Abstract
A spray-dried cholesterol free salad dressing powder was developed using mixed mono- and diglycerides (MG–DG) as emulsifier. The optimum conditions for enzymatic synthesis of the MG–DG from rice bran oil (RBO) and glycerol (Gly) using Candida antarctica lipase was investigated. The synthesis was done by glycerolysis of refined RBO and Gly at molar ratios of 2:1, 2.5:1 and 3:1 (Gly to RBO) and enzyme concentrations of 2% and 5%. Highest MG and DG yield (0.54 ± 0.01 and 0.49.03 ± 0.0 mg/mL) was obtained in sample prepared using 2:1 molar ratio and 5% enzyme concentration and this sample is considered optimum. Salad dressings prepared using 0.5, 1.0, and 1.5% MG–DG concentration (of optimum MG–DG) were spray dried at inlet temperatures of 150, 160 and 170 °C to find the best conditions. Salad dressing of 0.5% MG–DG spray-dried at 170 °C had the highest powder yield (42.70%), solubility (98.04%) and stability (100%). After reconstitution, this optimum sample was compared well next to a control salad dressing prepared using commercial distilled monoglycerides. These findings demonstrate the feasibility of preparing a spray dried salad dressing powder with the synthesized MG–DG as an emulsifier.
Keywords: Monoglycerides, Diglycerides, Rice bran oil, Enzymatic glycerolysis, Cholesterol free salad dressing, Spray drying
Introduction
Spray drying technology has been used in the food industry to transform emulsions into a dry powder with various properties that facilitate handling and storage (Mlalila et al. 2014). It is preferred for industrial application because of its unique properties, including simple, fast, efficient, continuous, reproducible, and scalable. Salad dressing is a common O/W emulsion which can have a fat content in the range of 20–65% (World Health Organization 2008). RBO has been recognized as a healthy oil, thus it is a desirable ingredient for preparation of salad dressings and synthesis of monoglycerides and diglycerides (MG and DG). It has a favourable ratio of saturated, monounsaturated and polyunsaturated fatty acids, as described in the recommendations of the World Health Organization (2008). The MG and DG are the most commonly used synthetic emulsifiers in the food and pharmaceutical industries (Paraskevopoulou et al. 2007) mainly produced through chemical or enzymatic glycerolysis of glycerol and fatty acid (FA) in the presence of catalyst or enzyme. The use of Candida antarctica lipase B (CALB) for enzymatic synthesis of MG and DG has been widely studied and was found to be effective catalyst for the synthesis of MG and DG. It has high catalytic activity and selectivity, thus used for various chemical processes such glycerolysis of lipids (Zieniuk et al. 2019). The glycerolysis involve migration of fatty acyl moiety from fat and oil to an acyl acceptor (glycerol) (Naik et al. 2014; Phuah et al. 2015). For the preparation of salad dressings, the MG and DG could better replace the popularly used emulsifiers such as egg and soy lecithin that has cholesterol and allergy associated with them.
This study investigates the optimum conditions (gly to RBO mole ratio and enzyme concentration) for the synthesis of mixed mono- and diglycerides (MG–DG) as well as the optimum spray drying conditions (inlet temperature and MG–DG concentration) for preparation of spray-dried reconstitutable cholesterol free (no egg in the formulation) salad dressing powder. The effect of the inlet temperature and MG–DG concentration on solid content, viscosity, powder yield, emulsion stability and solubility, and other properties are also evaluated.
Materials and methods
Materials
The refined RBO and C. antarctica lipase enzymes used in this study were kindly provided by Surin Rice Bran Oil Co., Ltd. (Thailand). Glycerol, maltodextrin, glucose syrup, and HPLC grade acetone and acetonitrile were purchased from Vejchakit Chemical Co., Ltd. (Thailand). The monoolein (1-oleoyl-rac-glycerol ≥ 99% purity) and diolein (1,3 Diolein ≥ 99% purity) used as HPLC standards were purchased from Sigma-Aldrich Co. (St. Louis, Mo., USA). Distilled monoglyceride was also kindly provided by Thanachem (Bangkok) Co., Ltd., Thailand. Other ingredients for salad dressing such as vinegar, salt and mustard were purchased from Tesco Lotus supermarket, Phitsanulok, Thailand.
Methods
Synthesis of mixed mono- and diglycerides
A method of Fregolente et al. (2008) was used for the synthesis of MG and DG, with minor modification. RBO and glycerol at three different mole ratios of glycerol to oil (2:1, 2.5:1 and 3:1) and two different enzyme concentration of 2% and 5% (by weight of RBO) were mixed separately and each mixture was placed in a thermostatic water bath set at 70 °C. The mixture was continuously stirred using a stirring probe at a speed of 200 rpm for 24 h. After 24 h, the temperature was increased to 80 °C to inactivate the enzymes, and the process was stopped. The enzymes were carefully filtered out by straining, and the samples were further analysed.
HPLC analysis of mono- and diglycerides
HPLC analysis of the samples for MG and DG concentration was carried out following the method described by Ergan and André (1989) with minor modification. A reversed-phase high performance liquid chromatography (RP-HPLC, Waters, America) system with UV–visible detector (ELSD, Alltech, USA) was used. The separation of MG and DG in the samples was achieved using a Luna (R) 5 µm C18 (2) 100 Å size, LC column 250 × 4.6 mm i.d., bought from Fortune Scientific Co., Ltd. (Thailand) at wavelength of 206 and column temperature of 45 °C through gradient elution. The mobile phase used consists of A (10% acetone) and B (90% acetonitrile) at a flow rate of 1.0 mL/min. The standards for MG and DG were dissolved in acetone and various concentrations were made to form a calibration chart. A 40 µL of standards and sample (with concentration of 5 mg/mL in acetone) were automatically injected after filtration through 0.45 µm nylon syringe filter. The process lasted for 45 min for each sample, and samples were analysed in duplicate.
