Abstract
Abstract
Salty taste is an important sensory attribute in many foods, which stimulates the appetite. But high-salt diets bring many health risks, and salty alternatives should be explored to solve this problem. The salt-reducing agents may impart new odors in food. Therefore, the research should focus on developing a novel agent, which would replace the salt without affecting the taste of the food. Generally, some yeast extracts taste salty and can be used for replacing salts in foods without imparting any additional odor. In this study, we fractionated salty peptides from FA31 (Angel Yeast) by ultrafiltration, gel permeation chromatography, preparative liquid chromatography (pre-HPLC), with the combination of sensory evaluation, and the peptide sequence was identified by ESI-Q-TOF LC/MS as Asp-Asp, Glu-Asp, Asp-Asp-Asp, Ser-Pro-Glu, and Phe-Ile.
Graphic abstract
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04836-1) contains supplementary material, which is available to authorized users.
Keywords: Yeast extract, Fractioned, Salty peptide, ESI-Q-TOF LC/MS
Introduction
Salty taste is an important sensory attribute in different foods, which is generally imparted by sodium chloride. The sodium salt not only increases the salty taste, but also reduces the bitterness, enhances the sweetness and other flavors, and stimulates the appetite (Inguglia et al. 2017; Ganesan et al. 2014; Liem et al. 2011). In many countries and regions in the world, people's demand for salt is very high, especially in China, there is a saying called "No salt is not happy". Salt is consumed not only from cooked foods at home but also mostly from processed foods (Gębski J et al. 2019) A high intake of sodium causes high blood pressure, cardiovascular disease, stomach cancer, decreased bone density, and possibly obesity (Israr et al. 2016). Therefore, researches should focus on reducing the salt content in foods while ensuring their quality, taste and functional properties. In 2013, the World Health Assembly officially aimed at reducing salt intake by 30% by 2025. "As one of the nine voluntary global goals for the prevention and control of non-communicable diseases."
Most countries have adopted various salt reduction measures during food-processing, food labeling, and publicity. One of the main ways to reduce the sodium content in food is to replace NaCl with other chloride salts or ingredients. The importance of sodium evolution is reflected in a taste quality that identifies sodium in food. Therefore reducing or enhancing other tastes is undesirable. Lithium tastes like pure salt, but it is toxic. Salts of potassium also can replace sodium salt (Chandrashekar et al. 2010; Van Der Klaauw and Smith 1995; Strazzullo et al. 2009). Although it has been found in use that a source of salty taste can be substituted, the resulting reduction or enhancement of other tastes is undesirable, but in order to ensure the flavor, it is necessary to adjust and optimize the sensory characteristics. Reducing sodium chloride in the food without affecting the flavor of the food is a recognized problem. Salty flavor enhancers (such as salty peptides) are also being investigated. Many studies have reported that the amount of salt in food can be gradually reduced, but this strategy can substitute only a small amount of salt over a long time span (Chandrashekar et al. 2010; Van Der Klaauw and Smith 1995; Strazzullo et al. 2009).
At present, researches are focused on searching alternatives to salt (such as potassium chloride).In this case, the way to maximize the amount of salt or NaCl in the substitute food and minimize the sensory perception after reducing the sodium content is to add flavor enhancers (Brandsma 2006a, b). Among the common flavor enhancers obtained from microbial sources, YE has outstanding functions. Several studies have evaluated that adding yeast extract to salt substitutes can reduce sensory effects and achieve salt reduction without reducing salt (Mitchell et al. 2011; Silva et al. 2017). Through a research report on YE (Li et al. 2016a), YE is used as a flavor ingredient, which is rich in flavor precursors (non-volatile compounds), including amino acids, nucleotides and peptides.
In 1984, Tada et al. discovered salty peptides. Studies have shown that Orn-β-Ala HCl and Orn-Tau HCl have a similar salty taste as NaCl (Makoto Tada 1984). Peng et al. obtained a salty peptide with the sequence Gly-Lys (Peng et al. 2011). Li et al. reported to analyze the stability of salty peptides (Li, 2016a). Alim and Liu isolated and identified sour peptides, bitter peptides, umami peptides, and peptides with a strong taste in yeast extracts (such as Arg-Leu, Phe-Thr, Phe-Gln, and Pro-Leu, respectively). (Alim et al. 2019). The use of salty peptides to increase the salty taste of food under the same NaCl content is an effective method.
