Abstract
Objective
Vascular endothelial growth factor (VEGF) plays a critical role in regulating trophoblast cell invasion and proliferation, involved in a variety of pregnancy complications, such as spontaneous abortion and pre-eclampsia. Numerous studies have revealed that microRNAs (miRNAs) are participated in a series of molecular processes that regulate cell function, such as cell invasion, proliferation, and apoptosis. Vascular endothelial growth factor receptor 2 (VEGFR2), a receptor of VEGF, has been shown to be involved in trophoblast function. However, the relation between miRNA and VEGFR2 and their role in trophoblast function remain to be elucidated.
Methods
The effect of miR-219a on the trophoblast function has been explored using luciferase reporter, transwell, qRT-PCR, western blot, bromodeoxyuridine (BrdU), ELISA, immunofluorescent staining, and tube formation assays.
Results
In the current study, we observed that through targeted inhibition of VEGFR2 expression by miR-219a, the function of VEGFR2 as well as the downstream PI3K/AKT/NF-κB signaling pathway were suppressed, leading to suppression of trophoblastic proliferation and invasion. Moreover, upregulation of VEGFR2 restored the miR-219a–inhibited cell proliferation, invasion, and tube formation.
Conclusions
These results revealed that miR-219a played crucial roles in negatively regulating trophoblastic proliferation and invasion by suppression of the PI3K/AKT/NF-κB signaling pathway by targeting VEGFR2, therefore serving as a potential treatment method for the complications of pregnancy caused by trophoblastic dysregulation.
Keywords: Trophoblast, miR-219a, VEGFR2, PI3K/AKT/NF-κB signaling, Proliferation, Invasion
Introduction
Trophoblasts are cells forming the outer layer of a blastocyst, which provide nutrients to the embryo [1, 2]. The extravillous trophoblast (EVT) plays an important role in the growth and development of the placenta and fetus [3, 4]. Abnormal patterns of trophoblastic proliferation and invasion are critical for the complications of pregnancy, including spontaneous abortion and pre-eclampsia [5, 6]. Cytotrophoblast (CTB) is one of the major subpopulations of trophoblasts, which can give rise to syncytiotrophoblast (STB) and EVT [7]. CTB is widely used to study trophoblast biology in vitro due to the characteristics of undifferentiation and epithelial stemness [8]. HTR-8/SVneo cells are one of the extravillous CTB (evCTB) cell lines, which generated from the normal tissues of the first trimester placenta [9]. HTR-8/SVneo cells are largely used to study trophoblast invasion, proliferation, and regulation in vitro [10, 11]. Previous studies have shown the inhibition effect of miR-219a on the cell invasion and proliferation in various cancer cells [12–14]. Evidence also suggests that expression of miR-219a should be upregulated in cases of spontaneous abortion comparing with the control [15]. However, whether miR-219a affects trophoblast proliferation and invasion in HTR-8/SVneo cells remains to be elucidated.
VEGF is an important cytokine in preserving optimal embryogenesis [16–18]. VEGF activates the downstream signaling through VEGFR1, VEGFR2, and VEGFR3 receptors [19, 20]. Previous study has shown that the VEGF signaling pathway is inhibited in spontaneous abortion, while VEGF treatment promotes the proliferation and invasion of trophoblasts [21, 22]. Studies have shown that VEGF is a highly potent mitogenic factor [23, 24]. VEGF can promote the angiogenesis in endometrial and decidual tissues and increase EVT proliferation in vitro [25, 26]. Soluble fms-like tyrosine kinase 1 (sFlt1), a splice variant of the VEGFR1 and a potent inhibitor of VEGF, has been shown to be involved in trophoblast function [27]. Park et al. demonstrated that inhibition of phosphatidyl-inositol 3-kinase/serine-threonine kinase (PI3K/AKT) signaling pathway decreased sFlt1 levels in pre-eclampsia [28]. PI3K/AKT pathway plays an important role in regulating cellular functions including metabolism, proliferation, transcription, and invasion [29, 30]. Dysregulation of the PI3K/AKT pathway is implicated in various diseases including cancer, diabetes, cardiovascular, and reproductive-related diseases [28, 31]. Cheng et al. demonstrated that long non-coding RNA (lncRNA) MIR503HG modulated trophoblast cell proliferation, invasion, and migration via NF-κB signaling [32]. Previous studies have shown that VEGFR-2 is involved in trophoblast proliferation, migration, and invasion through binding to Decorin [25]. In the current study, we have observed that through targeted inhibition of VEGFR2 expression by miR-219a, the function of VEGF as well as the downstream PI3K/AKT/NF-κB signaling pathway was suppressed, leading to suppression of trophoblast proliferation and invasion. Moreover, upregulation of VEGFR2 reversed the miR-219a–regulated cell proliferation, invasion, and tube formation. These results might provide a potential treatment method for the complications of pregnancy caused by trophoblastic dysregulation.
Materials and Methods
Cell culture
The human trophoblast cells HTR-8/SVneo (passages 35–40) were purchased from the cell bank of the Chinese Academy of Sciences (Shanghai, China). HTR-8/SVneo cells were cultured in the medium of RPMI 1640, with 10% fetal bovine serum, 100 μg/ml of streptomycin, 100 U/ml of penicillin, and maintained in the condition of 5% CO2 and air (20% O2) at 37 °C in a humidified incubator (Thermo Fisher Scientific, Cleveland, OH, USA).
Immunofluorescent staining
Of the 24-well plate, 2 × 105 cells were seeded per well to make the cell slide. The cells were fixed in 4% paraformaldehyde for 15 min, followed by permeabilization with 0.05% Triton X-100 for 5 min. After fixation and permeabilization, the cells were incubated with 0.1 % BSA in PBS for 1 h to block the non-specific sites and then incubated with the primary anti-Cytokeratin 7 (CK7, Cat#: ab181598, Abcam, MA, USA) antibody at 4 °C overnight. Thereafter, cells were washed and incubated with Alexa Fluor 594-conjugated antibody for 1 h at room temperature. The nuclei were stained with 4′,6-diami-dino-2-phenylindole (DAPI) for 10 min. The immunofluorescent staining was performed using an Olympus microscope (BX41, Japan).
