Abstract
Purpose
To evaluate the factors that affect the incidence of euploid balanced embryos and interchromosomal effect (ICE) in carriers of different structural rearrangements.
Methods
This retrospective study includes 95 couples with reciprocal translocations (RecT) and 36 couples with Robertsonian translocations (RobT) undergoing Preimplantation Genetic Testing for Structural Rearrangements (PGT-SR) between March 2016 and July 2019. Next-generation sequencing (NGS) was the technique used coupled with trophectoderm (TE) biopsy. Only cases with females under 38 years were included. A total of 532 blastocysts were evaluated.
Results
The euploidy rate was similar in RobT when compared with RecT carriers [57/156 (36.5%) vs. 112/376 (29.8%), p = 0.127]. The pure ICE rate was significantly higher in RobT carriers [48/156 (30.8%) vs. 53/376 (14.1%), p < 0.001] than it was in RecT carriers. Female age was the independent factor for the probability of obtaining a euploid embryo in RecT and RobT carriers, and increasing female age decreases the probability of obtaining a euploid embryo. In RecT carriers, no significant differences were observed in euploidy rates, pure ICE, or combined ICE according to the length of the translocated fragment and the chromosome group. However, total ICE was significantly lower when there was a breakpoint in the short chromosome arm together with a breakpoint in the long arm [(44/158 (27.8%) for pq or qp, 51/155 (32.9%) for pp and 30/63 (47.6%) for qq; p = 0.02].
Conclusion
The incidence of euploid/balanced blastocysts was similar in both types of translocations. However, there was a significant increase in pure ICE in RobT compared to RecT carriers. In RecT carriers, the presence of the breakpoints in the long arm of the chromosomes involved in the rearrangement resulted in a higher total ICE.
Keywords: Structural rearrangement, PGT-SR, Interchromosomal effect
Introduction
The exchange of chromosomal segments between two chromosomes without any segmental loss is called translocation and can be classified as either a reciprocal translocation (RecT) or Robertsonian translocation (RobT). RecT and RobT are the most common chromosomal rearrangements, and their estimated prevalence is about 0.40% in prenatal samples [1, 2]. The phenotype of the balanced translocation carriers both for RecT and RobT is usually normal. However, translocation carriers have an increased risk of producing unbalanced gametes which may cause fertility problems, and recurrent pregnancy loss (RPL) [3, 4]. Although balanced translocations cause infertility and recurrent pregnancy loss, couples with fertility problems and recurrent pregnancy loss do not commonly carry balanced translocation.
The management of parental translocation carriers is controversial. There is paucity of data on the comparison of the subsequent pregnancy outcomes in carriers of translocation, in cases with RPL, who were managed with expectant management or with in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) and preimplantation genetic diagnosis (PGT-SR). Moreover, there is no data comparing the effectiveness of standard IVF/ICSI vs PGT-SR in carriers of translocation suffering infertility. PGT-SR is a well-accepted option in current practice. PGT-SR provides an opportunity to detect chromosomally unbalanced embryos. In theory, it minimizes fetal aneuploidy risk and thus reduces the risk of miscarriage or termination of the pregnancy [5]. In recent years, fluorescence in situ hybridization (FISH) has been used for PGT-SR. However, a major limitation of FISH is its ability to identify only the involved chromosomes rather than the whole chromosome extent. In addition, overlapping FISH signals, hybridization failure, and fixation problems are other major concerns [6]. Nevertheless, clinical trials in which the FISH technique was used for PGT-SR reported improved outcomes for miscarriage and live birth rates [6–14].
The implementation of new technologies such as trophectoderm (TE) biopsy and comprehensive chromosomal screening (CCS) using array comprehensive genomic hybridization (aCGH) and next-generation sequencing (NGS) has been suggested to improve the clinical outcomes of preimplantation genetic testing for aneuploidy (PGT-A). These technologies are also used for PGT-SR [15–18].
During the meiosis process, chromosomal segregation on the spindle alignment in meiosis I is critical. In carriers of translocations, the chromosomes involved in the rearrangement could have a detrimental effect on the segregation of the other, structurally normal, chromosomes. This is defined as interchromosomal effect (ICE) [19]. Previous studies mostly utilized FISH, which cannot analyze all pairs of chromosomes and therefore cannot explore ICE. In addition, other FISH studies focused on sperm evaluation, which restricts the analysis to male carriers. There have been some studies using aCGH to evaluate ICE at the cleavage stage [20]. The percentage of total ICE was calculated as aneuploid balanced and unbalanced embryos divided to all embryos. The percentage of pure ICE was calculated as aneuploid balanced embryos divided to all embryos.
However, there are few studies evaluating ICE at the blastocyst stage with low sample sizes. The main factors suggested to affect the segregation pattern of balanced euploid embryos are the length of the translocated and interstitial segments, the position of the centromere, and the presence/absence of heterochromatic regions [21].
Our aim in this retrospective analysis is to evaluate the results of PGT-SR following NGS. We analyze the suggested possible effects of carrier- and rearrangement-specific characteristics on the ploidy status of embryos and the presence of pure and total ICE in young couples carrying translocations.
Materials and methods
Study population
In this retrospective analysis, PGT-SR results were reviewed for 124 cycles of 95 couples carrying RecT and 49 cycles of 36 couples carrying RobT between March 2016 and July 2019 in the Bahçeci Fulya IVF Centre. A conventional karyotype analysis was conducted on cultured peripheral blood lymphocytes for all couples before treatment. All translocation carriers had histories of infertility, recurrent spontaneous abortion, or chromosomal abnormality in products of conception. The NGS technique was used and coupled with TE biopsy. Only couples whose females’ ages were less than 38 years were included. Couples with both partners carrying a balanced structural rearrangement as well as complex translocations involving more than two chromosomes were excluded. A total of 532 blastocysts were evaluated (female RecT: 200; male RecT: 176; female RobT: 78, and male RobT: 78).
Ovarian stimulation, oocyte retrieval, intracytoplasmic sperm injection (ICSI), embryo culture, and TE biopsy
Ovarian stimulation (OS) began on day 2 of the menstrual period by employing antagonist protocol. The dosage of gonadotropins was attended based on the physician’s preferences. Patients received 250 μg of human chorionic gonadotropin (hCG; Ovitrelle, Serono) or 0.2 mg of triptorelin (Gonapeptyl, Ferring) for the final oocyte maturation when at least two follicles reached 18 mm in diameter, and TV-USG-guided follicle aspiration was performed for oocyte retrieval after 35 h [22].
