Abstract
We report a novel stabilized emulsified formulation containing omega-3 fatty acid (ω-3 FA) and micronutrient that can be readily used for food fortification. The emulsification methodology for producing a stabilized formulation containing both ω-3 FA and micronutrients is described. The formulation was developed considering the human requirement of ω-3 FA and recommended daily allowance of important micronutrients. This formulation was characterized for physical appearance, pH, specific gravity, color measurement etc. Fatty acid analysis revealed formulations (2, 4 and 5 g/serve) were able to provide 500—1300 mg of alpha linoleic acid. Oxidative stability assessment (peroxide value, free fatty acid content) of the formulations showed, stability of the formulation as non-significant alterations were noted in these parameters when the formulations were compared with raw flaxseed oil. Rheological evaluation indicated formulation followed Non-Newtonian system with shear thickening behavior. Particle size was found to be between 673.83 to 798.76 nm and poly-dispersity index was between 0.438 to 0.681. Microscopic analysis by Cryo-SEM analysis of the formulation showed stable homogeneous nature of formulation. Stability of the formulations was confirmed by freeze-thawing, dilution test and emulsion stability index. Acute oral toxicity study as per OECD guideline showed safety of the formulations. Most importantly, in vivo bioavailability study of ω-3 FA confirmed better bioavailability of the metabolites of ω-3 FA i.e. eicosapentaenoic and docosahexaenoic acids in formulation treated group as compared to flax oil and comparable bioavailability to that of fish oil.
Keywords: Emulsion, Fortification, Functional foods, Micronutrients, Omega-3-fatty acid, Lipid oxidation
Introduction
Omega-3 and omega-6 are essential fatty acids must be taken through food as they are not synthesized in the body. Balanced omega-6 to 3 ratio is very pivotal for human health (Simopoulos 2016). Omega-3-fatty acids (ω-3 FA) play a key role in the control and prevention of several pathological conditions including inflammation, cancer, obesity, diabetes, mitochondrial function, eye health, cardiovascular disorders, immune response, myocardial infarction, pregnancy outcome, psoriasis etc. (Hegde et al. 2016). Alpha-linolenic acid (ALA) is the major form of ω-3 FA available to vegetarians (Barceló-Coblijn and Murphy 2009). Further micronutrients are important and essential components of functional food. The deficiencies of the micronutrients lead to several health issues. Several health authorities across the globe, have given guidelines on micronutrients. Fortification of many micronutrients in common food has been part of health policies in various countries (Europe W. O. N. C. A. 2002).
Emulsification is most commonly used technology to address the stability of the oil in food/neutracetical industries. Recently Zhu et al. (2019) have reviewed various aspects of the emulsified formulations and their subsequent utility in various industries for various kinds of novel functional food applications. Panagiotou and Fisher (2012) have thoroughly reviewed various aspects of the emulsification methodology to enhance product quality and its potential for industrial applications. Pre-emulsification of an oil mixture prior to ingestion increases the absorption. Emulsification increases the lipid surface area thereby allowing enhanced digestion of the triacylglycerol by lingual and gastric lipase and thereby bioavailability (Garaiova et al. 2007).
Flaxseed/linseed oil is one of the richest vegetarian source of ω-3 FA and has become potential choice for incorporation of ALA in various food formulations/products (Duboisa et al. 2007). Goyal et al. 2015 reported flaxseed oil-based emulsion for developing functional food such as pastry, ice-creams, curd, milk, yogurt, cakes. Earlier we have reviewed importance of ALA in the milk, its impact on human health and various approaches for ω-3 FA milk fortifications (Zanwar et al. 2016). Some researchers have reported flaxseed oil based emulsified formulations; however concentration of ALA is very less (Carneiro et al. 2013; Chen et al. 2017; Kartal et al. 2016; Yi et al. 2015; Chen et al. 2017).
Presently there is paucity of reports on emulsified formulation containing optimum concentration of vegetarian ω-3 FA along with micronutrients for desired health effect. While selecting the micronutrients, the metabolic effects of individual micronutrients was taken into consideration. In view of this, we have selected vitamin B3 (niacin), vitamin B6 (pyridoxine), zinc, vitamin E and magnesium along with flaxseed oil for developing a novel formulation. The selected micronutrients/co-factors plays a key role in bioavailability of ALA (Saunders et al. 2012; Ued et al. 2019; Yary et al. 2017). So stabilization of such nutritionally dense product and its safety, bioavailability of important metabolites needs to be studied. Hence the objective of the present study was to develop stabilized formulation of ALA with essential micronutrients and carry out physicochemical characterization, stability, bioavailability and safety evaluation.