Preparation of salad dressing
The formulation for the salad dressing is as follows: RBO (23.0%); Maltodextrin (21.8–22.8%); Glucose syrup (7.6%); Sugar (7.9%); Vinegar (1.1%); Mustard (1.1%); Mixed MG–DG (0.5–1.5%); and Water (28.8%). The salad dressing was prepared by first dissolving the glucose syrup, maltodextrin, sugar, salt and vinegar in hot water (70 °C), and then homogenized in a high-speed blender for 1 min. The mixed MG–DG was added at a concentration of 0.5%, 1.0% or 1.5% of the total salad dressing (by weight) and further homogenized for 1 min. The RBO was then slowly added into the mixture while homogenizing for another 1 min to finally obtain the salad dressing emulsions. A commercial distilled MD-DG was used to prepared a salad dressing used as control sample.
Determination of salad dressing properties before spray drying
Emulsion stability
The stability of the salad dressing emulsion was determined using the method reported by Vikbjerg et al. (2006). For each salad dressing sample, a test tube was filled with 10 mL of the sample, closed with a cap and kept at room temperature for 24 h. After this period the height of the total emulsion (HE) and the height of the cream (HC) were observed and recorded. All samples were tested in duplicate. The stability index was calculated using the formula (1):
| 1 |
Viscosity and total solids
The viscosity of the salad dressing emulsion was measured using a viscometer (Brookfield digital viscometer model DV-1). The samples were measured using spindle number 1 at 20 rpm. The viscosity was recorded once the value was stable (Fauziah et al. 2016). This test, too, was done in duplicate. The total solid (°B) was measured using a hand refractometer immediately after sample preparation.
Spray drying of salad dressing
Spray drying of the salad dressing samples was performed using a pilot scale spray dryer L-8 Ohkawara Kakohki Co. Ltd, Japan, at different inlet temperatures of 150 °C, 160 °C and 170 °C and feed flow rate of 15 mL/min. The optimum conditions for the spray drying were determined in terms of the properties of the powder samples.
Determination of salad dressing powder yield
The yield of the salad dressing powder was determined after spray drying using the formula (2):
| 2 |
where W1 is the weight (g) of final salad dressing powder after spray drying and W2 the weight (g) of salad dressing emulsion before spray drying.
Determination of salad dressing powder composition and properties
Proximate composition
The proximate composition such as moisture, ash, protein, fat, CHO and fiber were analysed using the standard AOAC method (2005).
Acid value and peroxide value content
The acid value and peroxide value were determined according to the IUPAC standard official methods (1998).
Emulsion stability and solubility
The emulsion stability of the reconstituted powder was determined using the methods previously described for the salad dressing emulsion (Vikbjerg et al. 2006). The powder was first reconstituted by dissolving 15 g of powder in 5 mL of distilled water, and the emulsion was then tested for its emulsion stability.
The solubility index of the salad dressing powder was determined according to Syll et al. (2016). A 2 g sample of salad dressing powder was added to 20 mL of distilled water in a 50-mL test tube, mixed and vortexed for 1 min using a vortex mixer at high speed. The mixture was then centrifuged at 1500 rpm for 10 min at 20 °C. The supernatant was carefully removed, discarded and replaced with an equal amount of distilled water. The sample was then mixed manually and centrifuged again in the same way already described. After centrifuging, the supernatant was carefully discarded, the tube was dried, and the residue was weighed. The solubility index (SI) was expressed as the percentage insoluble powder (residue) compared to the total amount of powder used.
Color
The color of the salad dressing powders was analysed for L* lightness, ranging from 0 (black) to 100 (white), a* greenness (−)/redness (+) and b* blueness (−)/yellowness (+) values using a Hunter Lab, Color Flex, USA (Shishir et al. 2014).
Particle size measurement
The mean particle size of salad dressing powder was measured using Zitasizer Nano Zs (Malvern Panalytical, UK). The particle size was analysed in terms of mean diameter (Shishir et al. 2014). Values were recorded at a refractive index (RI) of 1.330 and viscosity (cP) of 0.887 for the dispersant (water) and 1.59 for the salad dressing.
Scanning electron microscopy (SEM)
The morphology of the salad dressing powder particles was observed using scanning electron microscope according to Hammes et al. (2015). The powder sample was deposited onto an aluminium sample holder covered with two-sided carbon tape and subsequently covered with a gold layer and analysed in a Leo1455VP scanning electron microscope (EDAX) operating a 1–30 kv.
Data analysis
The duplicate results obtained were analysed for statistical significance using IBM SPSS Statistics 25 software. The analysis of variance (ANOVA) was performed to determine the significant difference of the mean values at p <0.05 by using Tukey’s test.
Results and discussion
Mono- and diglyceride yield
The enzymatic glycerolysis for the synthesis of MG and DG involved the transfer of a fatty acyl group (moiety) from the triglycerides (RBO) to the glycerol (acyl acceptor) in the presence of lipase enzymes (Phuah et al. 2015). Initially, the mixture separated into an upper layer containing the oil and a lower layer containing the glycerol. However, after the esterification reaction, the mixture turned homogeneous due to the production of MG and DG, which were effective emulsifiers. In this study, RBO with initial MG and DG concentrations of 0.045 ± 0.0 mg/mL and 0.077 ± 0.0 mg/mL, respectively, (Table 2) was used as a starting material, and the final MG and DG concentrations were measured after the reaction. The highest MG (0.544 ± 0.0 mg/mL) and DG (0.487 ± 0.18 mg/mL) concentrations (Table 1) were obtained when a molar ratio of 2:1 glycerol to oil and 5% enzymes was used. That works out to a sevenfold increase in MG and an 11-fold increase in DG, compared to the starting material.
