Abstract
The aim of this research was to design a new product, in particular a watermelon-based jelly candy, without generating waste. The study was divided in two steps: (i) optimization of candy formulation in terms of amount of rind, pulp and juice; (ii) fortification of the jelly candy with different concentrations of orange by-products (albedo and flavedo flours). The fortified jelly samples were assessed for sensory quality and chemical properties, before and after digestion. The new candy product was greatly appreciated. The addition of albedo and flavedo flours significantly improved the chemical composition compared to jelly candy without by-products, before and after digestion. A whole quality index was also calculated to determine the best combination of by-products to be added. Fortification with albedo 1.2% and flavedo ranged between 0.6 and 1.2% allowed recording the most interesting jelly candy.
Keywords: Candy, Watermelon, Albedo, Flavedo, Zero waste
Introduction
Recently, food waste is widely discussed because it represents a huge problem in developed countries. Every year, million tonnes of food by-products are generated along the whole chain: from industrial production until household consumption, becoming a serious economic and environmental problem. These are commonly managed as waste, therefore sent to landfills, where turned into greenhouse gas by anaerobic digestion. This negatively impacts on the environment, causing climate changes (Melikoglu et al. 2013), and causes economic problems to the producers, being their disposal not free. Thus, in the last few years the challenge of researchers was to find a more eco-sustainable production to reduce waste generation. In this regard, the “zero waste” theory is very interesting. It is a waste management strategy whose aim is to recycle waste, being considered a resource to be reused in other productions (Song et al. 2015). Zero Waste Manufacturing involves designing of products and processes in which no trash is sent to landfills or incinerators (Singh et al. 2017).
In this context, this research study took into account the manufacturing of watermelon-based candy using each part of the watermelon (rind, pulp and juice) in order to make a zero-waste food. Candy was chosen as vehicle of bioactive compounds being a food intended to a wide group of consumers, from children to adults. According to the literature, watermelon was often used as source of bioactive substances as citrulline (Rimando and Perkins-Veazie 2005; Tarazona-Díaz et al. 2011) and lycopene (Oberoi and Sogi 2017; Perkins-Veazie and Collins 2004). Other authors have also studied the nutritional properties of this fruit in terms of active compounds and antioxidant activity (Dieng et al. 2017; Kim et al. 2014).
Recently there is a growing interest concerning the incorporation of vegetable by-products into foods eaten daily to increase the consumption of beneficent substances. This is also due to new market needs, because the consumers require foods with health-promoting properties, being increasingly aware of existing relationship between diet and health (Schieber et al. 2001). For instance, watermelon rind was used to make jam (Souad et al. 2012) or as flour substitute in cake preparation (Al-Sayed and Ahmed 2013).
Fruits, as orange, grape or olive, or vegetables as artichokes are known for their high content in bioactive compounds (Ciriminna et al. 2016; D’Antuono et al. 2015; Escobedo-Avellaneda et al. 2014; Russo et al. 2015; Peixoto et al. 2018). Therefore, albedo and flavedo from orange fruits were also used in the watermelon-based candy in order to further improve the nutritional value. These by-products were chosen among the numerous typologies of by-products, being produced in large quantities during orange juice production.
Materials and methods
Raw materials
Fruits used in this study were obtained from a local market (Ipercoop, Foggia, Italy). Watermelons (Cucurbita lanatus cv. Crimson) and oranges (Citrus sinensis cv. Navel) were thoroughly washed in tap water to remove residuals, dipped for 1 min in chlorinated water (20 mL L−1) and rinsed. After that, the fruits peel was removed manually using a home knife; in particular, flavedo (the orange peripheral surface) and albedo (the white part inside of peel) were obtained from orange peel. The watermelon pulp was then placed in a fruit extractor (Delonghi, Italy) to produce the juice. The obtained juice was stored at −20 °C until to be used, while watermelon (rind and exhaust pulp) and orange by-products (flavedo and albedo) were dried at 38 °C for 48 h using a vacuum stove and then ground in a laboratory blender to fine powder.
Chemicals
Folin-Ciocalteu reagent, anhydrous sodium carbonate (Na2CO3), gallic acid monohydrate, methanol, hydrochloric acid, aluminum chloride (AlCl3), sodium nitrite (NaNO2), sodium hydroxide solution (NaOH), quercetin, sodium acetate trihydrate (CH3COONa·3H2O), glacial acetic acid (CH3COOH), 2,4,6-Tripyridyl-s-Triazine (TPTZ), ferric chloride (FeCl3), ferrous sulfate heptahydrate (FeSO4 7H2O), the ingredients for HBSS (potassium chloride, sodium chloride, disodium hydrogen phosphate di-hydrate, di-potassium hydrogen phosphate, sodium hydrogen carbonate, and calcium chloride) and the enzymes for in vitro digestion (porcine pepsin, porcine bile acid, pancreatin, alpha-amylase from Bacillus sp.), were supplied from Sigma-Aldrich (Milan, Italy). Amylo-glucosidase was purchased from Megazyme (Wicklow, Ireland). All reagents were of analytical grade.