Material and methods
Fructose-oligosaccharide (FOS containing total oligofructose—90.13%) was obtained from Alkem Laboratories ltd, Ankaleshwar, India. Sucrose fatty acid ester (W/S) F160 was obtained from Euto Taiyou Food Chemical Co. Ltd Taiwan. Cold press flaxseed oil was obtained from Real World Nutrition Laboratory Foundation, Pune. Other chemicals, micronutrients, were purchased from Merck and Hi-Media, India. All the other chemicals/ingredients used were of analytical /food grade.
Preparation of formulation
Cold press flaxseed oil was used as source of ω-3 FA. All micronutrients and other solid form of ingredients were passed through Sieve no. 120 (ASTM Standard) to maintain the uniform particle size. Emulsifier was selected based on HLB value (sucrose fatty acid ester-HLB value 15).
Different concentrations of flaxseed oil, fructo-oligosaccharides (FOS), emulsifier and water were tried to arrive at a composition that does not permit phase separation. Further concentration of emulsifier (sucrose fatty acid ester) decided by determining particle size, zeta potential and polydispersity index (PDI). Initially 1%, 1.5% of emulsifier were tried, however phase separation was observed after few days. Hence higher concentrations of emulsifier 2% 2.5%, 3% were tried. It is well known that lower PDI indicates narrower particle size distribution and vice versa. PDI was lowest with of 2% sucrose ester, indicating formulation would have better dispersity for oral administration of formulation. Based on this data 2% concentration of emulsifier was selected.
Table 1 represents composition of the formulations. The emulsifier was triturated in few parts of flaxseed oil and water using homogenizer (IKA- T 10 basic ULTRA-TURRAX®) at 2500–3000 rpm at room temperature. N-acetyl-L-cystine (previously neutralized) was added to flaxseed oil and triturated. Addition of preheated FOS (60 °C) to mortar produced primary emulsion with crackling sound. To this micro-ingredients were added in the order of L-glutamine, vitamin B3, Vitamin B6, magnesium, zinc, edentate disodium (EDTA) to the mortar. Mixed well and sodium saccharin as sweetener was added. Remaining portion of flaxseed oil was added to the mortar and levigated. Finally, vitamin E and BHT was added to the preparation. Formulation was again subjected for homogenization. Three formulations were prepared i.e. 2 g/serve, 4 g/serve and 5 g/serve. Here 5 g/serve is equivalent to 1 teaspoon of formulation which is maximum dose recommended. The formulations prepared considering the human requirement of ω-3 FA per day i.e. 500 mg to 1300 mg and micronutrients were added as per 30% recommended daily allowance per serve. These formulations were filled in glass bottle and stored at 4–8 °C.
Table 1.
Composition of formulations
| Parameters | 2 g/serve (%) | 4 g/serve (%) | 5 g/serve (%) |
|---|---|---|---|
| Flaxseed oil | 50 | 50 | 50 |
| Sucrose ester | 2 | 2 | 2 |
| Fructose Oligosaccharide | 11.5 | 14.5 | 16 |
| N-acetyl cysteine | 2.5 | 1.5 | 1.25 |
| L-Glutamine | 5 | 3 | 2.5 |
| Vitamin B-3 | 0.2 | 0.1 | 0.07 |
| Vitamin B-6 | 0.02 | 0.01 | 0.008 |
| Zinc | 0.25 | 0.15 | 0.125 |
| EDTA | 0.5 | 0.3 | 0.25 |
| Magnesium | 17 | 13 | 10 |
| Vitamin E + BHT | 0.36 | 1.25 | 1.25 |
| Flavor | 0.3 | 0.3 | 0.3 |
| Saccharin sodium | 0.3 | 0.3 | 0.3 |
| Water | q. s | q. s | q. s |
Where q. s.—quantity sufficient. All concentrations given in %
Physicochemical characterization of formulations
Physical appearance, pH, specific gravity and color measurement
Physical appearance of the formulations was analyzed by visual inspection based on various parameters such as appearance, homogeneity, transparency, texture, color etc. The pH of the formulations was determined by using a pH meter (Metler Toledo, India). The specific gravity of the formulations was determined using 10 ml specific gravity bottle at room temperature (Puranik 2016; Jaiswal et al. 2015).
Colour quantification of formulations was determined by “tristimulus coordinates” using LLOYD / LR-5 K, measurement mode was reflectance. The analysis was carried out in triplicate using CIELAB system. Data was expressed in terms of L* (lightness): ranging from 0 (black) to 100 (white), a* (redness): ranging from + 60 (red) to − 60 (green), and b* (yellowness) ranging from + 60 (yellow) to − 60 (blue) values (Chantrapornchai et al. 1998).