The oocyte retrieval, denudation, and ICSI procedures were performed as previously described [23]. After microinjection, oocytes were cultured individually in a special, pre-equilibrated culture dish. In our study, single-step media—namely, continuous single culture complete (CSCM-C) with human serum albumin (Irvine Scientific, CA, USA)—was used for the embryo cultures throughout the culture period.
All embryos were kept in benchtop incubators (MIRI, ESCO Medical, Singapore) and cultured until day 5 or 6 of embryo development. On day 3, embryos were instantly taken out of the incubator, and the zona pellucida was breached by assisted hatching (AH; a hole with a diameter of approximately 20 μm was created) using laser pulses (OCTAX). Embryo biopsy was performed in modified human tubal fluid (mHTF) with gentamicin (mHTF, Irvine Scientific, CA, USA) containing 10% serum substitute supplement (SSS) (Irvine Scientific, CA, USA) using the pulling method, as previously described by Zhao et al. [24]. All embryos with proper blastocoelic expansion and showing trophectoderm herniation underwent biopsy on day 5 and day 6. The biopsied material consisted of five to eight cells. After biopsy, each biopsied material was individually transferred into a PCR tube and kept frozen at − 20 °C until genetic analysis.
Preimplantation genetic testing for structural rearrangement (PGT-SR)
The analysis of the TE biopsies was performed using NGS. Whole-genome amplification (WGA) and DNA barcoding were performed using the Ion ReproSeqTMPGS kit for 24 chromosome aneuploidy screening (Thermo Fisher Scientific, USA). WGA products were pooled, purified, and quantified with QubitTM (Qubit dsDNA HS Assay Kit ThermoFisher). The preparation of the library and templates as well as chip loading were carried out using Ion ChefTM, and sequencing was completed using the Ion S5 System instrument (Thermo Fisher Scientific, USA). The complete workflow from sample processing to reporting was completed in 12–14 h depending on the number of samples processed simultaneously. An internally validated algorithm was applied for the automated diagnosis of whole chromosome aneuploidies and partial deletions/duplications ≥ 6 Mb.
Statistical Analysis
All statistical analyses were performed using SPSS for Windows software package version 20 (SPSS, Chicago, USA). The Kolmogorov-Smirnov test was first performed on continuous independent variables to test whether each variable followed a normal distribution. As the patient and embryological characteristics do not follow a normal distribution, the values of such variables were reported as medians (quartile 1–quartile 3) in Table 1. As well as this, the independent median test was used in Table 1 to identify if there is any significant difference in continuous independent patient and embryological characteristics between the RecT and RobT carriers, Female RecT and Female RobT carriers, and Male RecT and Male RobT carriers respectively. The incidences of chromosomal abnormalities were compared between embryos derivate from RecT and embryos derivate from RobT carrier groups to test whether there is a statistically significant difference in the proportion of aneuploid embryos, euploid balanced embryos, aneuploid balanced embryos, euploid unbalanced embryos, aneuploid unbalanced embryos, chaotic embryos, and ICE between RecT and RobT carrier groups using Chi-square test. To find out which factors were affecting the outcome of embryo where the embryo resulted to be euploid or aneuploid, Generalized Linear Mixed Model (GLMM) was used the binary logistic link function and repeated statement. Owing to the clustered nature of the data (embryos originating from the same patient), the analysis of the data was performed with the use of GLMM to further investigate which factors affect the embryos to be euploid. The parameters used in this model were translocation type, carrier gender, female age, and male age for the whole population. GLMM binary logistic links function and repeated statement, to identify factors affecting the ICE condition, where with ICE or without ICE in the RecT group separately. The parameters used in this model were carrier gender, female age, male age, breakpoint length, chromosome group, and chromosome breakpoint arm.
Table 1.
Comparison of patient and embryological characteristics of RecT and RobT carriers
| RecT | RobT | p value | Female RecT | Female RobT | p value | Male RecT | Male RobT | p value | |
|---|---|---|---|---|---|---|---|---|---|
| No. of patients | 95 | 36 | - | 46 | 18 | - | 49 | 18 | - |
| No. of cycles | 124 | 49 | - | 55 | 21 | - | 69 | 21 | - |
| Female age (years) | 32 (28–34) | 31 (28–5) | 0.934 | 31 (28–33) | 32 (27–35) | 0.608 | 32 (28–34.5) | 31 (28.25–35) | 0.879 |
| Male age (years) | 34(31–37) | 34(31–37) | 0.599 | 34 (31–37) | 35 (31.5–39) | 0.057 | 34 (31–37) | 32.5 (31–35.75) | 0.543 |
| BMI (kg/m2) | 23.10 (20.77–25.10) | 24.31 (21.67–28.93) | 0.156 | 23.80 (22.08–26.04) | 23.83 (23.1–28.93) | 1.000 | 22.83 (20.3–24.61) | 25.95 (21.67–29) | 0.589 |
| Total gonadotropin dosage (IU) | 2475 (1846.5–3000) | 2325 (1800–3000) | 0.111 | 2400 (1827.75–3000) | 2400 (1837.5–3150) | 0.829 | 2512.5 (1950–3225) | 2362.5 (1943.75–2962.5) | 0.087 |
| Sperm concentration (million/ml) | 30 (10–60) | 20 (0.9–54.5) | 0.310 | 40 (25–70) | 55 (31–80) | 0.305 | 20 (5–35.5) | 2 (0.3–18.25) | 0.004 |
| No. of oocytes | 12 (7–18) | 10 (5.50–18.50) | 0.439 | 13 (9–20) | 10 (5–19) | 0.457 | 10 (6.5–17.5) | 11 (6–16.75) | 0.968 |
| No. of MII | 9 (6–13) | 9 (4–13) | 0.926 | 11 (6–16) | 9 (4–15.5) | 0.162 | 9 (5.5–12) | 9.5 (4–12) | 0.306 |
| No. of 2PN | 7 (4–7) | 7 (3.–10) | 0.932 | 9 (5–13) | 7 (3.5–10.5) | 0.264 | 6 (4–10) | 8.5 (3.25–10) | 0.584 |
| Fertilization rate (%) | 80 (66.67–92.37) | 81.82 (74.34–100) | 0.7 | 81.82 (72.73–92.86) | 81.82 (75–100) | 0.818 | 80 (66.67–92.33) | 85.91 (71.99–100) | 0.676 |
| Blastocyst rate (%) | 50 (28.14–64.15) | 50 (40–67.71) | 0.530 | 50 (31.58–66.67) | 57.14 (50–70.5) | 0.569 | 50 (27.05–61.25) | 48.08 (33.33–55.56) | 0.9 |
| No. of embryos analyzed | 3 (2–4) | 3 (2–4.50) | 0.593 | 3 (2–5) | 3 (2–5.5) | 0.843 | 2 (1.5–4) | 2 (2–4) | 0.763 |
IU, international unite; MII, metaphase 2 oocyte; 2PN, two pronuclei
Results
Clinical characteristics
One hundred thirty-one couples underwent 173 PGT-SR cycles; 95 were carriers of RecT (124 cycles), and 36 were carriers of RobT (49 cycles). Among the 131 couples, 79 had a history of recurrent pregnancy loss, 43 had a history of primary infertility, and nine experienced previous aneuploidy in products of conception. The mean maternal age was 30.6 years (range, 21–37 years). Table 1 illustrates the comparison of the demographic and embryological characteristics between the RecT and RobT carriers. There were no statistically significant differences between the groups. Furthermore, we divided the RecT and RobT carriers by gender and compared the abovementioned parameters between female RecT and female RobT carriers and between male RecT and male RobT carriers. There was no statistically significant difference between female RecT and female RobT carriers. However, median sperm concentration was significantly lower in male RobT carriers compared to male RecT carriers [2 (0.3–18.25) vs. 20 (5–35.5), p = 0.004].
