Abstract
Objective
Assisted oocyte activation (AOA) can restore fertilization rates after IVF/ICSI cycles with fertilization failure. AOA is an experimental technique, and its downstream effects remain poorly characterized. Clarifying the relationship between AOA and embryo, morphokinetics could offer complementary insights into the quality and viability of the embryos obtained with this technique. The aim of this study is to compare the preimplantation morphokinetic development of embryos derived from ICSI-AOA (experimental group) vs. ICSI cycles (control group).
Methods
A retrospective cohort study was carried out with 141 embryos from fresh oocyte donation cycles performed between 2013 and 2017; 41 embryos were derived from 7 ICSI-AOA cycles and 100 embryos from 18 ICSI cycles. Morphokinetic development of all embryos was followed using a time-lapse system.
Results
We show that embryos from both groups develop similarly for most milestones, with the exception of the time of second polar body extrusion (tPB2) and the time to second cell division (t3).
Conclusions
We conclude that ionomycin mediated AOA does not seem to affect the morphokinetic pattern of preimplantation embryo development, despite the alterations found in tPB2 and t3, which could directly reflect the use of a Ca2+ ionophore as a transient and quick non-physiologic increase of free intracytoplasmic Ca2+.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-020-02025-9.
Keywords: ICSI, AOA, Ionomycin, Time-lapse, Oocyte donation
Introduction
Fertilization is one of the most important processes to achieve reproductive success and depends on molecular and biochemical events [1]. Although not all the mechanisms of fertilization are known, there is scientific evidence that the activation of the oocyte during fertilization depends on both sperm and oocyte factors largely related to signaling mediated by calcium ion (Ca2+). In an in vivo situation, sperm PLCζ triggers oocyte activation by hydrolyzing PIP2 and producing IP3 which binds to IP3 receptors in the endoplasmic reticulum, thus facilitating the release of Ca2+ to the cytoplasm. This originates the subsequent calcium oscillations involved in CAMKII activation and MPF inactivation, and required for meiotic resumption, pronuclear formation, and the first mitotic division [2]. The variations in the intracellular concentration of Ca2+ generated in the oocyte control all the necessary processes to get the normal progression from oocyte to embryo transition [3–5]. Therefore, problems in Ca2+ signaling could be related to recurrent in vitro fertilization failures as well as have an impact in the embryo development from zygote to blastocyst.
While ICSI provides high fertilization rates on average, fertilization failure still occurs in 1–5% of ICSI cycles [6, 7]. Assisted oocyte activation (AOA) involves timed incubation of oocytes with Ca2+ ionophores such as ionomycin after the sperm has been injected [8]. Ionomycin acts by increasing Ca+2 permeability of the oocyte cell membrane, facilitating extracellular Ca+2 inflow into the cytoplasm, and inducing a single increase of cytoplasmic Ca+2 levels able to trigger oocyte activation [9]. In the clinic, AOA is indicated in oocyte activation deficiencies (OADs) [10] and in proven alterations in the activity of sperm-borne oocyte activation factors, such as loss-of-function mutations in PLCζ1 [11]. Although no concerns were apparent in the long-term follow-up of AOA children [12–16], this technique is still highly experimental. In fact, the application of AOA is still controversial, as the artificial activating agents do not mimic exactly the calcium signaling and downstream physiological processes observed in mammalian zygotes [17]. In particular, how calcium ionophores affect the morphokinetic pattern of the resulting embryos remains unknown. Morphokinetic parameters, measured using time-lapse systems (TLS), can help in monitoring whether an embryo is developing at the expected pace [18–20]. The timings of the first cell divisions and the events of embryonic development prior to the blastocyst stage are related to the embryo quality and the embryo chromosomal status [21], as well as inform about how likely is an embryo to result in a live birth [22].
The aim of this study is to investigate whether the AOA procedures could alter the morphokinetic development of the developing embryos from ICSI-AOA cycles. We have compared the preimplantation quantitative morphokinetic parameters of embryos derived from ICSI-AOA vs. ICSI cycles.