Quantification of ALA and other fatty acids by gas chromatography
Fat extraction from formulations was carried out by using Folch et al. 1957, with few modifications as reported by Goyal et al. (2015). Trans-esterification and gas chromatographic (GC) analysis was carried out as reported earlier (Zanwar et al. 2014), using Agilent GC 7820A equipped with flame ionization detector using a fused silica capillary column (HP-88). Column dimensions were 30 m (length) × 0.25 mm (film thickness) × 0.20 µm (internal diameter). Briefly GC analysis conditions were—initially temperature was held at—140 °C for 5 min and raised to 230 °C at 4 °C/min, and held for 2.5 min; injector and detector temperatures were 250 °C with 25:1 split ratio. Nitrogen gas was used as carrier gas (flow rate 2.2 ml/min), hydrogen gas and zero air were used for flame ignition. Fatty acids were identified using standard fatty acid methyl ester Mixture (FAME-Supelco, Bellefonte, PA. USA). Results were expressed as milligram per serve of fatty acid.
Oxidative stability assessment
Formulations were broken by using organic solvent such as hexane under continuous stirring leading to phase separation. n-hexane layer was collected using separating funnel and evaporated at room temperature. Comparative oxidative stability study of oil extracted from the formulations and raw flaxseed oil was carried out using various parameters such as peroxide value, acid value and free fatty acid content as per AOCS guidelines (AOAC 2000).
Rheological properties
The rheological properties of the formulations were assessed by using a rheometer (Anton Paar, MCR-52, Austria) at shear rate of 10 to 150 s−1 at room temperature.
Particle size analysis and zeta potential evaluation
Particle size measurement, polydispersity index (PDI) and zeta potential analysis of the formulations was carried out using Nano Partica SZ-100 (HORIBA Scientific, Japan).
Cryo-scanning electron microscopic (SEM) analysis
Cryo-SEM analysis was carried out using JEOL 7600F Field emission scanning electron microscope. 5 g/serve formulation (concentrated and diluted) was mounted on the carbon tape. Samples were frozen in liquid nitrogen slush (temperature − 190 °C to − 200 °C), frozen samples were freeze fractured in the preparation chamber at − 120 °C. Freeze fractured samples were sublimated at − 95 °C for 10 min in preparation chamber. Finally sputter coating of the samples was done with platinum for 30 s at 10 mA current and then samples were transferred to the SEM chamber which was maintained at − 150 °C temperature.
Evaluation of stability of formulations
Repeated freezing and thawing
Formulations were subjected to repeated freezing and thawing to understand the mechanical stability of formulations (phase separation). 25 ml of sample was incubated at 25 °C for 24 h followed by incubation for 24 h at − 50 °C. Cycle was repeated three times.
Dilution test
Dilution test was carried out to understand the nature/type of the emulsified formulations (o/w or w/o). In this test sample (formulations) always generally miscible with the external phase in the proportion of tenfold to 100-fold of external phase (oil/water). Hence if water added into w/o emulsion, it does not mix well but oil mixes well. Similarly, if oil added to the o/w type emulsion, does not mix well but water does (Puranik 2016). In the present study the formulations were dissolved in water and oil and observations were recoded to know the nature/type of formulations.
Emulsion stability index (ESI)
Accelerated stability study of the formulations was carried out as per the method of Huang et al. (2001), with some modifications as mentioned by Cho et al. (2003). Formulations were subjected to centrifugation at 8000 rpm for 15 min. This centrifugation resulted in aqueous layer at bottom, oil layer at top and emulsion concentrate at middle level. Only very unstable emulsions produced a significant oil layer. Stability of the emulsion was determined by visually measuring the extent of oil layer separation.
Acute oral toxicity study
Acute oral toxicity study of the formulations was conducted according to the Organization for Economic Cooperation and Development (OECD) revised up and-down procedure for acute toxicity testing (OECD guideline 425, OECD 2001). The toxicity study protocol was approved by the Institutional Animal Ethics Committee (IAEC) constituted in accordance with the rules and guidelines of the Committee for the Purpose of Control and Supervision on Experimental Animals (CPCSEA), India. Protocol approval no. BVDUMC/1888/2018/002/017.
Wistar rats were fasted overnight for food, but not water, prior to dosing. Formulations (2 g/serve, 4 g/serve and 5 g/serve) were dissolved in water and route of administration was oral by oral feeding needle. Dose was calculated as per body weight (n = 5). Initially dose was 175 mg/kg, dose progression factor and later multiply by 3.2, highest dose used was 2000 mg/kg and these rats were observed for mortality and clinical signs (as per OECD guidelines) for initial 1st hour and thereafter after every 3 h upto 48 h. All the animals were observed for the period of 14 days and any gross clinical observations/mortality was noted.
