Abstract
Cell-free DNAs (cfDNAs) are fragmented forms of DNA that are released into extracellular environments. Analyzing them, regarding either concentration or genetic/epigenetic status can provide helpful information about disorders, response to treatments, estimation of success rates, etc. Moreover, since they are presented in body fluids, evaluation of the aforementioned items would be achieved by less/non-invasive methods. In human reproduction field, it is required to have biomarkers for prediction of assisted reproduction techniques (ART) outcome, as well as some non-invasive procedures for genetic/epigenetic assessments. cfDNA is an appropriate candidate for providing the both approaches in ART. Recently, scientists attempted to investigate its application in distinct fields of reproductive medicine that resulted in discovering its applicability for biomarker and genetic/epigenetic analyses. However, due to some limitations, it has not reached to clinical administration yet. In this article, we have reviewed the current reported data with respect to advantages and limitations of cfDNA utilization in three fields of ART, reproduction of male and female, as well as in vitro developed embryos.
Keywords: Cell-free DNA, Biomarker, Genetic/epigenetic, Reproduction, Assisted reproductive techniques
Introduction
Cell-free DNAs (cfDNAs) were initially discovered in human serum by Mandel and Metais, in 1948 [1, 2]. They are DNA fragments (driven from both the nucleus and mitochondria) released through apoptosis, necrosis, and active releasing mechanisms [3]. Their average length is estimated to be about 40–200 base pairs (bp) [3–5]. However, larger fragments up to >3 0 kb have been detected [6]. They exist in both free circulating form and extracellular vesicles, e.g., exosomes, apoptotic bodies, and microvesicles [7]. Although cfDNA concentration in different body fluids and distinguished pathological situations can be varied, approximately 5–10 ng/ml is estimated as an average amount in healthy individuals [5]. Not only cfDNA concentration but also size distribution, genetic variation (e.g., single nucleotide polymorphism, mutation, and ploidy status), and epigenetic pattern provide a wide spectrum of data about individuals that can be subjected for screening, diagnosis, prediction of clinical outcome, response to treatment, etc. [2, 3]. All these plus cost-effectiveness, easy accessibility, and high stability of cfDNA have made it an appropriate biomarker in different fields of medicine [8–11]. For instance, studies on cfDNA profiling over the last decade resulted in the first clinical application in non-invasive prenatal testing (NIPT) for fetal sex determination and disorders through maternal blood [12–14]. Subsequently, plenty of research has been performed on extending the cfDNA utilization in various fields of medicine, such as oncology and transplantation [15, 16].
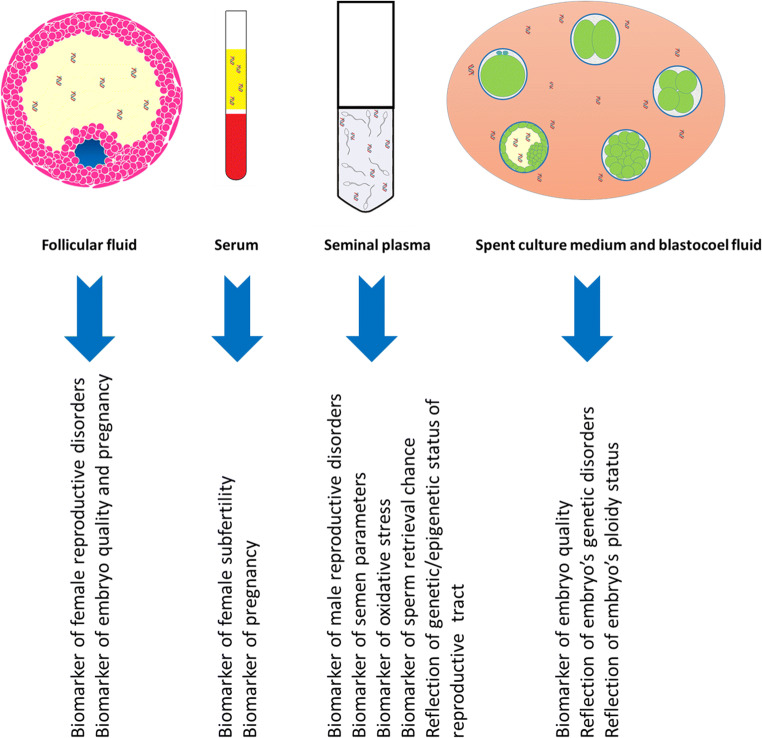
It goes without saying that in human reproductive medicine field, reliable applicable biomarkers as well as non-invasive diagnostic assays are highly required. Since DNA abnormalities, either on genetic or epigenetic status, play indispensable roles not only on fertility potential of individuals [17–19] but also on developmental competence and disorders of the in vitro developed embryos [20, 21], cfDNA might have the possibility to provide the non-invasive diagnostic assays, as well as practical biomarkers for prediction of ART procedure outcome. In this regard, during the recent years, scientists have explored the cfDNA profile within its main sources in reproductive medicine (seminal plasma (SP), follicular fluid (FF), serum, spent culture medium (SCM), and blastocoel fluid (BF)) (Fig. 1) to discover its potential for the desired applications. All this research obtained constructive information to confirm the applicability of cfDNA in some areas, as well as limitations in other fields.
Fig. 1.
cfDNA sources in assisted reproductive techniques and its applications that have been proved by some or all studies
Based on our knowledge, there has been no particular article gathering the cfDNA investigations’ reports in ART. Thus, the purpose of this review is to highlight the whole consequences of cfDNA research in all aspects of human reproductive medicine to elucidate the current achievements, gaps, and reliability for clinical utilization. With a particular emphasis on its application for biomarker and genetic assays, the reported data will be discussed under three sections: male and female reproductive systems, as well as in vitro developed embryos.
Search method
Data was extracted from Google Scholar and PubMed databases by searching the following terms: (“cell free DNA” OR “circulating DNA”) AND (“reproduction” OR “assisted reproductive technology” OR “ART” OR “infertility” OR “follicular fluid” OR “poly cystic ovary syndrome” OR “PCOS” OR “endometriosis” OR “semen” OR “seminal plasma” OR “azoospermia” OR “embryo” OR “culture media” OR “blastocoel fluid” OR “preimplantation genetic testing” OR “PGT-A” OR “PGD”). Published articles until August 2020 that were written in English language were checked, and those that were performed on human subjects were included to review.
cfDNA in male reproductive system
Initially, cfDNA in human reproduction field was discovered in semen by Chou and colleagues, in 2004 [22]. They proved cfDNA presence in SP (SP cfDNA) and categorized them into low (1 kb) and high (12 kb) molecular weight fragments. Semen parameters (motility and morphology) and capacitation index were demonstrated positively, whereas post wash hyper-activation was negatively correlated with the amounts of low and high molecular weight fragments, respectively. Thus, the low molecular weight cfDNA fragment was introduced as a marker of semen quality [22]. This report opened new doors to male infertility research field which resulted in discovering SP cfDNA correlations with different aspects of male reproduction. For instance, later size distribution analysis uncovered a wider range of SP cfDNA fragment size, from 180 bp to 15 kb [23]. In detail, fragments about 166 bp, especially with DNA ladder pattern, contribute to apoptosis mechanism, whereas fragments > 10 kb are ascribable to necrosis pathways. Moreover, fragments derived from active releasing are appraised to be between 1000 and 3000 bp, and the shortest fragment size, around 40–300 bp, belongs to cell-free mitochondrial DNA (cf-mtDNA) [24]. Therefore, through the newly discovered SP cfDNA size distribution, it could be concluded that a variety of mechanisms are involved in SP cfDNA secretion, and distinct concentrations might be capable to reflect pathological situations. As evidence, SP cfDNA comparison between azoospermia and normozoospermia men showed higher SP cfDNA concentration in azoospermia semen [23]. Higher concentration in addition to DNA ladder pattern of SP cfDNA fragments in azoospermia men implicated that apoptosis of germ cells may be considered as a mechanism of SP cfDNA secretion [23].