Materials and methods
Study design and ethical approval
This is a retrospective cohort study including 141 embryos from 25 ICSI cycles performed between 2013 and 2017 at a private fertility center. Inclusion criteria were: use of ICSI with donor oocytes and patient sperm. Cycles with severe male factor (i.e., use of sperm from testicular biopsy), or in which PGT-A was performed were excluded from the study. Also, cycles with autologous oocytes were also excluded to avoid the potential negative effect of maternal age and female infertility on our results.
The experimental group consisted of 41 embryos obtained from 7 ICSI-AOA cycles; in all these cases, the indication for AOA was the identification of at least one potentially pathogenic variant in PLCζ1 gene in the sperm gDNA after fertilization failure in a previous ICSI cycle. The control group consisted of 100 embryos obtained from 18 ICSI cycles, cultured under the same conditions.
Ethical approval by the Research Ethics Committee of the center was obtained before performing the study.
Ovarian stimulation and donor oocyte collection
In all cycles, controlled ovarian hyperstimulation (COH) was carried out with either highly purified hMG (Menopur®, Ferring, Spain) or Follitropin alpha (Gonal®, Merck Serono, Spain). GnRH antagonist (Cetrotide, Merck Serono Europe Limited, London, UK) was added from days 6 or 7 of stimulation. Multi-follicular development was evaluated by transvaginal ultrasonography during COH. Final oocyte maturation was triggered with 0.3 mg of GnRH agonist (Decapeptyl (Ipsen Pharma) S.A., L’Hospitalet de Llobregat, Spain) when 3 follicles of ≥ 18-mm diameter were detected. Cumulus oocyte complexes (COCs) collection was performed transvaginally, strictly 36 h after trigger. Thirty minutes after oocyte pickup (OPU), oocytes were denuded of cumulus cells by exposing the COCs to a solution of 80 IU/mL of hyaluronidase (HYASE-10Xw, Vitrolife) in G-MOPS medium, with gentle pipetting.
Semen analysis and preparation
All sperm samples were requested approximately 2 h after OPU, analyzed by SCA (Sperm Class Analyzer; Microptic, Spain), and graded according to the World Health Organization guidelines (WHO, 2010). Sperm selection was performed by centrifugation at 250 g for 5 min in 5 ml of sperm medium (PureSperm® Wash, Nidacon, Sweden), followed by swim up at 27 °C, 6% CO2, and 95% relative humidity in IVF medium (Vitrolife, Göteborg, Sweden).
ICSI and AOA procedures
ICSI was performed as previously described [23] from 2 to 4 h after OPU, independently of study group. AOA was performed immediately after ICSI on the inseminated oocytes of the ICSI-AOA group, according to Heindryckx [24], with modifications: Oocytes were allowed to recover for 30 min in G1™ PLUS (Vitrolife, Göteborg, Sweden) after ICSI and were then incubated for 7 min in a ionomycin (MP Biomedical, USA) solution 10 μmol/l in G1™ PLUS. Next, oocytes were washed in G1™ PLUS and incubated in fresh G1™ PLUS for 30 min; subsequently, the oocytes were exposed for a second round to the ionomycin solution at 10 μmol/l in G1™ PLUS for 7 min. Finally, the inseminated oocytes were washed in G1™ PLUS and incubated under Primo Vision® microscopes (Vitrolife, Göteborg, Sweden) to monitor embryo development, in standard incubator conditions (37 °C, 6%CO2, 5%O2, and 95% relative humidity).
Primo Vision® system procedures
The morphokinetic development of all embryos was recorded and analyzed with Primo Vision® Analyzer Software. Primo Vision® captured 11 focal planes over a 100-μm scan range every 20 min and one bright field image of the embryos every 5 min. Embryo development videos were recorded by Primo Vision Capture®, and the same operator analyzed with Primo Vision Analyzer® each embryo developmental video.
Morphokinetic data collection
The morphokinetic timing nomenclature was based on the guidelines of Ciray et al. [25]. The analyzed events were quantitative morphokinetic parameters: extrusion of the second polar body (tPB2), appearance of the pronuclei (tPN), pronuclear fading (tPNf), divisions to 2-cell through 8-cell stages (t2 to t8), start of blastulation (tSB), and full blastocyst stage (tB). All parameters were annotated considering time t0 as a time at the start of ICSI, i.e., the moment when the first oocyte of the cohort was injected (the injection was completed in less than 10 min in all cases). All these parameters are described in Supplementary Table 1.
