Abstract
A gas chromatography tandem mass spectrometry method was developed for simultaneous determination of 133 pesticides in Black pepper (Piper nigrum). QuEChERS (Quick, Easy, Cheap, Effective, Rugged and Safe) sample preparation method is preferred over multistep methods. Extraction was done by using acetonitrile followed by clean up using graphitized carbon, primary secondary amine and octadecyl silica (C18). Even after the cleanup, non-volatile co-extractives stick to the liner and column which results in affecting the performance of the instrument and volatile co-extractives impact the analysis by enhancing the analyte concentration. So we evaluated a dilution procedure to overcome the drawbacks. The limit of quantification of 0.01 mg kg−1 was achieved for fifty times diluted sample extract with S/N ≥ 10. The recovery was between 70 and 120% for 0.01, 0.025 and 0.05 mg kg−1 for fortified samples and corresponding precision was between 3 and 16% RSD. The seven-level calibration curve shows a regression co-efficient of 0.99.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04605-0) contains supplementary material, which is available to authorized users.
keywords: QuEChERS, Multi residue pesticides, Spices, Black pepper, GC-MS/MS
Introduction
India holds a prominent position in the world’s spice production. Black pepper is a popular spice produced in India and widely used for its culinary and medicinal properties. Pepper is used to improve appetite, digestion, to treat stomachache, heartburn, diarrhea and cholera. It is also used for lung problems including asthma, bronchitis and cough (Rama et al. 2018). The above properties make pepper, one of the most valuable spices in the world market and pepper accounts for 34% of the total spices traded internationally. India contributes 23% of the total world black pepper production and exports accounted to 2.7 billion US$ in 2017–2018(Spices board-India 2019). The quantity and value of pepper exported from India showed a fluctuating trend along with the price of pepper in domestic as well as international markets.
Being a crop cultivated in the tropical region of the country, a heavy infestation of pests and diseases cause a huge loss to the growers. For the management of such pests and diseases, growers are compelled to use various plant protection chemicals to safeguard their crop. In India, only 289 pesticides are registered with Central Insecticide Board and Registration Committee (CIB&RC 2020) for control of various pests and diseases with approved dosage. Out of these 289 pesticide formulations registered with CIB & RC, only one fungicides, Metalaxyl 8% + Mancozeb 64% is recommended in pepper for control of various fungal diseases and no insecticide is registered with CIB & RC for control of any insect pests in pepper. Hence, the growers are forced to use non recommended chemicals to protect their crop. This non-judicious use of different plant protection chemicals in pepper results in their residues above the regulatory limits in final produce and becomes a trade barrier. Further, being a spice produce generated after the processing involves drying, the residues in harvested produce may get further concentrated in final dry pepper, which aggravate the issue of the pesticide residues (Petersen et al. 1996; Shabeer et al. 2015; Sabale et al. 2014). Pesticides cause a potential health risk for consumers, resulting in food safety regulators across the globe setting up maximum residue limits (MRLs) for pesticides (EUR-Lex 2019a, b; JFSA 2019; USFDA 2019; UKFSA 2019). For example, European food safety authority pesticides database (European pesticide MRL) for black pepper, a list of 450 GC & LC amenable pesticide residues for monitoring and regulatory compliances are recommended (EUR-Lex 2019a, b). Hence, monitoring of pesticide residues is important to comply with the regulatory requirement and to ensure consumer safety which necessitate the requirement of comprehensive cost-effective matrix specific multi-residue analysis method in pepper.
Gas Chromatography-Tandem Mass spectrometry (GC–MS/MS) or Liquid Chromatography-Tandem Mass spectrometry (LC–MS/MS) are the most widely used technology for pesticide multi-residue analysis in various food matrices. Acetonitrile (QuEChERS method) (Anastassiades et al. 2003; Lehotay et al. 2005; Goon et al. 2019) or ethyl acetate (Banerjee et al. 2007) are the most widely used extraction solvent for multi-residue pesticide analysis in different matrices. However, in various spice matrices, the literature data preferred acetonitrile as the extraction solvent compared to ethyl acetate for various reasons. From literatures we find procedure for sample preparation and quantification methods for different matrices including various spices (Xue et al. 2013; Narong and Tiffany 2015; Angelika and Marek 2011; Jadhav et al. 2017; Shabeer et al. 2018; Chai and Elie 2013; Amate et al. 2010; Hakme et al. 2018). Spices in general and pepper in particular is considered as one of the dirtiest matrix in GC–MS/MS analysis due to its heavy matrix effect associated with co-extractives. Several researchers used different strategies to deal with matrix co-extractives in spices like dispersive solid phase extraction (d-SPE) clean-up (Shabeer et al. 2018), dilution of the sample extract (Amate et al. 2010), solvent exchange (Shabeer et al. 2018), freezing out prior to d-SPE cleanup (Rutkowska et al. 2018), SPE cleanup with hydrophilic-lipophilic-balance (HLB) cartridge (Goon et al. 2019) etc. either individually or in combination. However, the literature data are not adequate to deal with volatile and non-volatile co-extractives impacting on chromatographic performance in pepper matrix. During the experiment we observed frequent liner change, column trimming and to some extent source cleaning due to the presence of co-extractives. In this background, the current investigation aims to optimize and validate a multi-residue method for analysis of pesticide residues in pepper matrix by GC–MS/MS with special emphasis on reducing the matrix co-extractives (both volatile and non-volatile) by adopting various strategies.