Incidence of chromosomal abnormalities
The analysis of chromosomal abnormalities is shown in Table 2. The euploidy rate was comparable between RecT and RobT carriers [112/376 (29.8%) vs. 57/156 (36.5%), p = 0.127]. The pure ICE (aneuploid balanced) rate was significantly higher in RobT [48/156 (30.8%)] carriers versus RecT carriers [53/376 (14.1%)] (p < 0.001). However, no significant differences were observed with respect to the total percentage of ICE (aneuploid balanced and unbalanced) between RecT and RobT carriers [125/376 (33.2%) vs. 64/156 (41%); p = 0.088].
Table 2.
Incidence of chromosomal abnormalities according to type of rearrangement and gender
| RecT | RobT | p value | Female RecT | Female RobT | p value | Male RecT | Male RobT | p value | |
|---|---|---|---|---|---|---|---|---|---|
| No. of patients | 95 | 36 | - | 46 | 18 | - | 49 | 18 | - |
| No. of cycles | 124 | 49 | - | 55 | 21 | - | 69 | 21 | - |
| No. of analyzed embryos | 380 | 160 | - | 201 | 80 | - | 179 | 80 | - |
| No. of informative embryos | 376 | 156 | - | 200 | 78 | - | 176 | 78 | - |
| No. of aneuploid embryos (n, %) | 264/376 (70.2) | 99/156 (63.5) | 0.127 | 146/200 (73) | 49/78 (62.8) | 0.095 | 118/176 (67) | 50/78 (64.1) | 0.647 |
| No. of euploid balanced embryos (n, %) | 112/376 (29.8) | 57/156 (36.5) | 0.127 | 54/200 (27) | 29/78 (37.2) | 0.095 | 58/176 (33) | 28/78 (35.9) | 0.647 |
| No. of aneuploid balanced embryos (pure ICE) (n, %) | 53/376 (14.1) | 48/156 (30.8) | < 0.001 | 31/200 (15.5) | 23/78 (29.5) | 0.008 | 22/176 (12.5) | 25/78 (32.1) | < 0.001 |
| No. of euploid unbalanced embryos (n, %) | 131/376 (34.8) | 30/156 (19.2) | < 0.001 | 73/200 (36.5) | 18/78 (23.1) | 0.032 | 58/176 (33) | 12/78 (15.4) | 0.003 |
| No. of aneuploid unbalanced embryos (combined ICE) (n, %) | 72/376 (19.1) | 16/156 (10.3) | 0.012 | 37/200 (18.5) | 7/78 (9) | 0.050 | 35/176 (19.9) | 9/78 (11.5) | 0.104 |
| No. of chaotic embryos (n, %) | 8/376 (2.1) | 5/156 (3.2) | 0.463 | 5/200 (2.5) | 1/78 (1.3) | 0.530 | 3/176 (1.7) | 4/78 (5.1) | 0.124 |
| Total ICE (pure+combined ICE) (n, %) | 125/376 (33.2) | 64/156 (41) | 0.088 | 68/200 (34) | 30/78 (38.5) | 0.484 | 57/176 (32.4) | 34/78 (43.6) | 0.086 |
ICE, interchromosomal effect; RecT, reciprocal translocation; RobT, Robertsonian translocation
A gender-based subgroup analysis also showed no differences in euploidy rates between female RecT carriers and female RobT carriers [54/200 (27%) vs. 29/78 (37.2%), p = 0.095] or between male RecT carriers and male RobT carriers [58/176 (33%) vs. 28/78 (35.9%), p = 0.647]. The percentage of pure ICE was significantly higher in female Rob T carriers than in female RecT carriers [23/78 (29.5%) vs 31/200 (15.5%), p = 0.008]. Similarly, the percentage of pure ICE was significantly higher in male Rob T carriers than in male RecT carriers [25/78 (32.1%) vs 22/176 (12.5%) p <0.001]. No significant differences were observed in the total percentage of ICE between female RecT and female RobT carriers [68/200 (34%) vs. 30/78 (38.5%), p = 0.484] or between male RecT and male RobT carriers [57/176 (32.4%) vs. 34/78 (43.6%), p = 0.086]. A multi-level GLMM using binomial distribution revealed that female age was the sole independent factor related to the probability of obtaining a euploid embryo (OR: 1.088, 95% CI: 1.025–1.155; p = 0.006) (Table 3). The carrier gender was an insignificant parameter for obtaining an euploid embryo. A detailed analysis of the factors affecting total ICE was initially carried out in the entire cohort. Increasing female age was the independent factor related to total ICE and increases the probability of ICE (OR: 1.149, 95% CI: 1.081–1.221; p < 0.001) (Table 4).
Table 3.
Generalized linear mixed model using the binary logistic regression function and repeated statement: (euploid/aneuploid)
| Model term | Coefficient | p value | OR | 95% CI for OR lower | 95% CI for OR upper |
|---|---|---|---|---|---|
| Female age | 0.084 | 0.006 | 1.088 | 1.025 | 1.155 |
Dependent variable: euploid, aneuploid
Model parameters: (intercept) translocation type, carrier gender, female age, male age
Probability distribution: binomial
Link function: logit
Table 4.