Oxidative stress (OS) has been recommended as another source of SP cfDNA [25]. An in vitro study proved that treating semen by paraquat (a toxic compound causing oxidative stress by generating superoxide anion) caused elevated levels of double-strand SP cfDNA, decreasing sperms’ viability, motility, and normal morphology. In this regard, SP cfDNA concentration was suggested as an OS marker in the semen [25]. In contrast to positive relationship between SP cfDNA and OS, SP cf-mtDNA has been reported to have negative correlation with semen reactive oxygen species (ROS) level [16]. The authors justified the results in this manner that the lower SP cf-mtDNA copy number might represent insufficient mitochondria and mtDNA shedding from sperms’ cytoplasm during the normal spermatogenesis. Since mtDNA is the major source of ROS production, excess mtDNA content within the sperm (lower SP cf-mtDNA as well) triggers an increase in ROS level of the semen [16]. In the study, they also compared SP cf-mtDNA copy number in different phenotypes of semen. Results demonstrated that SP cf-mtDNA copy number was dramatically decreased through normo-, oligo-, astheno-, and oligoasthenoterato-zoospermia semen. Thus, they concluded that reduced level of SP cf-mtDNA might be owing to some spermatogenesis impairment events, such as decline in mitochondrial extrusion from the sperms, and subsequent OS formation [16].
To investigate whether sperm DNA fragmentation (SDF) was another source of SP cfDNA, Bounartzi and colleagues compared its levels with different SDF rates. As a result, no association between SP cfDNA concentration and sperm SDF was identified [26]. Hence, SP cfDNA does not depend on extrusion of sperm’s DNA fragments and therefore cannot reflect the sperm DNA damage.
Later research attempted to investigate the main origin organ of SP cfDNA secretion. In this respect, SP cfDNA concentration in normozoospermia and vasectomized men were compared. Based on the report, SP cfDNA concentration in normal semen was fourfold greater than vasectomized men [27]. Since vasectomized men semen does not contain testis and epididymis ejaculations, reduced SP cfDNA level in their semen represented that it is mainly originated from the desired organs. Thus, SP cfDNA may have the capacity to deliver the large amount of productive data about DNA status of these critical sexual organs, especially testis that directly regulates the fertility potential of men. In this regard, subsequent studies focused on exploring the more applicable aspects of SP cfDNA, especially for biomarker utilization in reproductive medicine procedures. As an illustration, to investigate the predictive value of SP cfDNA with ART outcome, a study assessed its correlation with embryo quality and pregnancy rate. However, it was shown that SP cfDNA concentration was not associated with the mentioned factors [26].
In addition to concentration, more characteristics of SP cfDNA have been assessed. For instance, research on SP cfDNA of azoospermia men indicated that the exact type of chromosome Y microdeletion (azoospermia factor a (AZFa), AZFb, and AZFc) could be detected by SP cfDNA polymerase chain reaction (PCR) results of the patients [23]. This consequence implied that SP cfDNA can possibly be reflective of the genetic abnormalities of male reproductive system, thus providing a non-invasive assessment for male infertility diagnosis. To confirm this hypothesis, careful studies were performed by Wu and colleagues corroborated that not only genetic abnormalities but also epigenetic patterns of male reproductive tract could be assessed by SP cfDNA analysis [27]. Briefly, they initially discovered 367 hypo- and 134 hyper-methylated promoters (that specifically related to male reproduction genes in the testis and epididymis) in SP cfDNA [27]. In the following study, they selected 5 of the previously detected promoters (acrosin-binding protein (ACRBP), doublesex and mab-3-related transcription factor 1 (DMRT1), calcium and integrin binding 1 (CIB1), heat shock factor 1 (HSF1), and cyclin-A1 (CCNA1)) and compared the methylation patterns between SP cfDNA and testicular biopsied tissues of non-obstructive azoospermia (NOA) patients. Results revealed that methylation status in SP cfDNA could directly illustrate the testicular DNA [28]. As epigenetic aberration is likely to be a possible etiology of male infertility [29, 30], this accomplishment confirmed the SP cfDNA capability to represent the epigenetic information of the testis, helping the diagnosis, as well as appraisal of some ART procedure outcome. As evidence, Wu et al. were able to use SP cfDNA methylation patterns to predict the sperm retrieval chance in NOA patients [28]. In detail, they analyzed the methylation status of mentioned promoters to evaluate their predictive value for sperm extraction rate through testicular biopsy of NOA patients. Among 5 candidate genes, CCNA1 and DMRT1 methylation levels were significantly different between sperm retrieved and un-retrieved groups. Thus, they recommended the methylation levels of CCNA1 and DMRT1 in SP cfDNA as biomarkers for the success of sperm extraction [28].
Taken together, research on SP cfDNA resulted in discovering numerous practical data. For instance, the primary investigations showed that different mechanisms, such as apoptosis and OS can influence the SP cfDNA level [23, 25]. Furthermore, the testis and epididymis were introduced as the main organs for SP cfDNA secretion [27]. Regarding SP cfDNA application in ART, its level seems to provide the biomarkers for some factors, e.g., capacitation index [22], but not SDF, embryo quality, and pregnancy outcome [26]. Based on the current issues, as the biomarker utilization, genetic/epigenetic status of SP cfDNA is more practical than the mere concentration, since this characteristic can be applied for diagnosis and prediction of success rate for sperm retrieval by testicular biopsy [28]. Nevertheless, future investigations in larger sample sizes are recommended to extend its utilization in wider aspects of ART.
cfDNA and female reproduction
The first report of cfDNA in the female reproduction field was submitted by Hart and colleagues, with the aim of analyzing the serum cfDNA value as a pregnancy marker, in 2004 [31]. In this regard, serums of women at first week post embryo transfer were analyzed. Based on the report, neither serum cfDNA concentration nor distributions of the fragment size correlated with pregnancy achievement [31]. Thus, serum cfDNA utilization as a biomarker in the female reproduction field could not be proved by the study. In compliance with this aspect, a recently performed research could not confirm the epigenetic status of serum cfDNA as the biomarker for polycystic ovarian syndrome (PCOS) disorder [32]. An investigation on endometriosis patients failed to establish the serum cfDNA biomarker possibility with regard to the disorder as well [33]. In detail, although the levels of 8-hydroxy-2′-deoxyguanosine (8-OHdG), a product of oxidative damaged DNA, was significantly higher in follicular fluids of endometriosis patients, no significant difference was observed between serums of patients and the control group [33].
In contrast, some studies pointed out serum cfDNA biomarker applicability in the female reproduction field. For instance, elevated levels of serum cfDNA were found to be correlated with reduced pregnancy rate [34, 35]. Serum cf-mtDNA level as well has been reported to be associated with subfertility of women, and copy number less than 105 was recommended as a cutoff point of subfertility situation [36]. However, it should be noticed that only subfertile pregnant women were included in that study and compared with fertile pregnant group. Thus, exploring the cf-mtDNA level in serums of non-pregnant subfertile women and in a larger sample size can make it a more reliable biomarker.
According to the current data, only a few studies have investigated the cfDNA biomarker potential in serums of women that three of which could not detect any correlation with the female reproductive status [31–33]. A possible justification for this would be that although the serum provides an easy accessible environment for biomarker investigation, the cfDNA within the serum is driven from all body organs and thus might not be specifically representative for the most aspects of women reproduction. However, due to the limited data, further studies are required to explore the more applications of cfDNA in women’s serum.
On the other side, FF that is considered as a biological waste material in ART procedures [37] makes additional environment available for cfDNA (FF cfDNA) in women. Several meticulous research has investigated FF cfDNA marker potential and led to extended cfDNA application in the female reproduction field. FF cfDNA within the studies have been analyzed either in individual follicles or pooled FFs of each patient that in the following we underlined the main results.
Assessments on pooled FFs of patients undergoing controlled ovarian stimulation (COS) divulged that larger number of retrieved oocytes were related to FFs with lower levels of FF cfDNA [38, 39]. In contrast, evidence on poor responder patients failed to identify this correlation [40]. However, it has to be noted that in the last study [40], oocytes were picked up from the natural cycle of the patients. Results from COS and natural cycles are not comparable because ovarian stimulation has an impact on FF cfDNA level. As evidence, analyses revealed that even among women undergoing COS, stimulation duration as well as total dose of gonadotropins can influence the FF cfDNA level [39, 41]. To the contrary, FF cf-mtDNA found to be not correlated with the aforementioned factors [41]. This implicates that FF cf-mtDNA might be more stable under distinct conditions and therefore providing the more authentic biomarker in the female reproduction field.
Regarding FF cfDNA association with oocyte characterization, studies unveiled that FF cfDNA level either within pooled FFs or individual follicles was not associated with oocyte’s maturity stage (metaphase II (MII) or metaphase I (MI)) [40, 42]. The main conclusion to be drawn from this result is that FF cfDNA level is not altered significantly through follicle developmental stages. In other words, it might be preferentially affected by pathological rather than physiological conditions that if this theory is accurate, the applicability of FF cfDNA biomarker in female reproductive medicine can be further reinforced.