Michelle et al. evaluated the effect of five salt substitutes and flavor enhancers of potassium chloride, potassium chloride + autolyzed yeast, potassium chloride + L-lysine, whole cell yeast, and nucleotide yeast extract through sensory analysis. Sensory tests have shown that the salt content in the commercial chili Bolognese can be reduced by up to 40% without detecting the difference in salty taste. Different commercial salt substitutes, especially commercial yeast extracts, can reduce salt up to 60% without affecting the salty taste. KCl-based salt substitutes can impart a bitter and metallic taste (Mitchell et al. 2011). Silva et al. compared the changes in the texture and functional properties of probiotic Prato cheese when added to potassium chloride, arginine, yeast extract, oregano extract (instead of sodium chloride). The addition of arginine, yeast extract and oregano extract to Cheese samples improved the robustness and elasticity significantly than traditional cheeses, but potassium chloride reduced these properties. Compared to the cheese, supplemented with arginine, sodium chloride and potassium chloride, the cheese supplemented with yeast extract and oregano extract exhibited less spatial voids and a more uniform and compact protein structure. The addition of arginine to the probiotic Prato cheese promotes the synthesis of antioxidant peptides and angiotensin-converting enzyme inhibitory peptides, while yeast extract and Oregano extract improve and produce antioxidant peptides (Silva et al. 2017).
Therefore, The full-flavored yeast extracts, containing amino acids and peptides can be used as salt substitutes. The salt-free yeast extract FA31 of Angel Yeast imparted a salty taste, and its peptide content was up to 80%; so this product was selected to isolate and identify salty peptides. In 1984, Tada et al. discovered salty peptides. Studies have shown that Orn-β-Ala HCl and Orn-Tau HCl have a similar salty taste as NaCl (Makoto Tada 1984). Peng et al. used Alcalase and pepsin to digest soybean protein, using layers after chromatographic separation, purification, and analyzing using liquid chromatography and other methods, the salty peptides with the sequence Gly-Lys were obtained (Peng et al. 2011). Li and others reported that bovine bone marrow (BBM) was enzymatically digested by a protease of animal origin and Flavourzyme™. The salty peptide was identified by MALDI-TOF/MS, which had a mass-to-charge ratio of 679.5109 (Li et al. 2016a). The stability of the salty peptides was analyzed. The results show that the addition of edible sugar did not affect the stability of salty peptides, but the addition of organic acids would reduce their stability (Li et al. 2016b). Due to its flavor-enhancing capacity, yeast extract is used in soy sauce, soybean paste, seasoning for instant noodles, instant soup base, and other foods for reducing the sodium chloride content (Alim et al. 2019). However, the researches of the salty substances in YE are rarely reported. So, this study was aimed at isolating and identifying salty substances in yeast extracts and providing useful information or scientific basis of saltiness in enhancing the feature of yeast extract.
This study aims to isolate and identify salty substances in yeast extract, find useful salty peptides, and evaluate the sensory characteristics of these peptides, so as to provide useful information or scientific basis for salty taste to improve the functionality of yeast extract.
Materials and methods
Materials and reagents
Yeast extract FA31 powder was supplied by Hubei Angel Yeast Co., Ltd. Ultrapure water was prepared by Pure water meter (Millipore, Waltham, MA, USA). Methanol and formic acid of chromatographic purity were purchased from Fisher Company, USA. Sodium hydroxide of analytical grade was purchased from Sinopharm Chemical Reagent Company.
Methods
Quantitative analysis of free amino acids
Sixteen free amino acids (Asp, Glu, Ser, His, Gly, Thr, Arg, Ala, Tyr, Cys, Val, Met, Phe, Ile, Leu, Lys) was quantified in the sample using Agilent's special pre-column derivatization method with boric acid, FMOC, and OPA. Amino acid mixed standard reagent (1 nmol/μL) was diluted with 0.1 mol/L HCl to prepare mixed standard injections with different concentrations, and the samples were quantified by external standard method. A 0.2 g/mL YE solution was prepared and filtered through a 0.22 μm filter membrane in a 2-mL liquid phase vial for future use.
