous tocilizumab were pooled.
Validation of study cohort
We validated the aforementioned process for identifying patients with new-onset systemic JIA at two PHIS participating centers, CHOP (ICD-9 and ICD-10 cohort) and Primary Children’s Hospital (PCH) (ICD-9 cohort only). Patient charts were reviewed at these two centers and determined to be either true positive or false positive new-onset systemic JIA admissions, using rheumatology provider diagnosis during the inpatient hospitalization as the reference standard. These values were then used to calculate the positive predictive values (PPV) separately for the ICD-9 and ICD-10 time periods.
Statistical analysis
All analyses were performed using data from the index admission. Standard descriptive statistics including range, mean and standard deviation (SD) for normally distributed variables or median and interquartile range for non-normally distributed variables were used, as appropriate. Trends in treatment over time were assessed using an extension of the Wilcoxon rank-sum test for trends and graphically displayed using simple linear regression. SD and coefficient of variation (CV) were used to describe variation in treatment between sites. In order to determine which clinical factors influenced the decision to treat with biologics and glucocorticoids, we fit a mixed-effects logistic regression model, accounting for within-hospital clustering by including a hospital-specific random effect. Separate models were fit for biologics and glucocorticoids. Other variables considered as fixed effects for each model included year of admission, demographics (age, sex, race, Medicaid insurance), hospital characteristics (region, mean systemic JIA volume per year, total annual patient volume, affiliated rheumatology fellowship), and disease severity indicators within first two hospital days prior to receipt of biologics or glucocorticoids. These disease severity indicators included intensive care unit [ICU] status, supplemental oxygen, laboratory billing code for blood gas, and multiple complete blood counts in a single hospital day. MAS was not included in the model as the onset of MAS during the hospitalization is unable to be determined in the PHIS database and may have occurred after treatment initiation. Stepwise selection was used to determine the final models. Likelihood ratio tests and Akaike information criterion (AIC) and Bayesian information criterion (BIC) were used to confirm final model selection.