Table 2.
Influence of inlet temperatures and MG–DG concentrations on the spray-dried salad dressing powder
| Mixed MG–DG concentration (%) | Inlet temperature (°C) | Outlet temperature (°C) ns | Moisture content (%) | Powder stability (%) ns | Powder solubility (%) |
|---|---|---|---|---|---|
| 0.5 | 150 | 90.5 ± 6.6 | 2.40 ± 0.13b | 100 ± 0.0 | 94.62 ± 0.38ab |
| 1.0 | 96.0 ± 1.0 | 2.11 ± 0.43b | 100 ± 0.0 | 94.89 ± 1.06ab | |
| 1.5 | 94.5 ± 0.5 | 2.06 ± 0.05b | 100 ± 0.0 | 92.27 ± 0.24b | |
| 0.5 | 160 | 92.0 ± 5.0 | 2.25 ± 0.39b | 100 ± 0.0 | 97.05 ± 0.38a |
| 1.0 | 92.0 ± 1.0 | 1.49 ± 0.03b | 100 ± 0.0 | 97.23 ± 1.05a | |
| 1.5 | 95.5 ± 1.5 | 1.37 ± 0.09b | 100 ± 0.0 | 98.07 ± 0.87a | |
| 0.5 | 170 | 101.0 ± 0.0 | 5.33 ± 0.64a | 100 ± 0.0 | 95.62 ± 0.38ab |
| 1.0 | 98.0 ± 3.0 | 1.88 ± 0.31b | 100 ± 0.0 | 97.05 ± 1.05a | |
| 1.5 | 103.5 ± 9.5 | 1.67 ± 0.06a | 100 ± 0.0 | 98.50 ± 1.05a |
Different alphabets within the same column indicates significant different (p <0.05) between the samples
Table 1.
Monoglyceride and diglyceride yields after glycerolysis
| Glycerol to RBO mole ratio | Enzyme concentration (%) | Concentration (mg/mL) | |
|---|---|---|---|
| Monoglycerides | Diglycerides | ||
| 2:1 | 2 | 0.311 ± 0.0b | 0.043 ± 0.001e |
| 2.5:1 | 0.147 ± 0.0d | 0.027 ± 0.001f | |
| 3:1 | 0.214 ± 0.0c | 0.200 ± 0.001b | |
| 2:1 | 5 | 0.544 ± 0.0a | 0.487 ± 0.001a |
| 2.5:1 | 0.319 ± 0.0b | 0.085 ± 0.001c | |
| 3:1 | 0.325 ± 0.0b | 0.204 ± 0.002b | |
| RBO | 0.044 ± 0.0e | 0.077 ± 0.0d | |
Different superscript letters within the same column indicate significant difference (p <0.05) between the samples
Using 5% enzymes significantly increased (p < 0.05) the MG and DG yield compared to using 2% enzymes. In all the cases, the use of 2:1 Gly to RBO yielded significantly higher (p < 0.05) MG and DG than any other ratios studied in this research. The amount (ratio) of glycerol in the higher molar ratios of 2.5:1 and 3:1 appeared to be too high as the amount of unreacted glycerol was noticed even after reaction time. This implies that, the lower mole ratio of 2:1 and 5% enzyme could be optimum for the synthesis of high MG and DG. Although, Hui (1996) observed that when high quantity of glycerol reacted with fatty acid, the end product showed higher content of MG. However, the quantity of glycerol relative to RBO in our study could relatively be high to consume all the fatty acid (RBO), and it was possible to observed more unreacted glycerol after the reaction even though high MG or DG were produced. In contrast to non-enzymatic glycerolysis of glycerol and palm stearin with 2% sodium hydroxide (by weight of glycerol (Chetpattananondh and Tongurai 2008), very low MG content was recorded when using 2:1 molar ratio compared to 2.5:1 and 3:1 molar ratios of glycerol to fatty acids.
Also, with the lower enzyme concentration of 2% in this study the reaction did not well proceed to produce the target ends product when compared to the higher enzyme concentration of 5%. In all the cases, the reaction was favoured toward the production of more MG than DG, which is more prefers as an effective emulsifier. This increasing MG and DG production at the lower molar ratio of 2:1 was similarly observed by Lee et al. (2007) who obtained a concentration of 0.14 mg/mL MG, 0.19 mg/mL DG and 0.93 mg/mL Triglyceride (TG) after 48 h of glycerolysis using RBO and glycerol with 12 g of Lipozyme RM IM in a solvent (hexane). The MG and DG yield obtained by the Lee et al. (2007) was lower than what was recorded in our study. However, when their reaction time was extended to 72 h, the resulted mixture contained 0.37 mg/mL DAG with concomitant reduction in TAG to 0.68 mg/mL. Dhara et al. (2012) also studied the glycerolysis reaction of RBO and glycerol using 1:2 mol ratio and enzymes concentration of 10% (w/w) of total substrate at 60 °C with stirring at 200 rpm for 5 h to produce diacyglycerol-rich (DAG) RBO. The resulting DAG-rich RBO contained 46.10% DAG higher than the starting RBO containing 1–5% DG.