HPLC conditions: Column: Zorbax Eclipse-AAA (4.6 mm × 150 mm, 5 μm); mobile phase: water phase A: 0.04 mol/L NaH2PO4 aqueous solution (pH 7.8), organic phase B: methanol: acetonitrile: water (45: 45: 10, V / V / V); gradient elution procedure: time / organic phase B (%): 0/0, 1.9/0, 18.1/37, 23.2/37, 24/100, 26/100, 27/0, 30/0; flow rate: 1 mL/min; injection method: program injection; injection volume: 1 μL; UV detection wavelength: 338 nm; column temperature: 40 °C.
Peptide distribution analysis
HPLC gel chromatography was employed to determine the peptide distribution in YE. Aprotinin (Mr = 6512 Da), vitamin B12 (Mr = 1355 Da), glycine-glycine-tyrosine-arginine (Mr = 451 Da), glutathione (Mr = 307 Da), glycine (Mr = 75 Da), the concentrations were 2 mg/mL. The calibration curve and its equation were obtained by plotting the logarithm of relative molecular mass (lg Mw) against retention time. HPLC conditions were: Zorbax Eclipse-AAA (4.6 mm × 150 mm, 5 μm) column; mobile phase was 20% acetonitrile in water, pH was adjusted to 3.0 with formic acid; flow rate: 0.5 mL/min; column temperature: 25 °C; UV detection wavelength: 220 nm; injection volume is 1 μL. A 0.2 g/mL YE solution was prepared and filtered through a 0.22 μm membrane-filter in a 2-mL liquid phase vial for further use.
Ultrafiltration separation
Forty grams of sample FA31 was weighed, dissolved in 2 L of ultrapure water, and it was filtered with a Buchner funnel. Then membranes of 1000 Da, 3000 Da, 5000 Da filters were utilized in the Mini Pellicon tangential flow ultrafiltration system for ultrafiltration. The filtrate was separated step by step. Retentate and filtrate of < 1000 Da, 1000–3000 Da, 3000–5000 Da, and > 5000 Da were obtained accordingly (Charve et al. 2018).
Freeze-drying
The filtrate and the retentate were frozen in a refrigerator at − 80 °C for more than 8 h, dried to a powder form using a freeze dryer, and stored at − 20 °C.
Gel permeation chromatography
The salty fraction was dispensed into a 0.1 g/mL solution and fractioned using an ÄKTA protein purifier equipped with a Superdex peptide 10/300 GL gel chromatography column. The elution conditions were maintained as follows: the mobile phase is a formic acid–water solution with pH = 4.0; UV detection wavelength: 280 nm, flow rate: 0.4 mL/min; injection volume is 500 μL. After multiple injections, the same components were combined together, freeze-dried and stored at 20 °C (Charve et al. 2018).
Preparative liquid chromatographic separation
The salty components were dissolved to prepare a 50 mg/mL solution, and further separated by preparative liquid chromatography equipped with an XDB-C18 column. The mobile phase was methanol and water, the UV detection wavelength was 280 nm, the flow rate was 10 mL/min, and the injection volume was 3 mL. The elution procedure was: methanol 5–15%, 0–15 min; methanol 15–25%, 15–17 min; methanol 25–60%, 17–30 min; methanol 60–5%, 30–32 min. The relevant components were collected according to the chromatographic peaks, and the components were freeze-dried and stored at − 20 °C (Charve et al. 2018).
Identification of the peptide sequences by ultrahigh performance liquid chromatography-quadrupole time-of-flight mass spectrometry
The above-mentioned salty components were dissolved in a certain amount of ultrapure water, and the Eclipse Plus C18 high-efficiency separation column was used for qualitative analysis by Q-TOF LC/MS. Methanol–water was used as the mobile phase. The flow rate was 0.2 mL/min, the injection volume was 2 μL, and the column temperature was 35 °C. The elution procedure is 3–20% of methanol, 0–20 min for 1 min; 80–100% of methanol, 21–30 min; 100–5% of methanol, 30–32 min. The mass spectrometry conditions are as follows (Kang 2017; Liu et al. 2015; Xu et al. 2018; Liu 2015):
Ion source: ESI positive ion mode; capillary voltage: 3500 V; nozzle voltage: 500 V; atomizing pressure: 35 psi, dryer temperature: 300 °C; flow rate: 5 L/min; carrier gas: N2; temperature: 150 °C; flow rate: 1 L/min; flow rate: 0.2 mL/min; fragmentor voltage: 135 V; collision energy: 100, 110, 120, 300 eV; ion scanning range: 50–1000 m/z.