All analyses were performed using Stata 15 (StataCorp. 2017, Stata Statistical Software. College Station, TX: StataCorp LP).
RESULTS
A total of 3,729 patients < 19 years of age with an ICD-9 or ICD-10 code consistent with systemic JIA (714.30, M08.2x) discharged between 1/1/2008 and 3/31/2019 were identified in the PHIS database. After applying the exclusion criteria, a cohort of 534 patients was identified for analysis from 51 US children’s hospitals distributed geographically across all five US regions (Figure 1). One PHIS hospital had no patients identified. The PPV of the study cohort identification process was 86.36% in the ICD-9 validation cohort (Table 1) and 100% in the ICD-10 validation cohort.
Figure 1. Study cohort selection.
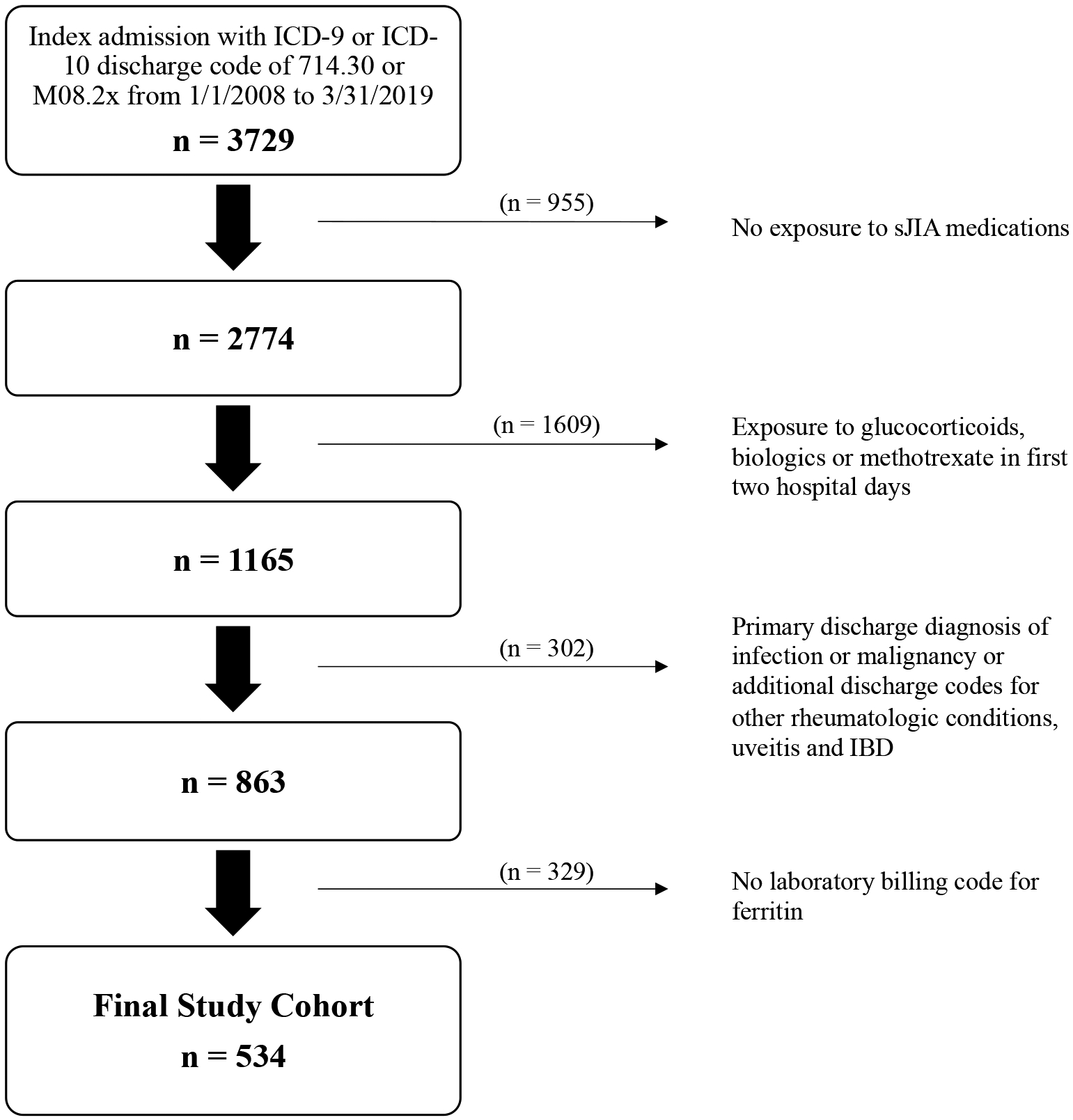
This figure shows the inclusion and exclusion criteria and selection of the cohort.
Table 1.
Positive Predictive Value Using Systemic JIA ICD-9 Codes Alone and in Combination with Additional Exclusion Criteria in CHOP and PCH Validation Cohorts
| Description | True Positives | False Positives | PPV |
|---|---|---|---|
| Step 1: ICD-9 code alone | |||
| CHOP | 16 | 120 | 11.76% |
| PCH | 12 | 127 | 8.63% |
| Overall (n = 275) | 28 | 247 | 10.18% |
| Step 2a: exclude admissions without CTC code for sJIA medications* | |||
| CHOP | 14 | 80 | 14.89% |
| PCH | 12 | 74 | 13.95% |
| Overall (n = 180) | 26 | 154 | 14.44% |
| Step 2b: same as step 2a, then exclude admissions with CTC code for glucocorticoids, biologics or methotrexate in first two hospital days | |||
| CHOP | 11 | 26 | 29.73% |
| PCH | 8 | 23 | 25.81% |
| Overall (n = 68) | 19 | 49 | 27.94% |
| Step 2c: same as step 2b, then exclude admission with a primary discharge diagnosis of infection or malignancy and additional discharge codes for other rheumatologic conditions, uveitis and IBD | |||
| CHOP | 11 | 22 | 33.33% |
| PCH | 8 | 12 | 40% |
| Overall (n = 53) | 19 | 34 | 35.85% |
| Step 2d (final algorithm): same as step 2c, then exclude admissions without a laboratory billing code for ferritin | |||
| CHOP | 11 | 1 | 91.67% |
| PCH | 8 | 2 | 80% |
| Overall (n = 22) | 19 | 3 | 86.36% |
Validation of ICD-9-CM codes done at 2 PHIS sites (CHOP and PCH).