ELISA
The β-hCG levels in the supernatants of HTR-8/SVneo cells were measured utilizing β-hCG ELISA kit (R&D system, Minneapolis, MN, USA). After the cells were cultured in different time points, the concentration of β-hCG was read at 450 nm using a Microplate Reader (Molecular Devices, Sunnyvale, USA).
Short tandem repeat analysis
The genomic DNA of HTR8/SVneo cell was isolated with a Genomic DNA Extraction kit (Takara Bio, Inc., Shiga, Japan) according to the manufacturer’s protocol. The short tandem repeat (STR) profiling PCR was carried out in the ABI 3100 genetic analyzer machine by using STR-multiplex kits (Promega, Madison, WI, USA). The STR profile of Amelogenin, CSF1PO, D13S317, D16S539, D5S818, D7S820, TH01, vWA, and TPOX was consistent with the ATCC STR database.
MiRNA and transfection
The miR-219a-3p inhibitor, inhibitor control, miR-219a-3p mimic, and mimic control were obtained from GenePharma (Shanghai, China). HTR-8/SVneo cells were transfected with miR-219a inhibitor, inhibitor control, miR-219a mimic, and mimic control by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the protocol.
RNA extraction and qRT-PCR
Total RNA was isolated from cells using TRIzol (Invitrogen) according to the instruction. An ABI PRISM 7500 real-time PCR System and SYBR Green Master Mix (Takara Bio, Inc.) were used to detect the result. GAPDH and U6 were used as the internal control. The PCR primers were as follows, miR-219a forward: 5′-CTCGCTTCGGCAGCACA-3′; reverse: 5′-GTCGTATCCAGTGCGTGTCGT-3′; sFlt1 forward: 5′-ACAATCAGAGGTGAGCACTGCAA-3′, reverse: 5′-TCCGAGC CTGAAAGTTAGCAA-3′; VEGFR2 forward: 5′-ATTCCTCCCCCGCATCA-3′, reverse: 5′-GCTCGTTGGCGCACTCTT-3′; VEGFR3 forward: 5′-CTGGCAAATG GTTACTCCATGA-3′, reverse: 5′-ACAACCCGTGTGTCTTCACTG-3′; Decorin forward: 5′-CGAGTGGTCCAGTGTTCTGA-3′, reverse: 5′-AAAGCCCCATTTTC AATTCC-3′; U6 forward: 5′-TGCGGGTGCTCGCTTCGCAGC-3′, reverse: 5′-CCA GTGCAGGGTCCGAGGT-3′; GAPDH forward: 5′-AGGTCGGTGTGAACGGATT TG-3′, reverse: 5′-TGTAGACCATGTAGTTGAGGTCA-3′. The relative expression levels were acquired using the comparative Ct method.
Bromodeoxyuridine assay
Bromodeoxyuridine (BrdU) was used to detect DNA synthesis in proliferating cells. HTR-8/SVneo cells were transfected with different treatments and incubated with a final concentration of 10 mM BrdU (BD Pharmingen, San Diego, CA, USA) for 2 h, and then fixed for 30 min. The peroxidase-conjugated BrdU antibody (1 μg/ml, Sigma-Aldrich, St Louis, USA) was added to cells and incubated for 1 h at room temperature (RT); 100 μl tetramethylbenzidine substrate dissolved in dimethylsulfoxide (15%) was added to cells and incubated for 30 min. The absorbance result was detected at 450 nm.
Transwell assay
Cell transwell invasion assay was performed following treatments in HTR-8/SVneo cells [33]. HTR-8/SVneo cells were seeded with mitomycin C (10 μg/ml, R&D Systems) in medium to inhibit cell proliferation and coated with Matrigel (9 mg/ml). The serum-free RPMI-1640 medium was added in the upper chamber. The RPMI-1640 medium with 10% FBS were added in the low chamber. The upper chamber was removed after 24 h incubation and HTR-8/SVneo cells in the low chamber were fixed with methanol, and then incubated with 1% crystal violet. The number of invaded cells was counted in three different microscopic fields.
Luciferase reporter assay
The sequence of wild-type (wt) or mutated (mut) 3′UTR of VEGFR2 were amplified, and then integrated in pGL3 luciferase reporter vector (Promega). HTR-8/SVneo cells were transfected with the reporter vector and miR-219a inhibitor or miR-219a mimic. Forty-eight hours after transfection, luciferase activities were detected by the dual luciferase assay (Promega).
Western blot assay
Cells were collected and lysed in buffer (Cat#: 9803, Cell Signaling Technology, Danvers, MA, USA) containing protease inhibitors. The lysates were heated at 98 °C for 10 min and proceeded to SDS-PAGE. The result was measured by enhanced chemiluminescence. The primary antibodies of p-AKT, AKT, NF-κB, and VEGFR2 were acquired from Cell Signaling Technology. After incubation with primary antibodies, membranes were washed and incubated with goat anti-mouse HRP-conjugated secondary antibodies (Santa Cruz Biotechnology, Dallas, TX, USA). Proteins were detected using chemiluminescence system (Roche, Basel, Switzerland) and visualized using autoradiography. Densitometry analysis of band intensity was digitized and analyzed using ImageJ software (National Institute of Health, NIH, Bethesda, MD, USA).
Tube formation assay
Tube formation assays were performed using a 24-well plate, and each well was coated with 40 μl Matrigel and incubated at 37 °C for 30 min. After solidification of the Matrigel matrix, 5-×-104–treated cells were seeded into each well. After 8 h of incubation, the tube formation was observed by a microscope at × 4 magnification (Nikon, Japan). The tube formation was quantified in three different microscopic fields.