Attempts to find FF cfDNA correlation with ART outcome failed to discover any interrelation with fertilization success rate [40, 42], but significant correlation with embryo quality. In details, of the first reports, although Dimopoulou and colleagues noted no relationship between FF cfDNA concentration of pooled FFs and embryo quality [38], Scalici et al. pointed out a significant association with day 3 embryo quality [42]. Briefly, results demonstrated that oocytes related to follicles with lower level of cfDNA developed to high-quality embryos and vice versa. They finally recommended FF cfDNA level as a biomarker of day 3 embryo quality [42]. Subsequent FF cfDNA research either within pooled FFs or individual follicles confirmed its negative association with day 3 embryo quality as well [39, 40, 43–45]. In contrast to these agreements, Liu and colleagues have recently reported that neither FF cf-nDNA nor FF cf-mtDNA levels correlated with day 3 embryo quality, but blastocyst developmental competence [41]. In fact, results indicated that FF cf-mtDNA was lower, whereas FF cf-nDNA was higher in follicles of the oocytes that developed to blastocysts than those that were not able to reach to the viable blastocysts. Since only FF cf-mtDNA difference was significant, it was suggested as a biomarker of blastocyst developmental potential [41]. The positive correlation between FF cf-nDNA and blastocyst developmental competence was explained in this manner that, since granulosa cell apoptosis is a normal physiological event at two follicle developmental stages (selection of dominant follicle and maturation at pre-ovulatory stages [46, 47]), elevated level of FF cf-nDNA might represent the higher quality of the oocyte, thus its appropriate developmental competence [41]. Although apoptosis has been introduced as a source of FF cfDNA by several studies [40, 44, 45, 48], the hypothesis was different from Liu et al.’s. For instance, an in vitro treatment of human granulosa cells by high concentrations of extracted FF cfDNA resulted in granulosa cells apoptosis [45]. Signaling pathway analysis indicated that FF cfDNA elevated the expression levels of FasL and caspase, as well as intracellular ROS. Thus, this theory was raised that the cause of decreasing embryo quality in the presence of high levels of FF cfDNA is apoptosis of intra-follicular cells, which is triggered by elevating the ROS levels [45]. Indeed, both theories seem rational. Liu et al. guessed the main source of FF cf-nDNA release [41], while the last study investigated the FF cfDNA mechanism of action [45]. FF cfDNA presence and function might be due to combination of several mechanisms that all together influence the oocyte developmental ability. Thus, since most of the studies admitted the significant adverse effect of high levels of FF cfDNA, and only one study considered a non-significant beneficial impact on oocyte developmental potential, a tenable conclusion to draw is that FF cfDNA level has a normal range which has not been discovered yet. Its presence up to a specific level reflects oocyte’s successful selection and maturation, while abundant levels impair its developmental potential. Therefore, additional investigations are needed to define a normal cutoff point that will bridge the current gaps.
The same previous scenario can be true for FF cf-mtDNA. In details, mitochondrial dysfunction has been identified as another source of FF cfDNA [49]. An in vitro treatment of porcine cumulus oocyte complex (COC) by carbonyl cyanide m-chlorophenyl hydrazone (CCCP) (a mitochondrial dysfunction inducer) increased the cfDNA concentration through elevation of cf-mtDNA copy number [49]. Based on the result, it can be concluded that high levels of FF cf-mtDNA might represent mitochondrial dysfunction that can directly affect oocyte’s developmental competence. Nonetheless, low levels of FF cf-mtDNA have been found to have adverse effects as well [41]. Comparison of FF cf-mtDNA level between follicles of with and without oocyte revealed that FF cf-mtDNA content was lower in follicles that did not contain oocytes [41]. This might be due to the insufficient intracellular mitochondria to provide oocyte maintenance. Thus, FF cf-mtDNA level seems to have a normal range as FF cf-nDNA, any amount higher or lower than that implies follicle impairment. However, the cutoff point of this normal range has not been identified and supplemental investigations are essential.
By continuous attempts on finding FF cfDNA biomarkers in the ART field, significant association with pregnancy outcome has been discovered. With the exception of one study [38], all accepted a negative correlation between FF cfDNA level and pregnancy, thus recommended as a highly sensitive and specific biomarker for prediction of pregnancy achievement [39, 43–45]. The same was proved for the levels of 8-OHDG within FFs of endometriosis patients as well [33].
Regarding female infertility disorders, only few studies have investigated FF cfDNA application for diagnostic biomarkers. Briefly, Traver and colleagues noted that FF cfDNA level was significantly higher in patients with ovarian reserve diseases (PCOS and poor ovarian reserve) than normal women [39]. However, another research reported no difference between FF cfDNA concentration of PCOS and the control group [43]. This controversy might be due to the distinctly applied methods, in addition to small sample sizes. For instance, Traver et al. assessed the FF cfDNA level by ALU qPCR [39], while the last study reported the concentrations of extracted FF cfDNAs only by spectrophotometer [43]. Hence, before clinical utilization of cfDNA as biomarker, a gold standard method should be determined to avoid these variations in results.
Taken together, research on FF cfDNA has been performed based on two strategies: biomarker for ART outcome and infertility disorder diagnosis. In respect to ART outcome, most studies have confirmed its association with oocytes’ developmental ability and pregnancy rate. The importance of biomarker utilization for these items is selection of the high-quality embryo via a reliable molecular method, as well as estimation of the ART procedure outcome. Moreover, in cases that only a few number of sperms (less than oocytes number) are retrieved by testicular biopsy, the most potent oocytes would be selected for intracytoplasmic sperm injection (ICSI), thus increasing the success rate. In this regard, FF cfDNA seems providing the biomarker for these aspects. However, there are some controversies for the exact type of correlations (positive/negative) between FF cfDNA level and embryo quality that, as above mentioned, it might be due to lack of the cutoff point definition. On the other side, since the impact of COS type on FF cfDNA level has been proved by some studies [39, 41], it has to be considered that distinct stimulation protocols might require their own specific cutoff point. Thus, future studies with the aim of cutoff point definition for different situations are required.
Unfortunately, there have been only few research on FF cfDNA biomarker potential for reproductive disorders that has not reached to a valid outcome. Furthermore, genetic and epigenetic status of FF cfDNA have not been assessed yet. Hence, research ahead in these respects can improve our knowledge about FF cfDNA, as well as extending its application in female reproductive medicine.
cfDNA and in vitro developed embryo
Studies on embryonic cfDNA are based on discovering two distinct applications: biomarker for embryo quality and embryonic genetic assays (preimplantation genetic testing for aneuploidy (PGT-A) and preimplantation genetic diagnosis (PGD)). Collection of SCM, BF, and both SCM-BF are three strategies for embryonic cfDNA assessments. SCM collection is completely a non-invasive method, while the two others require some semi-invasive procedures. Briefly, for BF collection, an ICSI pipette would be inserted between two trophectoderm (TE) cells of the expanded blastocyst and the fluid is aspirated for subsequent analysis [50, 51]. To collect SCM-BF, a laser pulse would be applied at a location far away from the inner cell mass (ICM) of the blastocyst for extruding the BF, followed by collecting the SCM that contained BF [52, 53]. Numerous research has evaluated the accuracy and reliability of these methods for utilization of embryonic cfDNA in ART that hereunder we summarized the main findings.
With regard to biomarker ability for embryo quality, Stigliani and colleagues for the first time discovered both cf-nDNA and cf-mtDNA in SCMs of day 3 embryos [15]. Since almost all SCMs contained detectable levels of cf-mtDNA, but not cf-nDNA, they focused on analysis of cf-mtDNA biomarker potential. Based on the results, cf-mtDNA level was correlated with embryo fragmentation rate [15], and the higher cf-mtDNA/cf-nDNA ratio was associated with blastocyst’s developmental competence, TE (but not ICM) quality, and implantation rate [54]; that different stimulation protocols and embryo culture systems (e.g., incubation systems and culture media) had no influence on its predictive value [55]. Lower cf-mtDNA level in SCM of low-quality embryos might reflect insufficient mtDNA reserve within regarded oocytes that resulted in impairment of its developmental ability. Studies on oocyte and cumulus cells have revealed that higher mtDNA content did associate with embryo quality [56, 57], and Stigliani and colleagues as well proved the same correlation for SCM cf-mtDNA level of day 3 embryos [15, 54, 55]. Since cf-mtDNA within SCM provides a non-invasive easy accessible biomarker, its combination with morphological assessment puts forward a practical molecular method to predict the day 3 embryo developmental competence, rather than merely morphological analysis.