The morphological score of the embryos assessed ET based on its developmental timing, the number and symmetry of the blastomeres, and their fragmentation [26].
Statistical analysis
Baseline characteristics have been compared using the Student’s t test for continuous variables and the Fisher exact test for categorical variables.
Median developmental times (tPB2, tPN, tPNf, t2, t3, t4, t5, t8, tSB, and tB) were calculated for experimental (ICSI-AOA) and control (ICSI) groups. A log-rank test (Mantel-Cox) was performed to test equality of survival distributions between groups; all time points were weighted equally in this test. In addition, a Kaplan-Meier curve with assisted AOA as a factor was plotted for each developmental time.
Fertilization rates and embryo morphological scores were compared between study groups using the Student’s t test and reproductive outcomes (biochemical pregnancy, clinical pregnancy, ongoing pregnancy, and live birth), after the first ET and cumulatively after all the performed ETs, were compared using the Fisher exact test.
Results
Baseline characteristics overall and by study group are presented in Table 1. The average age of the male patients included in the study was 42.85 ± 8.8 years. The mean age of the donors providing the oocytes for the cycles included in the study was 24.78 ± 2.36 years. Embryo transfers were mainly performed on day 3 of in vitro development. We observed that male patients in the experimental group (ICSI-AOA) were on average 3.4 years younger than in the control group (ICSI). Mean sperm sample concentration was higher in the control group, 59.6 million/ml vs. 30.6 million/ml.
Table 1.
Baseline characteristics, overall and for the experimental (ICSI-AOA) and the control (ICSI) groups
| Overall (n = 25 cycles) | ICSI-AOA (n = 7 cycles) | ICSI (n = 18 cycles) | p Value* | |
|---|---|---|---|---|
| Oocyte donor age, mean (SD) | 24.78 (2.36) | 24.68 (2.97) | 24.83 (2.07) | 0.73 |
| Male patient age, mean (SD) | 42.85 (8.8) | 40.41(4.18) | 43.86 (9.94) | 0.034 |
| Sperm sample concentration in million/ml, mean (SD) | 51.5 (43.22) | 30.6 (22.8) | 59.62 (46.9) | < 0.001 |
| Sperm motility, % of a + b, mean (SD) | 13.2 (12.6) | 10.5 (11.0) | 14.3 (13.2) | 0.11 |
| Embryo transfer day | ||||
| Day 3, n (%) | 20 (80%) | 5 (71.4%) | 15 (83.3%) | |
| Day 5, n (%) | 5 (20%) | 2 (28.5%) | 3 (16.6%) | 0.60 |
SD standard deviation
*Student’s t test or Fisher exact test
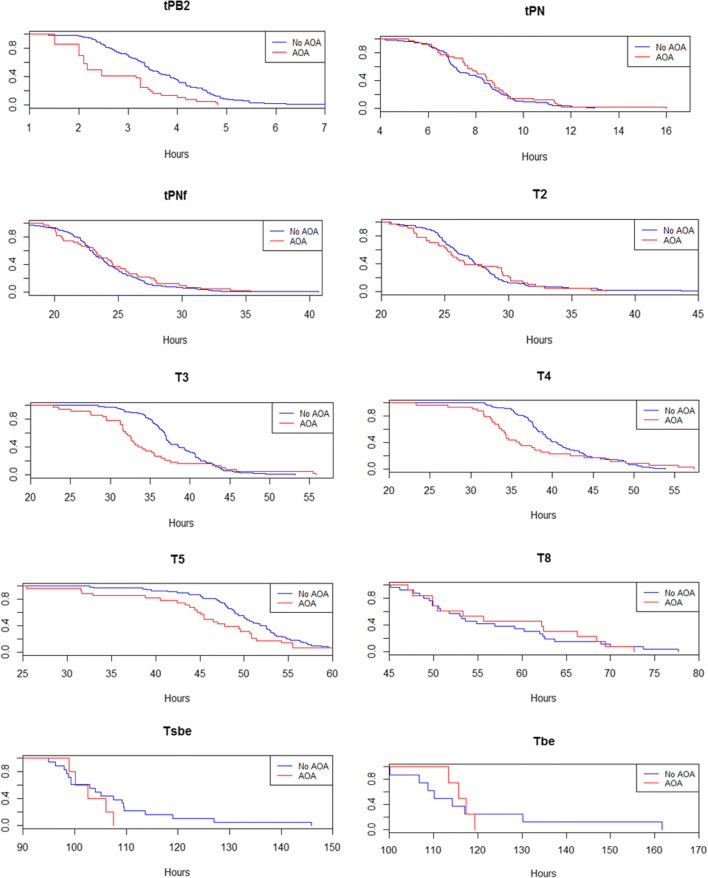