Experimental
Chemicals and reagents
The certified reference standards (CRMs) of pesticides (assay 98.1–99.9%) were procured from Dr. Ehrenstorfer GmbH (Augsburg, Germany) and Sigma–Aldrich (Saint Louis, USA). Residue analysis grade extraction solvents such as acetonitrile (MeCN), dried ethyl acetate (EtOAc), acetic acid and analytical grade salts such as Magnesium sulfate (MgSO4), sodium chloride (NaCl), and sodium acetate (CH3COONa) were purchased from Thomas Baker (Mumbai, India). Dispersive solid phase (d-SPE) cleanup agents such as primary secondary amine (PSA), graphitized carbon black (GCB), bonded octadecyl silica (C18) were procured from Agilent Technologies, Santa Clara, USA.
Apparatus
Analytical balance (Axis AGN2004PR, LC/GC, India) was used for sample weighing and 1.5 L capacity mixer grinder (GX7, India Ltd. India) was used for sample comminution and homogenization. A high-speed refrigerated centrifuge (Beckman Coulter, Allegra X-22RA, Atlanta, US), micro centrifuge (Eppendorf-5424, Germany), nitrogen flash evaporator (SPEEDOVAP-LV-Takase Analytical Instruments, India) and a high-speed blender (IKA, Bangalore, India) were used at various stages of sample preparation.
Preparation of standard solution
A stock solution (10,000 ng mL−1) of individual standards were prepared by dissolving 25 mg each in 25 mL acetonitrile. A total of seven intermediate mixtures (containing 20–30 compounds each) of 1000 ng mL−1 concentration was prepared by diluting adequate quantity of each compound in acetonitrile. An intermediate total standard mixture of 10 µg mL−1 was prepared by diluting an adequate quantity of each intermediate mixture in acetonitrile. A nine-point solvent calibration standard (0.78, 1.56, 3.13, 6.25, 12.5, 25,50, 100, 200 ng mL−1)was prepared by serial dilution of appropriate stock solution using acetonitrile. The matrix-matched calibration standards of concentration (0.78, 1.56, 3.13, 6.25, 12.5, 25, 50, 100, 200 ng mL−1) were prepared by successive dilutions in acetonitrile extract of the blank matrix.
Gravimetric determination of co-extracts
5 mL of each crude and purified sample extracts were evaporated to dryness using regulated nitrogen flash and estimated gravimetrically.
Gas chromatography mass spectrometry
Sample analysis was performed using a gas Chromatograph (GC 7890B; Agilent Technologies, Santa Clara, US) equipped with 7693A auto-injector and hyphenated to a triple quadruple mass spectrometer (7010B; Agilent Technologies, Santa Clara, US). The system was controlled and monitored by mass hunter software (version B.07.01). A 20.75 min acquisition, constant flow mode, mid-point post-run backflush method was configured. Chromatographic separation was done using two HP-5MS column (15 m × 0.25 mm internal diameter, 0.25 mm film thickness) connected using a pressure connector to perform backflush (Gray and Teale 2010, Katerina and Philip 2012). The inlet pressure was maintained at 2 psi whereas the backflush pressure was set at 35.322 psi. The backflush flow to the inlet was 3.6 mL min−1 for which additional helium flow was supplied through a purged ultimate union. The backflush was carried out for 2.5 min after the completion of the analytical run. A single tapered liner with glass wool (5190–2292, Agilent Technologies, Santa Clara, US) was used. Helium was used as carrier gas at a constant flow rate of 1.2 mL min−1 for the first column and 1.44 mL min−1 for second column. 1 µL was introduced using the autosampler. Oven temperature programming, the initial temperature of 70 °C (1 min hold), ramped to 170 °C at 40 °C min−1 (0 min hold), then at 10 °C min−1 up to 310 °C (hold 3 min) resulting in a total run time of 20.75 min. The transfer line temperature was maintained at 300 °C. The programmable temperature inlet was operated in cold splitless injection mode and set at the initial temperature of 70 °C (0.1 min hold), raised to 325 °C at 450 °C min−1 (5 min hold) followed by a decrease to 250 °C at 10 °C min−1. The mass spectrometer was operated in Multi Reaction Monitoring (MRM) mode with acquisition starting from 4.4 min. Electron impact ionization (EI +) was achieved at − 70 eV and the ion source temperature was set at 300 °C. Method retention time locked using chloripyrifosmethyl (Katerina and Philip 2012). Selection of two transitions was made by acquiring matrix-matched calibration standard with the available transitions in terms of abundance, ion ratio and recovery (Supplementary Table I).