In view of the current findings, it can be concluded that enzyme concentration has a significant effect on the glycerolysis process of the RBO and glycerol and that increasing the enzymes concentration could significantly increase the yield of MG and DG in the reaction mixture. The higher enzyme concentration of 5% gave the highest content of both MG and DG in the mixture. The production of MG and DG could be affected by various factors such as enzymes concentration, molar ratio of glycerol to oil, reaction time and vacuum condition (Phuah et al. 2015).
Characteristics of salad dressing before spray drying
To test the effectiveness of the mixed MG–DG synthesized in this research, various concentrations of the mixed MG–DG (0.5%, 1.0% and 1.5%) were used as emulsifier in preparation of salad dressings. These ranges of concentrations were selected after a series of trials using higher concentration of 2, 5, 10 and 15% which appeared to yield an emulsion with poor stability and inability to spray dried. The mixed MG–DG sample synthesized using 2:1 glycerol to oil mole ratio and 5% enzymes concentration having the highest MG and DG concentration was used for the preparation of the salad dressings. Emulsion stability, viscosity and total solids of the salad dressings emulsions were analysed.
The total solids of all the salad dressings were not significantly different (p > 0.05) and the values ranged between 50.5 and 51.0 °B. The emulsion stability of the salad dressings containing 0.5% and 1.0% MG–DG concentration were similar (67 ± 1.0a and 68 ± 0.0a, respectively) and significantly higher (p <0.05) than that salad dressing containing 1.5% which was 63 ± 1.0b. The viscosity of the salad dressings significantly increased (p <0.05) with increasing concentration of the MG–DG. The viscosities of the salad dressings were 254.0 ± 4.0c mPa s, 319.0 ± 4.0b mPa s, and 363.8 ± 1.3a mPa s for salad dressing containing 0.5%, 1.0% and 1.5% mixed MG–DG concentration respectively. Highest viscosity was recorded with the sample containing the highest mixed MG–DG concentration of 1.5%. It was observed that increasing the MG–DG concentration could lead to an increase in viscosity of the salad dressing probably due to better emulsification and formation of small droplets during the homogenization. The emulsion stability of salad dressing samples containing 0.5% and 1.0% MG–DG were not significantly different (p > 0.05) but were both significantly higher (p > 0.05) than that of the sample containing 1.5%. Although, higher stability and viscosity are attributed to the nature of the droplet size in an emulsion, the sample containing 1.5% could have slightly higher mean particle droplet sizes. Larger particle sizes could show a faster destabilizing process of coalescence and agglomeration which may ultimately decrease the salad dressing stability. This phenomena was confirmed by researchers such as Mohammed et al. (2016) and Hadnadev et al. (2013) who stated that lower emulsion droplet size lead to an increase emulsion viscosity as well as stability and vice versa. Another possible reason for the slightly higher stability observed in sample with 0.5% and 1.0% MG–DG could be due to the synergistic effect of the emulsifier and maltodextrin. It can be seen noted that there was a slight decrease in maltodextrin from 22.8% in sample containing 0.5% MG–DG to 21.8% in sample containing 1.5%. The viscosity of salad dressing before spray drying is of critical important for obtaining a good powder. A feed with high viscosity tends to be difficult to spray dried and might result in a powder with larger droplets, increased surface free fat level and larger particle size (Vignolles et al. 2007).
Yield of the spray-dried salad dressing powders
Figure 1 shows the yield of the salad dressing containing the different MG–DG concentration spray dried at the various inlet temperatures. The results showed that both the mixed MG–DG concentration and inlet temperatures have significant influence (p <0.05) on the yield of the powdered salad dressings. It was observed that the powder yield increases with an increase in the MG–DG concentration from 0.5 to 1.5% and inlet temperature from 150 to 170 °C. Maximum powder yield (42.70%) was obtained from salad dressing containing 1.5% MG–DG drying at 170 °C.
Fig. 1.
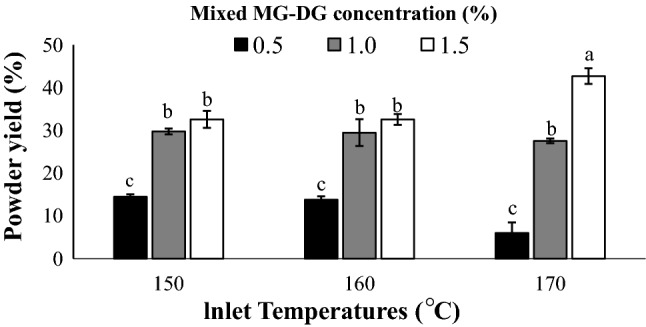
Yield of the salad dressing powder
The lower MG–DG concentration of 0.5% and 1.0% were probably not enough to The Lower MG–DG concentration of 0.5% and 1.0% were probably not sufficient to emulsify the salad dressing compared to the 1.5% and this could result in poor drying and lower yield. Additionally, at lower temperatures, the drying was not so quick to evaporate the water and thus the feed stuck on the surface of the drying chamber. These could be the reason for the lower powder yield, high moisture content and poor solubility of some samples.
Properties of spray dried salad dressing powder
The salad dressing emulsions containing the various mixed MG–DG concentrations (0.5, 1.0 and 1.5%) were spray dried at different inlet temperatures (150, 160 and 170 °C) and various properties of the powders were analysed, and the results are presented in Table 2. The properties of the salad dressing powders such as yield, moisture content, solubility, stability after reconstitution and color, were used to assess the optimum spray drying temperature to obtain the best sample. The optimum sample chosen was compared to the salad dressing powder prepared with a commercial DMG, spray dried at similar temperature.