Recently, another study has discovered a relationship between cfDNA level within the BF and blastocyst quality [58]. Interestingly, it showed that elevated level of BF cfDNA was correlated with higher quality of the embryo. Two theories were discussed about this relationship. First was that the higher level of BF cfDNA could be due to breaking and repairing of blastomeres’ DNAs through developing to a viable embryo. And the next justification was omitting the aneuploid cells by apoptosis induction during the embryogenesis to modify the genetic status of regarded embryo [58].
Based on the current issues, cfDNA seems to provide a reliable marker for embryo quality assessment. Although BF cfDNA analysis requires a minimally invasive procedure, cf-mtDNA within SCM is a non-invasive biomarker that has been evaluated with different aspects. Thus, according to the reports, SCM cf-mtDNA has the potential to be utilized assuredly in parallel with conventional morphological criteria to improve the embryo selection method.
Despite cf-mtDNA’s high potential at prediction of embryo’s developmental competence, cf-nDNA can perfectly reflect embryo’s genetic status, thus providing PGT-A/PGD analysis tools. However, it has to be noticed that cfDNA application for PTG-A/PGD is in its initial way and there are some challenges that would be discussed in the following.
SCM cfDNA applicable potential for non-invasive PGD analysis was mentioned initially by detecting X-link disorders, in 2014 [59]. In the following years, alpha thalassemia [60], thalassemia mutation [61], MTHFR C677T polymorphism [62], and cystic fibrosis (CFTR) [52] were diagnosed by SCM cfDNA assessments as well. Thus, SCM cfDNA seems capable to be applied for PGD assay, since no controversial result has been reported. However, it has to be considered that its application for clinical diagnosis requires more research in a wider spectrum of genes, as well as larger populations.
In parallel with discovering cfDNA utilization for PGD, most of the research has focused on PGT-A. However, because of its complexity it has not reached to a unique standard for clinical assays. For instance, for SCM cfDNA, DNA contamination is one of those important challenges that can arise from external (e.g., plastic wares and medium) [52, 63] or maternal sources [64, 65]. Polar bodies and cumulus cells are the sources of maternal DNA within the embryo culture media that interfere with embryonic cfDNA detection. To solve the problem, scientists attempted to find solutions to minimize/eliminate the maternal cfDNA in culture media. One recommended resolution was transferring the day 4, 5, or 6 embryo to a new individual culture media followed by collecting the SCM after 24 to 48 h, to decrease and increase the maternal DNA contamination and accuracy, respectively [53, 60, 62, 63, 66–68]. Although the exact unique period has not been defined, delay in SCM collection seems to provide a more reliable result. This might be due to elevating embryonic cfDNA load into SCM as a result of an increase in embryo’s cell number, as well as maternal cell degradation through initial stages. Performing ICSI instead of in vitro fertilization (IVF), and eliminating all cumulus cells before the sperm injection, was another solution [66]. However, results from a multicenter study on 1301 blastocysts demonstrated the similar specificity and sensitivity for PGT-A analysis from both ICSI and IVF techniques [69].
One other difficulty is very low amount of cfDNA that is varied between embryos and occasionally results in a non-informative output [64, 65]. As evidence, the average of SCM cfDNA concentration is estimated to be from 15.2 ± 2.7 to 58.03 ± 35.87 ng/μl after amplification step [70]. The large bias on reported concentrations might be due to the distinguished applied procedures, as well as difference in amount of cfDNA secretion by embryos themselves. Thus, to overcome the non-informative results, an efficient method has to be established to elevate and reach the cfDNA level to an optimal amount for subsequent analyses.
Mosaicism is an additional controversial complexity that has created different points of view for cfDNA reliability in PGT-A assay. Mosaicism arises from mitotic errors that occur during cleavage stage, resulting in distinct ploidy pattern of cells [71]. Since some embryos are capable to undergo “self-correction” through apoptosis induction of abnormal cells [72], SCM cfDNA analysis for PGT-A can represent a false-positive result for these cases. In this respect, some studies have recommended SCM cfDNA analysis as a screening assay rather than diagnosis of aneuploidy [73, 74]. In contrast, Huang et al. by determining a threshold noted that SCM cfDNA analysis disclosed the higher predictive value and specificity than the conventional TE biopsy method for PGT-A assay [66]. They stated that mosaicism diagnosis of embryo by TE biopsy depends on whether the both aneuploid and euploid cells are presented within the biopsied cells, or not, whereas SCM cfDNA is originated from all cells of the embryo, either euploid or aneuploid. Therefore, SCM cfDNA can provide a more practical utilization for PGT-A analysis than the conventional TE biopsy method [66]. The more complex aspect of SCM cfDNA for PGT-A was mentioned by Feichtinger and colleagues, in 2017 [64]. In most of the aneuploid detected embryos in that study, the involved chromosome was different between TE cells and SCM cfDNA. Surprisingly, results from whole-embryo analysis denoted that embryos were mosaic in which the biopsied TE cells and SCM cfDNA were reflecting the aneuploidy patterns of majority and minority of cells, respectively, and no justification was represented for this outcome [64]. All these controversial findings clarify the reason why the concordance rates between SCM cfDNA and TE biopsy method for both ploidy and sex determination were different in distinct reports (ranging from 62.1 to > 95% and 78.7 to 100% for embryo ploidy and sex, respectively) [63, 66, 67, 69, 75, 76]. Thus, with the current data, there is a long way to apply the SCM cfDNA in clinical utilization for PGT-A assay.
BF cfDNA, as another candidate for PGT-A, has been evaluated for the accuracy and reliability. An advantage of BF cfDNA is providing a more specific environment where DNA contamination is eliminated (was not reported by the studies). However, the same aforementioned challenges and controversies have been represented for its applicability. For instance, due to the low concordance rate (from 33.3 to 48%) between ploidy patterns of BF cfDNA and TE-ICM/TE cells by some studies, as well as its false-positive results, its application for clinical diagnosis has not been recommended by the researchers [51, 77]. The high discordance rate might be due to the “self-correction” mechanism of aneuploid cells that has been mentioned previously. In contrast, its successful application for embryo’s sex determination, high rate of informative results, and concordance of ploidy (from 84 to 97.4%) with TE-ICM/TE cells have been reported by the other studies that resulted in suggesting the BF cfDNA as a practical material for clinical application [50, 78–80]. This large bias on reported concordance rates between BF cfDNA and TE-ICM/TE cells might be due to small sample sizes, as well as bias in analyzed embryos, in which in some studies only discarded blastocysts (low quality), whereas in others, donated embryos with moderate to high quality were included for assessments. In other words, high concordance rates might belong to embryos without genetic abnormality or self-correction. Furthermore, distinguished applied methods as well as different aspirated volumes of BF may had influence on the results.
To investigate the preference of BF or SCM cfDNA for PGT-A/PGD assays, a careful study compared their authenticity with the corresponding TE biopsied cells [81]. The results demonstrated that higher discordance rate, as well as maternal DNA contamination, and allele drop out (ADO) occurred in SCM cfDNA, while BF cfDNA represented higher amplification failure rate [81], which might be due to the lower amount of cfDNA in BF [73]. Galluzzi and colleagues also reported a lower detection rate of polymorphism in BF cfDNA when compared to SCM [62]. Hence, based on the current data, both SCM and BF cfDNA have advantages and limitations that complicate the preference of one over the other.
To overcome the limitations of both SCM and BF cfDNA, a new strategy has been established in which combination of SCM-BF cfDNA would be assessed with the goal of increasing the validity. Although the first attempt of this strategy revealed to be non-beneficial because of the low amount of cfDNA [52], the following study reported sufficient cfDNA that could be amplified by the next-generation sequencing (NGS) method [82]. However, the same scenario about discordance of the cfDNA result with TE biopsy/whole-embryo genetic status was repeated [82]. In contrast, Kuznyetsov and colleagues noted the same concordance between SCM-BF cfDNA, TE biopsy, and whole-embryo analyses [83]. They also reported that SCM-BF collection after thawing of vitrified embryos would elevate the cfDNA level, thus increasing the amplification success rate up to 100% [83].
With the aim of solving/reducing the current limitations, optimizing the technical methods was the other side. In this respect, several improved methods have been introduced. For instance, a rapid boiling method for harvesting DNA from the medium was recommended by Yang et al., in which samples would be incubated at 56 °C for 30 min followed by 20 min at 100 °C. Finally, the samples could be stored at 4 °C for subsequent analysis [84]. Jiao and colleagues have established several modifications such as designing primers for pre-amplification step and reduction of the procedure time [85]. With respect to overcoming the low amount of embryonic cfDNA, double amplification by the whole genome amplification (WGA) method has been recommended [64]. Magli and colleagues also modified the techniques through 5 years and noted that direct transferring of collected BF from the ICSI pipette into cold PCR tubes followed by an immediate spinning would raise the DNA recovery rate and subsequent informative result [79]. Omitting cell lysis/extraction step for WGA has been also suggested for reducing the risk of maternal DNA contamination [53]. Although the recommended procedures have risen the cfDNA analysis efficiency within the desired studies, future evaluations, modifications, and comparison of techniques are required to find out the best gold standard method.