High pressure liquid chromatography (HPLC)
HPLC system equipped with a quaternary pump, variable wavelength detector (1290 Infinity II, Agilent Technologies, Santa Clara, US), C18 column with 100 mm length, 2.1 mm internal diameter and 1.8νm particle size ((P/N 959758-902, Agilent Technologies) was used. Mobile phase comprising acetonitrile: water (70:30) with 0.100 mL min−1 constant flow rate was set.
Sample preparation
Sample size and sample homogeneity
Control black pepper (2 kg) were crushed using the mixer grinder and 200 g of the blended sample was further homogenized in high-speed blender to get the homogenized fine powder (approximately 200 micron particle size) as per the procedure reported by (Shabeer et al. 2018) in cardamom. From this homogenized fine powder, 1.0 ± 0.1 g of the sample was taken, spiked with the targeted/test pesticide mixture for optimization of extraction procedure.
Procedure-I
1.0 ± 0.1 g of homogenized dried black pepper powder was weighed into a 50 mL plastic centrifuge tube, 10 mL of distilled water added, shook well for 1 min and allowed to stand for 30 min for matrix swelling. Then, 10 mL of acidified acetonitrile (1% acetic acid) was added and shaken for 1 min for extraction. To this sample,6 g MgSO4 (as dehydrating agent) and 1.5 g sodium acetate (as buffer also enables acetonitrile—water partition) was added. The samples were shaken vigorously for 1 min followed by centrifugation at 5000 rpm for 5 min to get the crude extract (Nestor et al. 2009; Stanislaw 2014).
Procedure-II
1.0 ± 0.1 g of homogenized dried black pepper powder into a 50 mL plastic centrifuge tube, added 10 mL distilled water and shaken well for 1 min. The sample was allowed to stand for 30 min for matrix swelling. 10 mL ethyl acetate and 10 g anhydrous MgSO4 added then shaken vigorously for 1 min and centrifuged at 5000 rpm for 5 min to get crude extract (Zeying et al. 2015, Steven et al. 2010).
Crude extract obtained from both the procedures were collected and further subjected to the following three dispersive solid phase (d-SPE) cleanup methods which differ in their sorbent.
Dispersive clean up optimization
Optimization-I
Each 1 mL of the crude extracts from the procedure I & II were transferred to a 2 mL tube containing 50 mg PSA, 50 mg C18, 7.5 mg GCB and 150 mg of MgSO4. Shook and vortexed for a minute, centrifuged at 10,000 rpm for 5 min. The purified upper layer sample extract was transferred into a vial for GC–MS/MS analysis.
Optimization-II
The polymer mix (Agilent 5982-1010) was activated by adding 5 mL of water and immediately transfer 5 mL of extract from extraction procedure-I. Since we used ethyl acetate in procedure-II, ethyl acetate and water are immiscible, so not considered for dispersive optimization. Extract from procedure-I was taken and vortexed for a minute and 5 mL of the upper layer was transferred to a 15 mL plastic centrifuge tube containing polish sorbent NaCl and MgSO4. Shaken vigorously for a minute, followed by centrifugation at 10,000 rpm for 5 min. The purified upper layer sample extract was transferred into a vial for GC–MS/MS analysis.
Optimization-III
1 mL of the crude extract from the procedure-I & II was transferred to 2 mL tube containing 50 mg PSA and 150 mg of MgSO4. Shook and vortexed for a minute followed by centrifugation at 10,000 rpm for 5 min. The purified upper layer sample extract was transferred into a vial for GC–MS/MS analysis.
Method validation
The performance of the optimized method was validated with respect to sensitivity in terms of LoD, LoQ, linearity, recovery, precision and matrix effect, as per SANTE guidelines (Agnesa et al. 2015).
Linearity and sensitivity
The linearity of all compounds in solvent standard, matrix standard, and diluted matrix standard was obtained by plotting the peak area against the concentration of the corresponding standards at 0.008, 0.016, 0.032, 0.062, 0.125,0.25, 0.5, 1 and 2 mg kg−1. The calibration curve was linear plotted on calibration curve fit weights by 1/x and origin by ignoring.
The sensitivity of the instrument was determined by LOD & LOQ. Limit of detection is the minimum concentration of each pesticide signal compared to blank matrix noise at same retention time giving S/N ≥ 3. The limit of quantification was determined by the same manner for the pesticides giving S/N ≥ 10.
