Abstract
Chironji fruit juice extraction using cellulase enzyme was performed at various concentrations of cellulase, incubation temperature, and time. Artificial neural network (ANN) conjugated with genetic algorithm (GA), and response surface methodology (RSM) were used for attaining the process conditions where the highest juice yield can be achieved. The recommended values of process parameters obtained using ANN-GA method were cellulase concentration of 0.093% (w/w), incubation temperature of 45.7 °C, and incubation time of 116 min. Using RSM method, the recommended values were cellulase concentration of 0.081% (w/w), incubation temperature of 39.6 °C, and incubation time of 99 min. However, the incubation time was found to be the most significant independent process parameter followed by cellulase concentration that affect the juice yield. The juice yield determined experimentally at RSM and ANN-GA recommended conditions was 69.77 ± 0.16% and 70.15 ± 0.12%, respectively. These values indicated that both RSM and ANN-GA methods have comparable accuracies. However, juice extracted using ANN-GA recommended conditions had better physicochemical properties than the juice extracted using RSM recommended conditions.
Keywords: Juice, Cellulase, Response surface methodology, Artificial neural network, Optimization
Introduction
Chironji (Buchanania lanzan Spreng.) is a member of the Anacardiaceae family, generally recognized as char, achar, and piyar in rural India (Siddiqui et al. 2016). In India, the tree is mostly found in the forest of Rajasthan, Madhya Pradesh, Jharkhand, Andhra Pradesh, Odisha, Chhattisgarh, Maharashtra, and Bihar (Khatoon et al. 2015). The tree is also found in several tropical Asian countries, as well as in the Pacific Islands and Australia (Siddiqui et al. 2016). Two species of the tree (Buchanania axillaries and Buchanania lanzan) produce edible fruits, out of the seven reported species in India (Rajput et al. 2018). The taste of the fruits of Buchanania lanzan is generally sweet and juicy (Srivastava et al. 2017). The fruit has around 39% of pulp and becomes black upon ripening. It has been reported that the pulp contains about 20.51% carbohydrate, 1.27% crude fiber, 0.67% fat, 1.82% ash, and 1.93% crude protein (Sahu 1998).
Chironji fruit is listed in the categories of minor forest produces, which are promoted through the Pradhan Mantri Van Dhan Yojana (PMVDY) under the Ministry of Tribal Affairs, Government of India, to generate livelihood for the tribal by harnessing the wealth of forest. This initiative promotes the value addition, branding, and marketing of such forest produces. As per the Tribal Cooperative Marketing Development Federation of India Limited (TRIFED), Chironji fruit has an estimated total potential of 10 million kilograms per annum and an estimated value of 230 Crores in Indian rupees (30.04 million US dollars). Therefore, the value addition of Chironji fruits may enhance the income of rural communities. According to TRIFED, value-added products like squash, RTS, and nectar can be prepared from the Chironji fruit pulp. Therefore, juice extraction from the Chironji fruit pulp is also possible and need of the hour. The juice has not been commercialized yet, and very little information is available about it on the literature. The process of cold extraction, hot extraction, and enzymatic extraction using pectinase enzyme for extraction of juice from Chironji fruit pulp has been reported in the literature (Sahu 1998). During juice extraction, specific polysaccharides (like pectin, hemicellulose, and cellulose) prevents the complete extraction of juices by blocking the release of water and water-soluble materials (Sandri et al. 2011; Singh et al. 2019). One feasible way to enhance the efficiency of juice extraction is to use an enzyme to degrade those polysaccharides. As 30% of cell wall dry matter is cellulosic polysaccharides, degradation of these polysaccharides using cellulase enzyme can reduce the cell wall strength and finally results in the maximum extraction of juice (Parameswaran et al. 2018).
Modeling and optimization of process parameters are the most significant steps for improving the effectiveness of the extraction process (Betiku and Taiwo 2015). Response Surface Methodology (RSM) is a technique utilized for developing empirical models that explain the relationship among independent process parameters and dependent parameters (Ghosh et al. 2012). RSM describes the influence of independent process parameters and their interaction on dependent parameters in a process. This approach produces a mathematical model that is useful in finding out the optimized conditions of independent process parameters during numerical optimization (Basri et al. 2007). Artificial neural network (ANN) is a tool widely used in the modeling of non-linear and complex processes (Tao et al. 2014). ANN in conjugation with the Genetic algorithm (GA), a popular optimization technique that follows the principle of biological evolution, has been used successfully for modeling and optimization in various food processing operations (Koc et al. 2007; Khawas et al. 2016; Mohebbi et al. 2011). Both RSM and ANN methods have been used simultaneously in several studies for a better understanding of the process (Sivamani et al. 2019; Youssefi et al. 2009; Maran et al. 2013). Therefore, both RSM and ANN-GA methods were used in this research for optimizing the process of cellulase-assisted extraction of Chironji fruit juice. Several physicochemical properties of the juices, extracted using optimized conditions obtained under RSM and ANN-GA methods, were also analyzed.
Materials and methods
Preparation of sample and extraction of juice
Ripened Chironji fruits were bought from the market of Rourkela, Odisha, India. The fruits were appropriately cleaned and washed with water. For further experiments, they were stored at −20 °C.