Inlet/outlet temperatures and moisture content
It can be observed in Table 2 that increasing the spray drying inlet air temperature did not significantly increase (p > 0.05) the outlet temperature which ranged between 90.5 and 103.5 °C. Most of the moisture contents of the spray dried salad dressing powders were not significantly different (p > 0.05) and the values ranged between 1.49 and 2.40%. Except for sample containing 0.5% MG–DG spray dried at 170 °C which had highest moisture content of 5.33%. The high moisture content in this sample was evidence of it is poor drying. Although there were no significant different (p > 0.05) between the moisture contents of most of the samples, all salad dressings containing 0.5% MG–DG concentration and spray dried at 170 °C showed relatively lower moisture content. The lower moisture content recorded at higher inlet temperatures was due to the greater heat transfer rate and rapid water vaporization resulting in the wider temperature gradients which ultimately resulted to a salad dressing powder with lower moisture content (Shishir et al. 2014).
Stability and solubility of salad dressing powder
The salad dressing powders were rehydrated by dissolving 15 g powder using 5 mL water and the stability was tested. All salad dressing powders containing the 0.5, 1.0 and 1.5%, spray dried at 150, 160 and 170 °C had shown a complete (100%) stability (Table 2) after reconstitution. This was confirmed by the absence of emulsion separation into two phases (water and oil phases) after storage for 24 h. No significant difference (p > 0.05) was observed in the mean values of the stability testing results obtained in this study.
Solubility is the ability of powder to dissolve in water, which indicates complete rehydration of powders. Table 2 shows the results for the solubility of the salad dressing powders. It was found that all powdered salad dressings exhibited relatively good solubility in the range of 92.27–98.50%. Powder solubility of the samples increased by increasing the inlet temperature from 150 to 170 °C and MG–DG concentration from 0.5 to 1.5%. This indicates that a better spray drying took place at higher temperature and MG–DG concentration. It can also be noted that samples with the best solubility had lower moisture content. This may be due to a lower agglomeration observed when the samples were spray dried at higher temperatures of 160 and 170 °C. In contrast, the lower solubility of the powder could be due to the higher powder agglomeration. The solubility of the samples containing 0.5% MD–DG spray-dried at both 160 and 170 °C were no significantly difference (p > 0.05).
Color of the spray-dried salad dressing powder
The L*, a* and b* color values of the various salad dressing powders obtained by spray drying at the various temperatures from 150 to 170 °C were analysed. All salad dressings powders produced were visually lighter with L* value in range of 72.66–78.10 and slightly yellow in color with b* value in the range of 22.25–25.24. The yellow color observed in the samples was contributed by mustard which was used as the part of the ingredients and the yellowness could be due the presence of carotenoids in the mustard. The yellow color was mostly lost during the spray drying process leading to an increased lightness of the samples. This result is in line with the findings of Shishir et al. (2014) who also observed a similar decrease in the yellowness at higher inlet temperature which they also attributed to the loss of the yellow pigment during spray drying. No visual redness could be seen in all the samples and the a* values were in range of − 6.02 to − 4.87.
Selection of the optimum concentration of mixed MG–DG and temperature
The sample spray dried at inlet temperature of 170 °C had shown the highest powder yield (42.7%), along with solubility (98.04%), stability (100%) and good appearance with L* (74.65), a* (25.24) and b* (− 5.28) values that no other sample surpassed. It can be concluded that spray drying of the salad dressing at this inlet temperature (170 °C) using 1.5% mixed MG–DG could be the best to obtained powder with best acceptable properties. This sample was thus selected for comparison with salad dressing powder prepared using commercial distilled monoglycerides (DMG) spray dried at 170 °C.
Properties of the selected salad dressings and control before spray drying
The physical properties of the salad dressing containing both mixed MG–DG and commercial DMG before spray drying is shown in Table 3.
Table 3.
Properties of selected salad dressing sample and control before spray drying
| Parameters | Mixed MG–DG | Commercial DMG |
|---|---|---|
| Total solids (°B) | 51.0 ± 0.0 | 50.3 ± 0.0 |
| Emulsion stability (%) | 63.0 ± 1.0 | 72.69 ± 0.14 |
| Viscosity (mPa s) | 363.75 ± 1.25 | 487.5 ± 2.5 |
| Emulsion droplet size (µm) | 0.85 ± 0.003 | 0.52 ± 0.03 |
Higher viscosity (487.5 mPa s) was observed for the sample prepared with commercial DMG compared to the one prepared using mixed MG–DG which has viscosity of (363.75 mPa s). The higher emulsion stability (72.69%) and viscosity of sample containing the commercial DMG could directly be attributed to the smaller mean droplet size (0.52 µm) of the salad dressing and similarly the lower stability and viscosity of the salad dressing containing the mixed MG–DG. This is phenomena was earlier discussed. Higher viscosity may yield a powder with larger droplets on atomization which could ultimately have an increased surface free fat level and larger particle size (Vignolles et al. 2007).
Physical properties and chemical composition of salad dressing powder with mixed MG–DG and commercial DMG
Table 4 also presents the physical properties and chemical composition of the salad dressing powder containing MG–DG and commercial DMG both at 1.5% and spray dried at an inlet temperature of 170 °C.
Table 4.