IL-1 inhibitor, IL-6 inhibitor, glucocorticoids, methotrexate, or scheduled NSAIDs
Demographics, clinical characteristics, and medication exposures of the cohort are shown in Table 2. The median age at diagnosis was six years (IQR 3–12). 29.2% of patients received a biologic (26.0% IL1i, 3.7% IL6i) and 57.9% received glucocorticoids. The median length of stay was six days (IQR 4.0–9.0) and the all-cause 90-day readmission rate was 14%. 7.9% of patients required ICU level of care at some point during the hospitalization. 11.8% of patients had a discharge diagnosis of macrophage activation syndrome (MAS), which was shown to be a reliable ICD-9 code in a prior PHIS study characterizing a cohort of JIA and systemic lupus erythematosus patients with MAS (6). Biologic use significantly increased (p < 0.001) and methotrexate use significantly decreased (p = < 0.01) from 2008 to 2019. Glucocorticoid use did not significantly change during this time period (p = 0.70) (Figure 2). There was significant variation between hospitals in the proportion of patients treated with biologics and glucocorticoids with a median of 0.26 (IQR 0.11–0.40; CV 74.5%) and 0.61 (IQR 0.46–0.75; CV 39%), respectively.
Table 2.
Patients
| N = 534 | |
|---|---|
| Demographics | |
| Age, Median (IQR) | 6.0 (3.0, 12.0) |
| Sex (male), N (%) | 281 (52.6) |
| Race, N (%) | |
| White | 354 (66.3) |
| Black | 68 (12.7) |
| Asian | 17 (3.2) |
| Other | 95 (17.8) |
| Medicaid insurance, N (%) | 214 (40.1) |
| Hospital Region, N (%) | |
| Northeast | 92 (17.2) |
| Southeast | 90 (16.9) |
| Southwest | 60 (11.2) |
| Midwest | 156 (29.2) |
| West | 136 (25.5) |
| Clinical Features and Medication Exposures | |
| Length of stay in days, Median (IQR) | 6.0 (4.0, 9.0) |
| ICU level of care, N (%) | 42 (7.9) |
| Macrophage activation syndrome*, N (%) | 63 (11.8) |
| Glucocorticoids, N (%) | 309 (57.9) |
| Biologics^, N (%) | 156 (29.2) |
| Anakinra, N (%) | 137 (25.7) |
| Canakinumab, N (%) | 11 (2.1) |
| Tocilizumab, N (%) | 20 (3.7) |
| Methotrexate, N (%) | 41 (7.7) |
| Scheduled NSAIDs, N (%) | 452 (84.6) |
| Readmission ≤ 90 days after discharge#, N (%) | 75 (14.0) |
Based on discharge ICD CM code. Diagnosis of MAS may have occurred at any point during hospitalization.
Not mutually exclusive. 9 patients received both canakinumab and anakinra and 3 patients received both anakinra and tocilizumab during hospitalization.
All-cause readmissions.
Figure 2. Change in use of treatments for children hospitalized with new-onset systemic JIA from 2008–2019.