Before experiments, fruits were taken out from the deep freezer and kept outside for 3 h at room temperature. The fruit pulp was separated by removing the nuts from the fruits. The thick pulp was then blended for 5 min using a mixer grinder (Model: GX-1, Bajaj, India). Some preliminary studies were carried out by incorporating water into the pulp in the proportion (w/w) of 1:2, 2:1, and 1:1. The juice yield was determined for all the three pulp-water mixtures (PWM) (data not reported). The juice yield was maximum in a PWM of 1:2 w/w, while it was minimum in a PWM of 2:1 w/w. So the proportion of PWM of 1:1 w/w and 1:2 w/w were only considered for juice extraction. However, the juice extracted from the PWM of 1:1 w/w was found to have better physicochemical properties (data not reported) in comparison to the juice extracted from a PWM of 1:2 w/w. Finally, water was added to the pulp in 1:1 w/w proportion for all the experiments in the experimental matrix.
In this study, Cellulase from Trichoderma reesei (Form: aqueous solution, activity: ≥ 700 units/g, density: 1.10–1.30 g/mL, molecular mass: 68 kDa, optimum pH: 6 and optimum temperature: 52 °C, Sigma-Aldrich Corporation) was used. Cellulase enzyme was mixed with 100 g of PWM for the extraction of juice. The pH of the PWM was 4.291 ± 0.011. Based on the preliminary experiments at a temperature above 50 °C, no significant increase in juice yield was observed (data not reported). This may be because of the pH value of the PWM was significantly lower than the optimum pH of cellulase from Trichoderma reesei. Therefore the incubation was carried out at a temperature range of 30–50 °C. An incubator (CIS-24 plus, REMI Instrument, India) with a shaking speed of 120 rpm was used for incubation at a specific temperature. The non-uniformity of temperature in incubator was around ± 0.5 °C whereas non-uniformity of cellulase concentration was around 2–4% of the desired value. After completion of incubation, the samples were pasteurized at 90 °C for 5 min for the inactivation of the cellulase enzyme (Mohanty et al. 2018). Then the juice extraction was carried out using the procedure followed by Majaliwa et al. (2019); Mohanty et al. (2018). Filtration and extraction of juice were done by pressing the pasteurized sample through a muslin cloth. Then the juice yield was computed using Eq. 1 (Handique et al. 2019).
| 1 |
Design of experiments
The software Design-Expert (version 11.0.5.0, Stat-Ease Inc.) was used in this research for preparing the central composite rotatable design (CCRD) 23 design. Cellulase concentration (0.01–0.1%w/w), incubation temperature (30–50 °C), and incubation time (20–120 min) were taken as the independent parameters, whereas the juice yield (Y) was taken as the dependent parameter. The number of experiments obtained from the CCRD design was 20, which were conducted in triplicates, and the results are presented in Table 1.
Table 1.
CCRD design of experiments with experimental, RSM predicted, and ANN predicted results of juice yield
| Run | Independent parameters | Juice yield (%) | ||||
|---|---|---|---|---|---|---|
| A | B | C | Experimental | RSM predicted | ANN predicted | |
| 1 | 0.06 (0) | 30 (−1.682) | 70 (0) | 65.94 ± 0.47 | 66.24 | 66.61 |
| 2 | 0.06 (0) | 40 (0) | 20 (−1.682) | 63.01 ± 0.29 | 62.89 | 62.95 |
| 3 | 0.08 (+ 1) | 34 (−1) | 40 (−1) | 64.38 ± 0.31 | 64.82 | 64.6 |
| 4 | 0.03 (−1) | 46 (+ 1) | 100 (+ 1) | 67.07 ± 0.37 | 66.64 | 66.9 |
| 5 | 0.08 (+ 1) | 34 (−1) | 100 (+ 1) | 69.46 ± 0.43 | 69.16 | 69.44 |
| 6 | 0.01 (−1.682) | 40 (0) | 70 (0) | 62.94 ± 0.19 | 63.4 | 63.22 |
| 7 | 0.03 (−1) | 46 (+ 1) | 40 (−1) | 63.54 ± 0.42 | 63.85 | 63.42 |
| 8 | 0.06 (0) | 50 (+ 1.682) | 70 (0) | 67.91 ± 0.25 | 67.59 | 67.95 |
| 9 | 0.08 (+ 1) | 46 (+ 1) | 100 (+ 1) | 69.7 ± 0.36 | 70.31 | 70.05 |
| 10 | 0.03 (−1) | 34 (−1) | 100 (+ 1) | 65.29 ± 0.21 | 65.25 | 64.87 |
| 11 | 0.08 (+ 1) | 46 (+ 1) | 40 (−1) | 64.99 ± 0.42 | 65.04 | 64.9 |
| 12 | 0.1 (+ 1.682) | 40 (0) | 70 (0) | 68.18 ± 0.33 | 67.7 | 68.21 |
| 13 | 0.06 (0) | 40 (0) | 120 (+ 1.682) | 68.79 ± 0.34 | 68.89 | 68.45 |
| 14 | 0.03 (−1) | 34 (−1) | 40 (−1) | 63.99 ± 0.27 | 63.39 | 63.98 |
| 15 | 0.06 (0) | 40 (0) | 70 (0) | 68.07 ± 0.27 | 67.11 | 67.16 |
| 16 | 0.06 (0) | 40 (0) | 70 (0) | 67.82 ± 0.35 | 67.11 | 67.16 |
| 17 | 0.06 (0) | 40 (0) | 70 (0) | 66.16 ± 0.39 | 67.11 | 67.16 |
| 18 | 0.06 (0) | 40 (0) | 70 (0) | 66.98 ± 0.23 | 67.11 | 67.16 |
| 19 | 0.06 (0) | 40 (0) | 70 (0) | 67.25 ± 0.38 | 67.11 | 67.16 |
| 20 | 0.06 (0) | 40 (0) | 70 (0) | 66.37 ± 0.25 | 67.11 | 67.16 |
A: Cellulase concentration (% w/w), B: Incubation temperature (°C), C: Incubation time (Minutes)
Response surface methodology
RSM was utilized for finding the recommended values of independent process parameters. A polynomial model was constructed that explained the relation between the juice yield and the independent process parameters. Regression analysis and analysis of variance (ANOVA) were done to find the significance of the developed model as well as the model terms that affect the juice yield. The influence of the interaction of independent process parameters on juice yield was evaluated by plotting the 3D response surfaces. Model development, regression analysis, response surface plotting, and ANOVA were carried out in the software Design-Expert.