Statistical analysis
The results were analyzed with GraphPad Prism 6 Software. The data were repeated in three independent experiments and presented as mean ± SD for the statistical analysis. Two-tailed Student’s t test was used to compare two groups. p < 0.05 was considered to be the statistical significance.
Results
Characterization of HTR-8/SVneo cells
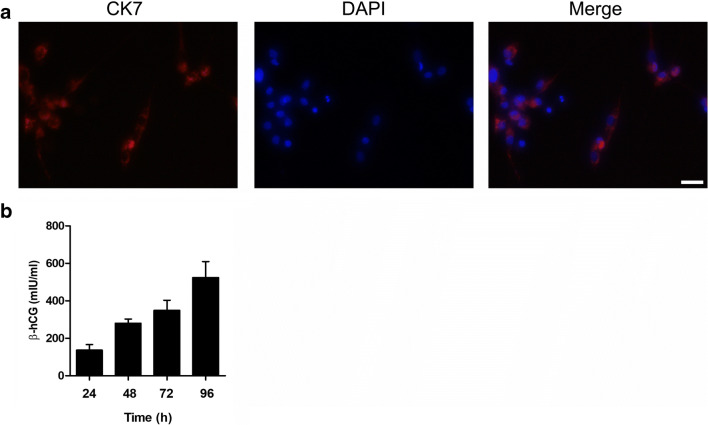
Cytokeratin 7 (CK7) is a generally accepted marker for trophoblast cells. The expression of CK7 was evaluated by immunofluorescence in HTR-8/SVneo cells. As shown in Fig. 1a, CK7 expression was present in almost all the HTR-8/SVneo cells. Fusion of cytotrophoblast cells can lead to formation of the multicleated syncytiotrophoblast cells, and β-hCG is the related marker. We found that the β-hCG levels gradually increased with the prolonged culture time points (Fig. 1c). In addition, the STR profile was an exact match between used HTR8/SVneo and the ATCC STR database: Amelogenin: X, CSF1PO: 12, D13S317: 9,12, D16S539: 13, D5S818: 12, D7S820: 12, TH01: 6,9.3, vWA: 13,18, TPOX: 8.
Fig. 1.
Characterization of HTR8/SVneo cells. a HTR-8/SVneo cells were stained with CK7, DAPI. Immunofluorescence staining of CK7 was detected in HTR8-SVneo cells. Scale bar, 25 μm. b The levels of β-hCG were determined in HTR8-SVneo cells for 24, 48, 72, and 96 h
MiR-219a–inhibited invasion and proliferation of HTR-8/SVneo cells
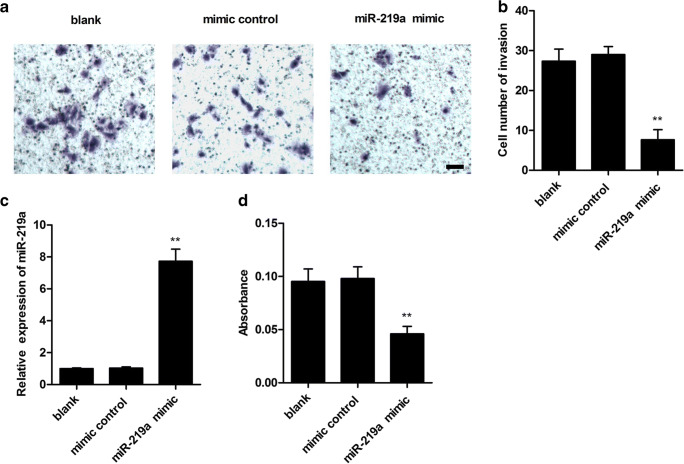
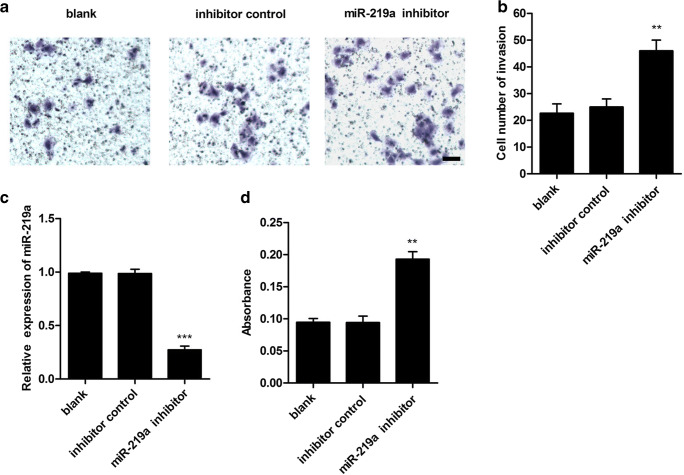
The relative expression level of miR-219a was measured using qPCR after transient transfection of miR-219a mimic or the mimic control in human HTR8/SVneo trophoblast cells. A significant increase of the miR-219a expression level was detected followed by the miR-219a mimic transfection compared with the control group (Fig. 2c). In order to examine the in vitro effects of miR-219a on invasion of trophoblast cells, a transwell invasion assay was performed following transfection with miR-219a. The results are shown in Fig. 2a, b; transfection of miR-219a mimic sufficiently reduced the number of invaded HTR-8/SVneo cells compared with the mimic control (p < 0.01). To further explore the effects of miR-219a on trophoblast cell proliferation, we detected the viability of trophoblast cells using BrdU assay (Fig. 2d), and the result showed miR-219a mimic significantly suppressed the cell proliferation in HTR-8/SVneo cells compared with the mimic control. To confirm the effects of miR-219a on regulating invasion and proliferation of traphoblast cells, the human HTR8/SVneo trophoblast cells were treated with miR-219a inhibitor or inhibitor control. As shown in Fig. 3c, miR-219a inhibitor suppressed the relative expression level of miR-219a (p < 0.001). In addition, miR-219 inhibitor significantly promoted cell invasion and proliferation (Fig. 3a, b, and d) compared with the control group in HTR8/SVneo cells (Fig. 3a, b, and d).