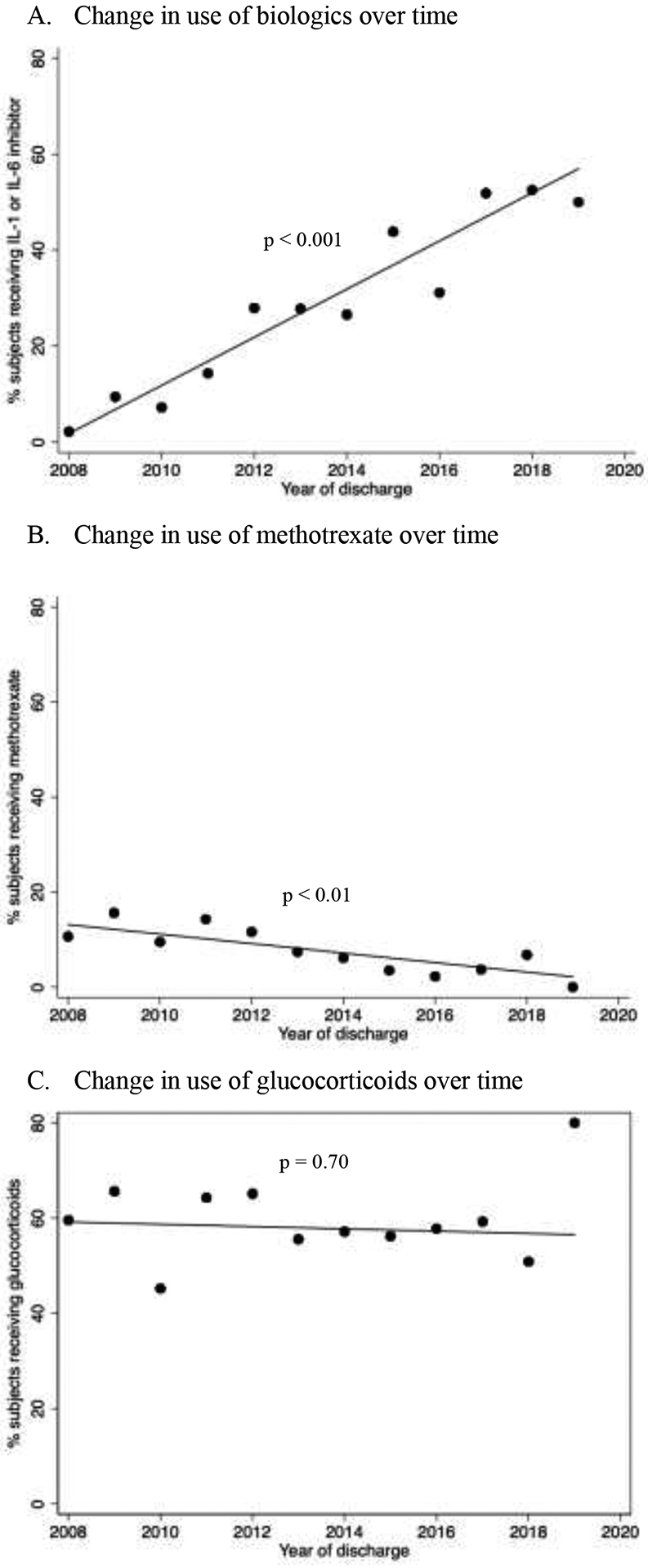
Each dot represents the raw percentage of patients in each year receiving A) biologics (IL-1 or IL-6 inhibitor), B) methotrexate, and C) glucocorticoids. The lines represent the best fit line. P-values for trends are shown on graphs.
The results of the multivariable mixed-effects logistic analysis to identify factors associated with biologic and glucocorticoid exposure at diagnosis are shown in Tables 3 and 4, respectively. Due to collinearity with annual hospital volume, average site systemic JIA volume per year was not included in variable selection for multivariable analysis for biologic or glucocorticoid exposure. High annual hospital volume (OR 6.68, 95% CI [1.54, 28.89]), ICU stay in first two hospital days (OR 4.94, 95% CI [1.64, 14.86]), and later discharge year were all significantly associated with biologic exposure. Annual hospital volume was the only variable found to be significantly associated with glucocorticoid exposure. This association was inversely proportional, with medium-high and high hospital volume associated with lower odds of glucocorticoid exposure (OR 0.15, 95% CI [0.05, 0.44] and OR 0.30, 95% CI [0.11, 0.79], respectively).
Table 3.