Artificial neural network and genetic algorithm
In this study, the neural net fitting (nftool) app in MATLAB (R2018a, MathWorks) was used for the construction of the ANN model. A feed-forward ANN model was constructed and trained with the Levenberg–Marquardt (LM) back-propagation algorithm. The network was comprised of an input layer having three neurons in it, a single hidden layer and an output layer containing one neuron in it. The trial and error technique was used for finding the number of neurons in the hidden layer. The transfer function used for the hidden and output layer of the network were hyperbolic tangent sigmoid (tansig) and linear (purelin), respectively.
The total number of experiments (20) obtained from the CCRD design were randomly divided for training (70%), testing (15%), and validation (15%) of the model. The construction of the model was completed when the minimum mean squared error (MSE) and the maximum coefficient of correlation (R) were achieved for the data sets used for training, testing, and validation. The final weights and bias values of the best ANN model were obtained, which were used in Eq. 2 for the prediction of juice yield.
| 2 |
where Y is ANN predicted juice yield, Xi is the independent process parameters, W1 and W2 are the weights of interconnecting lines between the input to the hidden layer and hidden to the output layer, respectively, B1 and B2 are the bias values of the neurons of hidden and output layer.
The optimization toolbox (optimtool) in MATLAB (R2018a, MathWorks) was used for performing GA optimization. The constructed ANN model was conjugated with GA for finding the optimized values of independent process parameters to maximize the juice yield. An objective function (Eq. 3) was developed by using the constructed ANN model. Since GA always minimizes the objective function (Kalathingal et al. 2020), the minimization of the developed objection function led to the attainment of the objective of the optimization.
| 3 |
The parameters used in GA optimization were a feasible population creation function with the population size of 50, rank scaling function, roulette selection function, adaptive feasible mutation function, two-point crossover function, and crossover fraction of 0.7.
Comparison of models
The developed ANN and RSM models were compared statistically based on three parameters, namely, mean squared error (MSE), coefficient of determination (R2), and mean absolute error (MAE). The mathematical expressions for these statistical parameters are given in Eqs. 4–6 (Nazghelichi et al. 2011).
| 4 |
| 5 |
| 6 |
where n is the total number of experiments, Ya is the average value of experimental juice yield, Ye and Yp are the experimental and predicted juice yield, respectively.
Analysis of physicochemical properties
Different physicochemical properties of the raw juice (untreated) along with the juice extracted at recommended values of independent process parameters attained through both ANN-GA and RSM technique were analyzed using the following methods.
A viscometer (DV2TLV, Brookfield, USA) was used for determining viscosity (unit: cP). Turbidity (unit: Nephelometric Turbidity Units, NTU) was determined using a digital turbidity meter (Model 335, Deluxe Company, India). Clarity was determined using a UV–visible spectrophotometer (AU2701, Systronics India Ltd., India) by measuring the transmittance (%T) of the juices at 660 nm (Ghosh et al. 2016). Total soluble solids (TSS, unit: degree Brix) and refractive index (RI) were measured using a digital refractometer (Model RFM 700, Bellingham and Stanley, UK). A pH meter (Eutech, India) was used for measuring the pH of the juice.
Hunter Lab calorimeter (ColorFlex EZ, Hunter Associates Laboratory Inc. USA) was used to determine the L*, a*, and b* values of the juice. The total color change (∆E) was computed using Eq. 7 (Kalathingal et al. 2020).
| 7 |
The total phenolic content of the juices was measured using the FCR method (Folin–Ciocalteau Reagent method) by keeping Gallic acid as standard and expressed as mg GAE/g dw (Ghani et al. 2018; Sagu et al. 2014). Antioxidant activity (DPPH radical scavenging, %) was found out as per the procedure proposed by (Kharazmi-Khorassani et al. 2019) using Eq. 8.
| 8 |
For finding any significant difference between the three samples, a Duncan homogeneity test was performed using SPSS Statistics 23.0 (IBM Corporation).
Results and discussion
RSM modeling and optimization
The results of the 20 experiments conducted are shown in Table 1. The regression model is provided in Eq. 9 in terms of the coded parameters that explained the process of juice extraction. The results of ANOVA, along with the significance of every model term, are provided in Table 2. The model's goodness of fit was examined by the coefficient of determination (R2). The R2 value of 0.941 of the model indicated that 94.1% variation in the sample for juice extraction was attributed to the independent process parameters, and the model did not explain the rest 5.9% of the total variation. The R2 value of more than 0.8 has been recommended for the good fit of a model (Akintunde et al. 2015). The adjusted R2 value was 0.888, which was good enough to confirm the model's significance. The generated quadratic model was significant, which was confirmed from its F and p-value of 17.73 and < 0.0001, respectively. The F-value and p-value of 0.68 and 0.6614, respectively, confirmed that the lack of fit was not significant, which is appropriate.
| 9 |
where 'Y' is juice yield, 'A' is the concentration of cellulase, 'B' is incubation temperature, and 'C' is incubation time in coded form.
Table 2.