Median times for each developmental event are detailed in Table 2. These times can be interpreted as the time at which 50% of the embryos have achieved each developmental stage and are further represented as Kaplan-Meier curves in Fig. 1. No statistically significant differences based on the log-rank test were observed between groups among most the morphokinetic parameters analyzed, except for tPB2, with a median of 2.17 h in the experimental group (ICSI-AOA) vs. 3.43 h in the control group (ICSI) (p < 0.001) and t3, with median values of 32.60 h vs. 37.07 h (p = 0.043).
Table 2.
Median times for developmental event in hours for the experimental group (ICSI-AOA) and the control group (ICSI)
| Developmental event | Group | N | Median | Standard error | 95% confidence interval | p Value* | |
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||
| tPB2 | ICSI | 90 | 3.43 | .14 | 3.17 | 3.70 | <0.001 |
| ICSI-AOA | 36 | 2.17 | .18 | 1.81 | 2.53 | ||
| tPN | ICSI | 100 | 7.72 | .43 | 6.87 | 8.56 | 0.33 |
| ICSI-AOA | 40 | 8.05 | .46 | 7.15 | 8.95 | ||
| tPNf | ICSI | 94 | 23.45 | .35 | 22.77 | 24.13 | 0.90 |
| ICSI-AOA | 40 | 23.67 | .79 | 22.12 | 25.22 | ||
| t2 | ICSI | 99 | 26.88 | .55 | 25.80 | 27.96 | 0.99 |
| ICSI-AOA | 38 | 25.62 | .52 | 24.59 | 26.64 | ||
| t3 | ICSI | 93 | 37.07 | .26 | 36.55 | 37.58 | 0.043 |
| ICSI-AOA | 37 | 32.60 | .58 | 31.47 | 33.73 | ||
| t4 | ICSI | 94 | 39.12 | .64 | 37.86 | 40.37 | 0.15 |
| ICSI-AOA | 34 | 34.28 | .36 | 33.57 | 35.00 | ||
| t5 | ICSI | 72 | 50.28 | 0.9 | 48.52 | 52.04 | 0.09 |
| ICSI-AOA | 30 | 45.48 | 1.26 | 43.02 | 47.95 | ||
| t8 | ICSI | 27 | 53.15 | 1.95 | 49.32 | 56.98 | 0.83 |
| ICSI-AOA | 15 | 55.63 | 7.07 | 41.78 | 69.49 | ||
| tSB | ICSI | 19 | 103.98 | 2.25 | 99.58 | 108.38 | 0.30 |
| ICSI-AOA | 6 | 102.53 | 2.59 | 97.45 | 107.61 | ||
| tB | ICSI | 9 | 110.07 | 3.85 | 102.51 | 117.62 | 0.88 |
| ICSI-AOA | 5 | 115.57 | 2.01 | 111.63 | 119.50 | ||
*Log-rank (Mantel-Cox) test
Fig. 1.
Median time point at which the embryos reached each developmental stage in the experimental (ICSI-AOA; red) and control group (ICSI; blue); x-axis, time in hours; y-axis, proportion of embryos reaching the developmental event
Regarding fertilization rates, we observed a statistically significant difference between the experimental group (ICSI-AOA) which was 66.2% on average (SD 12.5) and the ICSI group, which was 83.5% on average (SD 14.5) (p = 0.011). On the contrary, we did not observe any significant difference in the mean morphological score of embryos transferred on day 3, which was 6.4 (SD 1.3) in ICSI-AOA and 6.9 (SD 1.5) in ICSI (p = 0.16).