Recovery and precision
The recovery was evaluated for 0.010, 0.025 and 0.050 mg kg−1 fortified samples (each level n = 6) and calculated against matrix-matched standard. Each analyte relative standard deviation (RSD) in three levels (n = 6) was calculated as precision.
Matrix effect
Matrix effect was determined by comparing the peak area of matrix-matched standard with the corresponding area in solvent standard using the following formula.
Result and discussion
Black pepper is one of the most complex matrices for pesticide residue quantification because of high amounts of volatile and non-volatile co-extractives of 27.7%, piperine − 7.4%, starch − 49.0%,(OJEU 2017) and 0.01% of xanthophyll pigment (Ravindran 2012). Rapid sample preparation techniques such as QuEChERS is being preferred for its result consistency. Subsequent 50 injections of the purified extracts, non-volatile deposits were observed in the liner which results in loss of sensitivity (Aruna and Baskaran 2010). To overcome the loss of sensitivity and to enhance the chromatographic performance the following evaluations were carried out.
Crude and purified extracts evaluation
Volatile co-extractives
On sample acquisition volatile co-extractives evaporate along with pesticides and impact analyte concentration by enhancing the signal. This was overcome by choosing matrix and enhancement free two MRM (multireaction monitoring) transitions for each targeted analyte carefully. For example, ethion MRM transitions were optimized by acquiring a 250 ng mL−1 solvent standard. The peak abundance order was 152.9 96.9 (100%); 124.9 96.9 (90%); 230.9 175.0 (66%); 230.9 129.0 (65%); 120.9 65.0 (45%) these transitions were optimized by varying collision energy. In the matrix-matched standard, the abundance pattern was found to be from 98%, 60%, 66%, 100%, and 40% respectively. The following two MRM transitions 230. 9 175.0; 152.9 96.9 were chosen for identification since ion ratio was 78 ± 5% across calibration points and 94% recovery was obtained for 0.01 mg kg−1 fortified sample extract.
Non-volatile co-extractives
Non-volatile co-extractives stuck to the liner and to an extent beginning of the column. Quantitative estimation of these co-extractives was done gravimetrically to optimize sample preparation. Crude extract co-extractives were 23.62 mg mL−1 and 25.50 mg mL−1. After dispersive purification, the co-extractives were between 11.7 and 14.85 mg mL−1. The concentration of co-extractives from the three dispersive cleanup procedure doesn’t show significant variation. With this concentration of non-volatile co-extractives if we inject the samples, it affects the performance of the chromatograph, so we tried a dilution procedure to reduce the co-extractives concentration.
Dilution procedure
Diluted matrix-matched standard and purified sample extract
Diluted matrix-matched standards were prepared by diluting 100 µL of each standard with 400 µL of acetonitrile. Hence 200, 100, 50, 25, 12.5, 6.25, 3.13 ng mL−1 corresponds to 0.4, 0.2, 0.1, 0.05, 0.025, 0.013 and 0.006 mg kg−1 in sample. The 0.78 ng mL−1 standard were not considered for dilution. Similarly, black pepper sample was extracted by taking 1 g of sample in 10 mL of acetonitrile, followed by dispersive cleanup purification. 100 µL of this purified sample extract is further diluted by adding 100, 200, 300, 400 µL of acetonitrile which resulted in 20, 30,40 and 50 times diluted.
The purified extract (11.7 mg mL−1 co-extractives) from the dispersive optimization-I was taken for further dilution. Fortified sample extract of 0.01 mg kg−1 was diluted to 5 times with acetonitrile, the co-extractives concentration was reduced to 2.85 mg mL−1. But we observed variation in results for 0.01 mg kg−1 fortified sample extract quantification by matrix-matched standard. This is due to different amount of matrix in both diluted sample extract and matrix-matched standard. Hence both matrix-matched standard and sample extract was diluted.
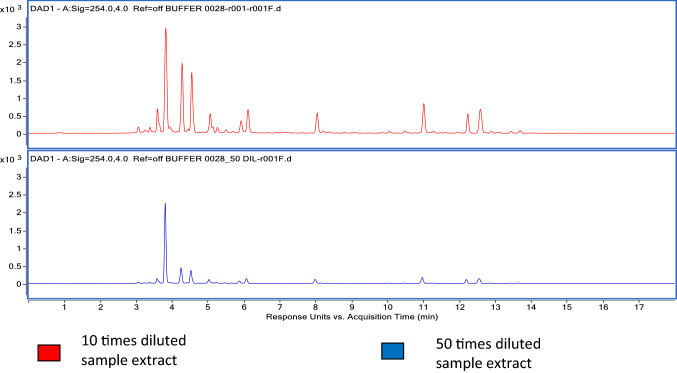
A comparison was done between crude extract, purified extract and 50 times diluted purified extract by acquiring in HPLC. In the case of non-volatiles co extractives, a 50% reduction of peak abundance was observed in purified extract compared to crude extract and after 50 times dilution of purified extract a further 60% reduction in peak abundance was observed (Fig. 1).