Jelly candy formulation
Jelly candy was prepared by mixing watermelon juice, freshly squeezed lemon juice and sugar. The mixture was boiled to dissolve completely the sugar and cooled to approximately 40 °C. Then, gelatine sheets, previously soaked in cold water, together with watermelon rind and pulp flours were added at the mixture. The latter was poured into a mould and left to solidify for 24 h under refrigeration conditions; afterward, the jelly was cut in pieces into dimension of 30 mm × 20 mm × 10 mm to realize candy samples. The details of formulation of watermelon jelly candy (WMC) are: watermelon juice (59.2%), lemon juice (11.8%), sugar (23.7%), gelatine foils (2.8%), watermelon rind flour (1.4%) and watermelon pulp flour (1.1%).
In the follow, the mass balance equation of the process that transforms fresh water melon into juice and by products (i.e. the flour obtained from watermelon rind and exhaust pulp) is reported:
| 1 |
where: WFM is the fresh melon weight, WW is the weight of water lost during the dehydration of watermelon rind and exhaust pulp, WF is the flour weight obtained from watermelon rind and exhaust pulp, and WJ is the juice weight.
To calculate the amount of watermelon by-products flours the following equations were used:
| 2 |
where x is the mass of flour obtained from watermelon rind and exhaust pulp per unit mass of obtained juice, it has been calculated as the average value deriving from the watermelon processing performed in triplicate.
It is evident that, as long as the amount of flour used in the candy formulation is equal to the amount of used juice times the factor 'x' (i.e. the mass of flour obtained from watermelon rind and exhaust pulp per unit mass of obtained juice), the production process that transforms fresh water melon into candy will have no waste.
In a subsequent experimental step, in order to improve the nutritional quality of the watermelon jelly candy (WMC) orange by-products were also added to the formulation. Their optimal concentration was defined by adopting a two steps optimization method. First different concentrations of albedo flour (1.2, 2.4, 3.6 and 4.8%) were used to individuate the optimal concentration to be added. The candies are labelled as following: AL1.2, AL2.4, AL3.6 and AL4.8. After that, on the basis of recorded sensory results, two albedo flour concentrations (1.2 and 2.4%) were combined with three different amounts of flavedo flour (0.6, 1.2 and 2.4%) thus obtaining six formulations of candies: AL1.2–FL0.6, AL1.2–FL1.2, AL1.2–FL2.4, AL2.4–FL0.6, AL2.4–FL1.2, AL2.4–FL2.4. The fortified candies were prepared with the same procedure used for the watermelon jelly candy.
Sensory evaluation
Sensory evaluation was designed to measure the degree of liking of samples according to a 9-point hedonic scale (1 = dislike extremely, 9 = like extremely). A panel formed by 8 members of the laboratory performed sensory evaluation of jelly candies in terms of appearance, colour, odour, taste, firmness and overall quality. The panellists had at least several years of experience in sensory evaluation prior to this study; however, they were retrained for this study in a session of 2 h to be experienced in the product and terminology. They were also instructed to rinse their mouth with plain water in between tasting.
In vitro digestion of candy
In vitro digestion was performed according to the protocol described by Gille et al. (2016) with some modifications. Briefly, 0.5 g of candy sample was subjected to an in vitro digestion process that consisted of two phases: gastric and intestinal. To simulate the gastric phase, porcine pepsin (40 mg mL−1 of 0.1 N HCl) was added and the pH adjusted to 2.2–2.4, followed by shaking at 37 °C in a water bath for 1 h. Subsequently, porcine bile acid (12 mg mL−1), pancreatin (11 mg mL−1), and alpha-amylase (3.3 mg mL−1) were added. The pH was adjusted to 7.2–7.6 and shaking at 37 °C in a water bath for 2 h. After digestion, the samples were centrifuged (4000 rpm × 10 min) and filtered. The filtrate was used for the analysis of the accessible fraction.