Synthetic peptide standards to verify saltiness
The identified peptide was synthesized, and the synthesized peptide was diluted with purified water, beef broth, and vegetable broth to verify whether each peptide tastes salty. Among them, the preparation methods for the beef and vegetable soups were following: 1 kg of beef (cabbage) was cut into small dices, 2 L of ultrapure water was added and boiled for 3 h, cooled to 2 °C, centrifuged to remove the fat, and then the supernatant was stored in a refrigerator at − 20 °C (Alim et al. 2019).
Sensory evaluation
Ten sensory panelists (7 females and 3 males) were from the Molecular Sensory Science Laboratory of Beijing Technology and Business University; all of them had more than one year of experience in sensory evaluation. The taste perception training was the following: 20 mmol/L citric acid for sour taste; 50 mmol/L sucrose for sweet taste; 1 mmol/L caffeine for bitter taste; 20 mmol/L NaCl for salty taste; 10 mmol/L monosodium glutamate (MSG) for umami taste; and 10 mmol/L glutathione (GSH) for kokumi taste.
Data processing
The data was processed using Agilent Masshunter B 07.00, and the chart was prepared using Origin 2018.
Results and discussion
Quantitative results of free amino acids
The concentrations of 16 amino acids in FA31 were measured by high-performance liquid chromatography, as shown in Table 1. α-Amino acids have four different tastes: sour, sweet, bitter and umami. Amino acids and their derivatives have a certain taste. The original sample has high levels of tyrosine (Tyr), leucine (Leu), and alanine (Ala). This is the same as the work of Münch et al. (1997). Besides, Glu presents in a considerable amount, it is umami in taste, and may be an enhancer for saltiness taste, as stated by Onuma et al. (2018). Amino acids have a typical reaction of amino and carboxyl groups, they are amphoteric substances and can form internal salts by themselves. This has a certain effect on taste.
Table 1.
Content of free amino acids in FA31 (mg/L)
| Asp | Glu | Ser | His | Gly | Thr | Arg | Ala |
|---|---|---|---|---|---|---|---|
| 18.02 ± 1.33 | 66.18 ± 2.94 | 15.34 ± 0.33 | 82.04 ± 3.1 | 60.32 ± 1.89 | 51.47 ± 1.19 | 38.59 ± 0.18 | 115.93 ± 0.89 |
| Tyr | Cys | Val | Met | Phe | Ile | Leu | Lys |
|---|---|---|---|---|---|---|---|
| 492.66 ± 9.06 | 25.58 ± 1.21 | 15.47 ± 0.3 | 25.11 ± 1.47 | 44.64 ± 0.21 | 16.48 ± 0.1 | 146.33 ± 7.87 | 39.51 ± 0.24 |
Peptide distribution
The percentage of distributions of different mass peptides in FA31 is shown in Table 2. Peptides are compounds formed by linking α-amino acids together by peptide bonds. It is also an intermediate product of protein hydrolysis. Generally speaking, their molecular weight is less than 10,000 Da. The percentage of peptide distribution in FA31 < 1000 Da component accounts for 77%, and the increase in the proportion of small molecule peptides leads to an increase in the concentration of certain amino acids.
Table 2.
Percentage distributions of different mass peptides in FA31 (%)
| > 5000 Da | 1000–5000 Da | < 1000 Da | |
|---|---|---|---|
| Percentage | 10.23 ± 0.19 | 12.37 ± 0.26 | 77.4 ± 0.57 |
Ultrafiltration separation results
The components of the retentate and filtrate after ultrafiltration were lyophilized and subjected to sensory evaluation; the results are shown in Table 3. The salty taste of the < 1000 Da component was the most intense, so the components of < 1000 Da were selected for subsequent separation and identification.
Table 3.
Intensity of salty taste in each retentate
| > 5000 Da | 3000–5000 Da | 1000–3000 Da | < 1000 Da | |
|---|---|---|---|---|
| Saltiness | − | − | − | + + |
Gel permeation chromatography results
According to Fig. 1, a total of 6 components (Gel-1–Gel-6) of < 1000 Da were obtained by gel permeation chromatography. The obtained fraction was freeze-dried and then dissolved in ultrapure water for sensory evaluation. The sensory evaluation showed that the second component in Gel-2 had a distinctly salty taste. The results are shown in Table 4.
Fig. 1.
