Abstract
The objective of this study was to optimize the extraction of oil from pre-dried roselle seeds using response surface methodology (RSM). We also determined the oxidative stability of oil extracted from oven and freeze-dried roselle seed in terms of iodine value (IV), free fatty acid (FFA) value, peroxide value (PV), P-anisidine and total oxidation values (TOTOX value). The RSM was designated based on the central composite design with the usage of three optimum parameters ranged from 8 to 16 g of sample weight, 250–350 mL of solvent volume, and 6–8 h of extraction time. The highest oil yielded from roselle seed using the optimization process was 22.11% with the parameters at sample weight of 14.4 g, solvent volume of 329.70 mL, and extraction time of 7.6 h. Besides, the oil extracted from the oven dried roselle seed had the values of 89.04, 2.11, 4.13, 3.76 and 12.03 for IV, FFA, PV, P-anisidine, and TOTOX values, respectively. While for the oil extracted from freeze-dried roselle seed showed IV of 90.31, FFA of 1.64, PV of 2.47, P-anisidine value of 3.48, and TOTOX value of 8.42. PV and TOTOX values showed significant differences whereas; IV, FFA, and P-anisidine values showed no significant differences between the oven and freeze-dried roselle seed oils.
Keywords: Roselle seed, Oil, RSM, Drying method, Oxidative stability
Introduction
Roselle (Hibiscus Sabdariffa L.) with more than 300 species are widely grown and distributed in tropical and subtropical regions around the world (Da-Costa-Rocha et al. 2014). Nowadays, roselle is widely cultivated in India, Saudi Arabia, China, Malaysia, Indonesia, Philippines, Vietnam, Sudan, Egypt, Nigeria and Mexico (Riaz and Chopra 2018). The flowers have five sepals which enclosed a capsule that contain five valves with three to four kidney-shaped seeds (Ismail et al. 2008). Roselle calyces are used for making food products such as jam, jellies, chocolate, ice cream, flavoring agents, confectionery, and herbal drinks (Riaz and Chopra 2018). Roselle has been used in the treatment of kidney and urinary bladder stones in Thailand and India. While in Mexico, the flower and leaves of the roselle plants are used together for curing of hypertension (Riaz and Chopra 2018).
Usually, the seeds are discharged as waste in the beverage industries. However, in some countries like Africa the roasted seeds are used in soup and sauces (Atta and Imaizumi 2002; Omobuwajo et al. 2000). The seed contains considerable amounts of protein (33.5%), fat (22.1%), total dietary fibre (18.3%), carbohydrate (13%), and moisture content (9.9%). Oleic (37.93%), linoleic (35.01%), and palmitic (19.65%) acids are the dominant fatty acids found in roselle seed oil (Akinoso and Suleiman 2011). Besides, the antimicrobial effect of the roselle seed oil (RSO) has also been proved to be efficient to reduce the microbial growth (Patel 2014). It also contains tocopherols as antioxidant and the plant sterols which are beneficial to human (Mohamed et al. 2007). Thus, the optimization of the RSO is crucial in order to achieve the highest yield and its quality as health beneficial oil for consumption. The effects of drying methods on the pistachio seed oil studied by Sena-Moreno et al. (2015). Their results showed that the drying methods affect the oil stability and quality. However, the effects of drying methods on RSO and their quality are still under unexplored area of research. Therefore, the differences in the oil stability between the oil yielded from the freeze and oven-dried seeds are being analyzed to determine the best pre-treatment method that yield the highest oxidative stability oil.
Optimization using RSM is used in the determination of optimum value of parameters in order to obtain the maximum yield or best quality products (Jaswir et al. 2016). The way to predict the optimization process is to study the interaction and optimum effects of the experimental responses recorded from different sets of experiment (Jaswir et al. 2016; Gharaibeh 2018). Different sets of experiment are arranged by a system which involves different independent variables (Mathiarasi and Partha 2018). In the optimization, the independent variables that normally involves are temperature, volume of solvent, weight of sample, duration of the extraction, pH value, particle size of the sample, degree of polarity of the solvents, extraction method, and the types of solvent used (Azmir et al. 2014; Ajala et al. 2016; Mathiarasi and Partha 2018). The aim of this study was to examine the effects of process conditions: sample weight; solvent volume, and extraction time on the oil yields in an optimization study using central composite design (CCD). For the first time, oxidative stability such as iodine value, free fatty acid value, peroxide value, and p-anisidine value of RSO yielded from different drying methods were also determined.