Chemical analyses
The extraction of polyphenols from watermelon rind and pulp, flavedo and albedo, and from candy samples was based on the method described by Cappa et al. (2015) with some modifications. Briefly, acidified methanol (80% MeOH acidified with 1% (w/w) HCl) was used as extraction solvent. An amount of 8 mL was added to 0.4 g of sample and shaken at room temperature in darkness for 2 h at 300 rpm using orbital shaker (HS 260 BASIC, IKA, Staufen, Germany). Next, the samples were centrifuged at 5 °C for 10 min at 10,000 rpm (5804R, Eppendorf, Milan, Italy) and the supernatant was stored while the pellet was re-extracted with 4 mL of solvent. The process was performed for three times. The supernatants were collected together and used for the analytical determinations. The extraction was carried out in triplicate. The evaluation of total phenols, flavonoids and antioxidant activity was performed on each part of watermelon, on orange by-products and on the experimental candy samples before and after digestion.
Determination of total phenolic compounds
The total phenols were determined according to the Folin-Ciocalteu method described by Spinelli et al. (2016). Total phenolic content (TPC) was expressed as mg of gallic acid equivalents (GAEs) per gram of sample. For each sample, the analysis was carried out in triplicate.
Determination of total flavonoids
Flavonoids were quantified by aluminum trichloride method as described by Spinelli et al. (2016), using quercetin as standard. Total flavonoid content (TFC) was expressed as mg of quercetin equivalents (QEs) per gram of sample. The analysis was carried out in triplicate for each sample.
Determination of antioxidant activity
The ferric reducing antioxidant power assay (FRAP assay) was carried out according to the procedure described by Benzie and Strain (1996). The antioxidant activity was expressed as μmoles of ferrous equivalent Fe(II) per gram of sample. All tests were carried out in triplicate.
Quality Index
A whole quality index (WQI) that accounted for both nutritional and sensory quality was proposed as following:
| 3 |
where: z is a variable related to the food formulation, it is the concentration of orange by-products; NQF(z) is the fortified food nutritional quality at a given value of z; NQC is the nutritional quality of the control sample (candy without orange by-products); OSQF(z) is the fortified food overall quality at a given value of z, OSQC is the overall quality of the control sample; OSQmin is the sensory threshold for food acceptability. Equation (3) is given by the product of two distinct terms: the former one takes into account the nutritional quality of the fortified food, it always increases with the concentration of the healthy ingredient; the latter one is related to the sensory quality of the fortified food, it always decreases with the healthy ingredient content.
Statistical analysis
All experimental data were subjected to one-way analysis of variance (ANOVA). To the aim, a Fischer test with the option of homogeneous groups (P < 0.05) was carried out to determinate significance differences among samples. To the aim, Statistica 7.1 for Windows was used.
Results and discussion
During the first part of this study, a watermelon-based jelly candy was realized using all the parts of the fruit including the by-products (rind and exhaust pulp) obtained in the juice production. Sensory and chemical quality were evaluated. Subsequently, in order to improve the candy nutritional content, orange by-products were added. First albedo flour was added at four different concentrations to the watermelon-based jelly candy formulation. Based on the sensory results two concentrations were chosen out of four, that were combined with four different amounts of flavedo flour. The fortified final candy samples were also assessed for sensory and chemical quality. In the subsequent paragraphs, the results recorded in each step were provided.
Quality of watermelon jelly candy
Sensory quality
Ratings for sensory attributes of watermelon jelly candies are presented in Table 1. As can be inferred, the WMC was attractive for the quality attribute of appearance (score 8.5) and similarly, for colour and odour, the product was marked as more than acceptable by all the panel members. The taste attribute was pleasant and the panellists recognized the fruity aroma typical of watermelon (score 8.1). The gelatine gave a gummy firmness characterized by suitable hardness and transparency, as confirmed also in the literature (Marfil et al. 2012). The firmness was slightly affected by the presence of watermelon flours that conferred a certain fibrous and graininess to the candy, even if most of the sensory scores were in the category of ‘like very much’ (score 8). Results of sensory analysis indicated that the product was appreciated for all the attributes with an overall quality more than 8.
Table 1.