The physical properties and chemical composition of salad dressing powder with mixed and commercial DMG
| Parameters | Samples | |
|---|---|---|
| Mixed MG–DG | Commercial DMG | |
| Yield (%) | 42.70 ± 0.83 | 24.65 ± 0.97 |
| Emulsion solubility (%) | 97.05 ± 1.05 | 98.28 ± 0.24 |
| Emulsion stability (%) | 100 ± 0.00 | 100 ± 0.00 |
| Mean particle size (µm) | 0.53 ± 0.03 | 0.27 ± 0.003 |
| Color | ||
| L* | 74.65 ± 0.01 | 70.34 ± 0.01 |
| a* | − 5.28 ± 0.04 | − 6.17 ± 0.02 |
| b* | 25.24 ± 0.09 | 26.41 ± 0.01 |
| Moisture (%) | 1.67 ± 0.06 | 2.48 ± 0.06 |
| Water activity | 0.20 ± 0.0 | 0.34 ± 0.00 |
| Ash (%) | 0.98 ± 0.00 | 0.99 ± 0.00 |
| Protein (%) | 0.14 ± 0.01 | 0.32 ± 0.07 |
| Total fat (%) | 28.95 ± 0.90 | 29.27 ± 0.15 |
| CHO (%) | 67.76 ± 0.63 | 66.81 ± 0.23 |
| Fibre (%) | 0.13 ± 0.04 | 0.36 ± 0.25 |
| Acid value (mg KOH/g) | 5.33 ± 0.84 | 5.61 ± 0.00 |
| Peroxide value (mEq/kg) | 5.24 ± 1.07 | 9.88 ± 0.67 |
Powder yield, moisture content and water activity
Highest powder yield (42.70%) was obtained for the salad dressing containing the MG–DG compared to the sample containing the commercial DMG (24.65%) (Table 4). This might be possible because although they are used at similar concentration of 1.5%, the commercial DMG is more pure and may be too high resulting to a more viscous salad dressing that was poorly spray-dried. Another possible reason could be deducted from the physical appearance of final powder which appeared to be oily and cohesive in nature. The oily nature could be due to an increased surface fat resulting from the migration of fat from the inner part of the fat globules to the surface of the particle during spray drying due to the higher temperature. This phenomenon together with the smaller particle size (Table 4) observed in the sample containing the commercial DMG could be the reason for the cohesiveness of the powder.
The moisture content of powder produced with mixed MG–DG and commercial DMG are 1.67% and 2.48%, respectively. The water activity of the samples ranged from 0.2 to 0.34. Sample with commercial DMG has a slightly higher moisture content due to poor drying resulting in higher water activity of 0.34 (Table 4). However, the water activity range of these samples is acceptance for this type of powder products since it is below the critical value of 0.37 (Hammes et al. 2015). Having this moisture and water activity levels, the powder salad dressing samples can relatively stable at room temperature during storage.
Powder solubility, stability and mean particle size
The results of the solubility and stability of both salad dressing containing the MG–DG and the commercial DMG are shown in Table 4. Both samples have shown a complete stability after reconstitution (100%) and the powder solubility were the relatively similar with 97.05 and 98.28% respectively. It should be noted that, the stability of powdered samples was 100% when the powder was reconstituted using 15 g powder with 5 mL water. The slightly lower solubility of the powder salad dressing sample containing MG–DG could be due to its larger mean particles sizes that may have greater agglomeration and ultimately lower solubility in water. The sample containing the commercial DMG had smaller mean particles size of 0.27 µm in contrast to the larger mean particles size of 0.53 µm recorded for the sample containing the mixed MG–DG.
Color
The L*, a*, and b* values were presented in Table 4. The salad dressing powder containing the mixed MG–DG was slightly lighter (L* value = 74.65) than the powder containing the commercial DMG. This could be evidence of good spray drying as observed in the higher yield recorded by this sample. In contrast, slightly higher b* value of 26.41 (indicating the yellowish) could be due to the adequate drying during the process and the sample may retain higher yellow color. The same trend could be observed with the a* value which was of less significance for these sample since the values were all in negative ranges.
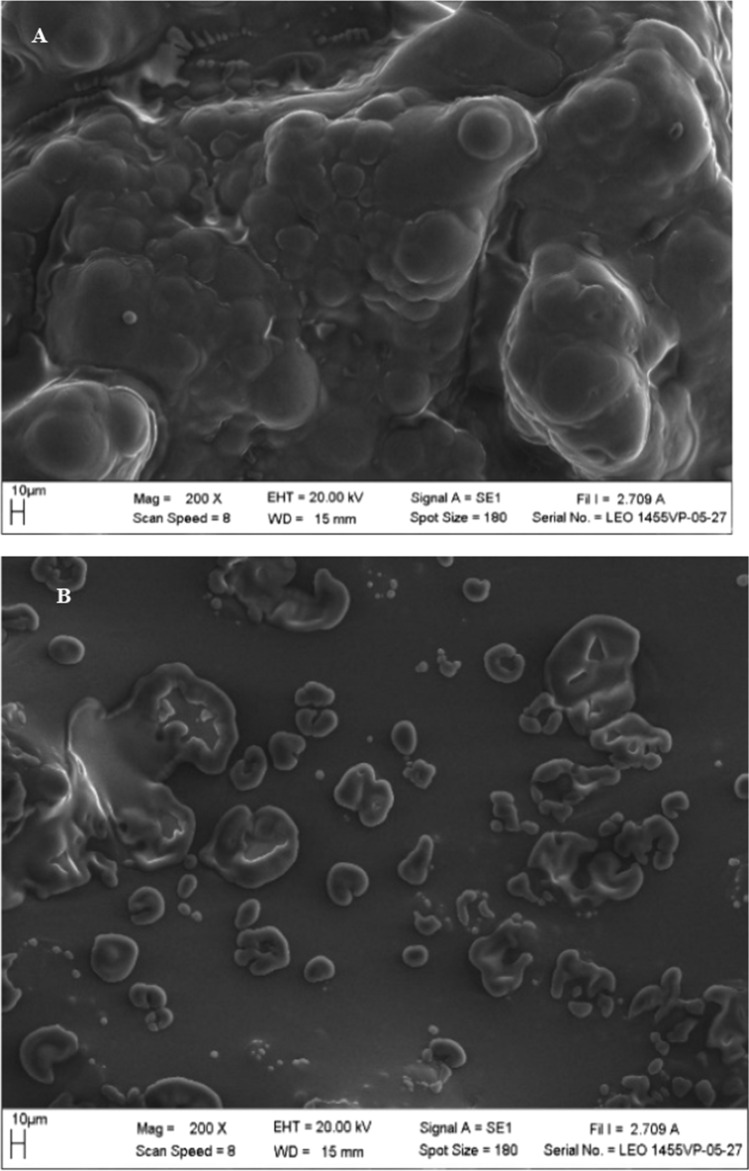
Scanning electron micrographs
The Fig. 2a, b illustrate the powder morphology (structure and shape) of the spray-dried salad dressing powder produced with mixed MG–DG and commercial DMG as emulsifiers. The powder particles of the commercial DMG particles (Fig. 2b) were more dispersed compared to the sample with mixed MG–DG sample in Fig. 2a.