In vivo bioavailability study by single dose oral administration of formulation
Being a single dose administration study, highest dose i.e. formulation 5 g/serve was selected for the study and its comparison with flax oil and fish oil was carried out. Male Wistar rats (200–220 g) were divided into three groups each group having 12 rats. 1st group rats received formulation (500 mg/kg from formulation 5 g/serve group) and rats from 2nd group received flaxseed oil and rats from 3rd group received fish oil. The concentration of total ω-3 FA was kept constant based on GC analysis in all the three groups. Blood was withdrawn at predefined time intervals i.e. at 0 (pre-dose) and 2, 4, 6, 8, 12 and 24 h time points after dose from retro orbital plexus under anesthesia. Animals were divided in 2 sets in each group. Each set were having 6 animals and blood was withdrawn from alternate set at pre-defined time points as per respective treatment group. Blood was collected in ice cool EDTA tubes. The whole blood was processed for esterification and subsequently subjected for fatty acid analysis by gas chromatography using Agilent GC model 7820A with flame ionization detector by using Supelco capillary column, Bellefonte, PA. USA (30 m × 0.25 mm × 0.20um). GC analysis conditions were—initially temperature was 110 °C and raised to 240 °C at 3 °C/min, and held for 5 min; injector and detector temperatures were 250 °C with 25:1 split ratio. Nitrogen gas was used as carrier gas (flow rate 2.2 ml/min), hydrogen gas and zero air were used for flame ignition. The methyl esters of the fatty acid (FAME Mixture, Supelco, Bellefonte, PA. USA) was used to identify the fatty acids.
Statistical analysis
Data was presented as mean ± standard deviation and statistical analysis was carried out by one-way ANOVA followed by post hoc Bonferroni test using GraphPad prism 5.00 for Windows 7, GraphPad Software, San Diego, CA, USA, www.graphpad.com. The p value was considered significant when p < 0.05.
Results and discussion
In the present study, emulsification technology was used to stabilize ω-3 FA along with important vitamins and micronutrients such as vitamin B3, vitamin B6, zinc, vitamin E and magnesium. Various combinations of flaxseed oil, FOS and emulsifier were tried and formulations were evaluated for various analytical parameters including particle size analysis, oxidative stability. Finally in vivo single dose bioavailability study and toxicological evaluations as per OECD guideline was carried. Emulsified formulation is most acceptable form to deliver both hydrophilic and hydrophobic ingredients together. Because of its water soluble nature the formulation can be administered orally as well as it can be used to fortify milk and other dairy products/dairy sweets and ice-creams. Due to its water miscible nature, the formulation can be used to fortify beverage such as fruit and milk juices.
Physicochemical characterization of formulations
Physical appearance, pH, specific gravity and color measurement
The appearance was cloudy, nature was homogenous and opaque, texture was smooth, yellow colored having sour taste and no oil separation was observed in all the formulations. The pH of the formulations was found to be approximately 7 which is generally stable and acceptable for food formulations (Table 2). The specific gravity of formulations was ranging from 1.06 to 1.22 (Table 2). The color of food product mainly depends on the kind of ingredients used in the formulations. As per CIELAB classification, L*value was approximately 74, a* value was ranging between 3.11 to 3.44 and b* approximately 45, indicating bright yellow coloured formulations (due to flax oil) and moreover there was non-significant alteration in colour co-ordinates in all three formulations indicating uniformity and homogeneity of formulations in spite of many ingredients present in the formulations in different concentrations (Table 2).
Table 2.
Characterization of formulation with respect to pH, specific gravity, colour analysis, particle size analysis, PDI, zeta potential evaluation and fatty acid analysis
| Parameters | 2 g/serve | 4 g/serve | 5 g/serve |
|---|---|---|---|
| pH | 6.43 ± 0.04 | 7.03 ± 0.13 | 6.92 ± 0.28 |
| Specific gravity | 1.22 ± 0.0 | 1.080 ± 0.0 | 1.06 ± 0.02 |
| Color measurement | |||
| L* | 73.99 ± 4.71 | 74.44 ± 3.93 | 74.44 ± 3.94 |
| a* | 3.44 ± 0.96 | 3.11 ± 0.38 | 3.12 ± 0.37 |
| b* | 44.71 ± 2.12 | 45.70 ± 3.71 | 45.65 ± 3.74 |
| dE*ab | 50.73 ± 3.89 | 51.33 ± 4.93 | 51.30 ± 4.96 |
| Particle size analysis, PDI and zeta potential evaluation | |||
| Particle size (nm) | 680.50 ± 88.86 | 673.83 ± 23.87 | 798.76 ± 44.51 |
| PDI | 0.681 ± 0.18 | 0.52 ± 0.07 | 0.438 ± 0.035 |
| Zeta potential (mv) | − 27.80 ± 1.38 | − 26.33 ± 2.20 | − 26.96 ± 2.25 |
| Fatty acid analysis (mg/serve) | |||