Sensory attributes of watermelon jelly candy with and without orange by-products
| Samples | Appearance | Colour | Odour | Taste | Firmness | Overall quality |
|---|---|---|---|---|---|---|
| WMC | 8.50 ± 0.50b | 8.00 ± 0.00a | 8.00 ± 0.00b | 8.14 ± 0.24c | 8.00 ± 0.00f | 8.14 ± 0.24f |
| AL1.2–FL0.6 | 7.79 ± 0.39a | 7.71 ± 0.49a | 7.28 ± 0.49a,b | 7.21 ± 0.48a,b | 6.78 ± 0.39e | 7.42 ± 0.35e |
| AL1.2–FL1.2 | 7.85 ± 0.24a | 7.85 ± 0.38a | 7.21 ± 0.49a,b | 6.64 ± 0.85b | 6.42 ± 0.35c,d | 6.85 ± 0.38d,e |
| AL1.2–FL2.4 | 7.64 ± 0.24a | 7.71 ± 0.39a | 7.28 ± 0.49a,b | 6.35 ± 1.03b | 5.85 ± 0.38b,c | 5.78 ± 0.49b,c |
| AL2.4–FL0.6 | 7.57 ± 0.35a | 7.64 ± 0.38a | 7.00 ± 0.58a | 6.64 ± 0.80b | 6.00 ± 0.58b,c | 6.42 ± 0.35c,d |
| AL2.4–FL1.2 | 7.50 ± 0.41a | 7.64 ± 0.38a | 7.00 ± 0.58a | 6.50 ± 0.65b | 5.35 ± 0.48b | 5.71 ± 0.57b |
| AL2.4–FL2.4 | 7.28 ± 0.39a | 7.35 ± 0.38a | 7.00 ± 0.58a | 4.92 ± 0.35a | 4.35 ± 0.24a | 4.50 ± 0.00a |
WMC: watermelon-based candy; AL1.2–FL0.6: watermelon candy enriched with 1.2% albedo and 0.6% flavedo; AL1.2–FL1.2: watermelon candy enriched with 1.2% albedo and 1.2% flavedo; AL1.2–FL2.4: watermelon candy enriched with 1.2% albedo and 2.4% flavedo; AL2.4–FL0.6: watermelon candy enriched with 2.4% albedo and 0.6% flavedo; AL2.2–FL1.2: watermelon candy enriched with 2.4% albedo and 1.2% flavedo; AL2.4–FL2.4: watermelon candy enriched with 2.4% albedo and 2.4% flavedo. Results are expressed as means ± SD for n = 3. a−eData in columns with different superscripts are significantly different (P < 0.05), as determined by ANOVA followed by the Fischer test
Chemical quality
Table 2 shows the total phenolic and flavonoid contents and the antioxidant activity of the watermelon candy (WMC) and its specific ingredients.
Table 2.
Chemical characterization of watermelon jelly candy and its ingredients
| Sample | TPC mg GAEs/g | TFC mg QEs/g | FRAP µmoles FeSO4 7H2O/g |
|---|---|---|---|
| Rind | 4.83 ± 0.06 | 1.87 ± 0.07 | 46.84 ± 3.17 |
| Pulp | 3.82 ± 0.12 | 1.67 ± 0.03 | 32.68 ± 2.43 |
| Watermelon juice | 0.20 ± 0.002 | 0.03 ± 0.001 | 1.09 ± 0.02 |
| Lemon juice | 0.71 ± 0.01 | 0.15 ± 0.001 | 10.5 ± 0.12 |
| WMC | 0.44 ± 0.05 | 0.16 ± 0.02 | 2.53 ± 0.10 |
| Orange Albedo | 17.4 ± 1.39 | 9.12 ± 0.44 | 166 ± 1.64 |
| Orange Flavedo | 22.6 ± 2.02 | 10.14 ± 0.18 | 319.7 ± 4.13 |
WMC: watermelon-based candy
According to our findings, the rind is the component with the highest contents of total polyphenols (TPC) and flavonoids (TFC) (4.83 ± 0.06 mg GAEs/g and 1.87 ± 0.07 mg QEs/g, respectively) as well as antioxidant activity (46.84 ± 3.17 µmoles FeSO4 7H2O/g), followed by the pulp. Compared to rind and pulp, the other ingredients are very poor in bioactive compounds. The values of TPC and TFC recorded for the developed candy, reported in Table 2, are higher than expected data (0.31 mg GAEs/g TPC and 0.08 mg QEs/g TFC). The same trend was also observed by Lee et al. (2010), who developed a jelly using banana peels and observed more polyphenols and flavonoids than calculated values. This behavior is probably due to cooking during candy making that promotes the release of active compounds by breaking the plant cell wall. According to Choi et al. (2006), who studied the influence of heat treatment on the antioxidant activity and polyphenol compounds of Shiitake mushroom, the phenol substances increased with increasing heating temperature. In terms of antioxidant activity, a value corresponding to that expected has been recorded. Taking into account the candy recipe and the chemical composition of its ingredients, each element influenced in different way the jelly chemical quality. The watermelon juice gives the highest phenolic contribution, equal to 39%, while the flavonoids are mostly attributed to the rind, which contributes for about 32.5%.