Generalized linear mixed model using the binary logistic regression function and repeated statement: (with ICE/without ICE)
| Model Term | Intercept | p value | OR | 95% CI for OR lower | 95% CI for OR upper |
|---|---|---|---|---|---|
| Female age | 0.139 | < 0.001 | 1.149 | 1.081 | 1.221 |
Dependent variable: with ICE, without ICE
Model parameters: (intercept) translocation type, carrier gender, female age, male age
Probability distribution: binomial
Link function: logit
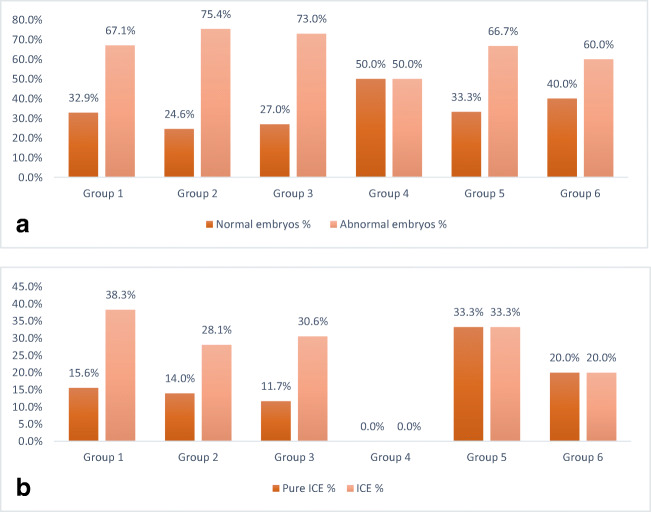
Further analyses were performed in RecT carriers to understand whether the length of the translocated fragment (Fig. 1), the placement of the breakpoint in the affected chromosome (Fig. 2) and/or the chromosome group (Fig. 3) determined the distribution of euploid/aneuploid embryos and the percentage of pure and total ICE. None of the parameters affected the ploidy status within the cohort. Although there was no significant difference between pure ICE and total ICE according to the length of the translocated fragment and chromosomal group, total ICE was significantly lower (p = 0.02) when the breakpoints of the translocated fragments were in different chromosome arms [44/158 (27.8%)] in pq + qp compared to cases in which the two breakpoints were placed in the same arm in pp arms [51/155 (32.9%)] and qq arms [30/63 (47.6%)]. RecT carriers were analyzed for the factors related to ICE in a multivariate analysis. Female age (OR: 1.193, 95% CI: 1.095–1.299; p < 0.001) and chromosome arms containing breakpoints (pq: reference, OR: 3.51 95% CI: 1.61–7.651; p = 0.002 and pp OR: 1.965, 95% CI: 1.076–3.589; p = 0.028) were determined as the independent factors related to ICE (Table 5). Increasing female age increased the incidence of total ICE. Contrarily, the chromosomes containing one breakpoint in the short and long arms resulted in a lower probability of ICE.
Fig. 1.
Percentage of euploid balanced and aneuploid embryos (a) and percentage of embryos pure ICE and ICE (aneuploid balanced and unbalanced) (b) according to translocated fragments length in carriers of reciprocal translocations. Fragment Length: T1 (6–40 Mb), T2 (41–80 Mb), or T3 (81–125 Mb). p = 0.328* T1T1: The fragment length of first breakpoint and second breakpoint are both T1 which is between 6 and 40 Mb. T1T2+T2T1: Either the fragment length of first breakpoint is T1 (6–40 Mb) and fragment length of second breakpoint is T2 (41–80 Mb) or the fragment length of first breakpoint is T2 (41–80 Mb) and fragment length of second breakpoint is T1 (6–40 Mb). T1T3+T3T1: Either the fragment length of first breakpoint is T1 (6–40 Mb) and fragment length of second breakpoint is T3 (81–125 Mb) or the fragment length of first breakpoint is T3 (81–125 Mb) and fragment length of second breakpoint is T1 (6–40 Mb). T2T2: The fragment length of first breakpoint and second breakpoint are both T2 which is between 41 and 80 Mb. T2T3+T3T2: Either the fragment length of first breakpoint is T2 (41–80 Mb) and fragment length of second breakpoint is T3 (81–125 Mb) or the fragment length of first breakpoint is T3 (81–125 Mb) and fragment length of second breakpoint is T2 (41–80 Mb). T3T3: The fragment length of first breakpoint and second breakpoint are both T3 which is between 81 and 125 Mb. *Chi-square test is used to obtain the p value. p = 0.64* for pure ICE, p = 0.312* for ICE. T1T1: The fragment length of first breakpoint and second breakpoint are both T1 which is between 6 and 40 Mb. T1T2+T2T1: Either the fragment length of first breakpoint is T1 (6–40 Mb) and fragment length of second breakpoint is T2 (41–80 Mb) or the fragment length of first breakpoint is T2 (41–80 Mb) and fragment length of second breakpoint is T1 (6–40 Mb). T1T3+T3T1: Either the fragment length of first breakpoint is T1 (6–40 Mb) and fragment length of second breakpoint is T3 (81–125 Mb) or the fragment length of first breakpoint is T3 (81–125 Mb) and fragment length of second breakpoint is T1 (6–40 Mb). T2T2: The fragment length of first breakpoint and second breakpoint are both T2 which is between 41 and 80 Mb. T2T3+T3T2: Either the fragment length of first breakpoint is T2 (41–80 Mb) and fragment length of second breakpoint is T3 (81–125 Mb) or the fragment length of first breakpoint is T3 (81–125 Mb) and fragment length of second breakpoint is T2 (41–80 Mb). T3T3: The fragment length of first breakpoint and second breakpoint are both T3 which is between 81 and 125 Mb. *Chi-square test is used to obtain the p value
Fig. 2.

Percentage of euploid balanced and aneuploid embryos (a) and percentage of embryos pure ICE and ICE (aneuploid balanced and unbalanced) (b) according to translocated arms containing breakpoint in carriers of reciprocal translocations. p = 0.876*; *Chi-square test is used to obtain the p value. p = 0.05* for pure ICE, p = 0.02* for ICE. *Chi-square test is used to obtain the p value
Fig. 3.