Fig. 2.
MiR-219a–inhibited HTR8/SVneo cell proliferation and invasion. a, b MiR-219a suppressed cell invasion. HTR8/SVneo cells were treated with miR-219a mimic or mimic control. At 48 h after transfection, transwell assay was performed to assay invasion ability. Scale bar, 25 μm. c miR-219a mimic transfection enhanced miR-219a expression level. Cells were transfected with miR-219a mimic or mimic control. The miR-219a expression level was detected. d miR-219a–inhibited cell proliferation. Cells were transfected with miR-219a mimic or mimic control. BrdU assay was performed after transfection. Data represent mean ± SD. **p < 0.01 vs. control
Fig. 3.
Inhibition of miR-219a enhanced proliferation and invasion in human HTR8/SVneo cells. a, b Inhibition of miR-219a enhanced cell invasion. Cells were treated with miR-219a inhibitor or inhibitor control for 48 h. Transwell assay was performed to assay invasion ability. Scale bar, 25 um. c miR-219a inhibitor treatment inhibited miR-219a expression level. Cells were transfected with miR-219a inhibitor or inhibitor control. The miR-219a expression level was detected. d miR-219a inhibitor increased cell proliferation. Cells were treated with miR-219a inhibitor or inhibitor control. BrdU assay was performed. Data represent mean ± SD. **p < 0.01; ***p < 0.001 vs. control
MiR-219a–regulated VEGFR2 expression in HRT-8/Svneo cells
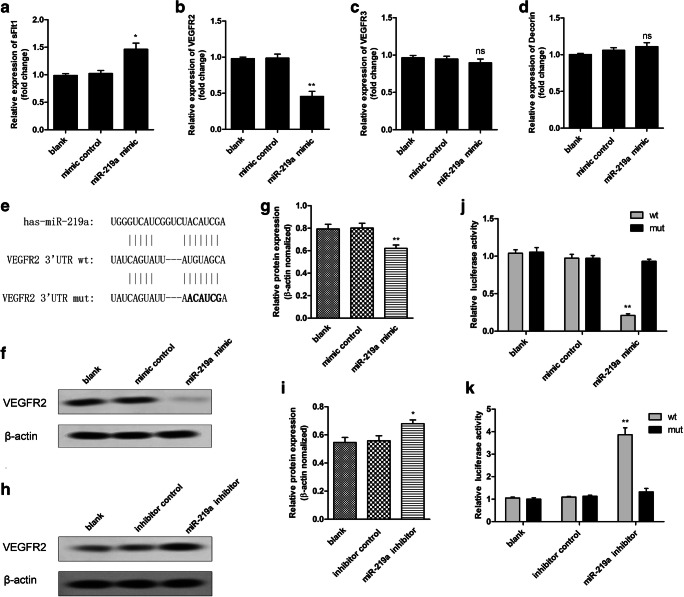
To explore the effect of miR-219a on VEGF signaling, the sFlt1, VEGF2, and VEGF3 expression levels were determined followed by the miR-219a mimic transfection in HRT-8/Svneo cells. The result showed that the expression levels of sFlt1 were significantly increased after miR-219a mimic treatment compared with the control (Fig. 4a). Interestingly, miR-219a mimic treatment lead to a significant decrease in VEGF2 levels, while barely changing the VEGF3 levels in HRT-8/Svneo cells (Fig. 4b, c). Decorin, involved in VEGFR2 and trophoblast proliferation, was not significantly changed following transfection with miR-219a mimic (Fig. 4d). To further examine the relation between VEGFR2 and miR-219a, the target prediction was performed using the sequence of miR-219a and VEGFR2 3′UTR (Fig. 4e). The relation between of miR-219a and VEGFR2 was determined using a dual luciferase reporter assay in HTR-8/SVneo cells. wt and mut VEGFR2 3′UTR luciferase reporter constructs were generated and were co-transfected with either miR-219a or mimic control into the trophoblast cells, respectively. The relative luciferase activity of wt 3′UTR was significantly reduced by the treatment of miR-219a mimic (Fig. 4j), and the activity was restored with co-transfection of miR-219a inhibitor (Fig. 4k). Similar changes were not observed with mut 3′UTR (Fig. 4j, k). In addition, VEGFR2 protein expression was obviously inhibited by miR-219a mimic treatment compared with the control group (Fig. 4f, g), while inhibition of miR-219a remarkably enhanced the protein expression of VEGFR2 (Fig. 4h, i).
Fig. 4.