To sum up, since the current conventional assays for PGT-A/PGD require biopsy procedure which is an invasive method, some less/non-invasive techniques are highly required. Assessment of embryonic DNA within the extracellular environments (cfDNA) seems to be able to meet this need. Utilization of embryonic cfDNA for PGT-A has some advantages, e.g., providing minimally or non-invasive methods and reflecting the genetic status of the whole embryo rather than few biopsied cells. Nonetheless, it has some limitations. For instance, due to representation of the embryo’s genetic status during the whole period of dynamic genetic errors and modification, it may illustrate false-negative/positive results for some cases. Low amount of embryonic cfDNA is another challenge that can cause the non-informative results. However, future technical modifications may be able to overcome these limitations. Research on cfDNA in oncology field demonstrated that cfDNA level within the EVs is significantly higher than the free circulating form [7]. Moreover, EV-derived cfDNA has shown higher specificity and sensitivity, as well as higher accordance rate with tumor cells’ genotyping, when compared with free circulating form [86, 87]. Thus, separated assessment of embryonic cfDNA through these two distinct fractions may discover new practical data to overcome the current limitations.
According to the reports, none of the three types of embryonic cfDNA (SCM, BF, SCM-BF) has reached to a standard for clinical assays. However, cfDNA analysis in SCM and BF, in parallel with the conventional TE biopsy methods, may be an ideal option that has been recommended by Ben-Nagi and colleagues [88].
Conclusion and future perspectives
During the recent years, investigations on cfDNA application in different fields of medicine have been developed. Although ART is in its initial stages of research, practical results have been achieved in reproduction of male, female, and relatively in in vitro developed embryo fields (summarized in Fig. 1 and Table 1). These findings can improve the methods for infertility diagnosis, estimation of the ART outcome, embryo selection, etc., through the non-invasive procedures.
Table 1.
Summary of reports according to distinguished aspects of ART
| Category | Type of fluid | Evaluated with | Result(s) | Reference(s) |
|---|---|---|---|---|
| Male | Semen | Parameters | Correlated with sperms’ viability, motility, morphology, and capacitation index | [22, 25] |
| Not correlated with SDF | [26] | |||
| Disorder(s) | Correlated with azoospermia, oligo-, astheno-, and oligoasthenoterato-zoospermia | [16, 23] | ||
| ART outcome | Correlated with sperm retrieval chance in NOA patients | [28] | ||
| Not correlated with embryo quality and pregnancy | [26] | |||
| Genetic | Correlated with microdeletions (AZFa, AZFb, and AZFc) | [23] | ||
| Epigenetic | Correlated with testis and epididymis-specific promoters | [28] | ||
| Female | Serum | Disorder(s) | Correlated with subfertility | [36] |
| Not correlated with PCOS and endometriosis | [32, 33] | |||
| Epigenetic | Not correlated | [32] | ||
| ART outcome | Controversial results regarding pregnancy | [31, 34, 35] | ||
| Follicular fluid | Disorder(s) | Correlated with endometriosis and poor ovarian reserve, but controversialresults for PCOS | [33, 39, 43] | |
| ART outcome | Not correlated with fertilization rate | [40, 42] | ||
| Correlated with embryo quality, except one study | [38–45] | |||
| Correlated with pregnancy, except one study | [33, 38, 39, 43–45] | |||
| Embryo | SCM | ART outcome | Correlated with embryo quality | [15, 54, 55] |
| Genetic disorder | Correlated with X-link disorder, thalassemia, MTHFR C677T polymorphism,and cystic fibrosis | [52, 59–62] | ||
| Aneuploidy | Distinguished concordance rates with regarded TE/whole embryo (from 56.3to > 95%) | [63, 66, 67, 69, 75, 76] | ||
| BF | ART outcome | Correlated with embryo quality | [58] | |
| Aneuploidy | Distinguished concordance rates with regarded TE/whole embryo (from 33.3to 97.4%) | [50, 51, 77–80] | ||
| SCM-BF | Aneuploidy | Distinguished concordance rates with regarded TE/whole embryo (from 87.5to 97.8%) | [52, 53, 82, 83, 85] |
In male reproduction field, constructive data regarding SP cfDNA applicability in ART has been proved. Its most important characteristic is representation of genetic/epigenetic profile of the testis. Despite the vital regulatory role of the epigenetic pattern in reproductive status, any strategy for its clinical assessment has not been considered yet. Reports indicated that a non-invasive method for the desired operation can be provided by SP cfDNA. Moreover, it has been confirmed that methylation levels of CCNA1 and DMRT1 genes’ promoters are associated with sperm retrieval chance by testicular biopsy in NOA patients [28], thus supplying the biomarker for prediction of success rate. With this point of view, future research on exploring the wider spectrum of genes’ epigenetic pattern may help in detecting the more potent biomarkers, as well as in discovering the etiology of some idiopathic/unexplained infertility cases. Regarding the other aspects of SP cfDNA biomarker potential, it seems it can be applied for representation of some parameters such as OS and sperm capacitation index [22, 25], whereas there is no evidence on its applicability for prediction of some ART procedure outcome, such as embryo quality and pregnancy rate [26], which might be due to the more important role of female reproductive status rather than male.
In female reproductive medicine, cfDNA has been assessed in the serum and FF. Studies on serum cfDNA are limited and its functional applicability could not be verified. This outcome might be due to its unspecified environment regarding reproductive events. However, future investigations may come forward to discover relationships between serum cfDNA and some infertility disorders such as endometriosis; in this manner, the current invasive diagnostic procedures will be replaced by non-invasive methods.
Research on FF cfDNA has emphasized on discovering associations between its level and ART outcome parameters; based on the current evidences, its reliability for biomarker utilization in some aspects of ART (e.g., embryo quality and pregnancy) has been proved. In contrast, genetic and epigenetic investigations on FF cfDNA have not been developed. Since practical data with respect to applicability of SP cfDNA epigenetic status in male reproduction field has been reported, future FF cfDNA analysis in this aspect may open new directions for further discoveries in female reproductive medicine.
As previously mentioned, most research as well as most challenges belong to embryonic cfDNA with respect to PGT-A application. Although numerous studies that have been performed during the years improved the results, it has not reached to a sufficient standard for clinical assays. This might be due to the mosaicism pattern, as well as dynamic errors/modification of genetic status in some embryos that complicate the results. Based on the current reports, embryonic cfDNA can be utilized more reliably for PGD, sex determination, and screening rather than the diagnosis of aneuploidy. However, even clinical applications of these options require more investigations, especially in larger sample sizes and multicenter studies, to prove the safety and reliability of the methods. In controversial results between cfDNA and the conventional TE biopsy method for PGT-A assay, we recommend the comparison of the outcomes with NIPT tests, if the embryo is transferred and results in physiological pregnancy.
Regarding the gaps in cfDNA studies, only a few research has focused on techniques. In fact, practical utilization of cfDNA requires a standard procedure that its preference to the other ones has been confirmed. Thus, we suggest performing more research to set up standard methods, in parallel with cfDNA application discoveries in ART. In fields where the level of cfDNA can be applied as a biomarker as well, standard cutoff points have not been determined. In most studies, cfDNA level within different situations was comparative, while exact reliable cutoff points have to be determined for distinguishing between conditions. And for the last consideration, all achievements, either for technique or cutoff point, as well as evaluation of cfDNA potential for use in ART, require evaluation in larger sample sizes to prove their veracity for all cases.
To sum up, based on the current evidences, cfDNA has the potential of being reliably applied in the following aspects of ART: biomarker of sperm extraction success rate through testicular biopsy, as well as representation of genetic/epigenetic status of the testis via SP cfDNA; biomarker of oocyte’s developmental competence and pregnancy outcome by FF cfDNA; and in addition to biomarker of embryo quality and PGD utilization through embryonic cfDNA. It must be taken into consideration that due to limitations and controversies in other fields, its practical application requires future research.
Compliance with ethical standards
Conflict of interest
The authors declare they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mandel P, Metais P. Les acides nucleiques du plasma sanguine chez l’homme. 1948. [PubMed] [Google Scholar]
- 2.Meddeb R, et al. Quantifying circulating cell-free DNA in humans. Sci Rep. 2019;9(1):1–16. doi: 10.1038/s41598-019-41593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun Y, An K, Yang C. Circulating cell-free DNA, in Liquid biopsy: IntechOpen; 2019.