Percentage of euploid balanced and aneuploid embryos (a) and percentage of embryos pure ICE and ICE (aneuploid balanced and unbalanced) (b) according to chromosomal group of translocated chromosomes. p = 0.699*; *Chi-square test is used to obtain the p value. p = 0.57* for Pure ICE, p = 0.49* for ICE. Group 1: Chromosomes 1, 2, and 3. Group 2: Chromosomes 4 and 5. Group 3: Chromosomes 6, 7, 8, 9, 10, 11, 12, and X. Group 4: Chromosomes 16, 17, and 18. Group 5: Chromosomes 19 and 20. Group 6: Chromosomes 21, 22, and Y. *Chi-square test is used to obtain the p value
Table 5.
Generalized linear mixed model using the binary logistic regression function and repeated statement for reciprocal translocations: (with ICE/without ICE)
| Model term | Intercept | p value | OR | 95% CI for OR lower | 95% CI for OR upper |
|---|---|---|---|---|---|
| Female age | 0.176 | < 0.001 | 1.193 | 1.095 | 1.299 |
| Chromosome breakpoint arm | |||||
| pq + qp: reference | |||||
| pp | 1.2 | 0.002 | 3.51 | 1.61 | 7.651 |
| 0.675 | 0.028 | 1.965 | 1.076 | 3.589 | |
Dependent variable: with ICE, without ICE
Model parameters: (intercept) carrier gender, female age, male age, breakpoint length, chromosome group, and chromosome breakpoint arm
Probability distribution: binomial
Link function: logit
pp: between to long arms
qq: between to short arms
pq+qp: between long and short arm
Discussion
Meiotic segregation patterns and ICE, which are encountered in translocation carriers’ gametes and embryos, have long been studied. However, in the literature, there is a paucity of data regarding ICE evaluation in blastocysts. In this study, we evaluated chromosome abnormalities using in 532 informative blastocysts stage embryos generated from 95 RecT carriers and 36 RobT carriers. Our aim was to determine the relation between the translocation type, rearrangement characteristics, carrier gender and carrier age with the ICE and ploidy status encountered within blastocysts. We found that there was a trend toward higher aneuploidy rates in RecT carriers compared to RobT carriers, but the difference was not statistically significant. Pure ICE was significantly higher in RobT carriers. Female age was the only independent factor impacting the probability of obtaining an euploid embryo. In addition, RecT carriers were separately analyzed, and chromosome breakpoint arm(s) and female age were the independent factors impacting the probability of total ICE.
RecT carriers can produce gametes from different types of segregation patterns. It has been described that carriers of RecT can generate at least 18 different gamete types during meiosis, of which only one type is normal and only one type is balanced [25]. As RobT occurs in acrocentric chromosomes, RobT carriers can produce six gamete types during meiosis, of which only one type is normal and only one type is balanced. Regarding these, RobT carriers are believed to have a higher euploidy rate in their embryos. A previous study reported a higher percentage of abnormal embryos in RecT carriers than in RobT carriers [19]. However, in this study, most of the embryos included in the study were day-3 embryos. The study has demonstrated significantly higher percentage of euploid unbalanced and aneuploid unbalanced embryos in RecT carriers. These results support the suggested different segregation patterns in RecT and RobT carriers. In our study, there was a trend toward higher aneuploidy rates in RecT, although the trend was statistically non-significant. A multi-level GLMM using binominal distribution revealed that the translocation type was not a significant determinant of the probability of obtaining a euploid balanced embryo. The natural selection process between day 3 and days 5 and 6 may explain the differences between the results of our study and those of previous studies those reported a higher percentage of abnormal embryos in RecT carriers than in RobT carrier. This could be attributed to delayed development of embryos carrying unbalanced chromosomal translocations.
ICE has long been evaluated in sperm samples from male translocation carriers, and results are conflicting. Some studies observed a significant increase in numerical abnormalities for many of the chromosomes analyzed [25–47], whereas others did not [48–51]. In oocytes, the numerical abnormalities were evaluated in only one study containing polar body biopsy and reporting aneuploidies for chromosomes not involved in the chromosome rearrangements [52]. In the abovementioned studies, FISH was used for the analyses, and probes for one to up to eight extra autosomes were used. It is important to take into account that detecting a small set of chromosomes, as in previous studies, cannot be considered a substitute for full karyotyping in current practice. In addition, the consequences of this phenomenon are still controversial at the embryo level. Although most of the previous studies in which cleavage-stage embryos were evaluated by FISH suggest that ICE exists within the gametes of translocation carriers [44, 53], there are contradictory studies among RobT carriers [54]. Alfarawati et al. reported a CCS analysis consisting of both oocytes and embryos obtained from translocation carriers [55]. The study revealed no evidence of an ICE in oocytes, but a significant effect was observed in the cleavage-stage embryos of female RobT carriers. Regarding this result, the authors suggested the possibility that ICE was of mitotic rather than meiotic origin. In this context, TE biopsy can be more determinant than blastomere biopsy to evaluate ICE, as it is studied with more than one cell in PGT-SR cycles. Our study compared the percentage of rearrangement types in RecT and RobT carriers. We limited the female age to less than 38 years. As female age is a major determinant of aneuploidy, it might affect the total ICE in cases with advanced maternal age by increasing the percentage of aneuploid balanced embryos. Although we observed a high percentage of pure ICE (aneuploid balanced) and a trend toward a higher percentage of total ICE (aneuploid balanced and unbalanced) in RobT than in RecT, the difference was statistically non-significant. The multivariate regression analysis with the whole study population revealed that, even in the study group including female individuals younger than 38 years old, female age was the only independent factor that affected the total ICE.
In the present study, there was no significant difference in the percentage of euploid balanced embryos between male and female RecT carriers or between male and female RobT carriers. Previous studies reported a similar frequency of abnormal embryos in male and female translocation carriers, but the incidences of each segregation mode were significantly different [9, 52, 54]. In a more recent study, an increase in the percentage of abnormal embryos was reported in female translocation carriers [25]. However, this study evaluated 1789 RecT and Rob T carriers’ embryos, and of these, 1567 were day 3 embryos. In our study, we evaluated the embryos until the blastocyst stage based on the idea of the natural selection process, which has the utmost importance beyond the cleavage stage.
We evaluated the relationship of translocated fragments lengths, breakpoint positions, chromosomes involved in the translocation, and the arms of the translocated chromosomes with the incidence of euploid balanced embryos. We could not find an effect of any of the aforementioned parameters [49, 55]. Regarding ICE, its incidence depends on the breakpoints and regions of the translocated chromosomes. However, we found no significant differences in euploidy rates according to the length of the translocated fragment, the chromosome group, or the positions of the breakpoints in RecT cases. A multi-level GLMM using a binomial distribution revealed that female age was the only factor related to the incidence of euploid embryos. The incidence of ICE was significantly lower in pq/qp breakpoints than in qq and pp breakpoints. Disturbed and/or altered segregation patterns during meiotic division may be the reason for ICE in embryos due to the positioning and pairing of the rearranged chromosomes with the structurally normal, homologous chromosomes.