Fig. 1.
Non-volatile co-extractives by HPLC–UV
In volatile co-extractives a 40% reduction of peak abundance in purified extract was observed compared to crude extract but on further 50 times dilution of the purified extract the peak abundance reduction was witnessed in definite retention time region of 5–6.5, 10.5–11.0, 15–17 (Supplementary Fig. I).
Method validation
Linearity, LOD and LOQ
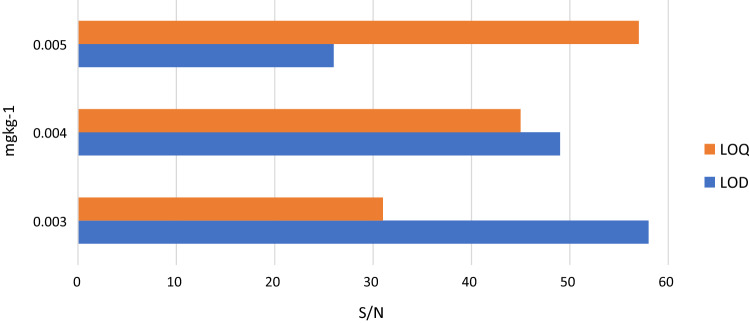
The seven-level linearity of corresponding sample concentration of diluted matrix-matched standard between 0.006 and 0.4 mg kg−1 was more than 0.9900 r2 for 89% compounds and between 0.9500 to 0.9800 r2 for 11% compounds. The instrument sensitivity (S/N 10:1) for all 50 times diluted targeted compounds were in the range of 0.003–0.005 mg kg−1 which was sufficient to achieve the LOD and LOQ of 0.01 mg kg−1 (Fig. 2).
Fig. 2.
Limit of detection and limit of quantification
Recovery and precision
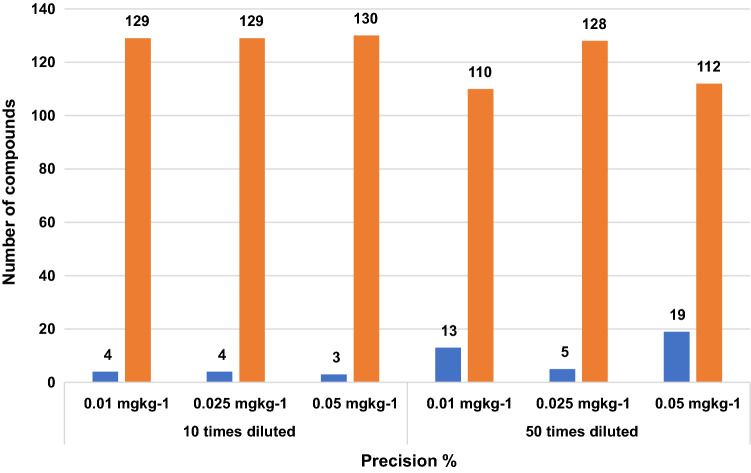
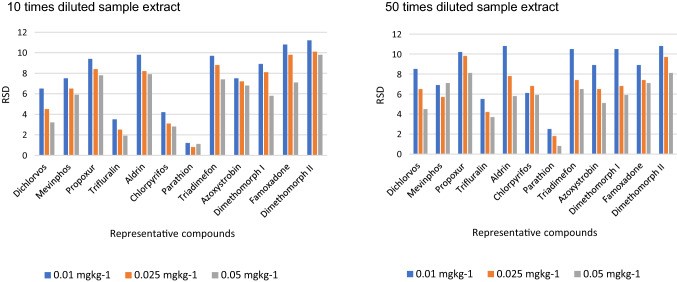
Recovery evaluation for 10 and 50 times diluted purified sample extract was compared. More number of compounds were shown lesser recovery in all three-level fortified study in 50 times diluted extract than 10 times diluted extract. The recovery below 70% and 70–120% for both 10 times & 50-times diluted extract was shown in (Fig. 3). The precision in terms of RSD for both dilution was compared and given in (Fig. 4) for first, middle and last eluted compounds. It was observed 10% more compounds shown lesser recovery in 50-times dilution on comparison with 10-times dilution but its corresponding precision for each compound was ≤ 20 RSD. The recovery of the pesticides edifenphos, permethrin, cyfluthrin and deltamethrin was lesser by 70% were as in 50 times dilution the recovery improved from 70 to 120%. In the case of heptachlor and fenvalerate the recovery was lesser by 70% even in 50 times dilution.