Odds ratios for association of biologic exposure with patient and hospital level factors
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|
| Patient level factors | ||
| Age | 1.04 (0.70–1.54) | ------- |
| Female gender | 0.88 (0.58–1.34) | 0.69 (0.42–1.14) |
| Race | ||
| White | [Reference group] | ------- |
| Black | 1.48 (0.80–2.76) | ------- |
| Asian | 1.88 (0.62–5.69) | ------- |
| Other | 1.07 (0.60–1.94) | ------- |
| Medicaid insurance | 1.43 (0.93–2.19) | ------- |
| Discharge year | ** | ** |
| Laboratory billing code for >1 complete blood count per day* | 2.59 (1.24–5.41) | 2.35 (0.94–5.91) |
| Laboratory billing code for venous or arterial blood gas* | 2.84 (1.18–6.88) | ------- |
| ICU level care* | 4.33 (1.70–11.06) | 4.94 (1.64–14.86) |
| Hospital level factors | ||
| Systemic JIA volume# | 1.47 (0.83–2.60) | ------- |
| Hospital inpatient admissions per year | ||
| Low (≤ 12,000) | [Reference group] | [Reference group] |
| Medium-low (12,000–15,999) | 1.35 (0.38–4.87) | 1.64 (0.37–7.24) |
| Medium-high (16,000–21,000) | 1.90 (0.57–6.39) | 3.44 (0.82–14.41) |
| High (≥21,000) | 3.59 (1.05–12.25) | 6.68 (1.54–28.89) |
| Pediatric Rheumatology fellowship | 1.36 (0.63–2.97) | ------- |
| Region | ||
| Northeast | [Reference group] | ------- |
| Southeast | 1.18 (0.32–4.28) | ------- |
| Southwest | 2.39 (0.58–9.81) | ------- |
| Midwest | 1.14 (0.35–3.77) | ------- |
| West | 1.03 (0.30–3.49) | ------- |
Within first two hospital days of admission
Systemic JIA volume characterized as mean number of systemic JIA patients per year at each site. Not included in multivariable model due to collinearity with annual hospital volume.
Discharge year was a significant positive predictor of biologic exposure and included in multivariable model (see Figure 2).
Table 4.
Odds ratios for association of glucocorticoid exposure with patient and hospital level factors
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|
| Patient level factors | ||
| Age | 1.02 (0.99–1.06) | ------- |
| Female gender | 1.02 (0.70–1.49) | ------- |
| Race | ||
| White | [Reference group] | ------- |
| Black | 0.92 (0.51–1.65) | ------- |
| Asian | 0.93 (0.31–2.76) | ------- |
| Other | 1.15 (0.68–1.95) | ------- |
| Medicaid insurance | 0.99 (0.67–1.47) | ------- |
| Discharge year | ** | ------- |
| Laboratory billing code for >1 complete blood count per day* | 1.42 (0.68–2.94) | ------- |
| Laboratory billing code for venous or arterial blood gas* | 2.07 (0.84–5.09) | ------- |
| ICU level care* | 1.66 (0.65–4.24) | ------- |
| Hospital level factors | ||
| Systemic JIA volume# | 0.78 (0.49–1.24) | ------- |
| Hospital inpatient admissions per year | ||
| Low (≤ 12,000) | [Reference group] | [Reference group] |
| Medium-low (12,000–15,999) | 0.68 (0.24–1.94) | 0.47 (0.16–1.37) |
| Medium-high (16,000–21,000) | 0.30 (0.11–0.81) | 0.15 (0.05–0.44) |
| High (≥21,000) | 0.35 (0.13–0.95) | 0.30 (0.11–0.79) |
| Pediatric Rheumatology fellowship | 0.72 (0.38–1.35) | ------- |
| Region | ||
| Northeast | [Reference group] | ------- |
| Southeast | 0.59 (0.21–1.68) | 0.75 (0.28–2.06) |
| Southwest | 1.18 (0.36–3.85) | 1.35 (0.46–3.92) |
| Midwest | 0.97 (0.37–2.52) | 1.24 (0.46–3.36) |
| West | 1.27 (0.47–3.39) | 3.11 (0.98–9.89) |
Within first two hospital days of admission
Systemic JIA volume characterized as mean number of systemic JIA patients per year at each site. Not included in multivariable model due to collinearity with annual hospital volume.