The sample size, retrospective nature, and absence of age-matched controls in this study are its main limitations. However, our data provide deeper insights into the effect of the translocation type, carrier gender, the chromosomes involved in rearrangement, and the position of breakpoints on PGT-SR results, since few studies have yet been published related to PGT-SR based on TE biopsy and 24-chromosome analysis.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to report related with this manuscript.
Ethical approval
This study was approved by the Institutional Review Board with a reference number of 55.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Van Dyke DL, Weiss L, Roberson JR, Babu VR. The frequency and mutation rate of balanced autosomal rearrangements in man estimated from prenatal genetic studies for advanced maternal age. Am J Hum Genet. 1983;35(2):301–308. [PMC free article] [PubMed] [Google Scholar]
- 2.Vasilevska M, Ivanovska E, Kubelka Sabit K, Sukarova-Angelovska E, Dimeska G. The incidence and type of chromosomal translocations from prenatal diagnosis of 3800 patients in the republic of macedonia. Balkan J Med Genet: BJMG. 2013;16(2):23–28. doi: 10.2478/bjmg-2013-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scriven PN, Bint SM, Davies AF, Ogilvie CM. Meiotic outcomes of three-way translocations ascertained in cleavage-stage embryos: refinement of reproductive risks and implications for PGD. Eur J Human Genet: EJHG. 2014;22(6):748–753. doi: 10.1038/ejhg.2013.237.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neri G, Serra A, Campana M, Tedeschi B. Reproductive risks for translocation carriers: cytogenetic study and analysis of pregnancy outcome in 58 families. Am J Med Genet. 1983;16(4):535–561. doi: 10.1002/ajmg.1320160412. [DOI] [PubMed] [Google Scholar]
- 5.Scriven PN, Flinter FA, Khalaf Y, Lashwood A, Mackie Ogilvie C. Benefits and drawbacks of preimplantation genetic diagnosis (PGD) for reciprocal translocations: lessons from a prospective cohort study. Eur J Human Genet: EJHG. 2013;21(10):1035–1041. doi: 10.1038/ejhg.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilton L, Thornhill A, Traeger-Synodinos J, Sermon KD, Harper JC. The causes of misdiagnosis and adverse outcomes in PGD. Hum Reprod. 2009;24(5):1221–1228. doi: 10.1093/humrep/den488. [DOI] [PubMed] [Google Scholar]
- 7.Fischer J, Colls P, Escudero T, Munne S. Preimplantation genetic diagnosis (PGD) improves pregnancy outcome for translocation carriers with a history of recurrent losses. Fertil Steril. 2010;94(1):283–289. doi: 10.1016/j.fertnstert.2009.02.060. [DOI] [PubMed] [Google Scholar]
- 8.Keymolen K, Staessen C, Verpoest W, Liebaers I, Bonduelle M. Preimplantation genetic diagnosis in female and male carriers of reciprocal translocations: clinical outcome until delivery of 312 cycles. Eur J Human Genet: EJHG. 2012;20(4):376–380. doi: 10.1038/ejhg.2011.208.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko DS, Cho JW, Park SY, Kim JY, Koong MK, Song IO, et al. Clinical outcomes of preimplantation genetic diagnosis (PGD) and analysis of meiotic segregation modes in reciprocal translocation carriers. Am J Med Genet Part A. 2010;152A(6):1428–1433. doi: 10.1002/ajmg.a.33368. [DOI] [PubMed] [Google Scholar]
- 10.Kyu Lim C, Hyun Jun J, Mi Min D, Lee HS, Young Kim J, Koong MK, et al. Efficacy and clinical outcome of preimplantation genetic diagnosis using FISH for couples of reciprocal and Robertsonian translocations: the Korean experience. Prenat Diagn. 2004;24(7):556–561. doi: 10.1002/pd.923. [DOI] [PubMed] [Google Scholar]
- 11.Munne S, Morrison L, Fung J, Marquez C, Weier U, Bahce M, et al. Spontaneous abortions are reduced after preconception diagnosis of translocations. J Assist Reprod Genet. 1998;15(5):290–296. doi: 10.1023/a:1022544511198.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munne S, Sandalinas M, Escudero T, Fung J, Gianaroli L, Cohen J. Outcome of preimplantation genetic diagnosis of translocations. Fertil Steril. 2000;73(6):1209–1218. doi: 10.1016/s0015-0282(00)00495-7. [DOI] [PubMed] [Google Scholar]
- 13.Otani T, Roche M, Mizuike M, Colls P, Escudero T, Munné S. Preimplantation genetic diagnosis significantly improves the pregnancy outcome of translocation carriers with a history of recurrent miscarriage and unsuccessful pregnancies. Reprod BioMed Online. 2006;13(6):869–874. doi: 10.1016/S1472-6483(10)61037-1. [DOI] [PubMed] [Google Scholar]
- 14.Verlinsky Y, Tur-Kaspa I, Cieslak J, Bernal A, Morris R, Taranissi M, et al. Preimplantation testing for chromosomal disorders improves reproductive outcome of poor-prognosis patients. Reprod BioMed Online. 2005;11(2):219–225. doi: 10.1016/s1472-6483(10)60961-3. [DOI] [PubMed] [Google Scholar]
- 15.Bono S, Biricik A, Spizzichino L, Nuccitelli A, Minasi MG, Greco E, et al. Validation of a semiconductor next-generation sequencing-based protocol for preimplantation genetic diagnosis of reciprocal translocations. Prenat Diagn. 2015;35(10):938–944. doi: 10.1002/pd.4665.. [DOI] [PubMed] [Google Scholar]
- 16.Fiorentino F, Spizzichino L, Bono S, Biricik A, Kokkali G, Rienzi L, et al. PGD for reciprocal and Robertsonian translocations using array comparative genomic hybridization. Hum Reprod. 2011;26(7):1925–1935. doi: 10.1093/humrep/der082. [DOI] [PubMed] [Google Scholar]