- 4.Kustanovich A, Schwartz R, Peretz T, Grinshpun A. Life and death of circulating cell-free DNA. Cancer Biol Ther. 2019;20(8):1057–1067. doi: 10.1080/15384047.2019.1598759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johann DJ, Jr, Steliga M, Shin IJ, Yoon D, Arnaoutakis K, Hutchins L, Liu M, Liem J, Walker K, Pereira A, Yang M, Jeffus SK, Peterson E, Xu J. Liquid biopsy and its role in an advanced clinical trial for lung cancer. Exp Biol Med. 2018;243(3):262–271. doi: 10.1177/1535370217750087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thierry A, et al. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016;35(3):347–376. doi: 10.1007/s10555-016-9629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klump J, et al. Extracellular vesicles or free circulating DNA: where to search for BRAF and cKIT mutations? Nanomedicine: Nanotechnol, Biol Med. 2018;14(3):875–882. doi: 10.1016/j.nano.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Barrett AN, Thadani HA, Laureano-Asibal C, Ponnusamy S, Choolani M. Stability of cell-free DNA from maternal plasma isolated following a single centrifugation step. Prenat Diagn. 2014;34(13):1283–1288. doi: 10.1002/pd.4468. [DOI] [PubMed] [Google Scholar]
- 9.Kim K, Shin DG, Park MK, Baik SH, Kim TH, Kim S, Lee SY. Circulating cell-free DNA as a promising biomarker in patients with gastric cancer: diagnostic validity and significant reduction of cfDNA after surgical resection. Ann Surg Treatment Res. 2014;86(3):136–142. doi: 10.4174/astr.2014.86.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spindler KLG, et al. Circulating free DNA as biomarker and source for mutation detection in metastatic colorectal cancer. PLoS One. 2015:10(4). [DOI] [PMC free article] [PubMed]
- 11.Olsen JA, Kenna LA, Tipon RC, Spelios MG, Stecker MM, Akirav EM. A minimally-invasive blood-derived biomarker of oligodendrocyte cell-loss in multiple sclerosis. EBioMedicine. 2016;10:227–235. doi: 10.1016/j.ebiom.2016.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo YD, et al. Quantitative analysis of fetal DNA in maternal plasma and serum: implications for noninvasive prenatal diagnosis. Am J Hum Genet. 1998;62(4):768–775. doi: 10.1086/301800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo YD. Fetal DNA in maternal plasma: biology and diagnostic applications. Clin Chem. 2000;46(12):1903–1906. [PubMed] [Google Scholar]
- 14.Norton ME, Jacobsson B, Swamy GK, Laurent LC, Ranzini AC, Brar H, Tomlinson MW, Pereira L, Spitz JL, Hollemon D, Cuckle H, Musci TJ, Wapner RJ. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372(17):1589–1597. doi: 10.1056/NEJMoa1407349. [DOI] [PubMed] [Google Scholar]
- 15.Stigliani S, Anserini P, Venturini PL, Scaruffi P. Mitochondrial DNA content in embryo culture medium is significantly associated with human embryo fragmentation. Hum Reprod. 2013;28(10):2652–2660. doi: 10.1093/humrep/det314. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Liao T, Zhu L, Lin X, Wu R, Jin L. Seminal plasma cell-free mitochondrial DNA copy number is associated with human semen quality. Eur J Obstet Gynecol Reprod Biol. 2018;231:164–168. doi: 10.1016/j.ejogrb.2018.10.048. [DOI] [PubMed] [Google Scholar]
- 17.Pourmasumi S, Sabeti P, Rahiminia T, Mangoli E, Tabibnejad N, Talebi AR. The etiologies of DNA abnormalities in male infertility: an assessment and review. Int J Reprod BioMed. 2017;15(6):331–344. [PMC free article] [PubMed] [Google Scholar]
- 18.Papachristou F, Simopoulou M, Touloupidis S, Tsalikidis C, Sofikitis N, Lialiaris T. DNA damage and chromosomal aberrations in various types of male factor infertility. Fertil Steril. 2008;90(5):1774–1781. doi: 10.1016/j.fertnstert.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Gajbhiye R, Fung JN, Montgomery GW. Complex genetics of female fertility. NPJ Genomic Med. 2018;3(1):1–10. doi: 10.1038/s41525-018-0068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ménézo YJ. Paternal and maternal factors in preimplantation embryogenesis: interaction with the biochemical environment. Reprod BioMed Online. 2006;12(5):616–621. doi: 10.1016/s1472-6483(10)61188-1. [DOI] [PubMed] [Google Scholar]
- 21.Kort DH, Chia G, Treff NR, Tanaka AJ, Xing T, Vensand LB, Micucci S, Prosser R, Lobo RA, Sauer MV, Egli D. Human embryos commonly form abnormal nuclei during development: a mechanism of DNA damage, embryonic aneuploidy, and developmental arrest. Hum Reprod. 2016;31(2):312–323. doi: 10.1093/humrep/dev281. [DOI] [PubMed] [Google Scholar]
- 22.Chou JS, Jacobson JD, Patton WC, King A, Chan PJ. Modified isocratic capillary electrophoresis detection of cell-free DNA in semen. J Assist Reprod Genet. 2004;21(11):397–400. doi: 10.1007/s10815-004-7527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H-G, Huang SY, Zhou H, Liao AH, Xiong CL. Quick recovery and characterization of cell-free DNA in seminal plasma of normozoospermia and azoospermia: implications for non-invasive genetic utilities. Asian J Androl. 2009;11(6):703–709. doi: 10.1038/aja.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bronkhorst AJ, Ungerer V, Holdenrieder S. The emerging role of cell-free DNA as a molecular marker for cancer management. Biomolecular Detection and Quantification. 2019;17:100087. doi: 10.1016/j.bdq.2019.100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costa F, Barbisan F, Assmann CE, Araújo NKF, de Oliveira AR, Signori JP, Rogalski F, Bonadiman B, Fernandes MS, da Cruz IBM. Seminal cell-free DNA levels measured by PicoGreen fluorochrome are associated with sperm fertility criteria. Zygote. 2017;25(2):111–119. doi: 10.1017/S0967199416000307. [DOI] [PubMed] [Google Scholar]
- 26.Bounartzi T, Dafopoulos K, Anifandis G, Messini CI, Koutsonikou C, Kouris S, Satra M, Sotiriou S, Vamvakopoulos N, Messinis IE. Pregnancy prediction by free sperm DNA and sperm DNA fragmentation in semen specimens of IVF/ICSI-ET patients. Hum Fertil. 2016;19(1):56–62. doi: 10.3109/14647273.2016.1157629. [DOI] [PubMed] [Google Scholar]
- 27.Wu C, Ding X, Li H, Zhu C, Xiong C. Genome-wide promoter methylation profile of human testis and epididymis: identified from cell-free seminal DNA. BMC Genomics. 2013;14(1):288. doi: 10.1186/1471-2164-14-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu C, Ding X, Tan H, Li H, Xiong C. Alterations of testis-specific promoter methylation in cell-free seminal deoxyribonucleic acid of idiopathic nonobstructive azoospermic men with different testicular phenotypes. Fertil Steril. 2016;106(6):1331–1337. doi: 10.1016/j.fertnstert.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Gunes S, Arslan MA, Hekim GNT, Asci R. The role of epigenetics in idiopathic male infertility. J Assist Reprod Genet. 2016;33(5):553–569. doi: 10.1007/s10815-016-0682-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shamsi M, Kumar K, Dada R. Genetic and epigenetic factors: role in male infertility. Indian Journal of Urology: IJU: journal of the Urological Society of India. 2011;27(1):110–120. doi: 10.4103/0970-1591.78436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hart EA, Patton WC, Jacobson JD, King A, Corselli J, Chan PJ. Luteal phase serum cell-free DNA as a marker of failed pregnancy after assisted reproductive technology. J Assist Reprod Genet. 2005;22(5):213–217. doi: 10.1007/s10815-005-4924-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Udesen PB, Sørensen AE, Joglekar MV, Hardikar AA, Wissing MLM, Englund ALM, Dalgaard LT. Levels of circulating insulin cell-free DNA in women with polycystic ovary syndrome–a longitudinal cohort study. Reprod Biol Endocrinol. 2019;17(1):34. doi: 10.1186/s12958-019-0478-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Da Broi MG, et al. Increased concentration of 8-hydroxy-2′-deoxyguanosine in follicular fluid of infertile women with endometriosis. Cell Tissue Res. 2016;366(1):231–242. doi: 10.1007/s00441-016-2428-4. [DOI] [PubMed] [Google Scholar]