Analysis of variance (ANOVA) for the quadratic model
| Source | Sum of Squares | df | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 77.87 | 9 | 8.65 | 17.73 | < 0.0001 | significant |
| A-Cellulase Concentration | 22.30 | 1 | 22.30 | 45.71 | < 0.0001 | |
| B-Temperature | 2.21 | 1 | 2.21 | 4.53 | 0.0592 | |
| C-Time | 43.38 | 1 | 43.38 | 88.91 | < 0.0001 | |
| AB | 0.0288 | 1 | 0.0288 | 0.0590 | 0.8130 | |
| AC | 3.08 | 1 | 3.08 | 6.30 | 0.0309 | |
| BC | 0.4324 | 1 | 0.4324 | 0.8863 | 0.3687 | |
| A2 | 4.37 | 1 | 4.37 | 8.96 | 0.0135 | |
| B2 | 0.0672 | 1 | 0.0672 | 0.1377 | 0.7183 | |
| C2 | 2.67 | 1 | 2.67 | 5.48 | 0.0413 | |
| Residual | 4.88 | 10 | 0.4879 | |||
| Lack of Fit | 1.97 | 5 | 0.3934 | 0.6754 | 0.6614 | Not significant |
| Pure Error | 2.91 | 5 | 0.5825 | |||
| Cor Total | 82.75 | 19 |
p-values less than 0.05 indicate model terms are significant
The significance of the model terms was investigated in a confidence interval of 95% (p-value < 0.05). The model terms that were found to be significant were A, C, AC, A2, and C2. The linear and interaction model terms A, C, and AC had a positive influence while the quadratic model terms A2 and C2 had a negative influence on the response Y. The linear model terms 'A' and 'C' were found to have the most significant influence on the juice yield, as indicated from their p-value of less than 0.0001. Furthermore, the coefficient of 'C' (+ 1.78) was higher than the coefficient of 'A' (1.28), which made 'C' as the most critical model term.
The influence of interactions of independent process parameters on the juice yield was examined using the 3D response surface plots. Figure 1a shows the influence of the interaction of concentration of cellulase and incubation temperature on juice yield. The juice yield improved with the rise in the level of cellulase, but no significant effect on the juice yield was seen with the increase of incubation temperature. The graph showed no significant effect on the yield of juice due to the interaction of cellulase concentration and incubation temperature. Figure 1b illustrates the interaction of incubation time and cellulase concentration on the yield of juice. The graph shows that their interaction had a substantial positive impact on the yield of juice. At cellulase concentration and an incubation time of 0.08% (w/w) and 100 min, respectively, the highest juice yield was recorded, approximately 69%. The increase in juice yield might be because the primary constituent of a fruit cell wall polysaccharide (around 30% of dry matter) is cellulose (Parameswaran et al. 2018). During incubation, the cellulase enzymes hydrolyze cellulose, which in turn reduces the cell wall strength and results in maximum extraction of juice (Kumar et al. 2019). Hence, the more incubation time and enzyme concentration, the more is the extraction yield. Similar results were also reported for banana (Sagu et al. 2014) and apple juices (Srivastava and Tyagi 2013). The influence of the interaction of incubation time and incubation temperature on juice yield is demonstrated in Fig. 1c. The graph confirms that the juice yield had risen substantially with the rise in the incubation time, which maybe because of the longer exposure time available for enzymatic actions (Mohanty et al. 2018); however, its interaction with the incubation temperature did not affect the yield of juice.
Fig. 1.
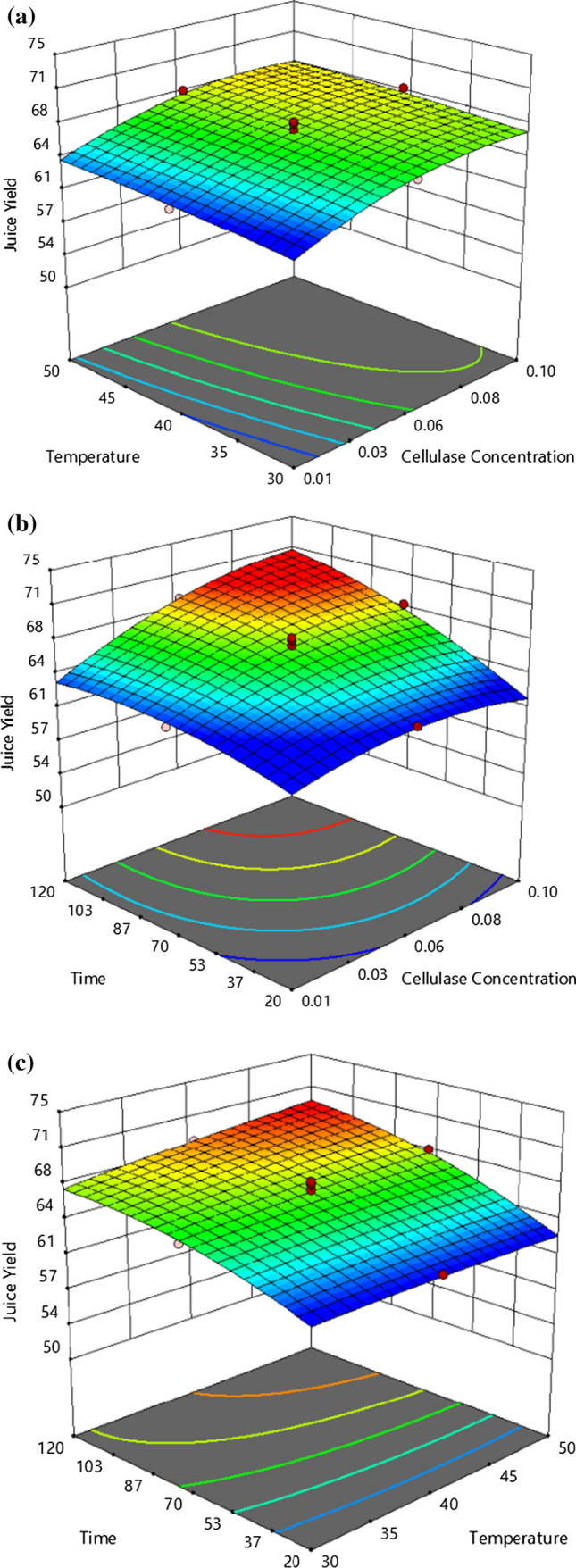
Effect of independent process parameters on juice yield (%), a temperature (°C) and cellulase concentration (%, w/w), b time (minutes) and cellulase concentration, c time, and temperature
The recommended conditions of independent process parameters were obtained by performing numerical optimization in the Design-Expert software (version 11.0.5.0, Stat-Ease Inc.). The concentration of cellulase, incubation time and temperature were kept in range while juice yield was maximized during optimization. The recommended combination of process parameters with a desirability value of one was a cellulase concentration of 0.081% (w/w), incubation temperature of 39.6 °C, and an incubation time of 99 min. The juice yield predicted by the RSM model at this recommended condition was 69.71%.