- 17.Tobler KJ, Brezina PR, Benner AT, Du L, Xu X, Kearns WG. Two different microarray technologies for preimplantation genetic diagnosis and screening, due to reciprocal translocation imbalances, demonstrate equivalent euploidy and clinical pregnancy rates. J Assist Reprod Genet. 2014;31(7):843–850. doi: 10.1007/s10815-014-0230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Cram DS, Shen J, Wang X, Zhang J, Song Z, et al. Validation of copy number variation sequencing for detecting chromosome imbalances in human preimplantation embryos. Biol Reprod. 2014;91(2):37. doi: 10.1095/biolreprod.114.120576.. [DOI] [PubMed] [Google Scholar]
- 19.Lejeune J. Autosomal disorders. Pediatrics. 1963;32:326–337. [PubMed] [Google Scholar]
- 20.Mateu-Brull E, Rodrigo L, Peinado V, Mercader A, Campos-Galindo I, Bronet F, et al. Interchromosomal effect in carriers of translocations and inversions assessed by preimplantation genetic testing for structural rearrangements (PGT-SR) J Assist Reprod Genet. 2019;36(12):2547–2555. doi: 10.1007/s10815-019-01593-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jalbert P, Sele B, Jalbert H. Reciprocal translocations: a way to predict the mode of imbalanced segregation by pachytene-diagram drawing. Hum Genet. 1980;55(2):209–222. doi: 10.1007/BF00291769. [DOI] [PubMed] [Google Scholar]
- 22.Borm G, Mannaerts B. Treatment with the gonadotrophin-releasing hormone antagonist ganirelix in women undergoing ovarian stimulation with recombinant follicle stimulating hormone is effective, safe and convenient: results of a controlled, randomized, multicentre trial. The European Orgalutran Study Group. Human Reprod. 2000;15(7):1490–1498. doi: 10.1093/humrep/15.7.1490. [DOI] [PubMed] [Google Scholar]
- 23.Serdarogullari M, Coban O, Boynukalin FK, Bilgin EM, Findikli N, Bahceci M. Successful application of a single warming protocol for embryos cryopreserved by either slow freezing or vitrification techniques. Syst Biol Reprod Med. 2019;65(1):12–19. doi: 10.1080/19396368.2018.1487477. [DOI] [PubMed] [Google Scholar]
- 24.Zhao H, Tao W, Li M, Liu H, Wu K, Ma S. Comparison of two protocols of blastocyst biopsy submitted to preimplantation genetic testing for aneuploidies: a randomized controlled trial. Arch Gynecol Obstet. 2019;299(5):1487–1493. doi: 10.1007/s00404-019-05084-1. [DOI] [PubMed] [Google Scholar]
- 25.Faraut T, Mermet MA, Demongeot J, Cohen O. Cooperation of selection and meiotic mechanisms in the production of imbalances in reciprocal translocations. Cytogenet Cell Genet. 2000;88(1-2):15–21. doi: 10.1159/000015476. [DOI] [PubMed] [Google Scholar]
- 26.Anton E, Blanco J, Egozcue J, Vidal F. Sperm FISH studies in seven male carriers of Robertsonian translocation t(13;14)(q10;q10) Human Reprod. 2004;19(6):1345–1351. doi: 10.1093/humrep/deh232. [DOI] [PubMed] [Google Scholar]
- 27.Anton E, Blanco J, Vidal F. Meiotic behavior of three D;G Robertsonian translocations: segregation and interchromosomal effect. J Hum Genet. 2010;55(8):541–545. doi: 10.1038/jhg.2010.67. [DOI] [PubMed] [Google Scholar]
- 28.Anton E, Vidal F, Blanco J. Role of sperm FISH studies in the genetic reproductive advice of structural reorganization carriers. Hum Reprod. 2007;22(8):2088–2092. doi: 10.1093/humrep/dem152. [DOI] [PubMed] [Google Scholar]
- 29.Anton E, Vidal F, Blanco J. Reciprocal translocations: tracing their meiotic behavior. Genet Med Off J Am Coll Med Genet. 2008;10(10):730–738. doi: 10.1097/GIM.0b013e318187760f.. [DOI] [PubMed] [Google Scholar]
- 30.Anton E, Vidal F, Egozcue J, Blanco J. Preferential alternate segregation in the common t(11;22)(q23;q11) reciprocal translocation: sperm FISH analysis in two brothers. Reprod BioMed Online. 2004;9(6):637–644. doi: 10.1016/s1472-6483(10)61774-9. [DOI] [PubMed] [Google Scholar]
- 31.Baccetti B, Capitani S, Collodel G, Estenoz M, Gambera L, Piomboni P. Infertile spermatozoa in a human carrier of robertsonian translocation 14;22. Fertil Steril. 2002;78(5):1127–1130. doi: 10.1016/s0015-0282(02)03379-4. [DOI] [PubMed] [Google Scholar]
- 32.Baccetti B, Collodel G, Marzella R, Moretti E, Piomboni P, Scapigliati G, et al. Ultrastructural studies of spermatozoa from infertile males with Robertsonian translocations and 18, X, Y aneuploidies. Hum Reprod. 2005;20(8):2295–2300. doi: 10.1093/humrep/dei050. [DOI] [PubMed] [Google Scholar]
- 33.Blanco J, Egozcue J, Clusellas N, Vidal F. FISH on sperm heads allows the analysis of chromosome segregation and interchromosomal effects in carriers of structural rearrangements: results in a translocation carrier, t(5;8)(q33;q13) Cytogenet Cell Genet. 1998;83(3-4):275–280. doi: 10.1159/000015170. [DOI] [PubMed] [Google Scholar]
- 34.Blanco J, Egozcue J, Vidal F. Interchromosomal effects for chromosome 21 in carriers of structural chromosome reorganizations determined by fluorescence in situ hybridization on sperm nuclei. Hum Genet. 2000;106(5):500–505. doi: 10.1007/s004390000295. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Huang J, Liu P, Qiao J. Analysis of meiotic segregation patterns and interchromosomal effects in sperm from six males with Robertsonian translocations. J Assist Reprod Genet. 2007;24(9):406–411. doi: 10.1007/s10815-007-9137-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machev N, Gosset P, Warter S, Treger M, Schillinger M, Viville S. Fluorescence in situ hybridization sperm analysis of six translocation carriers provides evidence of an interchromosomal effect. Fertil Steril. 2005;84(2):365–373. doi: 10.1016/j.fertnstert.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 37.Mercier S, Morel F, Fellman F, Roux C, Bresson JL. Molecular analysis of the chromosomal equipment in spermatozoa of a 46, XY, t(7;8) (q11.21;cen) carrier by using fluorescence in situ hybridization. Hum Genet. 1998;102(4):446–451. doi: 10.1007/s004390050719. [DOI] [PubMed] [Google Scholar]