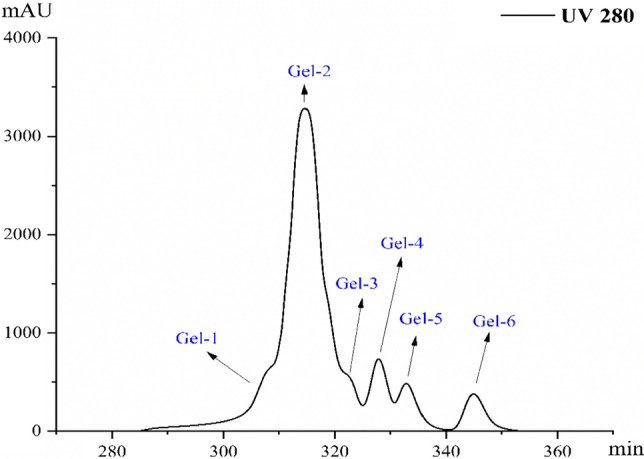
Gel permeation chromatogram of peptide < 1000 Da
Table 4.
Salty taste intensity of each component in gel filtration chromatography
| Gel-1 | Gel-2 | Gel-3 | Gel-4 | Gel-5 | Gel-6 | |
|---|---|---|---|---|---|---|
| Saltiness | − | + + | − | − | − | − |
Preparative liquid chromatography results
Gel-2 was separated by preparative liquid chromatography, and 9 components (F-1–F-9) were obtained (Fig. 2). The sensory evaluation results indicated that F-1 and F-2 had an intense salty taste, and the intensity of F-2 was higher than that of F-1. The results are shown in Table 5.
Fig. 2.
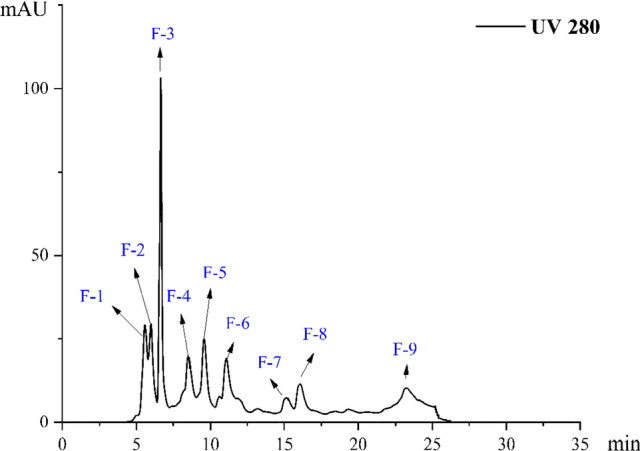
Preparation of liquid chromatography of the peptide component in Gel-2
Table 5.
The intensity of Salty taste of each component in preparative liquid chromatography
| F-1 | F-2 | F-3 | F-4 | F-5 | F-6 | F-7 | F-8 | F-9 | |
|---|---|---|---|---|---|---|---|---|---|
| Saltiness | + | + + | − | − | − | − | − | − | − |
Identification of salty peptide sequences
According to different studies, the peptide chains generally break at lower chemical energies during collision-induced dissociation of polypeptides. Figure 3 shows that a polypeptide obtained by dehydration condensation of n-amino acids breaks between the mth amino acid and the (m + 1)th amino acid starting from the N-terminus, depending upon the relationship between the cleavage site and the peptide bond. Three kinds of fracture modes (ax, by and cz) were produced, among which the sub-fragments of the N-terminus of the peptide chain were successively named as am, bm and cm ions, and the C-terminal sub-fragments were successively named as xm, ym and zm ions. In the Q-TOF two-stage tandem mass spectrometry, the by-type fracture is a common fracture mode. In addition, the am ion, am-NH3, yn-m-H2O, etc., which differ from the bm ion by 27.9949 Da, are also common sub-ion fragment types (Steen and Mann 2004).
Fig. 3.