Materials and methods
Sample preparation
The roselle seeds are removed from the capsules by crushing the capsules. Then, the seeds are collected, washed and dried. The seeds are separated into two portions which are treated in different ways. One portion was dried using freeze dryer at constant temperature of 50 °C for 24 h under vacuum pressure of 0.0145 kPa and a condenser temperature of − 48 °C (Gutierrez et al. 2008) and another portion with oven dryer at 30 °C for 24 h (Sena-Moreno et al. 2015). After that, the seeds were grounded into powder using warring blender. The moisture content was determined to ensure it is below 10%. Then, the powder was sieved (particle size < 200 μm) and stored in freezer (− 20 °C) until further analysis.
Extraction of roselle seed oil (RSO)
The oil from pre-treated roselle seed was extracted using petroleum benzene with modified standard Soxhlet extraction method at 60 °C and 6–8 h. The formula for total oil yield was expressed by the following equation:
| 1 |
Experimental design
The independent variables in the optimization of RSO yield studied were sample weight (X1), solvent volume (X2), and extraction time (X3). The temperature (60 °C), solvent type (petroleum benzene), and particle size (< 200 µm) were kept constant. A sample weight of 8–16 g, solvent volume of 250–350 mL and extraction time of 6–8 h were selected. This study had experimental design which consists of three parameters and 20 random runs based on the CCD. Six replicates with centre point were used for the prediction of “pure error”. Randomized experimental order was used in order to reduce the unexpected extraneous variables in the response. Second order polynomial regression equation was applied for the purpose of predicting the results or response variable (Y). The equation is as follow:
| 2 |
where Y = predicted response variables (yield of RSO in %), β0 = constant, βi = linear coefficient, βii = quadratic coefficient, βij = interactive coefficient, Xi and Xj = independent variables.
Oxidative stability under pre-treated drying
The oils were extracted from pre-treated (oven and freeze) roselle seed and determined their oxidative stability.
Determination of iodine, peroxide, and free fatty acid values
Official method established by American Oil Chemists’ Society (AOCS 1997) was used to determine iodine, peroxide, and free fatty acid values of the oven and freeze-dried RSO.
P-anisidine value
P-anisidine value of pre-treated (oven and freeze) RSO was determined according to AOCS method (1997).
Total oxidation (TOTOX) value assay
TOTOX value was calculated from the peroxide value and p-anisidine value of pre-dried RSO using the following Eq. 3 adapted from Ng et al. (2014).
| 3 |
Statistical analysis
The multiple regression analysis, analysis of variances, correlation coefficients, goodness of fit of regression model, and coefficient R2 for the optimization process were determined using Minitab software (version 18, Minitab Inc, USA). For the determination of the independent t test of oil stability from different drying methods, the Minitab programme was used. In the test of the statistical significance, a confident level of 95% was set as it is the basis for the determination of significance difference.
Results and discussion
Optimization of roselle seed oil
The observed oil yields from each run order are shown in Table 1. The highest oil yielded (22.11%) from the roselle seed in this optimization process was recorded at solvent volume of 329.7 mL, sample weight of 14.4 g and extraction time of 7.6 h. On the other hand, lowest oil yield (13.18%) was obtained from the run with the parameters at solvent volume of 270.3 mL, sample weight of 9.6 g and extraction time of 6.4 h (Table 1). From the Table 1, it showed the difference in magnitude of the independent variables that might cause differences in the results (Jahurul et al. 2018). This further indicated the importance of different magnitude of independent variables chosen which provides difference in the conditions and were related to the oil yields of the roselle seed in solvent extraction, especially in the optimization of oil extraction.
Table 1.