Quality of watermelon jelly candy fortified with orange by-products
Sensory quality
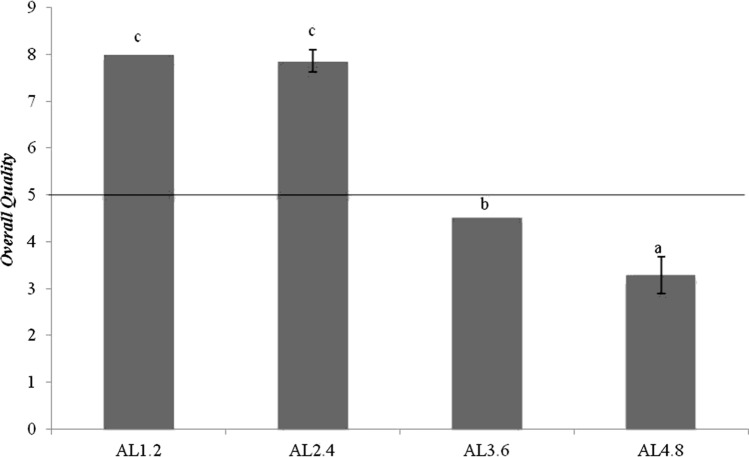
The overall quality referred to candies added with four albedo concentrations are reported in Fig. 1. As can be observed, the two highest concentrations of albedo flour (AL3.6 and AL4.8) compromised the sensory quality (score below 5). Comparable and acceptable results were recorded with both 1.2 and 2.4% of albedo addition. In particular, the firmness was the main cause responsible for the unacceptability of jelly candies, due to the pasty conferred by the excessive presence of flour that increased the feeling sticky on the palate. The other attributes (appearance, colour, odour) were not influenced by the albedo addition and exhibited values ranging around 8. The taste in all the samples was slightly reduced due to the bitterness conferred by the albedo, even though it remained agreeable (data not shown). There was no significant difference in the overall quality values and in the other parameters of both AL1.2 and AL2.4 samples. All sensory attributes of these candy samples showed scores of about 8.
Fig. 1.
Overall quality of watermelon jelly candy added with different albedo flour concentrations. AL1.2: watermelon candy enriched with 1.2% albedo; AL2.4: watermelon candy enriched with 2.4% albedo; AL3.6: watermelon candy enriched with 3.6% albedo; AL4.8: watermelon candy enriched with 4.8% albedo
On the basis of the results recorded, AL1.2 and AL2.4 were chosen and combined with the flavedo flour. In Table 1 are reported the sensory attributes for the six formulations of fortified jelly candies, compared to the original jelly product. Data highlight that all types of fortified candy samples, except AL2.4–FL2.4, show an overall quality values above the threshold. Sample AL2.4–FL2.4 were found unacceptable in terms of firmness and taste. As expected, an increase in flavedo concentration brought about a reduction in overall quality. Taste and firmness are the attributes mainly responsible of this reduction. In fact, increasing the flavedo flour concentration, a different undesirable intensity of the taste was perceived. Many studies report that an increase in viscosity or hardness globally reduces both perceived taste and aroma intensities (Boland et al. 2006; Kälviäinen et al. 2000). The addition of albedo and flavedo flours did not significantly influence the appearance, colour and odour attributes of jelly candy samples. It is worth noting that comparing all the samples, the combination AL1.2–FL0.6 did not affect at great level the whole sensory quality of the candy.
Chemical quality
Albedo and flavedo were characterized in terms of total phenolic content (TPC), total flavonoid content (TFC) and antioxidant activity by means FRAP assay. According to obtained data, flavedo had higher total phenol and flavonoid amounts and consequently higher antioxidant activity compared to albedo (Table 2). The chemical composition of both orange by-products in terms of antioxidants was better than watermelon fruit also reported in the same table, thus justifying their addition to watermelon-based jelly candy.
Chemical characterization of fortified samples is shown in Table 3. Only five of the six formulations of enriched products accepted from the sensory point of view were also studied for chemical properties (AL1.2–FL0.6, AL1.2–FL1.2, AL1.2–FL2.4, AL2.4–FL0.6, AL2.4–FL1.2). As can be observed from data listed in Table 3, very comparable results were recorded. Specifically, the AL1.2-FL2.4 sample was the best one, characterized by 1.26 ± 0.03 mg GAEs/g, 0.76 ± 0.03 mg QEs/g and 10.49 ± 0.46 µmoles FeSO4 ∙7H2O/g, followed by AL2.4-FL1.2, AL2.4-FL0.6, AL1.2-FL1.2, AL1.2-FL0.6. If compared these data with those reported for WMC sample in Table 2, it’s possible to infer that enrichment of jelly candy with albedo and flavedo greatly improved the chemical quality.