Finally, we could not find a statistically significant difference in reproductive outcomes in ICSI-AOA vs. ICSI after the first ET (Table 3) and cumulatively: 5 (83.3%) vs. 14 (77.8%) pregnancies and 4 (66.7%) vs. 11 (61.1%) live births.
Table 3.
Reproductive outcomes for the experimental (ICSI-AOA) and the control (ICSI) group, after the first embryo transfer
| First transfer results | ICSI-AOA (n = 7) | ICSI (n = 18) | p Value* |
|---|---|---|---|
| Biochemical pregnancy, % (n) | 57.1% (4) | 66.7% (12) | 0.65 |
| Clinical pregnancy, % (n) | 4.92% (3) | 50% (9) | 0.74 |
| Ongoing pregnancy, % (n) | 28.6% (2) | 44.4% (8) | 0.62 |
| Live birth, % (n) | 28.6% (2) | 44.4% (8) | 0.62 |
*Fisher exact test
Discussion
Assisted oocyte activation (AOA) is an experimental technique involving exposure of inseminated oocytes to calcium ionophore. However, it is unclear whether a few transient Ca2+ spikes induced over a short period of time effectively recapitulate the signaling effects of the long-lasting Ca2+ oscillatory signature produced in the oocyte upon fertilization by the sperm [27].
A greater understanding of how AOA affects embryo development is needed. In this study, we wanted to assess if and how the artificial peaks of Ca2+ generated by ionomycin exposure alter the preimplantation development of the resulting embryos.
Overall, we found that embryos derived from ICSI-AOA cycles present similar developmental time points when compared to embryos obtained from ICSI cycles. Nevertheless, two parameters diverge significantly in embryos obtained by ICSI-AOA cycles: tPB2 and t3.
The extrusion of the second PB is the first morphological event of meiotic resumption and is directly driven by the early Ca2+ CaMKII-dependent events [28]. It has been shown that 30% of inseminated oocytes extrude their second PB as early as 45 min post ICSI, while most oocytes (about 80%) have extrude their polar body by 3 h post-ICSI [29, 30].
During AOA, the exposure of the oocyte to ionomycin produces a transient and quick increase of free intracytoplasmic Ca2+, resulting from extracellular Ca2+ influx as well as from Ca2+ release from the ER (reviewed in [31]), compared to the sperm injection alone. Altogether, our results suggest that AOA could accelerate tPB2 mainly due to the ability of ionomycin to produce a quick increase of cytoplasmic Ca2+ that would induce an earlier inactivation of MPF, with a slightly earlier meiotic resumption and a faster PB2 extrusion.
Around 8 h after meiotic resumption, the majority of activated oocytes display two pronuclei [32]. Although tPB2 occurred earlier in ICSI-AOA embryos, tPN was similar in both groups, occurring at around 8 h after t0. Moreover, PN formation in mouse occurs even when Ca2+ spikes are not sufficient (in intensity and/or frequency) to completely resume meiosis. In summary, tPB2 and tPN seem to be mostly independent events, coinciding with a longer G1 phase from tPB2 to tPN.
Despite the acceleration of tPB2, the appearance and fading of the pronuclei (tPN, tPNf) and the first cell division (t2) occur in a similar pattern between groups, and in agreement with what reported in the literature [18, 22]. We found significant differences in t3 between groups; the second cell cycle starts significantly earlier in ICSI-AOA group (p = 0.043). This could be explained by the relationship between artificial exposure to Ca2+ and the acceleration of the mitotic processes [28]. Although this acceleration in the ICSI-AOA group remains evident up to t5, we did not find any significant differences between groups at t8 coinciding with the stages when the human gene expression dramatically increases [33, 34]. Our results could indicate that the morphokinetics of embryos from ICSI-AOA cycles are reasonably comparable with the morphokinetics of embryos from ICSI cycles [18, 35]. However, we recognize that our conclusions are preliminary due to the low sample size of the ICSI-AOA group. In the same way, we did not find significant differences between the two groups on morphokinetic parameters tSB and tB, related to the probability of aneuploidy [36] and chromosomal status of the embryos [21].