The law of peptide chain break
Three peptides containing aspartic acid were identified in F-1 and F-2, including two dipeptides and one tripeptide. Using high-precision mass spectrometry, the chemical formulas of 1, 2 and 3 are detected as C8H12N2O7, C9H14N2O7, C12H17N3O10.The secondary mass spectrum of each peptide component obtained by fractionation (Fig. 4a–e). Ion fragment [Asp + H]+(m/z = 133.9871), [M-Asp + H]+(m/z = 112.9923), [Asp + Asp-H2O + H]+(m/z = 248.0168), [Asp + Asp-H2O-CO2 + H]+(m/z = 205.0150), indicating that there are aspartic acid residues in 1, 2 and 3. In addition to the aspartic acid residue, [Glu + H] + (m/z = 144.5726) in 2 indicates that there is another glutamic acid residue. Since FA31 is powdered after protease, peptidase, flavor enzyme, and nuclease digestion, the spectrum is decomposed manually based on the digestion sites and mass fragments of these enzymes. The protease digestion sites are Ala and Pro, and the peptidase digestion sites are Arg, Asp, Glu and Lys. According to the amino acid composition and ionic fragments, 1, 2, 3 were identified as Asp-Asp, Glu-Asp, and Asp-Asp-Asp. Similarly, in 4 and 5, the ion spectra show fragment ions m/z 86.0716, 121.0544, 148.9887, 216.1170, corresponding to ammonia loss or decarboxylation. Combine the fragments [Ser + H] + (m/z = 103.0955), [Ser + Pro + H]+ (m/z = 216.1178), [Glu + Pro- H2O + H] + (m/z = 244.1972), The structure of 4 is determined as Ser-Pro-Glu. The molecular weight of LC–MS analysis 5 is 279.1102, [Phe-NH3 + H]+(m/z = 148.9887), [Ile-CO2 + H]+(m/z = 86.0713) shows that it is a dipeptide composed of phenylalanine and isoleucine (leucine). In this study, the fragmentation of each ion was analyzed by de novo sequencing according to the peptide chain fragmentation law and the method described by Bruni et al. (2005). By confirming the pure standard, the peptide molecules of 1, 2, 3, 4, 5 are clearly Asp-Asp, Glu-Asp, Asp-Asp-Asp, Ser-Pro-Glu, Phe-Ile (Table 6). According to Table 6, five salty peptides were detected in two components, including two tripeptides and two tripeptides. The amino acid sequences were Asp-Asp, Glu-Asp, Asp-Asp-Asp, Ser-Pro-Glu, and Phe-Ile.
Fig. 4.
a Secondary mass spectrum of Q-TOF of Asp-Asp in F-1 fraction. b Secondary mass spectrum of Q-TOF of Glu-Asp in F-1 fraction. c Secondary mass spectrum of Q-TOF of Asp-Asp-Asp in F-2 fraction. d Secondary mass spectrum of Q-TOF of Ser-Pro-Glu in F-2 component. e Secondary mass spectrum of Q-TOF of Phe-Ile in F-2 component
Table 6.
Amino acid sequence of the salty peptide and its ion fragmentation
| No | Component | Sequence | Precursor ion (m/z) | Fragment ion information |
|---|---|---|---|---|
| 1 | F-1 | Asp-Asp | 247.0848 |
130.5711 → [Aspc1 + –H] + 112.9923161.0568 → [M –Asp + Hx1 + H]+ 187.0697 → [M–CO2OH-NH3 + H]+ |
| 2 | F-1 | Glu-Asp | 261.0985 |
10,332..09595706 → [Aspa1 + + H]+ 144.5726 → [Gluc1 + –H] + 201.0479 → [M-CO2OH-NH3 + H]+ |
| 3 | F-2 | Asp-Asp-Asp | 365.0484 |
116.0416 → [Asp-NH3 + H] +b1 133.9871 → [Aspy1 + H]+ 205.0150 → [Asp + Aspz2-H2ONH3 –CO2 + H] 248.0168 → [Asp + Asp-H2O + Hy2 + H]+ 319.0797 → [M-CO2 + HOH] + |
| 4 | F-2 | Ser-Pro-Glu | 331.1558 |
86.0716 → [Serc1-NH3 + H] + 103.0955 → [Ser + H] +c1 244.1972121.0555 → [a2Glu + Pro- NH3-H2O + -H] + 216.1178 → [y2-COSer + Pro + H]+ |
| 5 | F-2 | Phe-Ile | 279.1102 |
86.0713121.0544 → [a1Ile-CO2 + H]+ 148.9887 → [b1Phe-NH3 + H]+ 163.0969 → [Phe + H] +c1 216.1170 → [M-COO2H-NH3 + H]+ |
Verification of synthetic peptide standards
The results of the sensory evaluation of the taste characteristics after dissolving each of the peptide standards in water, beef soup, and vegetable soup respectively are shown in Table 7. The five peptides imparted salty taste in the three matrices. Among them, Asp-Asp and Glu-Asp had salty, umami and sour flavors, which were consistent with the results of Masahiro and Kuramitsu. Asp-Asp-Asp has a salty and umami taste, which was consistent with the results of Takashi's study. Ser-Pro-Glu imparted a salty and sour taste, which was consistent with the results of Kenji's research. Phe-Ile had a salty and bitter taste, which is consistent with the findings of Zhu and Ishibashi. In addition, at the same concentration, the blank broth in which the peptide standard was dissolved had the strongest salty taste, and the aqueous solution of the peptide standard had the weakest salty taste.