MiR-219a directly targeted VEGFR2. The expression levels of sFlt1 (a), VEGF2 (b), and VEGF3 (c) were determined following transfection with miR-219a mimic. d The expression levels of Decorin were determined following transfection with miR-219a mimic. e Luciferase reporter assays. The predicted miR-219a targeting site is present at nucleotide of the 3′URT of VEGFR2. wt, wild-type reporter construct containing VEGFR2 3′UTR; mut, constructs with mutations in the predicted miR-219a binding regions; miR-219a inhibited the luciferase activities of the wide type but not the mutant. f, g miR-219a decreased VEGFR2 expression. The lysates were acquired from HTR-8/SVneo cells transfected with miR-219a mimic or its mimic control; the VEGFR2 protein expression level was detected. h, i miR-219a inhibitor increased VEGFR2 expression. The lysates were acquired from HTR-8/SVneo cells treated with miR-219a inhibitor or its control; the VEGFR2 protein expression level was detected. j miR-219a decreased the luciferase activity of VEGFR2 wt, but not the mut. k miR-219a inhibitor increased the luciferase activity of VEGFR2 wt, but not the mut. *p < 0.05; **p < 0.01 vs. control. ns, not significant
MiR-219a–inhibited VEGF/PI3K/AKT/NF-κB signaling pathway
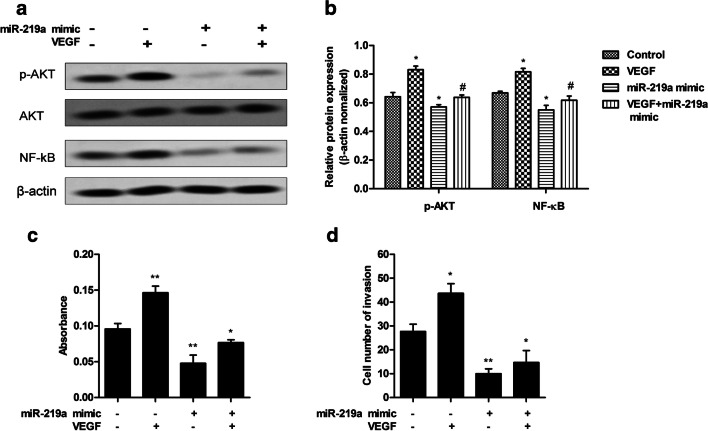
The changes of the VEGF/NF-κB signaling pathway induced by miR-219a was also investigated in human HTR-8/SVneo trophoblast cells. Western blot analysis revealed that the downstream p-AKT and NF-κB expressions were regulated by miR-219a mimic (Fig. 5a, b). The relative protein expressions of p-AKT and NF-κB decreased compared with those of the negative control after miR-219a treatment. While VEGF promoted the expressions of both p-AKT and NF-κB, treatment of miR-219a repressed the VEGF-upregulated protein expressions of p-AKT and NF-κB. We examined cell proliferation and invasion of HTR-8/SVneo after miR-219a treatment or VEGF upregulation. We detected using BrdU assay that miR-219a remarkably suppressed trophoblast cell proliferation (Fig. 5c). While VEGF upregulation induced proliferation of trophoblast cells, treatment of miR-219a resulted in a significant drop of trophoblast cell proliferation after VEGF upregulation compared with the control. Moreover, a transwell assay detecting changes in trophoblast cell invasion revealed a similar trend that the cell number of invasion induced by VEGF was repressed by the miR-219a mimic (Fig. 5d).
Fig. 5.
MiR-219a suppressed PI3K/AKT signaling in human HTR8/SVneo cells. a miR-219a–inhibited PI3K/AKT signaling related protein expression. Cells were treated with miR-219a mimic and/or VEGF at 50 ng/ml concentration. At 48 h after transfection, the protein expression of p-AKT, AKT, and NF-κB was detected. b The relative protein expression was normalized with β-actin. c miR-219a and VEGF treatment inhibited cell proliferation. Cells were treated with miR-219a mimic or VEGF at 50 ng/ml concentration. BrdU assay was performed. d miR-219a and VEGF treatment inhibited cell invasion. Cells were treated with miR-219a mimic or VEGF at 50 ng/ml concentration. Transwell assay was performed to assay invasion ability. Data represent mean ± SD. *p < 0.05 vs. control; **p < 0.01 vs. control; #p < 0.05 vs. VEGF treatment group
The impact of VEGFR2 on miR-219a–regulated HTR-8/SVneo suppression
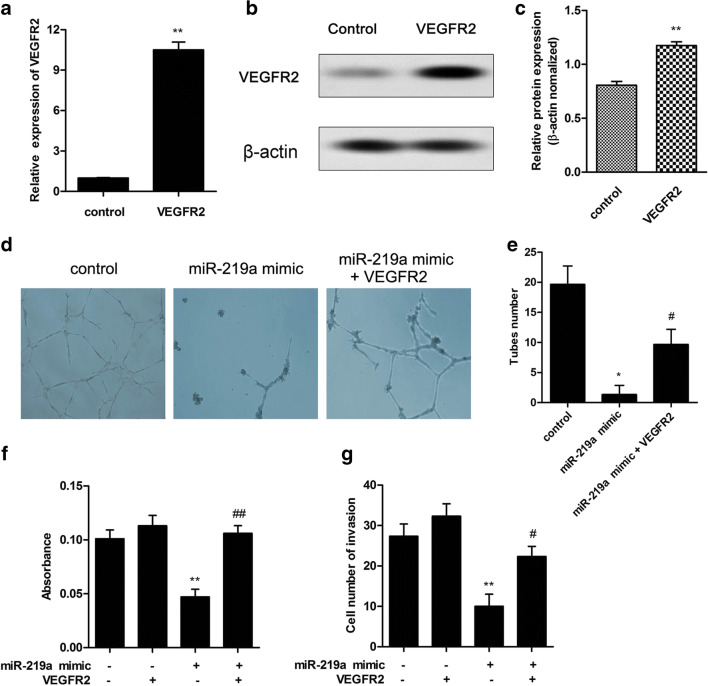
To examine the role of VEGFR2 on miR-219a–regulated trophoblast cell suppression, we investigated how cell invasion and proliferation of trophoblast cells were altered after VEGFR2 and miR-219a upregulation. We first determined the efficiency of VEGFR2 upregulation using qPCR (Fig. 6a), and a 10-fold increase was observed in the relative expression of VEGFR2. Besides, the protein expression level of VEGFR2 was consistently enhanced following VEGFR2 lentivirus vector transfection (Fig. 6b, c). Moreover, the tube formation assay was performed to evaluate the angiogenic response of HTR8/SVneo cells in the presence of miR-219a and VEGFR2. The result showed that miR-219a mimic significantly inhibited the tube formation processes compared with those in control, whereas VEGFR2 upregulation significantly restored the miR-219a–inhibited ability of tube formation in HTR8/SVneo cells (Fig. 6d, e). The BrdU and transwell assays exhibited that miR-219a mimic significantly decreased the cell invasion and proliferation of trophoblast cells (Fig. 6f, g). In addition, VEGFR2 transfection alone did not enhance trophoblastic proliferation or invasion, but restore the miR-219a–mediated suppression on trophoblastic proliferation and invasion after VEGFR2 transfection comparing with the control (p < 0.01 and p < 0.05, respectively).