We recognize some limitations in our study, mainly related to the sample size: Due to the fact that AOA is an infrequent technique rarely performed, studies with larger cohorts are needed to confirm our findings. Further, all ICSI-AOA cycles came from couples with a diagnosed male factor (presence of genetic alterations in PLCζ1) and no apparent female factor, so we cannot ascertain whether it is AOA that directly affects embryo morphokinetics or if the male factor per se also plays a role.
In conclusion, ionomycin mediated AOA does not seem to affect the general morphokinetic pattern of preimplantation embryo development, despite the alterations found in tPB2 and t3. These alterations could be explained by the transient and quick non-physiologic increase of free intracytoplasmic Ca2+ after the use of Ca2+ionophore.
Supplementary information
(DOCX 13 kb)
Acknowledgments
The authors wish to thank Désirée García and Francesc Figueras for the statistical support and to Montserrat Barragan for the helpful discussion and comments.
Authors’ contributions
M. Martínez involved in study design, video analysis, data compilation and analysis, and manuscript preparation. M. Durban involved in study design. A. Rodriguez involved in manuscript supervision and expert knowledge. J. Santaló and R.Vassena involved in the study design, implementation and supervision, expert knowledge, and manuscript preparation.
Funding
This study was carried out with intramural fundings from Clinica Eugin.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Plachot M, Mandelbaum J. Oocyte maturation, fertilization and embryonic growth in vitro. Br Med Bull. 1990;46(3):675–694. doi: 10.1093/oxfordjournals.bmb.a072424. [DOI] [PubMed] [Google Scholar]
- 2.Neri QV, Lee B, Rosenwaks Z, Machaca K, Palermo GD. Understanding fertilization through intracytoplasmic sperm injection (ICSI) Cell Calcium. 2014;55(1):24–37. doi: 10.1016/j.ceca.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 4.Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 2002;129(15):3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- 5.Malcuit C, Kurokawa M, Fissore RA. Calcium oscillations and mammalian egg activation. J Cell Physiol. 2006;206(3):565–573. doi: 10.1002/jcp.20471. [DOI] [PubMed] [Google Scholar]
- 6.Esfandiari N, Javed MH, Gotlieb L, Casper RF. Complete failed fertilization after intracytoplasmic sperm injection--analysis of 10 years' data. Int J Fertil Women's Med. 2005;50(4):187–192. [PubMed] [Google Scholar]
- 7.Combelles CM, Morozumi K, Yanagimachi R, Zhu L, Fox JH, Racowsky C. Diagnosing cellular defects in an unexplained case of total fertilization failure. Hum Reprod. 2010;25(7):1666–1671. doi: 10.1093/humrep/deq064. [DOI] [PubMed] [Google Scholar]
- 8.Heindryckx B, Van der Elst J, De Sutter P, Dhont M. Treatment option for sperm- or oocyte-related fertilization failure: assisted oocyte activation following diagnostic heterologous ICSI. Hum Reprod. 2005;20(8):2237–2241. doi: 10.1093/humrep/dei029. [DOI] [PubMed] [Google Scholar]
- 9.Borges E, Jr, de Almeida Ferreira Braga DP, de Sousa Bonetti TC, Iaconelli A, Jr, Franco JG., Jr Artificial oocyte activation using calcium ionophore in ICSI cycles with spermatozoa from different sources. Reprod BioMed Online. 2009;18(1):45–52. doi: 10.1016/s1472-6483(10)60423-3. [DOI] [PubMed] [Google Scholar]
- 10.Bonte D, Ferrer-Buitrago M, Dhaenens L, Popovic M, Thys V, De Croo I, et al. Assisted oocyte activation significantly increases fertilization and pregnancy outcome in patients with low and total failed fertilization after intracytoplasmic sperm injection: a 17-year retrospective study. Fertil Steril. 2019;112(2):266–274. doi: 10.1016/j.fertnstert.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Torra-Massana M, Cornet-Bartolome D, Barragan M, Durban M, Ferrer-Vaquer A, Zambelli F, et al. Novel phospholipase C zeta 1 mutations associated with fertilization failures after ICSI. Hum Reprod. 2019;34(8):1494–1504. doi: 10.1093/humrep/dez094. [DOI] [PubMed] [Google Scholar]