Fig. 3.
Recovery of ten times and fifty times diluted fortified samples
Fig. 4.
Precision of ten times and fifty times diluted extract
The method fit for purpose was evaluated by 0.01 mg kg−1 fortified sample of the instrument was expressed in terms of method limit of quantification (mLoQ), determined for each compound by comparing the area of pesticides spiked as signal (S) and blank matrix signal as noise (N). Supplementary Table II. Low responsive compounds in EI mass spectrometry such as BHC-Delta, imazalil, oxyfluorfen and fenthion sulfoxide, chlorfenapyr were smoothened for calculating S/N.
Repeated 100 acquisition each from 10 and 50 times diluted 0.05 mg kg−1 fortified sample extract in two different liner’s were done and the result was evaluated for peak shape and result consistency. Four first eluted, middle and last eluted pesticides were compared at 1st, 50th and 100th injection. 50-times diluted sample extract gives lesser non-volatiles in liner, better peak shape, result consistency in on 100th injection compared to10-times diluted sample extract given in (Fig. 5).
Fig. 5.
A—Appearance of liner after 100th injection a—10 times diluted, b—50 times diluted; B—Peak shape of cypermetrin and dieldrin after 100th injection; C—Response on continues 100 injection of 0.05 mg kg−1 fortified sample extract of ten times diluted and fifty times diluted
Matrix effect
The matrix effect of 50 ng mL−1 of diluted matrix matched standard with its corresponding diluted solvent standard was calculated. In comparison with matrix matched standard the matrix effect of diluted matrix standard was 5–38% lower than matrix match standard. Despite lower matrix effect due to dilution the ion ratio was proportional and within ± 30% for the same transition (Table 1). For example matrix standard of parathion the matrix effect was reduced by 50 times dilution whereas in dimethomorph matrix effect increased by 50 times dilution, the respective ion ratio was 55 ± 10% and 72 ± 9% across calibration standard. Since ion ratio was with in allowed limit of (± 30%) for all 133 compounds, further change in MRM, sample preparation optimization was not considered.
Table 1.
Matrix Effect impact on ion ratio by dilution
| Compounds | Rt | Compound | Matrix effect (ME %) | Ion ratio | ||
|---|---|---|---|---|---|---|
| 12.5 ng mL−1 matrix std. (10 times diluted) | 12.5 ng mL−1 matrix std. (50 times diluted) | 50 ng mL−1 matrix std. | 50 ng mL−1 matrix std. (50 times diluted) | |||
| Early eluting compounds | 4.66 | Dichlorvos | 63.21 | 60.04 | 81.9 | 83.0 |
| 5.58 | Mevinphos | 36.58 | 24.65 | 92.4 | 94.8 | |
| 6.82 | Propoxur | 25.98 | 18.95 | 69.2 | 71.0 | |
| 7.22 | Trifluralin | 32.65 | 27.98 | 81.1 | 84.6 | |
| Middle eluting compounds | 9.92 | Aldrin | 97.45 | 60.42 | 59.87 | 54.21 |
| 9.93 | Chlorpyrifos | 48.98 | 35.98 | 42.8 | 42.7 | |
| 9.96 | Parathion | 32.85 | 28.24 | 55.3 | 53.8 | |
| 9.99 | Triadimefon | 72.98 | 60.85 | 52.25 | 58.87 | |
| Late eluting compounds | 18.55 | Azoxystrobin | 65.85 | 51.85 | 80.26 | 81.32 |
| 18.60 | Dimethomorph I | 25.98 | 20.59 | 67.85 | 63.27 | |
| 18.62 | Famoxadone | 65.87 | 57.24 | 50.26 | 58.98 | |
| 18.88 | Dimethomorph II | 75.98 | 63.85 | 72.54 | 79.85 | |
This method was applied to real time market samples. 25 number of black pepper samples collected from open market and a homogenized sample extract was analyzed. 50% of samples contained residues of pendimethalin (0.01–0.02 mg kg−1), 40% contained chlorpyriphos (0.02–0.025 mg kg−1), and 35% contained ethion (0.04–0.065 mg kg−1). In all cases, the other residues were below their corresponding EU-MRL (Supplementary Fig. II). The 6.25 ng mL−1 diluted matrix matched standard extracted ion chromatogram of 133 pesticides is shown. (Supplementary Fig. III).