Discharge year was not a significant predictor of glucocorticoid exposure and not included in multivariable model (see Figure 2).
DISCUSSION
This study reports initial hospitalization characteristics and treatment approaches in 534 children diagnosed with systemic JIA across 52 geographically diverse US children’s hospitals. The demographics and clinical characteristics of our cohort, particularly age, gender, and diagnosis of MAS at disease onset are comparable to previously published systemic JIA cohorts (7,8,9,10). Our study highlights several important findings regarding treatment of patients with new-onset systemic JIA. First, use of biologics has steadily increased from 2008 to 2019 while glucocorticoid exposure has remained unchanged. Second, disease severity in the first two hospital days was associated with the decision to treat with biologics but was not associated with glucocorticoid exposure. Lastly, there was significant treatment variation between US children’s hospitals with higher utilization of biologics at high volume hospitals and conversely, higher utilization of glucocorticoids at low volume hospitals.
The first reports of successful use of IL-1 and IL-6 inhibition in systemic JIA were in 2005 (11,12,13). Randomized controlled trials over the next seven years demonstrated the safety and efficacy of tocilizumab, anakinra, and canakinumab (2,3,14). In 2013, the American College of Rheumatology (ACR) published an update to treatment recommendations for systemic JIA, recommending consideration of anakinra as initial therapy for patients with physician global assessment ≥ 5 as well as canakinumab or tocilizumab for patients with persistent disease activity, marking a major turning point in treatment approach to systemic JIA (15). Our findings that treatment with biologics in new-onset systemic JIA has increased from 2008 to 2019 parallels the emerging evidence and treatment guideline changes over this time period. Conversely, we found that glucocorticoid use has remained unchanged over time despite prospective studies demonstrating positive clinical outcomes with biologic monotherapy (5, 16). These temporal trends are consistent with previously published reports in other smaller observational cohort studies of patients with systemic JIA (7,8,10). Interestingly, we also found that decision to treat with glucocorticoids did not seem to be affected by markers of disease severity. Many providers may choose to treat new-onset systemic JIA with glucocorticoids as standard of care regardless of disease severity or anticipated response to alternative therapies such as biologics. While MAS is an additional factor that contributes significantly to medication choice in new-onset systemic JIA, this was unable to incorporated into the regression model due to unknown timing of MAS onset during the hospitalization.
The finding of treatment variation between US children’s hospitals in new-onset systemic JIA is not entirely unexpected. Provider surveys informing the creation of the Childhood Arthritis and Rheumatology Research Alliance (CARRA) consensus treatment plans (CTPs) as well as the initial CTP utilization highlighted significant inter-provider and inter-site variability (17). The association of hospital volume with glucocorticoid and biologic exposure, however, is a novel finding. Even after correcting for discharge year and markers of disease severity prior to treatment administration, hospitals with higher patient volume were more likely to prescribe biologics for new-onset systemic JIA patients and less likely to prescribe glucocorticoids. While there is the potential for residual confounding by indication that our models did not adjust for, we would have anticipated that high volume hospitals cared for sicker patients. Therefore, if residual confounding by indication persisted, this would have artificially increased the reported likelihood of glucocorticoid exposure at high volume hospitals. Additionally, high volume hospitals did not have a significantly different rate of discharge ICD code for MAS compared to low volume hospitals suggesting that the case mix amongst hospitals is comparable (p = 0.37). We hypothesize that physicians at higher volume hospitals may have more experience and comfort with using biologic medications. These sites may also have easier access to biologics through the inpatient pharmacy, whereas smaller sites may rely more on outpatient pharmacies and patient assistance programs, which would not have been captured in the PHIS database. Further studies are needed to describe the specific causes of treatment variation and identify potential barriers to first-line biologic use in new-onset systemic JIA.