Fig. 2. a.
Scanning electron micrograph of salad dressing powder containing 1.5% of a mixed MG–DG and b 1.5% commercial DMG spray dried at 170 °C
Chemical composition of powder
The differences between the micrograph of the two samples were mainly manifested in the particles structure and the extent of the particle’s agglomeration caused by cohesion/sticking of the particles. Powder containing the mixed MG–DG (Fig. 2a) appeared to smooth, spherical and relatively bigger particles while the powder containing the commercial DMG (Fig. 2b) has smooth, irregular dented particles that are relatively smaller. Vignolles et al. (2007) stated that during spray drying the smaller particles that got dried faster collide with still viscous larger particles thereby resulting in an agglomerated particle with smaller particles trapped on the surface of the bigger ones. Additionally, the viscosity resulting from the used of emulsifier, atomization and drying temperature could be responsible for the surface dent. Higher temperature gradient between the feed and drying temperature was responsible for the smoother particle surface. Although the sample containing MG–DG had slightly lower fat content, the presence of the teeth-like structure surrounding the agglomerated particle in Fig. 2a might be associated to the broken oil globules resulted due to the higher spray drying temperature. Similar phenomenon was observed for a spray dried infant formula emulsion powder containing citric acid esters of mono- and di-glycerides and lecithin (Drapala et al. 2016). The difference in the micrograph of the sample containing the mixed MG–DG and commercial DMG was probably due to the differences in the emulsifier type since both samples contained similar ingredients except the emulsifier and similar spray drying condition. The higher purity of the commercial DMG compared to our mixed MG–DG could be another possible reason for the variation in the properties of the salad dressing powders.
Chemical composition
Table 4 shows the results for the chemical composition of both salad dressing containing the MG–DG and commercial DMG. The compositional analysis of salad dressing powders showed that the ash content, total fat and acid value were relatively similar. The slight differences observed in the CHO, fibre, protein, fat, and peroxide value could be due to the differences in the nature and purity of mixed MG–DG and commercial DMG used.
The sample containing the commercial DMG had slightly higher acid value but substantially higher peroxide value. As stated earlier this sample was physically oily in appearance and that may be due the higher surface fat which could have resulted in oxidation and consequently higher peroxide value. Although the quantity of RBO used was the same, commercial DMG might have contributed to the slight increase in fat content. Hebishy et al. (2017) observed that increase in fat content of an O/W emulsion contributed to an increased in peroxide value, which supported the findings of this research.
Conclusion
This study demonstrated the feasibility of producing a spray-dried cholesterol free salad dressing powder which can be reconstituted. The use of mixed MG–DG synthesized from glycerol and RBO in a molar ratio of 2:1 with 5% enzymes (C. antarctica lipase) as emulsifier for the salad dressing was also demonstrated. A salad dressing powder having higher yield of 42.70% compared to 24.64% for salad dressing prepared with DMG was obtained. The powder had good properties such as solubility, stability, color and low moisture content. This indicated the viable potential for the commercial production of monoglyceride and diglycerides from the enzymatic glycerolysis of RBO and glycerol and their effectiveness as emulsifier for production of salad dressing powder.