- 12.Tejera A, Molla M, Muriel L, Remohi J, Pellicer A, De Pablo JL. Successful pregnancy and childbirth after intracytoplasmic sperm injection with calcium ionophore oocyte activation in a globozoospermic patient. Fertil Steril. 2008;90(4):1202 e1–5. doi: 10.1016/j.fertnstert.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 13.Murugesu S, Saso S, Jones BP, Bracewell-Milnes T, Athanasiou T, Mania A, Serhal P, Ben-Nagi J. Does the use of calcium ionophore during artificial oocyte activation demonstrate an effect on pregnancy rate? A meta-analysis. Fertil Steril. 2017;108(3):468–482. doi: 10.1016/j.fertnstert.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 14.D'Haeseleer E, Vanden Meerschaut F, Bettens K, Luyten A, Gysels H, Thienpont Y, et al. Language development of children born following intracytoplasmic sperm injection (ICSI) combined with assisted oocyte activation (AOA) Int J Language Commun Disord. 2014;49(6):702–709. doi: 10.1111/1460-6984.12100. [DOI] [PubMed] [Google Scholar]
- 15.Vanden Meerschaut F, Nikiforaki D, Heindryckx B, De Sutter P. Assisted oocyte activation following ICSI fertilization failure. Reprod BioMed Online. 2014;28(5):560–571. doi: 10.1016/j.rbmo.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Deemeh MR, Tavalaee M, Nasr-Esfahani MH. Health of children born through artificial oocyte activation: a pilot study. Reprod Sci. 2015;22(3):322–328. doi: 10.1177/1933719114542017. [DOI] [PubMed] [Google Scholar]
- 17.Vanden Meerschaut F, D'Haeseleer E, Gysels H, Thienpont Y, Dewitte G, Heindryckx B, et al. Neonatal and neurodevelopmental outcome of children aged 3-10 years born following assisted oocyte activation. Reprod BioMed Online. 2014;28(1):54–63. doi: 10.1016/j.rbmo.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Meseguer M, Herrero J, Tejera A, Hilligsoe KM, Ramsing NB, Remohi J. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;26(10):2658–2671. doi: 10.1093/humrep/der256. [DOI] [PubMed] [Google Scholar]
- 19.Herrero J, Tejera A, Albert C, Vidal C, de los Santos MJ, Meseguer M. A time to look back: analysis of morphokinetic characteristics of human embryo development. Fertil Steril. 2013;100(6):1602–9 e1–4. doi: 10.1016/j.fertnstert.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 20.Chen AA, Tan L, Suraj V, Reijo Pera R, Shen S. Biomarkers identified with time-lapse imaging: discovery, validation, and practical application. Fertil Steril. 2013;99(4):1035–1043. doi: 10.1016/j.fertnstert.2013.01.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desai N, Goldberg JM, Austin C, Falcone T. Are cleavage anomalies, multinucleation, or specific cell cycle kinetics observed with time-lapse imaging predictive of embryo developmental capacity or ploidy? Fertil Steril. 2018;109(4):665–674. doi: 10.1016/j.fertnstert.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 22.Azzarello A, Hoest T, Mikkelsen AL. The impact of pronuclei morphology and dynamicity on live birth outcome after time-lapse culture. Hum Reprod. 2012;27(9):2649–2657. doi: 10.1093/humrep/des210. [DOI] [PubMed] [Google Scholar]
- 23.Pujol A, Garcia D, Obradors A, Rodriguez A, Vassena R. Is there a relation between the time to ICSI and the reproductive outcomes? Hum Reprod. 2018;33(5):797–806. doi: 10.1093/humrep/dey067. [DOI] [PubMed] [Google Scholar]