There were several limitations to our study. First, it is important to note that this study was unable to evaluate continuation or dose of glucocorticoid therapy after hospital discharge. The duration of outpatient glucocorticoid treatment may be decreasing over time with improving clinical outcomes in systemic JIA, which was shown in a recently published single-site cohort study of new-onset systemic JIA patients diagnosed between 1995 and 2015 (10). Second, there was the potential for misclassification of the cohort, which is an inherent risk in epidemiologic studies utilizing administrative claims databases in which identification of patients is based on diagnostic codes. We attempted to overcome this misclassification by incorporating additional criteria for cohort inclusion. This process resulted in acceptable PPVs in the ICD-9 and ICD-10 cohorts suggesting that most of the patients included in the cohort were truly new-onset systemic JIA patients. It is important to note that we were unable to assess the sensitivity of our patient identification process. The restrictive inclusion and exclusion criteria may have led to omission of a small subset of patients with new-onset disease, particularly those in whom the diagnosis was certain on admission and received treatment within the first two hospital days. However, this approach was thought to be necessary to avoid including patients admitted with systemic JIA flares in the final cohort. Third, there may have been confounding by center in which treatment choice strongly clustered with individual site. We incorporated analytic techniques to address this by including site as a random effect in our mixed effects logistic regression model but residual confounding by center may have persisted. Fourth, due to small numbers of patients receiving IL-6 inhibition at diagnosis (n = 20), comparisons were unable to be made between IL-1 and IL-6 inhibition and these two patient groups were analyzed together in the biologic exposure group. Finally, this study was limited to children who required hospitalization at pediatric centers for diagnosis and initiation of treatment for systemic JIA so our results may not be generalizable to all children with systemic JIA or patients diagnosed in the outpatient setting. However, children who require hospitalization represent the highest acuity new-onset systemic JIA population, for whom the risk of morbidity and mortality is the highest. This patient population is the most likely to require first-line treatment with therapies such as biologics and glucocorticoids and thus, would benefit the most from movement towards more standardized care.
As increasing evidence emerges demonstrating improved outcomes with first-line treatment with biologics, it is critical to identify barriers to implementation of evidence-based care. There remains significant treatment variation across hospitals with a large proportion of children not receiving biologics and a persistence of high rates of glucocorticoid exposure over time. These results point to the need for more robust data regarding comparative efficacy of treatment strategies at diagnosis in the highest acuity hospitalized systemic JIA population, followed by improved treatment standardization.
Supplementary Material
Significance and Innovations:
This study is the largest cohort to evaluate treatment of hospitalized patients with new-onset systemic JIA
Use of biologics has steadily increased from 2008 to 2019 while glucocorticoid exposure has remained unchanged
Disease severity in the first two hospital days was associated with the decision to treat with biologics but was not associated with glucocorticoid exposure
There was significant treatment variation between US children’s hospitals with higher utilization of biologics at high volume hospitals and conversely, higher utilization of glucocorticoids at low volume hospitals
Acknowledgments
This work is not supported by any financial or other benefits from commercial sources and the authors do not have any financial disclosures or potential conflicts of interest.