Running order of roselle seed oil extraction based on central composite design using RSM and its corresponding observed oil yield (%)
| Run | Sample weight (g) | Solvent volume (ml) | Time (h) | Yield (%) |
|---|---|---|---|---|
| 1 | 12.0 | 250.0 | 7.0 | 14.07 |
| 2 | 9.6 | 329.7 | 7.6 | 14.14 |
| 3 | 12.0 | 300.0 | 7.0 | 15.06 |
| 4 | 12.0 | 300.0 | 7.0 | 15.06 |
| 5 | 12.0 | 300.0 | 7.0 | 15.06 |
| 6 | 16.0 | 300.0 | 7.0 | 16.45 |
| 7 | 14.4 | 270.3 | 6.4 | 13.98 |
| 8 | 12.0 | 300.0 | 7.0 | 15.06 |
| 9 | 12.0 | 300.0 | 8.0 | 19.54 |
| 10 | 14.4 | 329.7 | 7.6 | 22.11 |
| 11 | 12.0 | 350.0 | 7.0 | 17.13 |
| 12 | 14.4 | 270.3 | 7.6 | 19.48 |
| 13 | 9.6 | 329.7 | 6.4 | 14.04 |
| 14 | 14.4 | 329.7 | 6.4 | 15.86 |
| 15 | 9.6 | 270.3 | 6.4 | 13.18 |
| 16 | 8.0 | 300.0 | 7.0 | 13.85 |
| 17 | 9.6 | 270.3 | 7.6 | 15.18 |
| 18 | 12.0 | 300.0 | 6.0 | 14.89 |
| 19 | 12.0 | 300.0 | 7.0 | 15.06 |
| 20 | 12.0 | 300.0 | 7.0 | 15.06 |
Table 2 shows the significant probability of the regression coefficients, R2, p value and lack of fit for the reduced response surface model. The determination of whether the reduced response surface model is the best fit for data was tested with the lack of fit test which has a null hypothesis showed that the RSM is the best fit model to specify the relationship between the response and the variables (Owolabi et al. 2018). The significance level that was determined which has a value of less than 0.05 indicated the lack of fit of the model, whereas when the significance level is higher than 0.05, it indicated that the model is fit to the examination of data (Owolabi et al. 2018). The accuracy and the general availability of the models are depended on the lack of fit value. In this study, the lack of fit value was larger than 0.05 which showed non-significance effect and also indicated the well fit of the models to the observed data (Jahurul et al. 2018). Furthermore, the regression, R2 was used in the description and determination of the acceptability of the response and the degree of fit of the regression model to the dataset. In order to be considered as a good fit model, the regression coefficient of determination that was determined from this dataset is suggested to be higher or equal to 0.80 (Jahurul et al. 2018). The regression coefficient of determination in this study was found to be 0.9283. This value indicated the adequacy of the adjustment of reduced response models in determining the oil yields of roselle seed.
Table 2.
Significanta probability (p values and F-ratio) of the solvent volume, samples weight, and extraction time (independent variables), regression coefficients, R2, p value and lack of fit for the reduced response surface model
| Regression coefficients | Oil yield |
|---|---|
| β0 | 179.2 |
| βi | − 7.86 |
| βj | − 0.0762 |
| βc | − 34.77 |
| βij | 0.00829 |
| βic | 0.853 |
| βjc | – |
| β2i | – |
| β2j | – |
| β2c | 1.943 |
| Main effects | Interaction effects | Quadratic effect | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | xi | xj | xc | xixj | xixc | xjxc | x2i | x2j | x2c |
| p value | 0.000 | 0.004 | 0.000 | 0.041 | 0.000 | – | – | – | 0.003 |
| F-ratio | 51.09 | 12.37 | 64.66 | 5.17 | 21.89 | – | – | – | 13.02 |
| Regression | R2 | p value | Lack of fit (F value) | Lack of fit (p value) | |||||
| Oil yield | 0.9283 | 0.000 | 4.56 | 0.056b | |||||
aOnly the terms with statistical significance are included
bNon-significant (p > 0.05)
The main effects or the linear parameters shows that the sample weight (xi, g), volume of solvent (xj, mL), and the extraction time (xc, h) exhibited significant effect (p < 0.05) on the oil yields. While the interaction effects between the sample weight, volume of solvent (xixj) and sample weight, extraction time (xixc) showed the synergistic effects (P < 0.05), the volume of solvent, extraction time (xjxc) exhibited no significant effects (p > 0.05) on the oil yields. On the other hand, the quadratic effects indicates only extraction time (x2c) showed significant positive effect (p < 0.05) on the oil yield, whereas the other two parameters, sample weight (x2i) and volume of solvent (x2x) had no significant effect (p > 0.05).