Table 3.
Chemical characterization of watermelon-based candies fortified with orange by-products
| Sample | TPC mg GAEs/g | TFC mg QEs/g | FRAP µmoles FeSO4 7H2O/g |
|---|---|---|---|
| AL1.2–FL0.6 | 0.94 ± 0.03a | 0.46 ± 0.02a | 6.55 ± 0.27a |
| AL1.2–FL1.2 | 0.94 ± 0.03a | 0.54 ± 0.03b | 7.82 ± 0.41b |
| AL1.2–FL2.4 | 1.26 ± 0.03d | 0.76 ± 0.03e | 10.49 ± 0.46d |
| AL2.4–FL0.6 | 1.13 ± 0.03b | 0.58 ± 0.06c | 8.02 ± 0.22b |
| AL2.4–FL1.2 | 1.16 ± 0.03c | 0.68 ± 0.03d | 9.62 ± 0.13c |
AL1.2–FL0.6: watermelon candy enriched with 1.2% albedo and 0.6% flavedo; AL1.2–FL1.2: watermelon candy enriched with 1.2% albedo and 1.2% flavedo; AL1.2–FL2.4: watermelon candy enriched with 1.2% albedo and 2.4% flavedo; AL2.4–FL0.6: watermelon candy enriched with 2.4% albedo and 0.6% flavedo; AL2.4–FL1.2: watermelon candy enriched with 2.4% albedo and 1.2%. Results are expressed as means ± SD for n = 3. a−eData in columns with different superscripts are significantly different (P < 0.05), as determined by ANOVA followed by the Fischer test
In order to have positive effect on human health, it is important that the bioactive compounds in candy are available for absorption in the gastrointestinal tract. Therefore, to understand if the jelly chemical properties are compromised by digestion conditions, in this study in vitro digestion of the jellies was also performed. Table 4 shows the total phenols, flavonoids and antioxidant activity of both control and fortified jellies after digestion. By comparing data of each sample before and after digestion (Tables 2, 3 and 4), some differences can be highlighted, mainly ascribed to food related factors and to interactions with other compounds (D’Archivio et al. 2010). As proved from abundant scientific literature on the topic, to establish conclusive evidence, a deeper investigation on the factors that affect the bioavailability of the various phenolic compounds could be necessary. Anyhow, to the aim of the current study, it is worth noting that comparing data in the Table 4 the results in terms of TPC, TFC and antioxidant activity of samples fortified with orange by-products are statistically higher than those refereed to the control candy. The AL1.2-FL2.4 sample resulted again the best one, with the highest TPC, TFC and FRAP value. Therefore, these last experimental findings are adequate to consider the new fortified food promising products from the nutritional point of view.
Table 4.
Chemical characterization of candy with and without orange by-products after digestion
| Sample | TPC mg GAEs/g | TFC mg QEs/g | FRAP µmoles FeSO4 7H2O/g |
|---|---|---|---|
| WMC | 0.53 ± 0.27a | 0.03 ± 0.02a | 2.87 ± 0.56a |
| AL1.2–FL0.6 | 0.88 ± 0.06b,c | 0.25 ± 0.03b | 5.73 ± 0.20b |
| AL1.2–FL1.2 | 0.93 ± 0.15b,c | 0.35 ± 0.05c | 6.33 ± 0.48c,d |
| AL1.2–FL2.4 | 1.00 ± 0.26c | 0.54 ± 0.06d | 8.23 ± 0.69e |
| AL2.4–FL0.6 | 0.85 ± 0.16b | 0.28 ± 0.05b | 5.89 ± 0.46b,c |
| AL2.4–FL1.2 | 0.97 ± 0.23b,c | 0.37 ± 0.09c | 6.72 ± 0.81d |
WMC: watermelon-based candy; AL1.2–FL0.6: watermelon candy enriched with 1.2% albedo and 0.6% flavedo; AL1.2–FL1.2: watermelon candy enriched with 1.2% albedo and 1.2% flavedo; AL1.2–FL2.4: watermelon candy enriched with 1.2% albedo and 2.4% flavedo; AL2.4–FL0.6: watermelon candy enriched with 2.4% albedo and 0.6% flavedo; AL2.4–FL1.2: watermelon candy enriched with 2.4% albedo and 1.2%. Results are expressed as means ± SD for n = 3. a−eData in columns with different superscripts are significantly different (P < 0.05), as determined by ANOVA followed by the Fischer test
With the aim to develop an acceptable new candy product, both sensory quality and chemical composition need to be taken into account. For this reason, a whole quality index (WQI) was also calculated to find the optimal amount of orange by-products to be added to reach a product with additional chemical properties without compromising its acceptability. This index took into account the score of overall quality and the TPC value recorded after digestion. Table 5 reports the WQI values calculated according to Eq. (3). As can be seen, the WQI greatly changed among samples and allowed to select the best samples. In fact, the highest WQI value was referred to the AL1.2–FL0.6 sample but it is also possible to infer that the flavedo amount could be increased up to 1.2% without compromising the product quality.
