Abstract
Purpose
Intrafollicular fluid (IFF) melatonin plays a decisive role in maintaining granulosa cells’ DNA integrity and protects them against apoptosis. It reduces oxidative stress and improves the oocyte quality with a higher fertilization rate.
Method
This prospective study investigated the antioxidant property of IFF melatonin and its impact on IVF outcome parameters. We also explored the relative expression of five microRNAs (miR-663b, miR-320a, miR-766-3p, miR-132-3p, miR-16-5p) and levels of cell-free DNA (cfDNA) by real-time PCR in unexplained infertile patients. We collected 425 follicular fluid (FF) samples containing mature oocytes from 295 patients undergoing IVF.
Results
Patients were subgrouped based on IFF melatonin concentration (group A ≤ 30 pg/mL, group B > 70 to ≤ 110 pg/mL, group C > 111 to ≤ 385 pg/mL). Our results showed that patients with ≤ 30 pg/mL IFF melatonin levels have significantly higher oxidative stress markers, cfDNA levels, and lower relative expression of miR-663b, miR-320a, miR-766-3p, miR-132-3p, and miR-16-5p compared to other subgroups (p < 0.001). Similarly, they have a low fertilization rate and a reduced number of high-quality day 3 embryos.
Conclusion
Findings suggest that the therapeutic use of melatonin produces a considerable rise in the number of mature oocytes retrieved, fertilization rate, and good-quality embryo selection. Furthermore, miRNA signature enhances the quality of embryo selection, thus, may allow us to classify them as non-invasive biomarkers to identify good-quality embryos.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-020-02010-2.
Keywords: Melatonin, miRNA expression, Cell-free DNA, Follicular fluid, Embryo quality, Unexplained infertility
Introduction
Infertility is a non-contracepting couple’s inability to achieve a clinical pregnancy after a year or more of regular unprotected sexual intercourse [1]. Infertility is said to be idiopathic when standard investigation such as tests for ovulation, tubal patency, and semen quality has not been seen abnormal, and no pathological condition is reported [2]. The exact causes of unexplained infertility are still under debate; however, factors such as oxidative imbalance extensively contribute to female infertility. In fact, an excessive quantity of reactive oxygen species (ROS) in the follicular fluid (FF) of unexplained infertile patients has been described previously [3]. Other studies have revealed low concentrations of antioxidants in the FF of unexplained infertile patients [4, 5]. Additionally, the increased ROS levels in the FF of infertile patients are negatively associated with oocyte maturation and embryo quality [6].
Oxidative stress is an incessant cause of DNA damage that modulates the expression of apoptosis-associated-microRNAs (miRNAs) in the granulosa cells of developing oocytes [7]. Owing to their wide plethora of functional activities and dynamic stability, miRNAs have a promising function as diagnostic and prognostic biomarkers [8]. MiRNAs are the most abundant form of extracellular RNAs in FF that can regulate the gene expression by directly targeting the messenger RNA (mRNA). More specifically, miR-663b, miR-766-3p, miR-132-3p, and miR-16-5p were involved in folliculogenesis, fertilization, oocyte maturation, and ovulation [9–16]. Prevailing evidence indicates that miR-320a is associated with embryo quality in the human FF and can influence the in vitro development of mouse embryos [17]. In spent culture media, miR-320a was significantly lower in poor-quality embryos than good-quality embryos [18]. It has been reported previously that miR-663b, miR-766-3p, miR-132-3p, and miR-16-5p were downregulated in FF retrieved from follicles which developed into poor-quality embryos compared with the high-quality embryos [10, 19]. Moreover, Sang et al. reported that miR-132-3p and miR-320a were associated with steroidogenesis and metabolic pathways in human FF [20].