In the analyzing of the experimental oil yields to determine the constant and coefficients of the variables for the second order polynomial model, the multiple regression equation was applied. In second order polynomial model, the effects such as the main or linear, the interaction, and the quadratic effects of the variables were correlated to each other. The second order polynomial model equation exhibited two equations which included the initial and the final reduced model. Both of the equations were expressed with coefficient in the following Eq. 4a and 4b:
| 4a |
| 4b |
Effect of volume and weight on the RSO yield
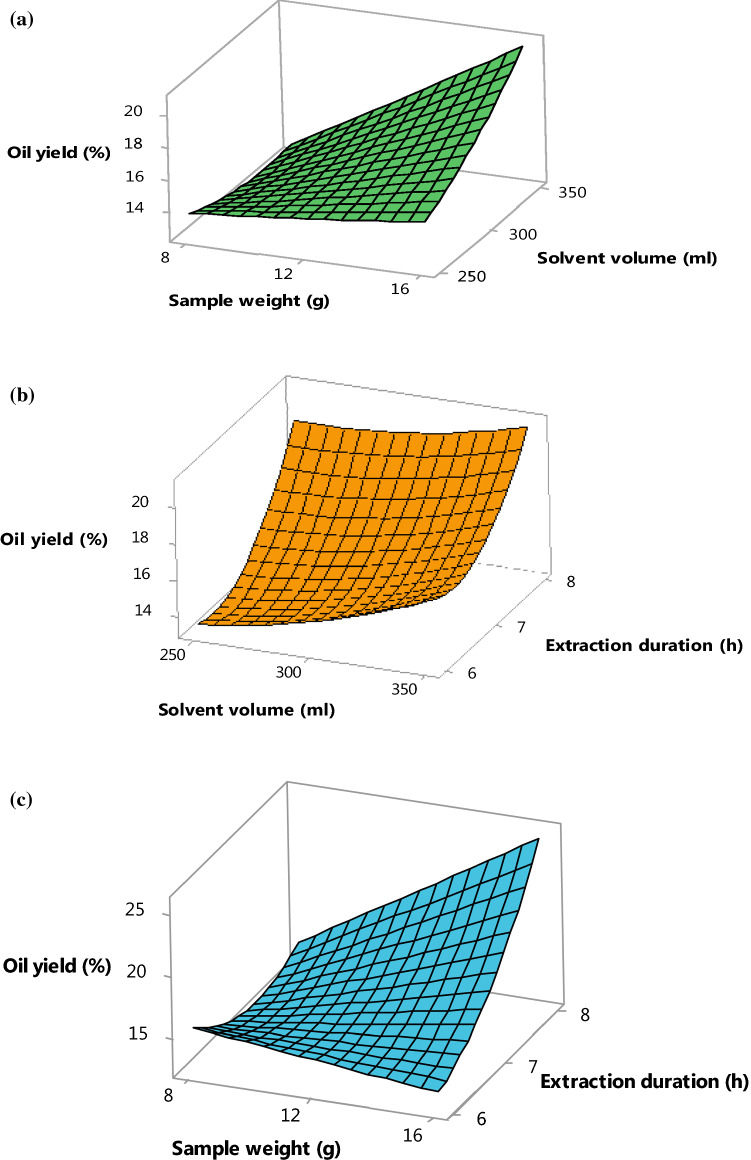
Figure 1a shows the effect of the solvent volume and sample weight to the oil yields with the extraction time maintained at 7 h. The oil yields slightly increased with the sample weight from 8 to 15 g. This was exhibited in Table 1, run order 5, 6, and 16. In these runs, the solvent and the time were set to be 300 mL and 7 h, respectively with the manipulation of the sample weight. From these runs, the oil yield was recorded to be 13.85% when sample weight was 8 g and the oil yields continuously increased from 15.06 to 16.45% with the sample weight from 12 to 16 g. The independent parameter of sample weight showed significant positive effect (p < 0.05) on the percentage of oil yields which was supported by the study performed by Mabayo et al. (2018), who stated that high number of feeds increased the oil yield in optimization process.
Fig. 1.
3D surface plot of oil yield of roselle seed as a function of sample weight, solvent volume and extraction time at a fixed a extraction time of 7 h, b sample weight of 12 kg and c solvent volume of 300 ml
Besides, the volume of solvent used in the extraction also exhibited a synergistic effect on the percentage of oil yield. This result was supported by the study conducted by Oniya et al. (2017), who stated that the increases in the solvent increased the oil yield. These can be seen from the run orders number 1, 11, and 19 in Table 1. In these run orders, the sample weight and time were held at constant, the changes in the solvent affected the percentage of oil yield. In run order 1, with the solvent volume of 250.0 mL, the oil yield recorded was 14.07%, further increment of the solvent in run order 11, the percentage of oil yield increased to 17.13% with the other condition remain constant. This indicated that the solvent volume has a positive effect (p < 0.05) on the oil yield (Table 1). This effect can be explained as high solvent volume used in the extraction, the rate of extraction, the solubility of oils and also the rate of diffusion of the solvent to the solid increases, thus the percentage of oil yield increased (Jahurul et al. 2018). Figure 1a shows the interaction effect of the sample weight and solvent volume that exhibited a significant positive effect (p < 0.05) on the oil yields. The percentage of oil yield increased as the sample weight and solvent volume increased. The run order 14 and 15 showed the increases of oil yield as the volume and sample weight increased (Table 1). Similar trends were observed by Ajala et al. (2016), who optimized shea butter using RSM.