- 38.Morel F, Douet-Guilbert N, Roux C, Tripogney C, Le Bris MJ, De Braekeleer M, et al. Meiotic segregation of a t(7;8)(q11.21;cen) translocation in two carrier brothers. Fertil Steril. 2004;81(3):682–685. doi: 10.1016/j.fertnstert.2003.07.034. [DOI] [PubMed] [Google Scholar]
- 39.Morel F, Roux C, Bresson JL. FISH analysis of the chromosomal status of spermatozoa from three men with 45,XY,der(13;14)(q10;q10) karyotype. Mol Hum Reprod. 2001;7(5):483–488. doi: 10.1093/molehr/7.5.483. [DOI] [PubMed] [Google Scholar]
- 40.Oliver-Bonet M, Navarro J, Codina-Pascual M, Carrera M, Egozcue J, Benet J. Meiotic segregation analysis in a t(4;8) carrier: comparison of FISH methods on sperm chromosome metaphases and interphase sperm nuclei. Eur J Human Genet: EJHG. 2001;9(6):395–403. doi: 10.1038/sj.ejhg.5200654.. [DOI] [PubMed] [Google Scholar]
- 41.Rousseaux S, Chevret E, Monteil M, Cozzi J, Pelletier R, Delafontaine D, et al. Sperm nuclei analysis of a Robertsonian t(14q21q) carrier, by FISH, using three plasmids and two YAC probes. Hum Genet. 1995;96(6):655–660. doi: 10.1007/BF00210294. [DOI] [PubMed] [Google Scholar]
- 42.Rousseaux S, Chevret E, Monteil M, Cozzi J, Pelletier R, Devillard F, et al. Meiotic segregation in males heterozygote for reciprocal translocations: analysis of sperm nuclei by two and three colour fluorescence in situ hybridization. Cytogenet Cell Genet. 1995;71(3):240–246. doi: 10.1159/000134118. [DOI] [PubMed] [Google Scholar]
- 43.Van Hummelen P, Manchester D, Lowe X, Wyrobek AJ. Meiotic segregation, recombination, and gamete aneuploidy assessed in a t(1;10)(p22.1;q22.3) reciprocal translocation carrier by three- and four-probe multicolor FISH in sperm. Am J Hum Genet. 1997;61(3):651–659. doi: 10.1086/515516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vozdova M, Oracova E, Musilova P, Kasikova K, Prinosilova P, Gaillyova R, et al. Sperm and embryo analysis of similar t(7;10) translocations transmitted in two families. Fertil Steril. 2011;96(1):e66–e70. doi: 10.1016/j.fertnstert.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 45.Wiland E, Midro AT, Panasiuk B, Kurpisz M. The analysis of meiotic segregation patterns and aneuploidy in the spermatozoa of father and son with translocation t(4;5)(p15.1;p12) and the prediction of the individual probability rate for unbalanced progeny at birth. J Androl. 2007;28(2):262–272. doi: 10.2164/jandrol.106.000919.. [DOI] [PubMed] [Google Scholar]
- 46.Juchniuk de Vozzi MS, Santos SA, Pereira CS, Cuzzi JF, Laureano LA, Franco JG, Jr, et al. Meiotic segregation and interchromosomal effect in the sperm of a double translocation carrier: a case report. Mol Cytogenet. 2009;2:24. doi: 10.1186/1755-8166-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pellestor F, Imbert I, Andreo B, Lefort G. Study of the occurrence of interchromosomal effect in spermatozoa of chromosomal rearrangement carriers by fluorescence in-situ hybridization and primed in-situ labelling techniques. Human Reproduction. 2001;16(6):1155–1164. doi: 10.1093/humrep/16.6.1155. [DOI] [PubMed] [Google Scholar]
- 48.Chelli MH, Ferfouri F, Boitrelle F, Albert M, Molina-Gomes D, Selva J, et al. High-magnification sperm selection does not decrease the aneuploidy rate in patients who are heterozygous for reciprocal translocations. J Assist Reprod Genet. 2013;30(4):525–530. doi: 10.1007/s10815-013-9959-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Estop AM, Cieply K, Munne S, Surti U, Wakim A, Feingold E. Is there an interchromosomal effect in reciprocal translocation carriers? Sperm FISH studies. Hum Genet. 2000;106(5):517–524. doi: 10.1007/s004390000275. [DOI] [PubMed] [Google Scholar]
- 50.Godo A, Blanco J, Vidal F, Sandalinas M, Garcia-Guixe E, Anton E. Altered segregation pattern and numerical chromosome abnormalities interrelate in spermatozoa from Robertsonian translocation carriers. Reprod BioMed Online. 2015;31(1):79–88. doi: 10.1016/j.rbmo.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 51.Honda H, Miharu N, Ohashi Y, Honda N, Hara T, Ohama K. Analysis of segregation and aneuploidy in two reciprocal translocation carriers, t(3;9)(q26.2;q32) and t(3;9)(p25;q32), by triple-color fluorescence in situ hybridization. Hum Genet. 1999;105(5):428–436. doi: 10.1007/s004390051126. [DOI] [PubMed] [Google Scholar]
- 52.Pujol A, Durban M, Benet J, Boiso I, Calafell JM, Egozcue J, et al. Multiple aneuploidies in the oocytes of balanced translocation carriers: a preimplantation genetic diagnosis study using first polar body. Reproduction. 2003;126(6):701–711. doi: 10.1530/rep.0.1260701. [DOI] [PubMed] [Google Scholar]
- 53.Tulay P, Gultomruk M, Findikli N, Bahceci M. Number of embryos biopsied as a predictive indicator for the outcome of preimplantation genetic diagnosis by fluorescence in situ hybridisation in translocation cases. Zygote. 2016;24(1):107–114. doi: 10.1017/S0967199414000793. [DOI] [PubMed] [Google Scholar]
- 54.Munne S, Escudero T, Fischer J, Chen S, Hill J, Stelling JR, et al. Negligible interchromosomal effect in embryos of Robertsonian translocation carriers. Reprod BioMed Online. 2005;10(3):363–369. doi: 10.1016/s1472-6483(10)61797-x. [DOI] [PubMed] [Google Scholar]
- 55.Alfarawati S, Fragouli E, Colls P, Wells D. Embryos of robertsonian translocation carriers exhibit a mitotic interchromosomal effect that enhances genetic instability during early development. PLoS Genet. 2012;8(10):e1003025. doi: 10.1371/journal.pgen.1003025. [DOI] [PMC free article] [PubMed] [Google Scholar]