Fig. 6.
MiR-219a suppressed cell proliferation and invasion by targeting VEGFR2. a VEGFR2 lentivirus vector transfection enhanced VEGFR2 expression level. Cells were transfected with VEGFR2 vector or control. The VEGFR2 expression level was detected. b, c VEGFR2 lentivirus vector transfection enhanced VEGFR2 protein expression level. Cells were transfected with VEGFR2 vector or control. The VEGFR2 protein expression level was detected. d, e The effect of VEGFR2 on the ability of tube formation was determined in HTR8/SVneo cells. The number of tubes formed by HTR8/SVneo cells was quantified in three different fields (× 4 magnification). f VEGFR2 lentivirus vector transfection increased cell proliferation. Cells were treated with miR-219a mimic or VEGFR2 lentivirus vector. BrdU assay was performed. g VEGFR2 lentivirus vector transfection restored miR-219a–inhibited cell invasion. Cells were treated with miR-219a mimic or VEGFR2 lentivirus vector. Transwell assay was performed to assay invasion ability. Data represent mean ± SD. **p < 0.01 vs. control; #p < 0.05 vs. miR-219a mimic treatment group; ##p < 0.01 vs. miR-219a mimic treatment group
Discussion
Dysregulation of trophoblast cell proliferation and invasion has been reported to be associated with complications of pregnancy, such as spontaneous abortion and pre-eclampsia [34–37]. Previous studies have shown the critical role of miRNAs in regulating trophoblast cells, and miR-219a is found upregulated in cases of spontaneous abortion [38–40]. Thus, it is important to investigate the regulatory roles of miR-219a in trophoblast proliferation and invasion.
Previous literature has suggested that miR-219a should be able to suppress cell proliferation and invasion in multiple cell lines [12, 14, 41]. In the current study, we have further demonstrated that such suppression on cell proliferation as well as cell invasion by miR-219a is applicable to the human trophoblast cells HTR-8/SVneo. Moreover, downregulation of miR-219a sufficiently enhances HTR-8/SVneo trophoblastic proliferation and invasion. These results together indicate that miR-219a should be a critical player regulating trophoblastic proliferation and invasion, and therefore miR-219a appears to be potential treatment in complications of pregnancy.
VEGF is well documented in terms of promoting trophoblastic proliferation and invasion [21]. In this study, we investigated the impact of miR-219a on VEGFR2 expression to verify whether miR-219a regulated cell activities of human HTR-8SV/neo trophoblasts through targeting VEGFR2. Luciferase reporter assays have shown a repression of the luciferase activity after a treatment of miR-219a, as well as an increase of the luciferase activity after an inhibition of miR-219a, on wild-type VEGFR2 3′UTR but not the mutant. Furthermore, transfection of miR-219a has led to a profound reduction of the VEGFR2 expression. On the other hand, the VEGFR2 protein expression level is upregulated followed by an inhibition of miR-219a. These findings, accordingly, support that miR-219a targets VEGFR2 3′UTR directly and specifically and serves as an antagonist of VEGFR2.
The downstream VEGF signaling is further investigated for better understanding the underlying mechanisms of miR-219a–mediated suppression on trophoblastic activity. Besides that miR-219a transfection inhibits the expressions of downstream p-AKT and NF-κB when compared with the negative control (the baseline), miR-219a is able to suppress the downstream VEGF/NF-κB signaling after VEGF upregulation. Even though VEGF upregulation significantly promotes trophoblastic proliferation and invasion, miR-219a effectively represses the trophoblastic activity under the condition of VEGF upregulation, resulting in an even lower rate of proliferation and invasion than that of the negative control. As a result, it is very likely that one-way miR-219a suppresses trophoblastic proliferation and invasion is by targeting the VEGF/NF-κB signaling pathway.
As mentioned above, miR-219a appears to specifically target VEGFR2 in the VEGF signaling pathway. We therefore investigate the role of VEGFR2 in miR-219a–mediated suppression on trophoblastic activities. We do not find significant difference on the trophoblastic activities followed by VEGFR2 transfection. However, miR-219a fails to prohibit cell proliferation and invasion in trophoblast cells after VEGFR2 transfection. Interestingly, such recovery is not observed with VEGF upregulation. This may suggest that instead of inhibiting VEGF, miR-219a partly inhibits the VEGF signaling pathway through targeting VEGFR2. Moreover, the expression levels of sFlt1 were increased, whereas the VEGF3 levels were barely changed after miR-219a mimic treatment in HRT-8/Svneo cells. The current study has demonstrated a critical role of VEGFR2 in regulating the miR-219a suppression on trophoblastic activities. In conclusion, miR-219a plays crucial roles in negatively regulating trophoblastic activity, very likely by suppression of the VEGF/NF-κB signaling pathway by targeting VEGFR2, therefore serving as a potential treatment method for the complications of pregnancy caused by trophoblastic dysregulation.
Acknowledgments
This work was supported by the Natural Science Foundation of Anhui Province (1708085MH214).
Compliance with ethical standards
Conflict of interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bing Wei, Email: bingw1@sohu.com.
Yu Zhang, Email: zhangy_11@21cn.com.