- 34.Czamanski-Cohen J, Sarid O, Cwikel J, Lunenfeld E, Douvdevani A, Levitas E, Har-Vardi I. Increased plasma cell-free DNA is associated with low pregnancy rates among women undergoing IVF–embryo transfer. Reprod BioMed Online. 2013;26(1):36–41. doi: 10.1016/j.rbmo.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 35.Czamanski-Cohen J, Sarid O, Cwikel J, Levitas E, Lunenfeld E, Douvdevani A, Har-Vardi I. Decrease in cell free DNA levels following participation in stress reduction techniques among women undergoing infertility treatment. Archives of Women’s Mental Health. 2014;17(3):251–253. doi: 10.1007/s00737-013-0407-2. [DOI] [PubMed] [Google Scholar]
- 36.Busnelli A, Lattuada D, Rossetti R, Paffoni A, Persani L, Fedele L, Somigliana E. Mitochondrial DNA copy number in peripheral blood: a potential non-invasive biomarker for female subfertility. J Assist Reprod Genet. 2018;35(11):1987–1994. doi: 10.1007/s10815-018-1291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kenigsberg S, et al. Protocol for exosome isolation from small volume of ovarian follicular fluid: evaluation of ultracentrifugation and commercial kits, in Extracellular vesicles. Berlin: Springer; 2017. pp. 321–341. [DOI] [PubMed] [Google Scholar]
- 38.Dimopoulou M, Anifandis G, Messini CI, Dafopoulos K, Kouris S, Sotiriou S, Satra M, Vamvakopoulos N, Messinis IE. Follicular fluid oocyte/cumulus-free DNA concentrations as a potential biomolecular marker of embryo quality and IVF outcome. Biomed Res Int. 2014;2014:1–5. doi: 10.1155/2014/289306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Traver S, et al. Cell-free DNA in human follicular microenvironment: new prognostic biomarker to predict in vitro fertilization outcomes. PLoS One. 2015:10(8). [DOI] [PMC free article] [PubMed]
- 40.Konstantinos S, Petroula T, Evangelos M, Polina G, Argyro G, Sokratis G, Anna R, Andrianos N, Agni P, Michael K, Konstantinos P, George M, Mara S. Assessing the practice of LuPOR for poor responders: a prospective study evaluating follicular fluid cfDNA levels during natural IVF cycles. J Assist Reprod Genet. 2020;37:1183–1194. doi: 10.1007/s10815-020-01743-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y, Shen Q, Zhao X, Zou M, Shao S, Li J, Ren X, Zhang L. Cell-free mitochondrial DNA in human follicular fluid: a promising bio-marker of blastocyst developmental potential in women undergoing assisted reproductive technology. Reprod Biol Endocrinol. 2019;17(1):54. doi: 10.1186/s12958-019-0495-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scalici E, Traver S, Molinari N, Mullet T, Monforte M, Vintejoux E, Hamamah S. Cell-free DNA in human follicular fluid as a biomarker of embryo quality. Hum Reprod. 2014;29(12):2661–2669. doi: 10.1093/humrep/deu238. [DOI] [PubMed] [Google Scholar]
- 43.Kassim, H.R., H.L. AL-Omary, S.J. Ahmed, Evaluation of cell free DNA in follicular fluid and embryo quality in poly cystic ovarian syndrome of Iraqi women.
- 44.Khan HL, et al. Cell-free nucleic acids and melatonin levels in human follicular fluid predict embryo quality in patients undergoing in-vitro fertilization treatment. Journal of Gynecology Obstetrics and Human Reproduction. 2020;49(1):101624. doi: 10.1016/j.jogoh.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 45.Guan Y, Zhang W, Wang X, Cai P, Jia Q, Zhao W. Cell-free DNA induced apoptosis of granulosa cells by oxidative stress. Clin Chim Acta. 2017;473:213–217. doi: 10.1016/j.cca.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 46.Regan SL, et al. Granulosa cell apoptosis in the ovarian follicle—a changing view. Front Endocrinol. 2018;9:61. doi: 10.3389/fendo.2018.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Regan SL, et al. The effect of ovarian reserve and receptor signalling on granulosa cell apoptosis during human follicle development. Mol Cell Endocrinol. 2018;470:219–227. doi: 10.1016/j.mce.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Ichikawa K, Shibahara H, Shirasuna K, Kuwayama T, Iwata H. Cell-free DNA content in follicular fluid: a marker for the developmental ability of porcine oocytes. Reproductive Medicine and Biology. 2020;19(1):95–103. doi: 10.1002/rmb2.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kansaku K, et al. Mitochondrial dysfunction in cumulus-oocyte complexes increases cell-free mitochondrial DNA. J Reprod Dev. 2018;64(3):261–266. doi: 10.1262/jrd.2018-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gianaroli L, et al. Blastocentesis: a source of DNA for preimplantation genetic testing. Results from a pilot study. Fertility and sterility. 2014;102(6):1692–1699. e6. doi: 10.1016/j.fertnstert.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 51.Tobler KJ, Zhao Y, Ross R, Benner AT, Xu X, du L, Broman K, Thrift K, Brezina PR, Kearns WG. Blastocoel fluid from differentiated blastocysts harbors embryonic genomic material capable of a whole-genome deoxyribonucleic acid amplification and comprehensive chromosome microarray analysis. Fertil Steril. 2015;104(2):418–425. doi: 10.1016/j.fertnstert.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 52.Hammond ER, et al. Characterizing nuclear and mitochondrial DNA in spent embryo culture media: genetic contamination identified. Fertility and Sterility. 2017;107(1):220–228. e5. doi: 10.1016/j.fertnstert.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 53.Kuznyetsov V, et al. Minimally invasive cell-free human embryo aneuploidy testing (miPGT-A) utilizing combined spent embryo culture medium and blastocoel fluid–towards development of a clinical assay. Sci Rep. 2020;10(1):1–12. doi: 10.1038/s41598-020-64335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stigliani S, Persico L, Lagazio C, Anserini P, Venturini PL, Scaruffi P. Mitochondrial DNA in day 3 embryo culture medium is a novel, non-invasive biomarker of blastocyst potential and implantation outcome. Mol Hum Reprod. 2014;20(12):1238–1246. doi: 10.1093/molehr/gau086. [DOI] [PubMed] [Google Scholar]
- 55.Stigliani S, Orlando G, Massarotti C, Casciano I, Bovis F, Anserini P, Ubaldi FM, Remorgida V, Rienzi L, Scaruffi P. Non-invasive mitochondrial DNA quantification on day 3 predicts blastocyst development: a prospective, blinded, multi-centric study. Mol Hum Reprod. 2019;25(9):527–537. doi: 10.1093/molehr/gaz032. [DOI] [PubMed] [Google Scholar]
- 56.Desquiret-Dumas V, et al. The mitochondrial DNA content of cumulus granulosa cells is linked to embryo quality. Hum Reprod. 2017;32(3):607–614. doi: 10.1093/humrep/dew341. [DOI] [PubMed] [Google Scholar]
- 57.Chappel S. The role of mitochondria from mature oocyte to viable blastocyst. Obstet Gynecol Int. 2013;2013:1–10. doi: 10.1155/2013/183024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rule K, Chosed RJ, Arthur Chang T, David Wininger J, Roudebush WE. Relationship between blastocoel cell-free DNA and day-5 blastocyst morphology. J Assist Reprod Genet. 2018;35(8):1497–1501. doi: 10.1007/s10815-018-1223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Assou S, Aït-Ahmed O, el Messaoudi S, Thierry AR, Hamamah S. Non-invasive pre-implantation genetic diagnosis of X-linked disorders. Med Hypotheses. 2014;83(4):506–508. doi: 10.1016/j.mehy.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 60.Wu H, et al. Medium-based noninvasive preimplantation genetic diagnosis for human α-thalassemias-SEA. Medicine. 2015:94(12). [DOI] [PMC free article] [PubMed]
- 61.Liu W, Liu JQ, du HZ, Ling JW, Sun XF, Chen DJ. Non-invasive pre-implantation aneuploidy screening and diagnosis of beta thalassemia IVSII654 mutation using spent embryo culture medium. Ann Med. 2017;49(4):319–328. doi: 10.1080/07853890.2016.1254816. [DOI] [PubMed] [Google Scholar]