| Palmitic acid | 63.4 ± 0.6 | 124.86 ± 0.50 | 163.08 ± 0.62 |
| Stearic acid | 62.4 ± 3.4 | 126.4 ± 1.59 | 154.08 ± 1.37 |
| Oleic acid | 207 ± 5.60 | 419.8 ± 0.59 | 522.25 ± 3.25 |
| Linoleic acid | 142.6 ± 1 | 286.66 ± 1.10 | 361.5 ± 3.5 |
| Alpha-linolenic acid | 524.6 ± 8.60 | 1042.2 ± 1.60 | 1299.12 ± 1.62 |
| Saturated fatty acid | 125.8 ± 4 | 251.26 ± 2.10 | 317.25 ± 2 |
| Mono-saturated fatty acid | 207 ± 5.60 | 419.8 ± 0.59 | 522.25 ± 3.25 |
| Poly-unsaturated fatty acid | 667.2 ± 9.60 | 1328.86 ± 2.70 | 1660.58 ± 5.12 |
The analysis were carried out triplicate and data was presented as Mean ± SD
Quantification of ALA and other fatty acids by gas chromatography
Table 2 presents quantification of omega-3 and other fatty acids present in the formulation as per their respective serving size. The total ALA content was 524.6 ± 8.60 mg, 1042.2 ± 1.60 mg and 1299.12 ± 1.62 mg in 2 g/serve, 4 g/serve and 5 g/serve formulations respectively. The total poly-unsaturated fatty acid content was 667.2 ± 9.60 mg, 1328.86 ± 2.70 mg and 1660.58 ± 5.12 mg in 2 g/serve, 4 g/serve and 5 g/serve formulations respectively. Interestingly total saturated fatty acid content was significantly lower in the formulation was found to be 125.8 ± 4 mg, 251.26 ± 2.10 mg and 317.25 ± 2 mg in in 2 g/serve, 4 g/serve and 5 g/serve formulations respectively. All the formulations found to be nutritive as saturated fatty acid content was very less compared to that of poly-unsaturated fatty acid content (Table 2).
Oxidative stability assessment
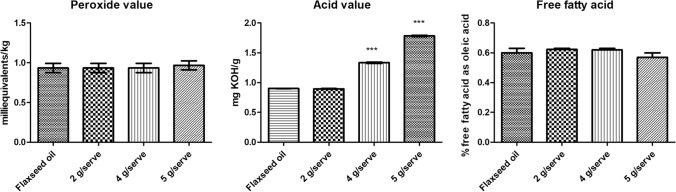
Peroxide value is used as an indicator of the quality and stability of fats and oils in emulsion. Peroxide value gives information about the oxidative degradation of fatty acid and subsequent rancidity of the oil (Matthäus 2010). Peroxide value of flaxseed oil was below 1 meq/kg and peroxide values of oil extracted from all the three formulations were similar to that of flaxseed oil, indicating no rancidification in the formulations. Rancidity starts after 10 meq /kg, indicating no oxidation has occurred during the preparation of the formulations. Acid value and free fatty acid content are other parameters for determining the oxidative susceptibility. There was non-significant change in free fatty acid content between flaxseed oil extracted from formulation and raw flaxseed oil, indicating oxidative stability of oil in the formulations. Although there was significant difference in case of acid value (p < 0.001) in 4 g/serve and 5 g/serve as compared to raw flaxseed oil (Fig. 1), however these values remained in acceptable limits as per Codex standards for fats and oil (Codex Alimentarius Commission).
Fig. 1.
Comparative oxidative stability assessment of the formulations
Rheological properties
In the present study, it was observed that, formulations followed Non-Newtonian nature having shear thickening behavior because shear rate increase was proportionate to viscosity (Fig. 2). This shear thickening nature of the formulations is mainly due to high fat content, FOS and solid micro-ingredients used in the formulations. These shear thickening components have unique application with respect to stability to the formulation especially when formulation is multi-ingredient. Especially role of polymer i.e. fructooligosaccharides-FOS was crucial here. FOS plays key role in formulation as FOS affects several physiological parameters such as viscosity, pH, solubility and taste (Ibrahim 2018; Mabel et al. 2008). The viscosity of FOS decreases droplet collisions, thus may be responsible for decreasing flocculation and coalescence. Higher levels of FOS may also form a protective layer around emulsion droplets.
Fig. 2.
Rheological behavior of formulations
Particle size analysis, PDI and zeta potential evaluation
All the formulations could produce nano sized globules on diluting with dissolution media with high zeta potential. There was increase in globule size but not in the zeta potential as the amount of fat in emulsion per serve increased. It is well known that, PDI is an indicator of polydispersity nature of the particles and it gives an idea about bimodal distribution profiles. PDI less than 0.7 indicates the homogenous nature of formulations and there is limited chance of aggregation of the emulsion droplets (Nidhin et al. 2018). In the present investigation, maximum PDI value noted was 0.681 ± 0.18 in 2 g/serve and lowest 0.438 ± 0.035 in 5 g/serve, indicating homogenous nature of all formulations. Further zeta potential values of formulations were − 26.33 to − 27.80 which is another good indicator of stability. High zeta values (more than − 30 mV), indicate more repulsion between particles which is likely to prevent possible aggregation/separation (Mosqueira et al. 2000). Formulations with 4 g/serve and 5 g/serve produced globules in nano size with optimum PDI (Table 2).