Conclusion
Simultaneous 133 pesticides quantification was demonstrated with improved method performance. The result consistency up to consecutive 100 injections, achieved by reducing the amount of co-extractives especially non-volatile co-extractives by dilution. This method improves the chromatographic performance by diluting the final extract but not involving reconstitution or evaporation. The 50 times diluted matrix control evident of lesser response as noise, was a better choice for S/N estimation since getting pesticide free blank is challenging. The limit of quantification for all 133 targeted pesticides is between 0.003 and 0.005 mg kg−1 and method fit for purpose was proven with S/N ≥ 10 a diluted 0.01 mg kg−1 fortified sample. The optimized GC–MS/MS method gave performance characteristics of recoveries within the acceptable range of 70–120% and repeatability (n = 6) was ≤ 20% at three spiking levels (0.01, 0.025, 0.05 mg kg−1). For pesticides lower than 70% recovery, consistent RSD (≤ 20% RSD) achieved.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agnesa P, Gerardo MD, Francisco JA, Roberto RG, Svetlana H, Antonia GF. Multifamily determination of pesticide residues in soya-based nutraceutical products by GC/MS–MS. Food Chem. 2015;173:796–807. doi: 10.1016/j.foodchem.2014.10.100. [DOI] [PubMed] [Google Scholar]
- Amate FC, Unterluggauer H, Fischer RJ, Fernández-Alba AR, Masselter S. Development and validation of a LC–MS/MS method for the simultaneous determination of aflatoxins, dyes and pesticides in spices. Anal Bioanal Chem. 2010;397(1):93–107. doi: 10.1007/s00216-010-3526-x. [DOI] [PubMed] [Google Scholar]
- Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int. 2003;86:412–431. doi: 10.1093/jaoac/86.2.412. [DOI] [PubMed] [Google Scholar]
- Angelika W, Marek B. Determiantion of pesticide residues in food matrices using the QuEChERS methodology. Food Chem. 2011;125:803–812. doi: 10.1016/j.foodchem.2010.09.094. [DOI] [Google Scholar]
- Aruna G, Baskaran V. Comparative study on the levels of carotenoids lutein, zeaxanthin and β-carotene in Indian spices of nutritional and medicinal importance. Food Chem. 2010;123:404–409. doi: 10.1016/j.foodchem.20. [DOI] [Google Scholar]
- Banerjee K, Oulkar DP, Dasgupta S, Patil SB, Patil SH, Savant R, Adsule PG. Validation and uncertainty analysis of a multi-residue method for pesticides in grapes using ethyl acetate extraction and liquid chromatography–tandem mass spectrometry. J Chromatogr A. 2007;1173:98–109. doi: 10.1016/j.chroma.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Chai LK, Elie F. A rapid multi-residue method for pesticide residues determination in white and black pepper (Piper nigrum L.) Food Control. 2013;32(1):322–326. doi: 10.1016/j.foodcont.2012.12.015. [DOI] [Google Scholar]
- CIB & RC (2020) Central insecticide board and registration committee: major use of pesticides. http://www.ppqs.gov.in/divisions/cib-rc/major-uses-of-pesticides. Accessed Apr 2020
- EUR-Lex (2019a) Official Journal of Regulation (2005), European union parliament, Regulation 396, Accessed 02 Jan 2019
- EUR-Lex (2019b) European union parliament. https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=homepage&language=EN. Accessed 03 Jan 2019
- Goon A, Shinde R, Ghosh B, Banerjee K. Application of automated mini–solid-phase extraction cleanup for the analysis of pesticides in complex spice matrixes by GC-MS/MS. J AOAC Int. 2019 doi: 10.5740/jaoacint.19-0202. [DOI] [PubMed] [Google Scholar]
- Gray BP, Teale P. The use of a simple back flush technology to improve sample throughput and system robustness in routine gas chromatography tandem mass spectrometry analysis of doping control samples. J Chromatogr A. 2010;1217:4749–4752. doi: 10.1016/j.chroma.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Hakme E, Lozano A, Uclés S, Gomez-Ramos MM, Fernández-Alba M. High-throughput gas chromatography-mass spectrometry analysis of pesticide residues in spices by using the Enhanced Matrix Removal-lipid and the sample dilution approach. J Chrom A. 2018;1573:28–41. doi: 10.1016/j.chroma.2018.08.046. [DOI] [PubMed] [Google Scholar]
- Jadhav M, Shabeer ATP, Nakade M, Gadgil M, Oulkar D, Arimboor R, Ramakrishna M, Banerjee K. Multiresidue method for targeted screening of pesticide residues in spice cardamom (Elettaria cardamomum) by liquid chromatography with tandem mass spectrometry. J AOAC Int. 2017;100:603–609. doi: 10.5740/jaoacint.17-0061. [DOI] [PubMed] [Google Scholar]