- 24.Heindryckx B, De Gheselle S, Gerris J, Dhont M, De Sutter P. Efficiency of assisted oocyte activation as a solution for failed intracytoplasmic sperm injection. Reprod BioMed Online. 2008;17(5):662–668. doi: 10.1016/s1472-6483(10)60313-6. [DOI] [PubMed] [Google Scholar]
- 25.Ciray HN, Campbell A, Agerholm IE, Aguilar J, Chamayou S, Esbert M, Sayed S, Time-Lapse User Group Proposed guidelines on the nomenclature and annotation of dynamic human embryo monitoring by a time-lapse user group. Hum Reprod. 2014;29(12):2650–2660. doi: 10.1093/humrep/deu278. [DOI] [PubMed] [Google Scholar]
- 26.Coroleu B, Barri PN, Carreras O, Belil I, Buxaderas R, Veiga A, Balasch J. Effect of using an echogenic catheter for ultrasound-guided embryo transfer in an IVF programme: a prospective, randomized, controlled study. Hum Reprod. 2006;21(7):1809–1815. doi: 10.1093/humrep/del045. [DOI] [PubMed] [Google Scholar]
- 27.Nikiforaki D, Vanden Meerschaut F, de Roo C, Lu Y, Ferrer-Buitrago M, de Sutter P, et al. Effect of two assisted oocyte activation protocols used to overcome fertilization failure on the activation potential and calcium releasing pattern. Fertil Steril. 2016;105(3):798–806. doi: 10.1016/j.fertnstert.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Miao YL, Stein P, Jefferson WN, Padilla-Banks E, Williams CJ. Calcium influx-mediated signaling is required for complete mouse egg activation. Proc Natl Acad Sci U S A. 2012;109(11):4169–4174. doi: 10.1073/pnas.1112333109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van den Bergh M, Bertrand E, Englert Y. Second polar body extrusion is highly predictive for oocyte fertilization as soon as 3 hr after intracytoplasmic sperm injection (ICSI) J Assist Reprod Genet. 1995;12(4):258–262. doi: 10.1007/BF02212928. [DOI] [PubMed] [Google Scholar]
- 30.Martinez M, Obradors A, Vernaeve V, Santalo J, Vassena R. Oocyte vitrification does not affect early developmental timings after intracytoplasmic sperm injection for women younger than 30 years old. Mol Reprod Dev. 2016;83(7):624–629. doi: 10.1002/mrd.22667. [DOI] [PubMed] [Google Scholar]
- 31.Ferrer-Buitrago M, Bonte D, De Sutter P, Leybaert L, Heindryckx B. Single Ca(2+) transients vs oscillatory Ca(2+) signaling for assisted oocyte activation: limitations and benefits. Reproduction. 2018;155(2):R105–RR19. doi: 10.1530/REP-17-0098. [DOI] [PubMed] [Google Scholar]
- 32.Nagy ZP, Liu J, Joris H, Devroey P, Van Steirteghem A. Time-course of oocyte activation, pronucleus formation and cleavage in human oocytes fertilized by intracytoplasmic sperm injection. Hum Reprod. 1994;9(9):1743–1748. doi: 10.1093/oxfordjournals.humrep.a138786. [DOI] [PubMed] [Google Scholar]
- 33.Vassena R, Boue S, Gonzalez-Roca E, Aran B, Auer H, Veiga A, Belmonte JCI. Waves of early transcriptional activation and pluripotency program initiation during human preimplantation development. Development. 2011;138(17):3699–3709. doi: 10.1242/dev.064741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braude P, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature. 1988;332(6163):459–461. doi: 10.1038/332459a0. [DOI] [PubMed] [Google Scholar]
- 35.Conaghan J, Chen AA, Willman SP, Ivani K, Chenette PE, Boostanfar R, Baker VL, Adamson GD, Abusief ME, Gvakharia M, Loewke KE, Shen S. Improving embryo selection using a computer-automated time-lapse image analysis test plus day 3 morphology: results from a prospective multicenter trial. Fertil Steril. 2013;100(2):412–419. doi: 10.1016/j.fertnstert.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 36.Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Hickman CF. Modelling a risk classification of aneuploidy in human embryos using non-invasive morphokinetics. Reprod BioMed Online. 2013;26(5):477–485. doi: 10.1016/j.rbmo.2013.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 13 kb)