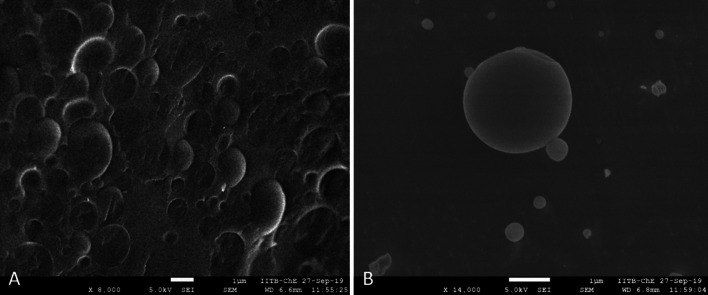
Cryo-scanning electron microscopic analysis
Cryo-SEM technique is most suitable for studying the morphology of viscous formulations (Klang et al. 2012). The oil content in the formulation (5 g/serve) was approximately 50% and there were various micronutrients which made the formulation more complex and viscous. Cryo-SEM technique was utilized to capture the image of the emulsified formulation. Figure 3 represents the concentrated formulation (Fig. 3a) and diluted form (Fig. 3b) of the same formulation, which indicated the uniform nature of the formulation both in concentrated and diluted state. Further cryo-SEM study showed stable nature of the formulation as no morphological/microscopic signs of emulsion breakdown/instability were noticed in both the concentrated and diluted samples.
Fig. 3.
Cryo-Scanning Electron Microscopic analysis of formulation (5 g/serve)
Evaluation of stability of formulation
Repeated freezing and thawing
There was no phase separation in the formulation even after repeated freeze thaw cycles indicating high physical/mechanical stability.
Dilution test
The formulation was found to be of o/w type, as when water was added the uniform solution was observed and on other side when oil is added, oil was insoluble in formulation. The formulation were found to be stable and does not showed any oil separation. In presence of 1% emulsifier, formulation showed oil separation but in case of 2% emulsifier, there was no such oil separation, indicating higher percentage of emulsifier (2%) prevented physical instability.
Emulsion stability index (ESI)
ESI provides information on the extent of droplet aggregation in the formulation. If level of aggregation is more then, larger flocks are formed which may lead to faster instability of the formulation. In the present study with 2% emulsifier, there was no droplet aggregation which indicated stability of formulations.
Acute oral toxicity study of formulation
Formulations showed no mortality upto the dose of 2000 mg/kg in all the three formulations. Further clinical signs (as per OECD guidelines) were noted and no gross clinical signs of toxic effects of the formulations were noted upto 14 days in the rats, indicating safety of the formulations.
In vivo bioavailability study by single dose oral administration of formulation
Bioavailability of plant derived ω-3 FA is an important issue with regards to its pharmacological effects. In the present study along with flax oil, micronutrients such as vitamin B2 (niacin), vitamin B6 (pyridoxine) and zinc, magnesium were added, these vitamins plays key a role in promoting Δ6 desaturase activity. This is an important enzyme involved in converting ALA to its metabolite such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Saunders et al. 2012; Ued et al. 2019; Yary et al. 2017). So in order to understand the bioavailability of ω-3 FA, single dose oral administration of 5 g/serve formulation and its comparison with flax oil and fish oil was carried by single dose in vivo model. Figure 4 shows ALA, EPA and DHA content in blood after 2, 4, 6, 8, 12 and 24 h of oral administration of formulation (5 g/serve), flax oil and fish oil. In the formulation group, ALA peak reaches at its highest concentration in 2 h whereas in case of flaxseed oil group peak reaches at highest concentration after 4 h. This possibly indicates better absorption and bioavailability in formulation group. Fish oil was devoid of ALA, therefore, ALA level remained at baseline only. Further it is also seen that, EPA appears earlier within 2 h in formulation group whereas it takes 4 h for the flax oil group. This possibly indicates better conversion because of the co-factors present in the formulation. In case of DHA, formulation group showed better conversion as compared to flax oil and comparable to the fish oil group, indicating improved bioavailability in the formulation treated group possibly due presence of micronutrients/co-factors such as zinc, magnesium, vitamin B3 and B6. However this is a single dose preliminary study and long term bioavailability is required to confirm the same.
Fig. 4.