ANN modeling and GA optimization
The same data used for the construction of the RSM model was used for building the ANN model for predicting the juice yield. The ANN predicted juice yield for all the 20 experiments is given in Table 1.
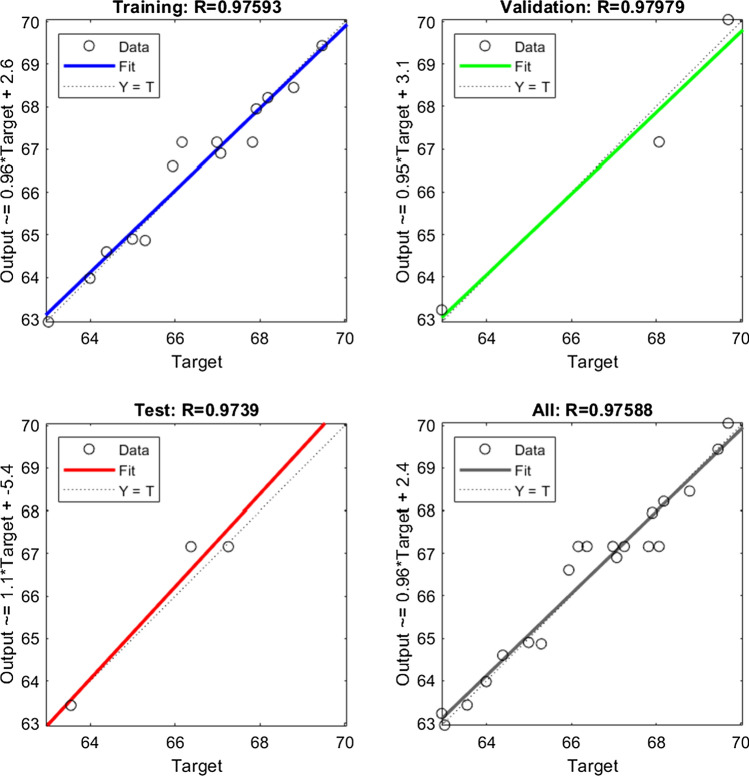
The optimum structure of the ANN model trained by the LM algorithm was three, ten, and one neuron in the input, hidden, and output layer, respectively. The ten neurons in the network's hidden layer were taken based on minimum MSE, and maximum R-value for data set used in training, validation, and testing of the network. The optimized weight and bias values obtained for the developed model are given in Eqs. 10–13. The network achieved its best performance at the 3rd epoch, where MSE was 0.34 for the validation data set. Also, the MSE for the data set used in the training and testing of the network at this stage was 0.164 and 0.217, respectively. The regression plot of the developed model is illustrated in Fig. 2. The R-values of 0.975, and 0.979 for the training and validation data set confirmed the excellent correlation between the experimental data used for model development and the predicted data. For the testing data set, the R-value was 0.973, which confirmed the excellent prediction ability of the ANN model for the unseen data. Overall, the R-value for all data set was found to be 0.975, which confirmed the excellent agreement between the experimental and predicted data of all the 20 experiments.
| 10 |
| 11 |
| 12 |
| 13 |
Fig. 2.
Regression plot of the ANN model for training, testing, validation, and all data sets
The ANN model was conjugated with GA for obtaining the optimized values of the independent process parameters for achieving maximum juice yield. The optimized values obtained after 51 generations of GA were 0.093% (w/w) concentration of cellulase, 45.7 °C incubation temperature, and time of 116 min. The juice yield predicted by the ANN model at this optimized combination of process parameters was 70.34%.
Validation and comparison of the models
Table 3 presents the optimized conditions of process parameters where experiments were performed in triplicates to validate RSM and ANN-GA method. For finding any significant (p < 0.05) difference among the predicted and experimental results, a paired samples t-test was performed. For both the models, there was no significant difference found between the model predicted and experimental results, as confirmed from the p-value of 0.58 for RSM and 0.07 for the ANN-GA method. Experimental juice yield was found to be 69.77 ± 0.16% and 70.15 ± 0.12% at the RSM and ANN-GA recommended conditions, respectively, which are insignificant to each other as confirmed from a paired samples t-test. Therefore, the ANN-GA and RSM methods have comparable accuracies.
Table 3.