Effect of solvent volume and time on the RSO yield
Figure 1b shows the effect of solvent volume and extraction time to the oil yields with the holding of sample weight at middle range (12 g). It can be seen from Fig. 1b that the total oil yields increased with the solvent volume and extraction time. The graph showed the similar linear effect of solvent volume as described earlier in the Fig. 1a, where the percentage of oil yields increased with the solvent volume. In this graph, the increases of the extraction time showed significant (p < 0.05) positive effects on the oil yield. The oil yield increased with time and this was supported by the results reported by Oniya et al. (2017), who stated that the increases in the extraction time increased the oil yield. Our results was also supported by Rodrigues-Miranda et al. (2014), who reported that the increases of the solvent contact time with the solid increased the oil yield. This trends can be seen in run order 8, 9, and 18 (Table 1). The oil yield was recorded to be 14.89% in run order 18, whereas it was 19.54% in run order 9 at fixed weight and solvent volume with manipulating times. This effect could be due to longer time needed for the solvent to penetrate into the matrix of the seed in order to extract the oil (Rodrigues-Miranda et al. 2014). Although, the increment of the extraction time causes the increases in the oil yield, the increment is not significant in the interaction effect of time and solvent volume. This was further shown in Table 2, where the interactive effects showed not significance (p > 0.05).
Effect of weight and time on the RSO yield
Figure 1c shows the effect of sample weight and extraction time on the oil yields, while maintain the solvent volume of 300 mL. The results showed that the percentage of oil yield was highly associated with the sample weight and extraction time. This can be seen in the run order 10 and 13 (Table 1). The lowest oil yield (14.04%) was achieved with sample weight of 9.6 g, time of 6.4 h, and solvent volume of 329.7 mL (run order 13). The highest oil yield (22.11%) was achieved when the sample weight and extraction time increased from 9.6 to 14.4 g and 6.4 to 7.6 h (run order 10). The run order 7 and 17 suggested that decreasing sample weight with increasing of extraction time increased the oil yields. This result was supported by the study performed by Ajala et al. (2016) and Jahurul et al. (2018), who stated that the oil yields decreased with the increased of the sample weight.
As shown in Table 1, the increases of the sample weight caused the decreased of oil yields. However, the total oil yields increased proportionally with extraction time. The longer the duration the higher the recovery of oil from roselle seed. Table 2 also showed that there was a significant (p < 0.05) interactive effect of the sample weight and the extraction duration to the percentage of oil yield.
Oxidative stability under pre-treated drying
Iodine value (IV)
The IV of oils extracted from pre-treated (oven and freeze-dried) roselle seeds were found to be 89.04 and 90.31 g iodine/100 g oil, respectively (Table 3). This value falls in the range of IV of RSO (81.45–98.32 g iodine/100 g oil), camellia oil (82.17), and chufa oil (82.48) reported by Nzikou et al. (2011), Lee et al. (2014) and Yoon (2016). However, Bamgboye and Adejumo (2010) and Eltayeb and Elaziz (2014) found high IV (111–119 g iodine/100 g oil) in RSO. The differences in the results could be due to the different growing region (Hainida et al. 2008a, b). The IV of oils extracted from oven and freeze-dried roselle seeds showed no significant differences (P > 0.05). This indicated that there was no difference in terms of degree of unsaturation between the oils extracted from oven and freeze-dried roselle seeds. This can also be explained as the oven and freeze-drying methods did not alter much on the degree of unsaturation of the RSO. The IV obtained in this study suggested that the oil did not oxidize easily and they are more stable and low chance to get rancid (Atta and Imaizumi 2002).
Table 3.
Iodine, free fatty acid, peroxide, P-anisidine, and TOTOX values of roselle seed oils extracted from different drying methods
| Oxidative stability | Drying methods | |
|---|---|---|
| Oven dried seed oil | Freeze dried seed oil | |
| Iodine value (g iodine/100 g oil) | 89.04a ± 0.80 | 90.31a ± 0.79 |
| Free fatty acid value (%) | 2.115b ± 0.705 | 1.645b ± 0.407 |
| Peroxide value (meq/kg) | 4.133c ± 0.503 | 2.467d ± 0.116 |
| P-anisidine value | 3.764e ± 0.405 | 3.484e ± 0.301 |
| TOTOX value | 12.031f ± 1.105 | 8.417 g ± 0.508 |
aNo significant difference between the value as p = 0.123 (P > 0.05)
bNo significant difference between the value as p = 0.391 (P > 0.05)
c,dSignificant difference between the value as p = 0.031 (P < 0.05)
eNo significant difference between the value as p = 0.407 (P > 0.05)
f,gSignificant difference between the value as p = 0.036 (P < 0.05)
Free fatty acid (FFA) value
The FFA value of the oils extracted from roselle seeds that undergone oven and freeze drying methods are shown in Table 3. The FFA values for oven and freeze-dried RSO were found to be 2.11 and 1.64%, respectively. The FFA value of freeze-dried RSO was found to be lower than oven-dried RSO. Although the result was slightly different, there was no significant difference (P > 0.05) which means they are same quality in terms of FFA. The FFA values were reported to be in the ranges of 0.43–2.4% for RSO and camellia oil by Atta and Imaizumi (2002), Mohamed et al. (2007), Nzikou et al. (2011) and Lee et al. (2014). The FFA value is an important indicator for the determination of oil quality. The low FFA value indicated low enzymatic hydrolysis, which controls the development of off flavour during storage, thus with low level of FFA, the oil can remain its quality in longer term. The high FFA value may cause the increase of acidity of the oil (Thomas 2000). Besides, it also increases the oxygen solubility and cause oxidation to take place which may cause the hydrolysis of triacylglycerols (Kochhar 2016). Thus, it is important to have FFA value as low as possible in order to have high quality oil in terms of stability and can retain its quality through storage.