Single dose bioavailability study of formulation (5 g/serve) as compared to flax and fish oil
Summary and conclusion
Stabilized ω-3 FA fortified, micronutrient enriched, formulation was prepared by using flaxseed oil, water and sucrose ester (emulsifier). The developed formulation contained plant based ω-3 FA along with important micronutrients and antioxidants. Emulsification method is a most widely used technology and industrially viable also which is used in to deliver the novel nutrients in the present study. The developed formulations were characterized by several parameters followed by stability studies. Further, formulations were tested for acute toxicity in animal model as per OECD guidelines to confirm the safety of the formulations. Finally, in vivo single dose bioavailability study of formulation (5 g/serve) was performed to understand the fate of bioactive metabolites (ALA, EPA and DHA) as compared to flax and fish oil. The developed product can be explored in food/neutraceutical industries to enhance nutritive quality of the food products as well as to enhance bioavailability of ω-3 FA.
Acknowledgements
Authors are thankful to Science and Engineering Research Board (SERB), New Delhi for providing financial support (Grant Numbers: ECR/2016/002024).
Funding
Science and Engineering Research Board (SERB), New Delhi (Grant Numbers: ECR/2016/002024).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Association of Official Analytical Chemists (2000). AOAC, Gaithersburg, MD
- Barceló-Coblijn G, Murphy EJ. Alpha-linolenic acid and its conversion to longer chain n− 3 fatty acids: Benefits for human health and a role in maintaining tissue n− 3 fatty acid levels. Prog Lipid Res. 2009;48(6):355–374. doi: 10.1016/j.plipres.2009.07.002. [DOI] [PubMed] [Google Scholar]
- C. Alimentarius (1999) Codex standard for named vegetable oils. Codex Stan 210. https://www.fao.org/3/y2774e/y2774e04.htm.
- Carneiro HC, Tonon RV, Grosso CR, Hubinger MD. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J Food Eng. 2013;115(4):443–451. doi: 10.1016/j.jfoodeng.2012.03.033. [DOI] [Google Scholar]
- Chantrapornchai W, Clydesdale F, McClements DJ. Influence of droplet size and concentration on the color of oil-in-water emulsions. J Agric Food Chem. 1998;46(8):2914–2920. doi: 10.1021/jf980278z. [DOI] [Google Scholar]
- Chen F, Liang L, Zhang Z, Deng Z, Decker EA, McClements DJ. Inhibition of lipid oxidation in nanoemulsions and filled microgels fortified with omega-3 fatty acids using casein as a natural antioxidant. Food Hydrocoll. 2017;63:240–248. doi: 10.1016/j.foodhyd.2016.09.001. [DOI] [Google Scholar]
- Cho YH, Shim HK, Park J. Encapsulation of fish oil by an enzymatic gelation process using transglutaminase cross-linked proteins. J Food Sci. 2003;68(9):2717–2723. doi: 10.1111/j.1365-2621.2003.tb05794.x. [DOI] [Google Scholar]
- Codex Alimentarius Commission (1999) Report of the sixteenth session of the Codex Committee on fats and oils. https://www.codexalimentarius.org/codex-home/en/
- Duboisa V, Bretonb S, Lindera M, Fannia J, Parmentiera M. Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur J Lipid Sci Technol. 2007;109:710–732. doi: 10.1002/ejlt.200700040. [DOI] [Google Scholar]
- Europe WONCA. The European definition of general practice/family medicine. Barcelona: WONCA Europe; 2002. [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. doi: 10.1016/S0021-9258(18)64849-5. [DOI] [PubMed] [Google Scholar]
- Garaiova I, Guschina IA, Plummer SF, Tang J, Wang D, Plummer NT. A randomised cross-over trial in healthy adults indicating improved absorption of omega-3 fatty acids by pre-emulsification. Nutr Res. 2007;6(1):4. doi: 10.1186/1475-2891-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal A, Sharma V, Upadhyay N, Singh AK, Arora S, Lal D, Sabikhi L. Development of stable flaxseed oil emulsions as a potential delivery system of ω-3 fatty acids. J Food Sci Technol. 2015;52(7):4256–4265. doi: 10.1007/s13197-014-1370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde MV, Zanwar AA, Adekar SP. Omega-3 fatty acids: keys to nutritional health. Switzerland: Springer; 2016. [Google Scholar]
- Huang X, Kakuda Y, Cui W. Hydrocolloids in emulsions: particle size distribution and interfacial activity. Food Hydrocoll. 2001;15(4–6):533–542. doi: 10.1016/S0268-005X(01)00091-1. [DOI] [Google Scholar]
- Ibrahim OO. Functional oligosaccharides: chemicals structure, manufacturing, health benefits, applications and regulations. J Food Chem Nanotechnol. 2018;4(4):65–76. doi: 10.17756/jfcn.2018-060. [DOI] [Google Scholar]