References
- 1.Baines KJ, Renaud SJ. Transcription factors that regulate trophoblast development and function. Prog Mol Biol Transl Sci. 2017;145:39–88. doi: 10.1016/bs.pmbts.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Pollheimer J, Vondra S, Baltayeva J, Beristain AG, Knofler M. Regulation of placental extravillous trophoblasts by the maternal uterine environment. Front Immunol. 2018;9:2597. doi: 10.3389/fimmu.2018.02597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moser G, Windsperger K, Pollheimer J, de Sousa Lopes SC, Huppertz B. Human trophoblast invasion: new and unexpected routes and functions. Histochem Cell Biol. 2018;150:361–370. doi: 10.1007/s00418-018-1699-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujiwara H, Matsumoto H, Sato Y, Horie A, Ono M, Nakamura M, Mizumoto Y, Kagami K, Fujiwara T, Hattori A, Maida Y, Daikoku T, Imakawa K, Araki Y. Factors regulating human extravillous trophoblast invasion: chemokine-peptidase and CD9-integrin systems. Curr Pharm Biotechnol. 2018;19:764–770. doi: 10.2174/1389201019666181029164906. [DOI] [PubMed] [Google Scholar]
- 5.Tian FJ, Qin CM, Li XC, Wu F, Liu XR, Xu WM, Lin Y. Decreased stathmin-1 expression inhibits trophoblast proliferation and invasion and is associated with recurrent miscarriage. Am J Pathol. 2015;185:2709–2721. doi: 10.1016/j.ajpath.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Xiang H, Yan H, Sun B, Feng F, Chen P. Decreased expression of long non-coding RNA SNHG7 cause recurrent spontaneous abortion through suppression proliferation and invasion of trophoblast cells via miR-34a. Am J Transl Res. 2019;11:463–472. [PMC free article] [PubMed] [Google Scholar]
- 7.Chang CW, Wakeland AK, Parast MM. Trophoblast lineage specification, differentiation and their regulation by oxygen tension. J Endocrinol. 2018;236:43–56. doi: 10.1530/JOE-17-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H, Wang L, Wang Y, Zhu Q, Aldo P, Ding J, Mor G, Liao A. Establishment and characterization of a new human first trimester trophoblast cell line, AL07. Placenta. 2020;100:122–132. doi: 10.1016/j.placenta.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abou-Kheir W, Barrak J, Hadadeh O, Daoud G. HTR-8/SVneo cell line contains a mixed population of cells. Placenta. 2017;50:1–7. doi: 10.1016/j.placenta.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Lunghi L, Frigato E, Ferretti ME, Biondi C, Bertolucci C. Circadian variation of cell proliferation in HTR-8/SVneo cell line. Hum Cell. 2011;24(4):161–164. doi: 10.1007/s13577-011-0032-1. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee P, Malik A, Malhotra SS, Gupta SK. Role of STAT signaling and autocrine action of chemokines during H(2)O(2) induced HTR-8/SVneo trophoblastic cells invasion. J Cell Physiol. 2019;234:1380–1397. doi: 10.1002/jcp.26934. [DOI] [PubMed] [Google Scholar]
- 12.Zhu X, Chen L, Lin J. miR-219a-5p represses migration and invasion of osteosarcoma cells via targeting EYA2. Artif Cells Nanomed Biotechnol. 2018;46:S1004–S1010. doi: 10.1080/21691401.2018.1525391. [DOI] [PubMed] [Google Scholar]
- 13.Zheng H, Tang R, Yao Y, Ji Z, Cao Y, Liu Z, Peng F, Wang W, Can D, Xing H, Bu G, Xu H, Zhang YW, Zheng W. MiR-219 protects against seizure in the kainic acid model of epilepsy. Mol Neurobiol. 2016;53:1–7. doi: 10.1007/s12035-014-8981-5. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Dong J, Han Z, Zhang K. MicroRNA-219-5p represses the proliferation, migration, and invasion of gastric cancer cells by targeting the LRH-1/Wnt/beta-catenin signaling pathway. Oncol Res. 2017;25:617–627. doi: 10.3727/096504016X14768374457986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Lv Y, Wang L, Gong C, Sun J, Chen X, Chen Y, Yang L, Zhang Y, Yang X, Bai C, Wei Z, Li G. MicroRNAome in decidua: a new approach to assess the maintenance of pregnancy. Fertil Steril. 2015;103:980–989. doi: 10.1016/j.fertnstert.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 16.De Angelis JE, Lagendijk AK, Chen H, Tromp A, Bower NI, Tunny KA, Brooks AJ, Bakkers J, Francois M, Yap AS, Simons C, Wicking C, Hogan BM, Smith KA. Tmem2 regulates embryonic VEGF signaling by controlling hyaluronic acid turnover. Dev Cell. 2017;40:123–136. doi: 10.1016/j.devcel.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto K, Ema M. Roles of VEGF-A signalling in development, regeneration, and tumours. J Biochem. 2014;156:1–10. doi: 10.1093/jb/mvu031. [DOI] [PubMed] [Google Scholar]
- 18.Llurba Olive E, Xiao E, Natale DR, Fisher SA. Oxygen and lack of oxygen in fetal and placental development, feto-placental coupling, and congenital heart defects. Birth Defects Res. 2018;110:1517–1530. doi: 10.1002/bdr2.1430. [DOI] [PubMed] [Google Scholar]
- 19.Stevens M, Oltean S. Modulation of receptor tyrosine kinase activity through alternative splicing of ligands and receptors in the VEGF-A/VEGFR Axis. Cells. 2019;8. [DOI] [PMC free article] [PubMed]
- 20.Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17:611–625. doi: 10.1038/nrm.2016.87. [DOI] [PubMed] [Google Scholar]
- 21.Chai L, Ling K, He X, Yang R. Expression of ATF4 and VEGF in chorionic villus tissue in early spontaneous abortion. Eur J Obstet Gynecol Reprod Biol. 2013;170:434–438. doi: 10.1016/j.ejogrb.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Lash GE, Cartwright JE, Whitley GS, Trew AJ, Baker PN. The effects of angiogenic growth factors on extravillous trophoblast invasion and motility. Placenta. 1999;20:661–667. doi: 10.1053/plac.1999.0427. [DOI] [PubMed] [Google Scholar]