Peroxide value (PV)
The PV of the oils extracted from oven and freeze-dried roselle seeds were found to be 4.13 and 2.47 (meq/kg), respectively (Table 3). The PV of the oil extracted from freeze-dried roselle seeds was found to be lower than oven-dried roselle seeds. The results showed significant different between the values as the p value was lower than 0.05. This indicated that the oils from freeze-dried roselle seed have high quality and low primary oxidation products. The PV falls in the range of the values (2.05–4.6) reported by Nzikou et al. (2011), Eltayeb and Elaziz (2014), Lee et al. (2014). However, the ranges were reported to be high in the other studies which achieved 6.0–9.0. This was still considered in the ranges of non-rancid oil as the rancid oil ranges from 20 to 40 meq/kg. However, this value cannot be used solely in the determination of quality of fats and oils as it has high decomposition rate of primary oxidation products into new hydroperoxide (Majchrzak et al. 2018). High PV had been reported by Sena-Moreno et al. (2015) for pistachio oil that undergone drying at high temperature.
P-anisidine value
P-anisidine value was used to measure the secondary oxidation products which formed from the decomposition of primary oxidation products, peroxide and hydroperoxide of fats and oils (Majchrzak et al. 2018). The P-anisidine values of oven and freeze-dried seeds were reported to be 3.76 and 3.48 (Table 3). The results were found to be no significant different between oven and freeze-dried RSO as the p-value was higher than 0.05. The P-anisidine values found in this study were lower than the P-anisidne values (6.17–8.52) reported by Gan et al. (2005) for edible oils. However, our results are in line with the results reported by Gan et at. (2005) for sunflower (4.52), palm (2.96), peanut (4.22), and canola (2.06) oils, respectively.
TOTOX value
TOTOX value or the total oxidation value is used for the determination of autoxidative state of fats and oils. It is calculated by the combination of peroxide and P-anisidine values (Thomas, 2000). TOTOX value can be determined the more accurate quality of oils in terms of the storage ability. The TOTOX values for the oils extracted from oven and freeze-dried seeds were 12.03 and 8.42, respectively (Table 3). There was a significant difference observed between the TOTOX values for oven and freeze-dried RSO. The lower TOTOX value of the freeze-dried seed oil indicated the high storage ability and high oil stability compared to the oil extracted from oven-dried seeds. Based on the results, it can be concluded that the stability and storability of oils from the freeze-dried seeds was better as less TOTOX value was found which also indicates less oxidation process occurred.
Conclusion
The designation of optimization model of RSO was based on the CCD with three variables using RSM. The highest yield (22.11%) was recorded at parameters of sample weight of 14.4 g, solvent volume of 329.7 mL, and extraction time of 7.6 h. It was found that the RSO increased as the solvent volume and extraction time increased, while decreased as the sample weight increased. The oil extracted from the oven-dried roselle seed had the values of 89.04, 2.12, 4.13, 3.76 and 12.03 for IV, FFA, PV, P-anisidine, and TOTOX values, respectively. While for the oil extracted from freeze-dried roselle seed showed IV of 90.31, FFA of 1.64, PV of 2.47, P-anisidine value of 3.48, and TOTOX value of 8.42, respectively. The results showed that the values of IV, FFA, and P-anisidine have no significant differences between the oils extracted from oven and freeze-dried roselle seeds. However, the PV and TOTOX values showed significant differences between the oven and freeze-dried RSO. The results showed that the freeze-dried roselle seed produced oil with higher quality in terms of stability and storage ability.
Acknowledgements
This research was supported by the Centre for Research and Innovation, Universiti Malaysia Sabah (UMS) (SDN0061-2019).