- Jaiswal M, Dudhe R, Sharma PK. Nanoemulsion: an advanced mode of drug delivery system. Biotech. 2015;5:123–127. doi: 10.1007/s13205-014-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartal C, Unal MK, Otles S. Flaxseed oil-in-water emulsions stabilized by multilayer membranes: oxidative stability and the effects of pH. J Disper Sci Technol. 2016;37(12):1683–1691. doi: 10.1080/01932691.2016.1141294. [DOI] [Google Scholar]
- Klang V, Matsko NB, Valenta C, Hofer F. Electron microscopy of nanoemulsions: an essential tool for characterisation and stability assessment. Micron. 2012;43(2–3):85–103. doi: 10.1016/j.micron.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Mabel MJ, Sangeetha PT, Platel K, Srinivasan K, Prapulla SG. Physicochemical characterization of fructo oligosaccharides and evaluation of their suitability as a potential sweetener for diabetics. Carbohydr Res. 2008;343(1):56–66. doi: 10.1016/j.carres.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Matthäus B. Oxidation of edible oils. In: Decker EA, Elias RJ, McClements DJ, editors. Oxidation in foods and beverages and antioxidant applications: management in different industry sectors. Sawston: Woodhead Publishing; 2010. pp. 183–238. [Google Scholar]
- Mosqueira VCF, Legrand P, Pinto-Alphandary H, Puisieux F, Barratt G. Poly (D, L-lactide) nanocapsules prepared by a solvent displacement process: Influence of the composition on physicochemical and structural properties. J Pharm Sci. 2000;89(5):614–626. doi: 10.1002/(SICI)1520-6017(200005)89:5<614::AID-JPS7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Nidhin M, Indumathy R, Sreeram KJ, Nair BU. Synthesis of iron oxide nanoparticles of narrow size distribution on polysaccharide templates. Bull Mater Sci. 2008;31(1):93–96. doi: 10.1007/s12034-008-0016-2. [DOI] [Google Scholar]
- Organization for Economic Co-operation Development . Guidance document on acute oral toxicity testing. Paris: Environment Directorate OECD; 2001. pp. 1–24. [Google Scholar]
- Panagiotou T, Fisher R. Improving product quality with entrapped stable emulsions: from theory to industrial application. Challenges. 2012;3(2):84–113. doi: 10.3390/challe3020084. [DOI] [Google Scholar]
- Puranik SS. Emulsions of omega-3 fatty acids for better bioavailability and beneficial health effects. In: Hegde MV, Zanwar AA, Adekar SP, editors. Omega-3 fatty acids. Switzerland: Springer; 2016. pp. 27–139. [Google Scholar]
- Saunders AV, Davis BC. Garg M L (2012) Omega-3 polyunsaturated fatty acids and vegetarian diets. MJA Open. 2012;1(2):22–26. doi: 10.5694/mjao11.11507. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients. 2016;8(3):128. doi: 10.3390/nu8030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ued FV, Mathias MG, Toffano RBD, Barros TT, Almada MORV, Salomão RG, Coelho-Landell CA, Hillesheim E, Camarneiro JM, Camelo-Junior JS, Aragon DC, Moco S, Kussmann M, Kaput J, Monteiro JP. Vitamin B2 and folate concentrations are associated with ARA, EPA and DHA fatty acids in red blood cells of Brazilian children and adolescents. Nutrients. 2019;11(12):2918. doi: 10.3390/nu11122918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yary T, Voutilainen S, Tuomainen TP, Ruusunen A, Nurmi T, Virtanen JK. Omega-6 polyunsaturated fatty acids, serum zinc, delta-5- and delta-6-desaturase activities and incident metabolic syndrome. J Hum Nutr Diet. 2017;30(4):506–514. doi: 10.1111/jhn.12437. [DOI] [PubMed] [Google Scholar]
- Yi H, Cho H, Hwang KT, Shin BS. Physical and oxidative stability of flaxseed oil-fructo oligosaccharide emulsion. J Food Process Pres. 2015;39(6):2348–2355. doi: 10.1111/jfpp.12482. [DOI] [Google Scholar]
- Zanwar AA, Badhe YS, Bodhankar SL, Ghorpade PB, Hegde MV (2016) Omega-3 milk. In: Hegde MV, Zanwar AA, Adekar SP (ed) Omega-3 fatty acids, Springer, Switzerland, pp 45–50 10.1007/978-3-319-40458-5_4
- Zanwar AA, Hegde MV, Rojatkar SR, Bodhankar SL. Antihyperlipidemic activity of concomitant administration of methanolic fraction of flax lignan concentrate and omega-3-fatty acid in poloxamer-407 induced experimental hyperlipidemia. Ind Crop Prod. 2014;1(52):656–663. doi: 10.1016/j.indcrop.2013.11.041. [DOI] [Google Scholar]
- Zhu Q, Pan Y, Jia X, Li J, Zhang M, Yin L. Review on the stability mechanism and application of water-in-oil emulsions encapsulating various additives. Compr Rev Food Sci F. 2019;18(6):1660–1675. doi: 10.1111/1541-4337.12482. [DOI] [PubMed] [Google Scholar]