Table 5.
Whole Quality Index (WQI) of enriched candies
| Sample | WQI |
|---|---|
| AL1.2–FL0.6 | 0.50 |
| AL1.2–FL1.2 | 0.44 |
| AL1.2–FL2.4 | 0.22 |
| AL2.4–FL0.6 | 0.27 |
| AL2.4–FL1.2 | 0.19 |
AL1.2–FL0.6: watermelon candy enriched with 1.2% albedo and 0.6% flavedo; AL1.2–FL1.2: watermelon candy enriched with 1.2% albedo and 1.2% flavedo; AL1.2–FL2.4: watermelon candy enriched with 1.2% albedo and 2.4% flavedo; AL2.4–FL0.6: watermelon candy enriched with 2.4% albedo and 0.6% flavedo; AL2.4–FL1.2: watermelon candy enriched with 2.4% albedo and 1.2%
Conclusion
In this study a zero-waste food product was designed by combining all the watermelon parts and also adding orange by-products to enhance the nutritional quality. The research was divided in two subsequent steps, the first aimed to develop an acceptable jelly candy and the second one to improve the antioxidant activity. Results showed that the watermelon-based candy was very appreciated, but it is very poor from the chemical point of view. The addition of albedo and flavedo flour, as valid chemical components, affected the sensory quality, but greatly improved the antioxidant activity of jelly candies. A balance between these two aspects was also estimated and a consequent quality index was calculated. This index highlighted that fortification with albedo at 1.2% and flavedo ranged between 0.6 and 1.2% allowed recording a new very interesting jelly candy. It would be interesting to study in the future how the new sustainable product impacts on the environment.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al-Sayed HMA, Ahmed AR. Utilization of watermelon rinds and sharlyn melon peels as a natural source of dietary fiber and antioxidants in cake. Ann Agric Sci. 2013;58:83–95. doi: 10.1016/j.aoas.2013.01.012. [DOI] [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Boland AB, Delahunty CM, van Ruth SM. Influence of texture of gelatin gels and pectin gels on strawberry flavour release and perception. Food Chem. 2006;96:452–460. doi: 10.1016/j.foodchem.2005.02.027. [DOI] [Google Scholar]
- Cappa C, Lavelli V, Mariotti M. Fruit candies enriched with grape skin powders: physicochemical properties. LWT Food Sci Technol. 2015;62:569–575. doi: 10.1016/j.lwt.2014.07.039. [DOI] [Google Scholar]
- Choi Y, Lee SM, Chun J, Lee HB, Lee J. Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom. Food Chem. 2006;99:381–387. doi: 10.1016/j.foodchem.2005.08.004. [DOI] [Google Scholar]
- Ciriminna R, Meneguzzo F, Fidalgo A, Ilharco LM, Pagliaro M. Extraction, benefits and valorization of olive polyphenols. Eur J Lipid Sci Technol. 2016;118:503–511. doi: 10.1002/ejlt.201500036. [DOI] [Google Scholar]
- D’Antuono I, Garbetta A, Linsalata V, Minervini F, Cardinali A. Polyphenols from artichoke heads (Cynara cardunculus (L.) subsp. scolymus Hayek): in vitro bio-accessibility, intestinal uptake and bioavailability. Food Funct. 2015;6:1268–1277. doi: 10.1039/C5FO00137D. [DOI] [PubMed] [Google Scholar]
- D’Archivio M, Filesi C, Varì R, Scazzocchio B, Masella R. Bioavailability of the polyphenols: status and controversies. Int J Mol Sci. 2010;11:1321–1342. doi: 10.3390/ijms11041321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieng SIM, Diallo AJ, Fall AD, Diatta-Badji K, Diatta W, Sarr A, Bassene E. Total polyphenols and flavonoids contents of aqueous extracts of watermelon red flesh and peels (Citrullus lanatus, Thunb) J Pharmac Phytochem. 2017;6:801–803. [Google Scholar]
- Escobedo-Avellaneda Z, Gutiérrez-Uribe J, Valdez-Fragoso A, Torres JA, Welti-Chanes J. Phytochemicals and antioxidant activity of juice, flavedo, albedo and comminuted orange. J Funct Foods. 2014;6:470–481. doi: 10.1016/j.jff.2013.11.013. [DOI] [Google Scholar]