Table 7.
Taste characteristics of peptide standards in different matrices
| Ultra-pure water | Beef broth | Vegetable broth | |
|---|---|---|---|
| Asp-Asp | salty, umami, sour | salty, umami, sour | salty, umami, sour |
| Glu-Asp | salty, umami, sour | salty, umami, sour | salty, umami, sour |
| Asp-Asp-Asp | salty, umami | salty, umami | salty, umami |
| Ser-Pro-Glu | salty, sour | salty, sour | salty, sour |
| Phe-Ile | salty, bitter | salty, bitter | salty, bitter |
Conclusion
In this study, the salty-salt-free yeast extract FA31 of Angel yeast was separated in a stepwise manner by ultrafiltration, gel permeation chromatography, and preparative liquid chromatography combined with the sensory evaluation to obtain a salty peptide component.
ESI-LC-Q-TOF MS/MS was used to fragment the peptide molecules, combined with the peptide chain break rule; 5 peptide sequences were manually analyzed, which were Asp-Asp, Glu-Asp, Asp-Asp-Asp, Ser-Pro- Glu, Phe-Ile. These five salty peptides were synthesized, and sensory evaluation was performed after dissolving them in water, vegetable soup and beef soup to determine the taste characteristics. All five peptides had a salty taste. Among them, Asp-Asp and Glu-Asp had salty, umami and sour taste, Asp-Asp-Asp had salty and umami taste, Ser-Pro-Glu had a salty and sour taste, and Phe-Ile had a salty and bitter taste.
Further study would focus on the saltiness verification of the salty peptides identified by E-tongue, calculation of their taste dilution (TD) factors, as well as their abilities of saltiness enhancement for replacing the table salt without compromising the saltiness.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alim A, Yang C, Song H, Liu Y, Zou T, Zhang Y, Zhang S. The behavior of umami components in thermally treated yeast extract. Food Res Int. 2019;120:534–543. doi: 10.1016/j.foodres.2018.11.002. [DOI] [PubMed] [Google Scholar]
- Brandsma I. Reducing sodium: a European perspective. Food Technol. 2006;60(3):25–29. [Google Scholar]
- Brandsma I. Reducing sodium: a European perspective[J] Food Technol. 2006;60(3):25–29. [Google Scholar]
- Bruni R, Gianfranceschi G, Koch G. On peptide de novo sequencing: a new approach. J Pept Sci. 2005;11:225–23410. doi: 10.1002/psc.595. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–3015. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charve J, Manganiello S, Glabasnia A. Analysis of umami taste compounds in a fermented corn sauce by means of sensory-guided fractionation. J Agric Food Chem. 2018;66:1863–1871. doi: 10.1021/acs.jafc.7b05633. [DOI] [PubMed] [Google Scholar]
- Ganesan B, Brown K, Irish DA, Brothersen C, McMahon DJ. Manufacture and sensory analysis of reduced-and low-sodium cheddar and mozzarella cheeses. J Dairy Sci. 2014;97:1970–1982. doi: 10.3168/jds.2013-7443. [DOI] [PubMed] [Google Scholar]
- Gębski J, Jezewska-Zychowicz M, Szlachciuk J, et al. Impact of nutritional claims on consumer preferences for bread with varied fiber and salt content. Food Qual Prefer. 2019;76:91–99. doi: 10.1016/j.foodqual.2019.03.012. [DOI] [Google Scholar]
- Inguglia ES, Zhang Z, Tiwari BK, Kerry JP, Burgess CM. Salt reduction strategies in processed meat products—a review. Trends Food Sci Technol. 2017;59:70–78. doi: 10.1016/j.tifs.2016.10.016. [DOI] [Google Scholar]
- Israr T, Rakha A, Sohail M, Rashid S, Shehzad A. Salt reduction in baked products: strategies and constraints. Trends Food Sci Technol. 2016;51:98–105. doi: 10.1016/j.tifs.2016.03.002. [DOI] [Google Scholar]