Compliance with ethical standards
Conflict of interest
No conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ajala EO, Aberuagba F, Olaniyan AM, Onifade KR. Optimization of solvent extraction of shea butter (Vitellaria paradoxa) using response surface methodology and its characterization. J Food Sci Technol. 2016;53(1):730–738. doi: 10.1007/s13197-015-2033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinoso R, Suleiman A. Heat treatment effects on extraction of roselle (Hibiscus sabdariffa L.) seed oil. Eur J Lipid Sci Technol. 2011;113:1527–1532. doi: 10.1002/ejlt.201100067. [DOI] [Google Scholar]
- American Oil Chemists’ Society (AOCS) Official methods and recommended practices of the american oil Chemists’ society. Champaign: AOCS Press; 1997. [Google Scholar]
- Atta MB, Imaizumi K. Some characteristics of crude oil extracted from Roselle (Hibiscus sabdariffa L.) seeds cultivated in Egypt. J Oleo Sci. 2002;51(7):457–461. doi: 10.5650/jos.51.457. [DOI] [Google Scholar]
- Azmir A, Zaidul ISM, Rahman MM, Sharif KM, Sahena F, Jahurul MHA, Mohamed A. Optimization of oil yield of Phaleria macrocarpa seed using response surface methodology and its fatty acid constituents. Indus Crops Prod. 2014;52:405–412. doi: 10.1016/j.indcrop.2013.11.009. [DOI] [Google Scholar]
- Bamgboye AI, Adejumo OI. Physicochemical properties of roselle seed oil. Nutr Food Sci. 2010;40(2):186–192. doi: 10.1108/00346651011029219. [DOI] [Google Scholar]
- Da-Costa-Rocha I, Bonnlaender B, Sievers H, Pischel I, Heinrich M. Hibiscus sabdariffa L.-A phytochemical ad pharmacological review. Food Chem. 2014;165:424–443. doi: 10.1016/j.foodchem.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Eltayeb AA, Elaziz AA. Physicochemical properties of Roselle (Hibiscus sabdariffa L.) seeds oil (Elrahad-1) in North Kordofan. Sudan. J Sci Innov Res. 2014;3(6):578–582. [Google Scholar]
- Gan HL, Man YBC, Tan CP, NorAini I, Nazimah SAH. Characterisation of vegetable oils by surface acoustic wave sensing electronic nose. Food Chem. 2005;89:507–518. doi: 10.1016/j.foodchem.2004.03.005. [DOI] [Google Scholar]
- Gharaibeh MA. Reliability analysis of vibrating electronic assemblies using analytical solutions and response surface methodology. Microelectron Reliab. 2018;84:238–247. doi: 10.1016/j.microrel.2018.03.029. [DOI] [Google Scholar]
- Gutierrez LF, Ratti C, Belkacemi K. Effects of drying method on the extraction yields and quality of oils from quebec sea buckthorn (Hippophae¨ rhamnoides L.) seeds and pulp. Food Chem. 2008;106:896–904. doi: 10.1016/j.foodchem.2007.06.058. [DOI] [Google Scholar]
- Hainida E, Ismail A, Hashim N, Mohd-Esa N, Zakiah A. Effects of defatted dried roselle (Hibiscus sabdariffa L.) seed powder on lipid profiles of hypercholesterolemia rats. J Sci Food Agric. 2008;88:1043–1050. doi: 10.1002/jsfa.3186. [DOI] [Google Scholar]
- Hainida E, Amin KI, Normah I, Mohd-Esa HN. Nutritional and amino acid contents of differently treated Roselle (Hibiscus sabdariffa L.) seeds. Food Chem. 2008;111:906–911. doi: 10.1016/j.foodchem.2008.04.070. [DOI] [Google Scholar]
- Ismail A, Ikram EHK, Nazri HSM. Roselle (Hibiscus sabdariffa L.) seeds nutritional composition protein quality and health benefits. Food. 2008;2(1):1–16. [Google Scholar]
- Jahurul MHA, Beh LK, Sharifudin MS, Hasmadi M, Zaidul ISM, Jinap S, Ali ME, Mohd Omar AK. Optimization of fat yield of bambangan (Mangifera pajang) kernel using response surface methodology and its antioxidant activities. J Food Measur Charact. 2018;12(2):1427–1438. doi: 10.1007/s11694-018-9758-8. [DOI] [Google Scholar]
- Jaswir I, Alotaibi A, Jamal P, Octavianti F, Lestari W, Hendri R, Alkahtani H. Optimization of extraction process of plant-based gelatin replacer. Int Food Res J. 2016;23(6):2519–2524. [Google Scholar]
- Kochhar P. Thermal stability of fats for high temperature applications. In: Sanders TAB, editor. Functional dietary lipids: food formulation, consumer issues and innovation for health. Sawston: Woodhead Publishing; 2016. pp. 103–148. [Google Scholar]