- 62.Galluzzi L, et al. Extracellular embryo genomic DNA and its potential for genotyping applications. Future Science OA. 2015;1(4). [DOI] [PMC free article] [PubMed]
- 63.Rubio C, Rienzi L, Navarro-Sánchez L, Cimadomo D, García-Pascual CM, Albricci L, Soscia D, Valbuena D, Capalbo A, Ubaldi F, Simón C. Embryonic cell-free DNA versus trophectoderm biopsy for aneuploidy testing: concordance rate and clinical implications. Fertil Steril. 2019;112(3):510–519. doi: 10.1016/j.fertnstert.2019.04.038. [DOI] [PubMed] [Google Scholar]
- 64.Vera-Rodriguez M, Diez-Juan A, Jimenez-Almazan J, Martinez S, Navarro R, Peinado V, Mercader A, Meseguer M, Blesa D, Moreno I, Valbuena D, Rubio C, Simon C. Origin and composition of cell-free DNA in spent medium from human embryo culture during preimplantation development. Hum Reprod. 2018;33(4):745–756. doi: 10.1093/humrep/dey028. [DOI] [PubMed] [Google Scholar]
- 65.Feichtinger M, Vaccari E, Carli L, Wallner E, Mädel U, Figl K, Palini S, Feichtinger W. Non-invasive preimplantation genetic screening using array comparative genomic hybridization on spent culture media: a proof-of-concept pilot study. Reprod BioMed Online. 2017;34(6):583–589. doi: 10.1016/j.rbmo.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 66.Huang L, Bogale B, Tang Y, Lu S, Xie XS, Racowsky C. Noninvasive preimplantation genetic testing for aneuploidy in spent medium may be more reliable than trophectoderm biopsy. Proc Natl Acad Sci. 2019;116(28):14105–14112. doi: 10.1073/pnas.1907472116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ho JR, et al. Pushing the limits of detection: investigation of cell-free DNA for aneuploidy screening in embryos. Fertil Steril. 2018;110(3):467–475. e2. doi: 10.1016/j.fertnstert.2018.03.036. [DOI] [PubMed] [Google Scholar]
- 68.Lane M, Zander-Fox DL, Hamilton H, Jasper MJ, Hodgson BL, Fraser M, Bell F. Ability to detect aneuploidy from cell free DNA collected from media is dependent on the stage of development of the embryo. Fertil Steril. 2017;108(3):e61. [Google Scholar]
- 69.Rubio C, Navarro-Sánchez L, García-Pascual CM, Ocali O, Cimadomo D, Venier W, Barroso G, Kopcow L, Bahçeci M, Kulmann MIR, López L, de la Fuente E, Navarro R, Valbuena D, Sakkas D, Rienzi L, Simón C. Multicenter prospective study of concordance between embryo cell-free DNA and trophectoderm biopsies from 1,301 human blastocysts. Am J Obstet Gynecol. 2020;223:751.e1–751.e13. doi: 10.1016/j.ajog.2020.04.035. [DOI] [PubMed] [Google Scholar]
- 70.Sophie, B., et al., Is cell-free DNA in spent embryo culture medium an alternative to embryo biopsy for preimplantation genetic testing? A systematic review. Reproductive BioMedicine Online, 2020. [DOI] [PubMed]
- 71.Kahraman S, Cetinkaya M, Yuksel B, Yesil M, Pirkevi Cetinkaya C. The birth of a baby with mosaicism resulting from a known mosaic embryo transfer: a case report. Hum Reprod. 2020;35(3):727–733. doi: 10.1093/humrep/dez309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sachdev NM, Maxwell SM, Besser AG, Grifo JA. Diagnosis and clinical management of embryonic mosaicism. Fertil Steril. 2017;107(1):6–11. doi: 10.1016/j.fertnstert.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 73.Fang R, Yang W, Zhao X, Xiong F, Guo C, Xiao J, Chen L, Song X, Wang H, Chen J, Xiao X, Yao B, Cai LY. Chromosome screening using culture medium of embryos fertilised in vitro: a pilot clinical study. J Transl Med. 2019;17(1):73. doi: 10.1186/s12967-019-1827-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu J, Fang R, Chen L, Chen D, Xiao JP, Yang W, Wang H, Song X, Ma T, Bo S, Shi C, Ren J, Huang L, Cai LY, Yao B, Xie XS, Lu S. Noninvasive chromosome screening of human embryos by genome sequencing of embryo culture medium for in vitro fertilization. Proc Natl Acad Sci. 2016;113(42):11907–11912. doi: 10.1073/pnas.1613294113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shamonki MI, Jin H, Haimowitz Z, Liu L. Proof of concept: preimplantation genetic screening without embryo biopsy through analysis of cell-free DNA in spent embryo culture media. Fertil Steril. 2016;106(6):1312–1318. doi: 10.1016/j.fertnstert.2016.07.1112. [DOI] [PubMed] [Google Scholar]
- 76.Yeung QS, et al. A prospective study of non-invasive preimplantation genetic testing for aneuploidies (NiPGT-A) using next-generation sequencing (NGS) on spent culture media (SCM) J Assist Reprod Genet. 2019;36(8):1609–1621. doi: 10.1007/s10815-019-01517-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perloe M, Welch C, Morton P, Venier W, Wells D, Palini S. Validation of blastocoele fluid aspiration for preimplantation genetic screening using array comparative genomic hybridization (aCGH) Fertil Steril. 2013;100(3):S208. [Google Scholar]
- 78.Zhang Y, Li N, Wang L, Sun H, Ma M, Wang H, Xu X, Zhang W, Liu Y, Cram DS, Sun B, Yao Y. Molecular analysis of DNA in blastocoele fluid using next-generation sequencing. J Assist Reprod Genet. 2016;33(5):637–645. doi: 10.1007/s10815-016-0667-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Magli MC, et al. Preimplantation genetic testing: polar bodies, blastomeres, trophectoderm cells, or blastocoelic fluid? Fertility and Sterility. 2016;105(3):676–683. e5. doi: 10.1016/j.fertnstert.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 80.Palini S, Galluzzi L, de Stefani S, Bianchi M, Wells D, Magnani M, Bulletti C. Genomic DNA in human blastocoele fluid. Reprod BioMed Online. 2013;26(6):603–610. doi: 10.1016/j.rbmo.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 81.Capalbo A, et al. Diagnostic efficacy of blastocoel fluid and spent media as sources of DNA for preimplantation genetic testing in standard clinical conditions. Fertility and Sterility. 2018;110(5):870–879. e5. doi: 10.1016/j.fertnstert.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 82.Li P, et al. Preimplantation genetic screening with spent culture medium/blastocoel fluid for in vitro fertilization. Sci Rep. 2018;8(1):1–10. doi: 10.1038/s41598-018-27367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kuznyetsov V, Madjunkova S, Antes R, Abramov R, Motamedi G, Ibarrientos Z, Librach C. Evaluation of a novel non-invasive preimplantation genetic screening approach. PLoS One. 2018;13(5):e0197262. doi: 10.1371/journal.pone.0197262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang L, Lv Q, Chen W, Sun J, Wu Y, Wang Y, Chen X, Chen X, Zhang Z. Presence of embryonic DNA in culture medium. Oncotarget. 2017;8(40):67805–67809. doi: 10.18632/oncotarget.18852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiao J, Shi B, Sagnelli M, Yang D, Yao Y, Li W, Shao L, Lu S, Li D, Wang X. Minimally invasive preimplantation genetic testing using blastocyst culture medium. Hum Reprod. 2019;34(7):1369–1379. doi: 10.1093/humrep/dez075. [DOI] [PubMed] [Google Scholar]
- 86.Wan Y, Liu B, Lei H, Zhang B, Wang Y, Huang H, Chen S, Feng Y, Zhu L, Gu Y, Zhang Q, Ma H, Zheng SY. Nanoscale extracellular vesicle-derived DNA is superior to circulating cell-free DNA for mutation detection in early-stage non-small-cell lung cancer. Ann Oncol. 2018;29(12):2379–2383. doi: 10.1093/annonc/mdy458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hur JY, Kim HJ, Lee JS, Choi CM, Lee JC, Jung MK, Pack CG, Lee KY. Extracellular vesicle-derived DNA for performing EGFR genotyping of NSCLC patients. Mol Cancer. 2018;17(1):15. doi: 10.1186/s12943-018-0772-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ben-Nagi J, et al. The first ongoing pregnancy following comprehensive aneuploidy assessment using a combined blastocenetesis, cell free DNA and trophectoderm biopsy strategy. J Reprod Infertil. 2019;20(1):57. [PMC free article] [PubMed] [Google Scholar]