- 23.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 24.Jozwiak-Bebenista M, Jasinska-Stroschein M, Kowalczyk E. Involvement of vascular endothelial growth factor (VEGF) and mitogen-activated protein kinases (MAPK) in the mechanism of neuroleptic drugs. Pharmacol Rep: PR. 2018;70:1032–1039. doi: 10.1016/j.pharep.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Khan GA, Girish GV, Lala N, Di Guglielmo GM, Lala PK. Decorin is a novel VEGFR-2-binding antagonist for the human extravillous trophoblast. Mol Endocrinol. 2011;25:1431–1443. doi: 10.1210/me.2010-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasilopoulou E, Loubiere LS, Lash GE, Ohizua O, McCabe CJ, Franklyn JA, Kilby MD, Chan SY. Triiodothyronine regulates angiogenic growth factor and cytokine secretion by isolated human decidual cells in a cell-type specific and gestational age-dependent manner. Hum Reprod. 2014;29:1161–1172. doi: 10.1093/humrep/deu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adamson SL. sFLT1 in preeclampsia: trophoblast defense against a decidual VEGFA barrage? J Clin Investig. 2014;124:4690–4692. doi: 10.1172/JCI78532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park JK, Jeong JW, Kang MY, Baek JC, Shin JK, Lee SA, Choi WS, Lee JH, Paik WY. Inhibition of the PI3K-Akt pathway suppresses sFlt1 expression in human placental hypoxia models in vitro. Placenta. 2010;31:621–629. doi: 10.1016/j.placenta.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Ao R, Guan L, Wang Y, Wang JN. Silencing of COL1A2, COL6A3, and THBS2 inhibits gastric cancer cell proliferation, migration, and invasion while promoting apoptosis through the PI3k-Akt signaling pathway. J Cell Biochem. 2018;119:4420–4434. doi: 10.1002/jcb.26524. [DOI] [PubMed] [Google Scholar]
- 30.Cui X, Qian DW, Jiang S, Shang EX, Zhu ZH, Duan JA. Scutellariae radix and Coptidis rhizoma improve glucose and lipid metabolism in T2DM rats via regulation of the metabolic profiling and MAPK/PI3K/Akt signaling pathway. Int J Mol Sci. 2018;19(11):3634. doi: 10.3390/ijms19113634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Very N, Vercoutter-Edouart AS, Lefebvre T, Hardivillé S, El Yazidi-Belkoura I. Cross-dysregulation of O-GlcNAcylation and PI3K/AKT/mTOR axis in human chronic diseases. Front Endocrinol. 2018;9:9–602. doi: 10.3389/fendo.2018.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng D, Jiang S, Chen J, Li J, Ao L, Zhang Y. The increased lncRNA MIR503HG in preeclampsia modulated trophoblast cell proliferation, invasion, and migration via regulating matrix metalloproteinases and NF-κB signaling. Dis Markers. 2019;2019:4976845. [DOI] [PMC free article] [PubMed]
- 33.Meng L, Wang X, Liao W, Liu J, Liao Y, He Q. BAF53a is a potential prognostic biomarker and promotes invasion and epithelial-mesenchymal transition of glioma cells. Oncol Rep. 2017;38:3327–3334. doi: 10.3892/or.2017.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zong S, Li C, Luo C, Zhao X, Liu C, Wang K, Jia W, Bai M, Yin M, Bao S, Guo J, Kang J, Duan T, Zhou Q. Dysregulated expression of IDO may cause unexplained recurrent spontaneous abortion through suppression of trophoblast cell proliferation and migration. Sci Rep. 2016;6:19916. doi: 10.1038/srep19916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Litwin S, Cortina ME, Barrientos GL, Prados MB, Roux ME, Miranda SE. Multiparity increases trophoblast invasion and vascular endothelial growth factor expression at the maternal-fetal interface in mice. J Reprod Immunol. 2010;85:161–167. doi: 10.1016/j.jri.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Dai X, Cai Y. Down-regulation of microRNA let-7d inhibits the proliferation and invasion of trophoblast cells in preeclampsia. J Cell Biochem. 2018;119:1141–1151. doi: 10.1002/jcb.26282. [DOI] [PubMed] [Google Scholar]
- 37.Zou Y, Li S, Wu D, Xu Y, Wang S, Jiang Y, Liu F, Jiang Z, Qu H, Yu X, Wang X, Wang Y, Sun L. Resveratrol promotes trophoblast invasion in pre-eclampsia by inducing epithelial-mesenchymal transition. J Cell Mol Med. 2019;23:2702–2710. doi: 10.1111/jcmm.14175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao Y, She R, Wang Q, Li Y, Zhang H. Up-regulation of miR-299 suppressed the invasion and migration of HTR-8/SVneo trophoblast cells partly via targeting HDAC2 in pre-eclampsia. Biomed Pharmacother = Biomed Pharmacother. 2018;97:1222–1228. doi: 10.1016/j.biopha.2017.11.053. [DOI] [PubMed] [Google Scholar]
- 39.Luo L, Ye G, Nadeem L, Fu G, Yang BB, Honarparvar E, Dunk C, Lye S, Peng C. MicroRNA-378a-5p promotes trophoblast cell survival, migration and invasion by targeting Nodal. J Cell Sci. 2012;125:3124–3132. doi: 10.1242/jcs.096412. [DOI] [PubMed] [Google Scholar]
- 40.Su MT, Tsai PY, Tsai HL, Chen YC, Kuo PL. miR-346 and miR-582-3p-regulated EG-VEGF expression and trophoblast invasion via matrix metalloproteinases 2 and 9. BioFactors. 2017;43:210–219. doi: 10.1002/biof.1325. [DOI] [PubMed] [Google Scholar]
- 41.Sun X, Xu M, Liu H, Ming K. MicroRNA-219 is downregulated in non-small cell lung cancer and inhibits cell growth and metastasis by targeting HMGA2. Mol Med Rep. 2017;16:3557–3564. doi: 10.3892/mmr.2017.7000. [DOI] [PubMed] [Google Scholar]