References:
- 1.Beukelman T Treatment Advances in Systemic Juvenile Idiopathic Arthritis. F1000Prime Reports 2014; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruperto N, Brunner HI, Quartier P, Constantin T, Wulffraat N, Horneff G, et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. New England Journal of Medicine 2012; 367: 2396–406. [DOI] [PubMed] [Google Scholar]
- 3.De Benedetti F, Brunner HI, Ruperto N, Kenwright A, Wright S, Calvo I, et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. New England Journal of Medicine 2012; 367:2385–95. [DOI] [PubMed] [Google Scholar]
- 4.DeWitt E, Kimura Y, Beukelman T, Nigrovic PA, Onel K, Prahalad S. Consensus treatment plans for new-onset systemic juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). 2012;64:1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haar T, van Diikhuizen EH, Swart JF, van Roven-Kerkhof A, El Idrissi A, Leek AP, et al. Treat‐to‐target Using First‐line Recombinant Interleukin‐1 Receptor Antagonist Monotherapy in New‐onset Systemic Juvenile Idiopathic Arthritis: Results from a Five Year Follow‐up Study. Arthritis & Rheumatology. 2019; 71:1163–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett TD, Fluchel M, Hersh AO, Hayward KN, Hersh AL, Brogan TV, et al. Macrophage Activation Syndrome in Children with Systemic Lupus Erythematosus and Juvenile Idiopathic Arthritis. Arthritis and Rheumatism. 2012; 64: 4135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janow G, Schanberg LE, Setoguchi S, Hasselblad V, Mellins ED, Schneider R, et al. The Systemic Juvenile Idiopathic Arthritis Cohort of the Childhood Arthritis and Rheumatology Research Alliance Registry: 2010–2013. The Journal of Rheumatology. 2016; 43: 1755–62. [DOI] [PubMed] [Google Scholar]
- 8.Klotsche J, Raab A, Niewerth M, Sengler C, Ganser G, Kallinich T, et al. Outcome and Trends in Treatment of Systemic Juvenile Idiopathic Arthritis in the German National Pediatric Rheumatologic Database, 2000–2013. Arthritis & Rheumatology. 2016; 68: 3023–34. [DOI] [PubMed] [Google Scholar]
- 9.Sawhney S, Woo P, Murray K. Macrophage Activation Syndrome: A Potentially Fatal Complication of Rheumatic Disorders. Archives of Disease in Childhood. 2001; 85: 421–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baris H, Anderson E, Sozeri B, Dedeoglu F. Impact of biologics on disease course in systemic onset juvenile idiopathic arthritis. Clinical Rheumatology. 2018;37: 3263–73. [DOI] [PubMed] [Google Scholar]
- 11.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of Interleukin-1 (IL-1) in the Pathogenesis of Systemic Onset Juvenile Idiopathic Arthritis and Clinical Response to IL-1 Blockade. The Journal of Experimental Medicine. 2005; 201: 1479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woo P, Wilkinson N, Prieur A, Southwood T, Leone V, Livermore P, et al. Open Label Phase II Trial of Single, Ascending Doses of MRA in Caucasian Children with Severe Systemic Juvenile Idiopathic Arthritis: Proof of Principle of the Efficacy of IL-6 Receptor Blockade in This Type of Arthritis and Demonstration of Prolonged Clinical Improvement. Arthritis Research & Therapy. 2005; 7: R1281–R1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokota S, Miyamae T, Imagawa T, Iwata N, Katakura S, Mori M, et al. Therapeutic Efficacy of Humanized Recombinant Anti-Interleukin-6 Receptor Antibody in Children with Systemic-Onset Juvenile Idiopathic Arthritis. Arthritis and Rheumatism. 2005; 52: 818–25. [DOI] [PubMed] [Google Scholar]
- 14.Yokota S, Imagawa T, Mori M, Miyamae T, Aihara Y, Takei S, et al. Efficacy and Safety of Tocilizumab in Patients with Systemic-Onset Juvenile Idiopathic Arthritis: A Randomised, Double-Blind, Placebo-Controlled, Withdrawal Phase III Trial. The Lancet. 2008; 371: 998–1006. [DOI] [PubMed] [Google Scholar]
- 15.Ringold S, Weiss P, Beukelman T, DeWitt EM, Ilowite NT, Kimura Y, et al. 2013 Update of the 2011 American College of Rheumatology Recommendations for the Treatment of Juvenile Idiopathic Arthritis. Arthritis and Rheumatism. 2013; 65: 2499–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pardeo M, Bracaglia C, Tulone A, Insalaco A, Marucci G, Nicolai R. Op0057 Early Treatment with Anakinra in Systemic Juvenile Idiopathic Arthritis. Annals of the Rheumatic Diseases. 2019; 78: 100.30026257 [Google Scholar]
- 17.Kimura Y, Grevich S, Beukelman T, et al. Pilot study comparing the Childhood Arthritis & Rheumatology Research Alliance (CARRA) systemic Juvenile Idiopathic Arthritis Consensus Treatment Plans. Pediatr Rheumatol Online J. 2017;15:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