RSM and ANN-GA model validation
| Methods | Optimum values | Juice yield (%) | ||||
|---|---|---|---|---|---|---|
| A | B | C | Predicted | Experimental | p-value | |
| Raw | – | – | – | – | 54.06 ± 0.23 | – |
| RSM | 0.081 | 39.6 | 99 | 69.71 | 69.77 ± 0.16 | 0.58 |
| ANN-GA | 0.093 | 45.7 | 116 | 70.34 | 70.15 ± 0.12 | 0.07 |
Raw: untreated sample, A: cellulase concentration (% w/w), B: incubation temperature (°C), C: incubation time (minutes), p-values < 0.05 indicates significance
To calculate the percentage increase in juice yield following the cellulase assisted extraction under RSM and ANN-GA optimized conditions, the juice yield for the raw sample (untreated) was determined. Juice yield of 54.06 ± 0.23% was measured for the raw sample. At RSM optimized conditions, a 15.71% increase in juice yield was observed, whereas a 16.09% increase was observed at ANN-GA optimized conditions. The higher juice yield at ANN-GA recommended conditions might be because of the higher cellulase concentration and longer incubation time.
The MSE, R2, and MAE of RSM and ANN model for all data sets were computed for model comparison. Lower the value of MSE and MAE, whereas higher the value of R2 is desirable for a good model. The MSE, R2, and MAE for the RSM model were 0.243, 0.941, and 0.409, respectively. For the ANN model, the MSE, R2, and MAE were 0.198, 0.952, and 0.322, respectively. The ANN model's MSE and MAE were lower than the RSM model, while the R2 was higher in comparison to the RSM model. The ANN model was superior to the RSM model for predicting juice yield was confirmed from the results of these three parameters.
Physicochemical properties of the juice
The results of different physicochemical properties of the raw juice (untreated) and the juice extracted at recommended values of independent process parameters are provided in Table 4. Significant (p < 0.05) decrease in viscosity, turbidity, and pH was noticed when the juice was extracted using recommended conditions of process parameters. For viscosity and turbidity, there was a significant difference between the juices extracted using RSM and ANN-GA optimized conditions; however, there was no significant difference observed between them for pH. All three properties, i.e., viscosity (1.316 ± 0.005 cP), turbidity (139 ± 1 NTU), and pH (4.163 ± 0.005), were found to be minimal for the juice extracted under the optimized conditions given by the ANN-GA method. The clarity of the juice increased as compared to raw juice by using the optimized conditions obtained from RSM and ANN-GA method. Cellulase enzyme degrades the cellulosic polysaccharides during the incubation period, which leads to the reduction of the water-holding capacity of the cell and subsequent release of all the free water into the juice. This results in the reduction in viscosity, turbidity, and improvement in clarity (Handique et al. 2019).
Table 4.
Physicochemical properties of the raw juice (untreated) and juice extracted at optimum conditions (RSM and ANN-GA) of process parameters
| Properties | Raw | RSM optimized | ANN-GA optimized |
|---|---|---|---|
| Viscosity (cP) | 1.433c ± 0.005 | 1.376b ± 0.005 | 1.316a ± 0.005 |
| Turbidity (NTU) | 269.33c ± 1.527 | 159b ± 1.732 | 139a ± 1 |
| Clarity (%T) | 0.666a ± 0.057 | 0.766ab ± 0.058 | 0.866b ± 0.057 |
| TSS (0Brix) | 13.533a ± 0.058 | 13.833b ± 0.058 | 13.934b ± 0.057 |
| Refractive index | 1.3531a ± 0 | 1.3545c ± 0 | 1.3543b ± 0 |
| L* | 15.083a ± 0.025 | 15.146a ± 0.046 | 16.426b ± 0.066 |
| a* | 12.953c ± 0.055 | 11.12b ± 0.043 | 10.47a ± 0.026 |
| b* | 5.533a ± 0.066 | 6.076b ± 0.037 | 6.88c ± 0.04 |
| ∆E | – | 1.914a ± 0.045 | 3.128b ± 0.071 |
| pH | 4.286b ± 0.015 | 4.164a ± 0.005 | 4.163a ± 0.005 |
| Total phenolic content (mg GAE /g dw) | 9.41a ± 0.28 | 11.08b ± 0.19 | 11.76c ± 0.21 |
| DPPH radical scavenging activity (%) | 77.072a ± 0.661 | 79.229b ± 0.226 | 79.426b ± 0.103 |
Means in a row with different superscripts are significantly different (p < 0.05)
A significant difference in TSS content between the raw juice and the enzymatically extracted juice was noted; however, there were no significant differences between the juices extracted at RSM and ANN-GA optimized conditions. Improvement in TSS content was observed in the enzymatically extracted juice. The release of components such as sugars during the incubation period contributes to the improvement in TSS (Sharma et al. 2014). The refractive index increased significantly with the treatment of cellulase at optimized conditions obtained through both RSM and ANN-GA method. The refractive index (1.3545) was higher in the juice extracted using RSM optimized conditions.
Cellulase treatment at optimized conditions obtained through both RSM and ANN-GA method significantly influenced the color properties of the juice. An increase in L* and b* value while a decrease in a* value was observed in the juices extracted using cellulase treatment. A significant difference in the total color change was observed between the juices extracted using RSM and ANN-GA optimized conditions. The maximum change in total color (3.128 ± 0.071) was recorded for the juice obtained using ANN-GA optimized conditions. The color change has also been reported for palm juice extracted using cellulase enzyme, and it may be attributed to the reduction in the viscosity of the juice after enzymatic extraction (Mohanty et al. 2018).