- Kang L (2017) Identification of Maillard Reactive peptides in beef and deducing the mechanism of its flavor products formation. Master Thesis. Beijing Technology and Business University
- Li YN, Liu WY, Zhang SL, Cheng XY. Separation, purification and analysis of salty peptides derived from bovine bone by chromatography and mass spectrometry. Meat Res. 2016;30(3):25–284. [Google Scholar]
- Li YN, Liu WY, Zhang SL, Qiao XL, Chen WH, Qu C, Cheng XY. Amino acid composition and stability analysis of salty peptides derived from bovine bone under simulated processing conditions. Meat Res. 2016;30(1):11–144. [Google Scholar]
- Liem DG, Miremadi F, Keast RS. Reducing sodium in foods: the effect on flavor. Nutrients. 2011;3:694–711. doi: 10.3390/nu3060694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J (2015) The mechanism of chicken peptide in flavor generation via Maillard reaction. Master Thesis. Beijing Technology and Business University
- Liu J, Song H, Liu Y, Li P, Yao J, Xiong J. Discovery of kokumi peptide from yeast extract by LC-Q-TOF-MS/MS and sensomics approach. J Sci Food Agric. 2015;95:3183–3194. doi: 10.1002/jsfa.7058. [DOI] [PubMed] [Google Scholar]
- Makoto Tada ISAH. L-ornithyltaurineOrnithyltaurine, a new salty peptide. J Agrie Food Chem. 1984;32:992–996. doi: 10.1021/jf00125a009. [DOI] [Google Scholar]
- Mitchell M, Brunton NP, Wilkinson MG. Current salt reduction strategies and their effect on sensory acceptability: a study with reduced salt ready-meals. Eur Food Res Technol. 2011;232:529–539. doi: 10.1007/s00217-010-1420-6. [DOI] [Google Scholar]
- Münch P, Hofmann T, Schieberle P. Comparison of key odorants generated by thermal treatment of commercial and self-prepared yeast extracts: influence of the amino acid composition on odorant formation. J Agric Food Chem. 1997;45(4):1338–1344. doi: 10.1021/jf960658p. [DOI] [Google Scholar]
- Onuma T, Maruyama H, Sakai N. Enhancement of saltiness perception by monosodium glutamate taste and soy sauce odor: a near infrared spectroscopy study. Chem Senses. 2018;43(3):151–167. doi: 10.1093/chemse/bjx084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng ZQ, Zhang YW, Guo XY (2011) Polypeptide common salt substitute and preparation method. CN102224921A
- Silva HLA, Balthazar CF, Esmerino EA, Vieira AH, Cappato LP, Neto RPC, Verruck S, Cavalcanti RN, Portela JB, Andrade MM, Moraes J, Franco RM, Tavares MIB, Prudencio ES, Freitas MQ, Nascimento JS, Silva MC, Raices RSL, Cruz AG. Effect of sodium reduction and flavor enhancer addition on probiotic prato cheese processing. Food Res Int. 2017;99:247–255. doi: 10.1016/j.foodres.2017.05.018. [DOI] [PubMed] [Google Scholar]
- Steen H, Mann M. The ABC's (and XYZ's) of peptide sequencing. Nat Rev Mol Cell Biol. 2004;5(9):699–711. doi: 10.1038/nrm1468. [DOI] [PubMed] [Google Scholar]
- Strazzullo P, D'Elia L, Ngianga-Bakwin K, Cappucccio FP. Salt intake, stroke and cardiovascular disease: metaanalysis of prospective studies. BMJ. 2009;339:b4567. doi: 10.1136/bmj.b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Klaauw NJ, Smith DV. Taste quality profiles for fifteen organic and inorganic salts. Physiol Behav. 1995;58(2):295–306. doi: 10.1016/0031-9384(95)00056-O. [DOI] [PubMed] [Google Scholar]
- Xu X, You M, Song H, Gong L, Pan W. Investigation of umami and kokumi taste-active components in bovine bone marrow extract produced during enzymatic hydrolysis and Maillard reaction. Int J Food Sci Technol. 2018;53:2465–2481. doi: 10.1111/ijfs.13893. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