- Gille A, Trautmann A, Posten C, Briviba K. Bioaccessibility of carotenoids from Chlorella vulgaris and Chlamydomonas reinhardtii. Int J Food Sci Nutr. 2016;67:507–513. doi: 10.1080/09637486.2016.1181158. [DOI] [PubMed] [Google Scholar]
- Kälviäinen N, Roininen K, Tuorila H. Sensory characterization of texture and flavor of high viscosity gels made with different thickeners. J Sens Stud. 2000;31:407–419. [Google Scholar]
- Kim SJ, Matsushita Y, Fukushima K, Aoki D, Yagami S, Yuk HG, Lee SC. Antioxidant activity of a hydrothermal extract from watermelons. LWT Food Sci Technol. 2014;59:361–368. doi: 10.1016/j.lwt.2014.04.041. [DOI] [Google Scholar]
- Lee EH, Yeom HJ, Ha MS, Bae DH. Development of banana peel jelly and its antioxidant and textural properties. Food Sci Biotechnol. 2010;19:449–455. doi: 10.1007/s10068-010-0063-5. [DOI] [Google Scholar]
- Marfil PHM, Anhê ACBM, Telis VRN. Texture and microstructure of gelatin/corn starch-based gummy confections. Food Biophys. 2012;7:236–243. doi: 10.1007/s11483-012-9262-3. [DOI] [Google Scholar]
- Melikoglu M, Lin CSK, Webb C. Analysing global food waste problem: Pinpointing the facts and estimating the energy content. Cent Eur J Eng. 2013;3:157–164. [Google Scholar]
- Oberoi DPS, Sogi DS. Utilization of watermelon pulp for lycopene extraction by response surface methodology. Food Chem. 2017;232:316–321. doi: 10.1016/j.foodchem.2017.04.038. [DOI] [PubMed] [Google Scholar]
- Peixoto CM, Dias MI, Alves MJ, Calhelha RC, Barros L, Pinho SP, Ferreira ICFR. Grape pomace as a source of phenolic compounds and diverse bioactive properties. Food Chem. 2018;253:132–138. doi: 10.1016/j.foodchem.2018.01.163. [DOI] [PubMed] [Google Scholar]
- Perkins-Veazie P, Collins JK. Flesh quality and lycopene stability of fresh-cut watermelon. Postharvest Biol Technol. 2004;31:159–166. doi: 10.1016/j.postharvbio.2003.08.005. [DOI] [Google Scholar]
- Rimando AM, Perkins-Veazie PM. Determination of citrulline in watermelon rind. J Chromatogr A. 2005;1078:196–200. doi: 10.1016/j.chroma.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Russo M, Bonaccorsi I, Inferrera V, Dugo P, Mondello L. Underestimated sources of flavonoids, limonoids and dietary fiber: availability in orange's by-products. J Funct Foods. 2015;12:150–157. doi: 10.1016/j.jff.2014.11.008. [DOI] [Google Scholar]
- Schieber A, Stintzing FC, Carle R. By-products of plant food processing as a source of functional compounds—recent developments. Trends Food Sci Technol. 2001;12(11):401–413. doi: 10.1016/S0924-2244(02)00012-2. [DOI] [Google Scholar]
- Singh S, Ramakrishna S, Gupta MK. Towards zero waste manufacturing: a multidisciplinary review. J Clean Prod. 2017;168:1230–1243. doi: 10.1016/j.jclepro.2017.09.108. [DOI] [Google Scholar]
- Song Q, Li J, Zeng X. Minimizing the increasing solid waste through zero waste strategy. J Clean Prod. 2015;104:199–210. doi: 10.1016/j.jclepro.2014.08.027. [DOI] [Google Scholar]
- Souad AM, Jamal P, Olorunnisola KS. Effective jam preparations from watermelon waste. Inter Food Res J. 2012;19:1545–1549. [Google Scholar]
- Spinelli S, Conte A, Lecce L, Padalino L, Del Nobile MA. Supercritical carbon dioxide extraction of brewer's spent grain. J Supercrit Fluids. 2016;107:69–74. doi: 10.1016/j.supflu.2015.08.017. [DOI] [Google Scholar]
- Tarazona-Díaz MP, Viegas J, Moldao-Martins M, Aguayo E. Bioactive compounds from flesh and by-product of fresh-cut watermelon cultivars. J Sci Food Agric. 2011;91:805–812. doi: 10.1002/jsfa.4250. [DOI] [PubMed] [Google Scholar]