A significant increase in phenolic content was noticed in the juice extracted using RSM as well as ANN-GA optimized condition as compared to the raw juice. The increase in phenolic content might be because the fruit cell wall contains phenolic compounds bonded with cell wall polysaccharides such as cellulose, hemicellulose, and pectin using hydrogen and hydrophobic linkages. The addition of cellulase enzyme during juice extraction leads to the breakdown of cellulose into cellobiose and glucose resulting in the destruction of cell wall structure, which leads to the release of the phenolic compounds into the juice (Ghandahari Yazdi et al. 2019; Fernández et al. 2015; Parameswaran et al. 2018). Antioxidant activity expressed as % DPPH radical scavenging activity, increased significantly in the juices extracted at optimized conditions; however, there was no significant difference observed between the juices extracted at RSM and ANN-GA optimized conditions. The increase in radical scavenging activity may be attributed to the rise in total phenolic content after enzymatic extraction, which increases the possibility of hydrogen donation to free radicals because of the increased number of hydroxyl groups in the reaction medium (Ghandahari Yazdi et al. 2019).
Conclusion
ANN in conjugation with GA and RSM was used to find the recommended values of cellulase concentration, incubation temperature, and time for attaining maximum yield of Chironji fruit juice. Recommended conditions were 0.081% (w/w) concentration of cellulase, 39.6 °C incubation temperature, and 99 min incubation time in the RSM method. In the ANN-GA method, 0.093% (w/w) concentration of cellulase, 45.7 °C incubation temperature, and time of 116 min were obtained as recommended values. Juice yield increased by 15.71% and 16.09% experimentally using the recommended conditions gotten from RSM and ANN-GA method, respectively. The physicochemical analysis confirmed that juice obtained using ANN-GA recommended conditions had better physicochemical properties than the juice obtained using RSM recommended conditions. In future, further research is required on juice extraction using cellulase enzyme by collecting Chironji fruits from different geographical locations.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dileswar Pradhan, Email: dileswarpradhan08@gmail.com.
S. Abdullah, Email: abdullahfpe@gmail.com
Rama Chandra Pradhan, Email: pradhanrc@nitrkl.ac.in.
References
- Akintunde AM, Ajala SO, Betiku E. Optimization of Bauhinia monandra seed oil extraction via artificial neural network and response surface methodology: a potential biofuel candidate. Ind Crops Prod. 2015;67:387–394. doi: 10.1016/j.indcrop.2015.01.056. [DOI] [Google Scholar]
- Basri M, Rahman RNZRA, Ebrahimpour A, Salleh AB, Gunawan ER, Rahman MBA. Comparison of estimation capabilities of response surface methodology (RSM) with artificial neural network (ANN) in lipase-catalyzed synthesis of palm-based wax ester. BMC Biotechnol. 2007;7(1):53. doi: 10.1186/1472-6750-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betiku E, Taiwo AE. Modeling and optimization of bioethanol production from breadfruit starch hydrolyzate vis-à-vis response surface methodology and artificial neural network. Renew Energy. 2015;74:87–94. doi: 10.1016/j.renene.2014.07.054. [DOI] [Google Scholar]
- Fernández K, Vega M, Aspé E. An enzymatic extraction of proanthocyanidins from País grape seeds and skins. Food Chem. 2015;168:7–13. doi: 10.1016/j.foodchem.2014.07.021. [DOI] [PubMed] [Google Scholar]
- Ghandahari Yazdi AP, Barzegar M, Sahari MA, Ahmadi Gavlighi H. Optimization of the enzyme-assisted aqueous extraction of phenolic compounds from pistachio green hull. Food Sci Nutr. 2019;7(1):356–366. doi: 10.1002/fsn3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghani NAA, Channip AA, Chok Hwee Hwa P, Ja'afar F, Yasin HM, Usman A. Physicochemical properties, antioxidant capacities, and metal contents of virgin coconut oil produced by wet and dry processes. Food Sci Nutr. 2018;6(5):1298–1306. doi: 10.1002/fsn3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Chakraborty R, Chatterjee G, Raychaudhuri U. Study on fermentation conditions of palm juice vinegar by response surface methodology and development of a kinetic model. Braz J Chem Eng. 2012;29(3):461–472. doi: 10.1590/S0104-66322012000300003. [DOI] [Google Scholar]
- Ghosh P, Pradhan RC, Mishra S. Optimization of process parameters for enhanced production of Jamun juice using Pectinase (Aspergillus aculeatus) enzyme and its characterization. 3 Biotech. 2016;6(2):241. doi: 10.1007/s13205-016-0561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handique J, Bora SJ, Sit N. Optimization of banana juice extraction using combination of enzymes. J Food Sci Technol. 2019;56(8):3732–3743. doi: 10.1007/s13197-019-03845-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalathingal MSH, Basak S, Mitra J. Artificial neural network modeling and genetic algorithm optimization of process parameters in fluidized bed drying of green tea leaves. J Food Process Eng. 2020;43:e13128. doi: 10.1111/jfpe.13128. [DOI] [Google Scholar]
- Kharazmi-Khorassani J, Asoodeh A, Tanzadehpanah H. Antioxidant and angiotensin-converting enzyme (ACE) inhibitory activity of thymosin alpha-1 (Thα1) peptide. Bioorg Chem. 2019;87:743–752. doi: 10.1016/j.bioorg.2019.04.003. [DOI] [PubMed] [Google Scholar]