- JFSA(2019) Japanese food safety authority. https://www.mhlw.go.jp/english/topics/foodsafety/. Accessed 02 Jan 2019
- Katerina M, Philip LW. Strategies for the multi-residue analysis of 100 pesticides by liquid chromatography-triple quadrupole mass spectrometry. J Chromatogr A. 2012;1265:155–164. doi: 10.1016/j.chroma.2012.06.028. [DOI] [PubMed] [Google Scholar]
- Lehotay SJ, Mastovska K, Lightfield AR. Use of buffering and other means to improve results of problematic pesticides in a fast and easy method for residue analysis of fruits and vegetables. J AOAC Int. 2005;88:615–629. doi: 10.1093/jaoac/88.2.615. [DOI] [PubMed] [Google Scholar]
- Narong C, Tiffany H. Analysis of pesticides in olive oil using a modified QuEChERS method with LC-MS/MS and GC-MS/MS. J Regul Sci. 2015;01:16–35. [Google Scholar]
- Nestor E, Olatz Zuloaga MO, Luis JB, Patricia N. Retention-time locked methods in gas chromatography. J Chromatogr A. 2009;1216:1624–1629. doi: 10.1016/j.chroma.2008.12.038. [DOI] [PubMed] [Google Scholar]
- Official Journal of Guidance (2017), EUROPEAN COMMISSION, Document 11813
- Petersen B, Tomerlin JR, Barraj L. Pesticide degradation: exceptions to the rule. Food Tech. 1996;50:221–223. [Google Scholar]
- Rama S, Neeta R, Manu S, Akhlesh S. A magical medicinal fruit of piper Nigrum. World J Pharm Res. 2018;7:418–425. doi: 10.20959/wjpr20188-11748. [DOI] [Google Scholar]
- Ravindran PN. India handbook of herbs and spices. London: Woodhead; 2012. [Google Scholar]
- Rutkowska E, Lozowicka B, Kaczynski P. Modification of multiresidue QuEChERS protocol to minimize matrix effect and improve recoveries for determination of pesticide residues in dried herbs followed by GC–MS/MS. Food Anal Methods. 2018;11:709–724. doi: 10.1007/s12161-017-1047-3. [DOI] [Google Scholar]
- Sabale R, Shabeer TPA, Utture SC, Banerjee K, Jadhav MR, Oulkar DP, Adsule PG, Deshmukh MB. Dissipation kinetics, safety evaluation, and assessment of pre-harvest interval (PHI) and processing factor for kresoxim methyl residues in grape. Environ Monit Assess. 2014;186(4):2369–2374. doi: 10.1007/s10661-013-3544-1. [DOI] [PubMed] [Google Scholar]
- Shabeer TPA, Banerjee K, Jadhav M, Girame R, Utture S, Hingmire S, Oulkar D. Residue dissipation and processing factor for dimethomorph, famoxadone and cymoxanil during raisin preparation. Food Chem. 2015;170:180–185. doi: 10.1016/j.foodchem.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Shabeer TPA, Girame R, Utture S, Oulkar D, Banerjee K, Ajay D, Arimboor R, Menon KRK. Optimization of multi-residue method for targeted screening and quantitation of 243 pesticide residues in cardamom (Elettaria cardamomum) by gas chromatography tandem mass spectrometry (GC–MS/MS) analysis. Chemosphere. 2018;193:447–453. doi: 10.1016/j.chemosphere.2017.10.133. [DOI] [PubMed] [Google Scholar]
- Spices board-India (2019) (Indian union government spices promotion body). www.indianspices.com. Accessed Dec 2019
- Stanisław W. Validation and use of a QuEChERS-based gas chromatographic-tandem mass spectrometric method for multiresidue pesticide analysis in blackcurrants including studies of matrix effects and estimation of measurement uncertainty. Talanta. 2014;120:106–113. doi: 10.1016/j.talanta.2013.11.087. [DOI] [PubMed] [Google Scholar]
- Steven JL, Kyung AS, Hyeyoung K, Urairat K, Wusheng F, Katerina M, Eunha H, Natchanun L. Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J Chromatogr A. 2010;1217:2548–2560. doi: 10.1016/j.chroma.2010.01.044. [DOI] [PubMed] [Google Scholar]
- UKFSA (2019) United Kingdom food safety authority. http://www.hse.gov.uk/guidance/index.htm
- USFDA (2019) United States food and drug authority. https://www.fda.gov/default.htm
- Xue H, Mei H, XiaoHang D, XiaoFeng Y, Shengguo Y. A multi-residue method for the determination of 124 pesticides in rice by modified QuEChERS extraction and gas chromatography–tandem mass spectrometry. Food Chem. 2013;138:1198–1205. doi: 10.1016/j.foodchem.2012.11.089. [DOI] [PubMed] [Google Scholar]
- Zeying H, Lu W, Yi P, Ming L, Wenwen W, Xiaowei H, Xiaowei L. Determination of 255 pesticides in edible vegetable oils using QuEChERS method and gas chromatography tandem mass spectrometry. Food Chem. 2015;169:372–380. doi: 10.1007/s00216-016-0016-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.