Acknowledgements
The authors gratefully acknowledge the support of Surin Rice Bran Oil Co., Ltd., Thailand, for providing the Candida antarctica lipase enzyme and RBO as well as the Naresuan University (Thailand) for providing the Scholarship.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Association of Official Analytical Chemist . Cereal Foods. In: Firestone D, editor. Official Method 992–23. 5. Champaign: AOAC; 2005. [Google Scholar]
- Chetpattananondh P, Tongurai C. Synthesis of high purity monoglycerides from crude glycerol and palm stearin. Songklanakarin J Sci Technol. 2008;30:515–521. [Google Scholar]
- Dhara R, Dhar D, Ghosh M. Dietary effects of pure and diacylglycerol-rich rice bran oil on growth pattern and lipid profile of rats. J Oleo Sci. 2012;61:369–375. doi: 10.5650/jos.61.369. [DOI] [PubMed] [Google Scholar]
- Drapala KP, Auty MAE, Mulvihill DM, Mahony JAO. Influence of emulsifier type on the spray-drying properties of model infant formula emulsions. Food Hydrocoll. 2016;69:56–66. doi: 10.1016/j.foodhyd.2016.12.024. [DOI] [Google Scholar]
- Ergan F, André G. Simple high performance liquid chromatography methods for monitoring lipase reactions. Lipids. 1989;24:76–78. doi: 10.1007/BF02535268. [DOI] [PubMed] [Google Scholar]
- Fauziah CI, Zaibunnisa AH, Osman H, Wan Aida WM. Physicochemical analysis of cholesterol-reduced egg yolk powder and its application in mayonnaise. Int Food Res J. 2016;23:575–582. [Google Scholar]
- Fregolente PBL, Fregolente LV, Pinto GMF, Batistella BC, Wolf-Maciel MR, Filho RM. Monoglycerides and diglycerides synthesis in a solvent-free system by lipase-catalyzed glycerolysis. Appl Biochem Biotechnol. 2008;146:165–172. doi: 10.1007/s12010-008-8133-3. [DOI] [PubMed] [Google Scholar]
- Hadnadev TD, Dokić P, Krstonosic V, Hadnadev M. Influence of oil phase concentration on droplet size distribution and stability of oil-in-water emulsions. Eur J Lip Sci Technol. 2013;115:313–321. doi: 10.1002/ejlt.201100321. [DOI] [Google Scholar]
- Hammes MV, Englert AH, Noreña CPZ, Cardozo NSM. Study of the influence of soy lecithin addition on the wettability of buffalo milk powder obtained by spray drying. Powder Technol. 2015;277:237–243. doi: 10.1016/j.powtec.2015.02.047. [DOI] [Google Scholar]
- Hebishy E, Zamora A, Buffa M, Blasco-Moreno A, Trujillo A. Characterization of whey protein oil-in-water emulsions with different oil concentrations stabilized by ultra-high pressure homogenization. Process. 2017;5:1–18. doi: 10.3390/pr5010006. [DOI] [Google Scholar]
- Hui HY. Bailey’s industrial oil and fat products. 5. New York: Wiley; 1996. Edible oil and fat products: processing technology; pp. 569–585. [Google Scholar]
- International Union of Pure and Applied Chemistry Commission . Oils and Fats. In: Dieffenbacher A, Pocklington WD, editors. Standard methods for the analysis of oils, fats & derivatives. 7. Oxford: Blackwell Scientific Publications; 1998. [Google Scholar]
- Lee JH, Yu F, Vu PL, Choi MS, Akoh CC, Lee KT. Compositional study on rice bran oil after lipase-catalyzed glycerolysis and solvent fractionations. J Food Sci. 2007;72:163–167. doi: 10.1111/j.1750-3841.2007.00299.x. [DOI] [PubMed] [Google Scholar]
- Mlalila N, Swai H, Kalombo L, Hilonga A. Effects of spray-drying on w/o/w multiple emulsions prepared from a stearic acid matrix. Nanotechnol Sci Appl. 2014;7:105–112. doi: 10.2147/NSA.S72083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed A, Ijeoma S, Salisu J. Effect of dispersed phase viscosity on stability of emulsions produced by a rotor stator homogenizer. Int J Sci Basic Appl Res. 2016;25:256–268. [Google Scholar]
- Naik MK, Naik SN, Mohanty S. Enzymatic glycerolysis for conversion of sunflower oil to food based emulsifiers. Catal Today. 2014;237:145–149. doi: 10.1016/j.cattod.2013.11.005. [DOI] [Google Scholar]
- Paraskevopoulou D, Boskou D, Paraskevopoulou A. Oxidative stability of olive oil-lemon juice salad dressings stabilized with polysaccharides. Food Chem. 2007;101:1197–1204. doi: 10.1016/j.foodchem.2006.03.022. [DOI] [Google Scholar]
- Phuah ET, Tang TK, Lee YY, Choong TSY, Tan CP, Lai OM. Review on the current state of diacylglycerol production using enzymatic approach. Food Bioprocess Technol. 2015;8:1169–1186. doi: 10.1007/s11947-015-1505-0. [DOI] [Google Scholar]
- Shishir MRIRI, Taip FSS, Aziz NAA, Talib RAA. Physical properties of spray-dried pink guava (Psidium guajava) powder. Agric Agric Sci Procedia. 2014;2:74–81. [Google Scholar]
- Syll O, Khalloufi S, Méjean S, Schuck P. The effects of total protein/total solid ratio and pH on the spray drying process and rehydration properties of soy powder. Powder Technol. 2016;289:60–64. doi: 10.1016/j.powtec.2015.11.049. [DOI] [Google Scholar]
- Vignolles M, Jeantet R, Lopez C, Schuck P. Free fat, surface fat and dairy powders: interactions between process and product. A review. Le Lait, INRA Edn. 2007;87:187–236. doi: 10.1051/lait:2007010. [DOI] [Google Scholar]
- Vikbjerg AF, Rusig JY, Jonsson G, Mu H, Xu X. Comparative evaluation of the emulsifying properties of phosphatidylcholine after enzymatic acyl modification. J Agric Food Chem. 2006;54:3310–3316. doi: 10.1021/jf052665w. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2008) Interim conclusions and dietary recommendations on total fat & fatty acids from the join FAO/WHO expert consultation on fat and fatty acids in human nutrition, pp 10-14
- Zieniuk B, Fabiszewska A, Białecka-Florjańczyk E. Screening of solvents for favoring hydrolytic activity of Candida antarctica Lipase B. Bioproc Biosyst Eng. 2019;43(4):1–9. doi: 10.1007/s00449-019-02252-0. [DOI] [PubMed] [Google Scholar]