- Khatoon N, Gupta RK, Tyagi YK. Nutraceutical potential and phytochemical screening of Buchanania lanzan, an underutilized exotic Indian nut and its use as a source of functional food. J Pharmacognosy Phytochem. 2015;4(1):87–94. [Google Scholar]
- Khawas P, Dash KK, Das AJ, Deka SC. Modeling and optimization of the process parameters in vacuum drying of culinary banana (Musa ABB) slices by application of artificial neural network and genetic algorithm. Drying Technol. 2016;34(4):491–503. doi: 10.1080/07373937.2015.1060605. [DOI] [Google Scholar]
- Koc AB, Heinemann P, Ziegler G. Optimization of whole milk powder processing variables with neural networks and genetic algorithms. Food Bioprod Process. 2007;85(4):336–343. doi: 10.1205/fbp07074. [DOI] [Google Scholar]
- Kumar VA, Kurup RSC, Snishamol C & Prabhu GN (2019) Role of cellulases in food, feed, and beverage industries. In: Green bio-processes. Springer, pp 323–343
- Majaliwa N, Kibazohi O, Alminger M. Optimization of process parameters for mechanical extraction of banana juice using response surface methodology. J Food Sci Technol. 2019;56:4068–4075. doi: 10.1007/s13197-019-03875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maran JP, Sivakumar V, Thirugnanasambandham K, Sridhar R. Artificial neural network and response surface methodology modeling in mass transfer parameters predictions during osmotic dehydration of Carica papaya L. Alex Eng J. 2013;52(3):507–516. doi: 10.1016/j.aej.2013.06.007. [DOI] [Google Scholar]
- Mohanty S, Mishra S, Pradhan RC. Optimisation of enzymatic extraction and characterization of palm (Borassus flabellifer) juice. J Food Meas Charact. 2018;12(4):2644–2656. doi: 10.1007/s11694-018-9882-5. [DOI] [Google Scholar]
- Mohebbi M, Shahidi F, Fathi M, Ehtiati A, Noshad M. Prediction of moisture content in pre-osmosed and ultrasounded dried banana using genetic algorithm and neural network. Food Bioprod Process. 2011;89(4):362–366. doi: 10.1016/j.fbp.2010.08.001. [DOI] [Google Scholar]
- Nazghelichi T, Aghbashlo M, Kianmehr MH. Optimization of an artificial neural network topology using coupled response surface methodology and genetic algorithm for fluidized bed drying. Comput Electron Agric. 2011;75(1):84–91. doi: 10.1016/j.compag.2010.09.014. [DOI] [Google Scholar]
- Parameswaran B, Varjani S, Raveendran S. Green bio-processes: enzymes in industrial food processing. Singapore: Springer; 2018. [Google Scholar]
- Rajput BS, Gupta D, Kumar S, Singh K, Tiwari C. Buchanania lanzan Spreng (Chironji): a vulnerable multipurpose tree species in Vindhyan region. J Pharmacogn Phytochem. 2018;7(5):833–836. [Google Scholar]
- Sagu ST, Nso EJ, Karmakar S, De S. Optimisation of low temperature extraction of banana juice using commercial pectinase. Food Chem. 2014;151:182–190. doi: 10.1016/j.foodchem.2013.11.031. [DOI] [PubMed] [Google Scholar]
- Sahu BL. Effect of processing treatments on the quality characteristics of fruit pulp and seed kernel of Chironji (Buchanania lanzan) Jabalpur: JNKVV; 1998. [Google Scholar]
- Sandri IG, Fontana RC, Barfknecht DM, da Silveira MM. Clarification of fruit juices by fungal pectinases. LWT Food Sci Technol. 2011;44(10):2217–2222. doi: 10.1016/j.lwt.2011.02.008. [DOI] [Google Scholar]
- Sharma HP, Patel H, Sharma S. Enzymatic extraction and clarification of juice from various fruits—a review. Trends Post Harvest Technol. 2014;2(1):01–14. [Google Scholar]
- Siddiqui MZ, Chowdhury AR, Prasad N. Evaluation of phytochemicals, physico-chemical properties and antioxidant activity in gum exudates of Buchanania lanzan. Proc Natl Acad Sci India Sect B Biol Sci. 2016;86(4):817–822. doi: 10.1007/s40011-015-0539-4. [DOI] [Google Scholar]
- Singh SS, Abdullah S, Pradhan RC, Mishra S. Physical, chemical, textural, and thermal properties of cashew apple fruit. J Food Process Eng. 2019;42(5):e13094. doi: 10.1111/jfpe.13094. [DOI] [Google Scholar]
- Sivamani S, Selvakumar S, Rajendran K, Muthusamy S. Artificial neural network–genetic algorithm-based optimization of biodiesel production from Simarouba glauca. Biofuels. 2019;10(3):393–401. doi: 10.1080/17597269.2018.1432267. [DOI] [Google Scholar]
- Srivastava A, Bishnoi S, Sarkar P (2017) Value addition in minor fruits of eastern India: an opportunity to generate rural employment (Fruits for livelihood: production technology and management practices published by Agrobios (India), Agrobios), pp 395–417
- Srivastava S, Tyagi SK. Effect of enzymatic hydrolysis on the juice yield from apple fruit (Malus domestica) pulp. Int J Biotechnol Bioeng Res. 2013;4(4):299–306. [Google Scholar]
- Tao Y, Wu D, Zhang Q-A, Sun D-W. Ultrasound-assisted extraction of phenolics from wine lees: modeling, optimization and stability of extracts during storage. Ultrason Sonochem. 2014;21(2):706–715. doi: 10.1016/j.ultsonch.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Youssefi S, Emam-Djomeh Z, Mousavi S. Comparison of artificial neural network (ANN) and response surface methodology (RSM) in the prediction of quality parameters of spray-dried pomegranate juice. Dry Technol. 2009;27(7–8):910–917. doi: 10.1080/07373930902988247. [DOI] [Google Scholar]