- Lee SY, Jung MY, Yoon SH. Optimization of the refining process of camellia seed oil for edible purposes. Food Sci Biotechnol. 2014;23(1):65–73. doi: 10.1007/s10068-014-0009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabayo VIF, Aranas JRC, Cagas VJB, Cagas DPA, Ido AL, Arazo RO. Optimization of oil yield from Hevea brasiliensis seeds through ultrasonic-assisted solvent extraction via response surface methodology. Sustain Environ Res. 2018;28(1):39–46. doi: 10.1016/j.serj.2017.08.001. [DOI] [Google Scholar]
- Majchrzak T, Wojnowski W, Dymerski T, Gębicki J, Namieśnik J. Electronic noses in classification and quality control of edible oils: a review. Food Chem. 2018;246:192–201. doi: 10.1016/j.foodchem.2017.11.013. [DOI] [PubMed] [Google Scholar]
- Mathiarasi R, Partha N. Optimization, kinetics and thermodynamic studies on oil extraction from Daturametel Linn oil seed for biodiesel production. Renew Energ. 2018;96:583–590. doi: 10.1016/j.renene.2016.04.078. [DOI] [Google Scholar]
- Mohamed R, Fernandez J, Pineda M, Aguilar M. Roselle (Hibiscus sabdariffa) Seed oil is a rich source of γ-tocopherol. J Food Sci. 2007;72(3):207–211. doi: 10.1111/j.1750-3841.2007.00285.x. [DOI] [PubMed] [Google Scholar]
- Ng S-K, Choong Y-H, Tan C-P, Long K, Nyam K-L. Effect of total solids content in feed emulsion on the physical properties and oxidative stability of microencapsulated kenaf seed oil. LWT Food Sci Technol. 2014;58:627–632. doi: 10.1016/j.lwt.2014.03.010. [DOI] [Google Scholar]
- Nzikou JM, Bouanga-Kalou G, Matos L, Ganongo-Po FB, Mboungou-Mboussi PS, Montoula FE, Panyoo-Akdowa E, Silou TH, Desobry S. Characteristics and nutritional evaluation of seed oil from roselle (Hibiscus sabdariffa L.) in Congo-Brazzaville. Curr Res J Biol Sci. 2011;3(2):141–146. [Google Scholar]
- Omobuwajo TO, Sanni LA, Balami YA. Physical properties of sorrel (Hibiscus sabdariffa) seeds. J Food Eng. 2000;45:37–41. doi: 10.1016/S0260-8774(00)00039-X. [DOI] [Google Scholar]
- Oniya OO, Oyelade JO, Ogunkunle O, Idowu DO. Optimization of solvent extraction of oil from sandbox kernels (Hura crepitans L.) by a response surface method. Energ Policy Res. 2017;4(1):36–43. doi: 10.1080/23815639.2017.1324332. [DOI] [Google Scholar]
- Owolabi RU, Usman MA, Kehinde AJ. Modelling and optimization of process variables for the solution polymerization of styrene using response surface methodology. J King Saud Univ Eng Sci. 2018;30:22–30. [Google Scholar]
- Patel S. Hibiscus sabdariffa: an ideal yet under-exploited candidate for nutraceutical applications. Biomed Preven Nutr. 2014;4:23–27. doi: 10.1016/j.bionut.2013.10.004. [DOI] [Google Scholar]
- Riaz G, Chopra RA. review on phytochemical and therapeutic uses of Hibiscus Sabdariffa L. Biomed Pharmacother. 2018;102:575–586. doi: 10.1016/j.biopha.2018.03.023. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Miranda J, Hernandez-Santos B, Herman-Lara E, Gomez-Aldapa CA, Garcia HS, Martinez-Sanchez CE. Effect of some variables on oil extraction yield from Mexican pumpkin seeds. CyTA J Food. 2014;12(1):9–15. doi: 10.1080/19476337.2013.777123. [DOI] [Google Scholar]
- Sena-Moreno E, Pardo JE, Catalán L, Gómez R, Pardo-Giménez A, Alvarez-Ortí M. Drying temperature and extraction method influence physicochemical and sensory characteristics of pistachio oils. Eur J Lipid Sci Technol. 2015;117(5):684–691. doi: 10.1002/ejlt.201400366. [DOI] [Google Scholar]
- Thomas A. Fats and fatty oils. Ullmann’s encyclopedia of industrial chemistry. Hamburg: Unimills International; 2000. [Google Scholar]
- Yoon SH. Optimization of the refining process and oxidative stability of chufa (Cyperus esculentus L.) oil for edible purposes. Food Sci Biotechnol. 2016;25(1):85–90. doi: 10.1007/s10068-016-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]