Melatonin (N-acetyl-5-methoxytryptamine) is a potent immunomodulatory synthetic product of the pineal gland that is engaged in the management of circadian rhythm [21], sleep-wake cycle [22], blood pressure [23], and monitors mammalian reproductive activities [24]. Melatonin synthetic machinery is also present in extra-pineal organs such as muscles, adipose tissue, pancreatic beta cells, spleen, kidney, liver, heart, and reproductive organs, especially in the ovary and placenta [25–28]. More importantly, this ubiquitous indoleamine compound serves as a potent free radical scavenger [29]. Melatonin has been reported to neutralize oxidative stress by scavenging ROS and directly activating various endogenous antioxidant enzymes [30, 31]. Under moderate concentrations, ROS are responsible for mediating intercellular and intracellular signaling cascades, providing protection against apoptosis, while massive ROS production results in oxidative stress [32]. The oxidative damage in the FF microenvironment releases the cell-free DNA (cfDNA) fragments, reflecting the apoptotic events inside the ovarian follicles. The amount of cell-free DNA (cfDNA) in FF is significantly related to the oocyte’s quality and reflects the apoptotic events leading to cell damage [33].
Melatonin plays a vital role in the DNA integrity of granulosa cells, protects them against apoptosis, and stimulates estradiol and progesterone production, improving oocyte quality with a higher fertilization rate [34]. In human ovarian FF, melatonin’s concentration was almost threefold higher than blood plasma [35]. The concentration was reported highest in follicles ≥ 18-mm diameter than intermediate 15–16 mm and small follicles 9–12 mm [36]. Thus, an increased intrafollicular fluid (IFF) melatonin level protects oocyte from oxidative damage within the ovarian follicle. Moreover, melatonin supplementation (10−8, 10−7, and 10−9 M) in culture medium promotes embryo development in various species such as mice, bovine, and porcine [37–39].
Similarly, supplementation of in vitro maturation medium (IVM) with melatonin improves the oocyte maturation rate in human and subsequent IVF outcomes [40, 41]. Previous studies have revealed that this indoleamine compound has a vital role in the management of human fertility. In this context, some recent studies demonstrated the positive impacts of oral supplementation of melatonin during controlled ovarian stimulation (COS) on oocyte developmental competence and embryo quality [5, 42–45]. It has been demonstrated in many studies that exogenous melatonin has no harmful impact on rodents’ model during toxicity tests and is safe for humans, even in high doses [46–49]. Currently, limited studies have focused on the therapeutical potential of exogenous melatonin on IVF outcome parameters and miRNAs related to oocyte maturation and embryo quality in the human FF, which might serve as a potent non-invasive tool for predicting oocyte development capability in unexplained infertile patients.
Our study highlights a promising association between oral melatonin supplementation and oxidative stress. We also intended to test whether melatonin concentration in the follicular ambient microenvironment affects oocyte quality and embryo-developing potential. Moreover, we sought to explore five miRNAs’ relative expression (miR-663b, miR-320a, miR-766-3p, miR-132-3p, miR-16-5p) between high-quality and impaired-quality embryos. These miRNAs were selected based on previous studies demonstrating their role in oocyte maturation and embryo quality [10, 17].
Materials and methods
Participant’s selection
Subjects
This prospective study included 425 individual FF samples related to mature oocytes from 295 women (mean age: 33.87 ± 1.98 years) with unexplained infertility. The subjects were registered in the assisted reproductive center (Lahore Institute of Fertility and Endocrinology) affiliated with a tertiary-care hospital (Hameed Latif Hospital) between January 2017 and December 2018. The participants were subgrouped based on our data of IFF melatonin concentration: group A: ≤ 30 pg/mL (mean ± SD, 15.3 ± 9.3); group B: > 70 to ≤ 110 pg/mL (mean ± SD, 87.32 ± 25.3); and group C: > 111 to ≤ 385 pg/mL (mean ± SD, 181 ± 79). Patients of group A were not given oral melatonin supplementation, while groups B and C were received 3 and 6 mg/day melatonin orally. The intake of oral melatonin was started from the first visit to controlled ovarian stimulation until the oocyte retrieval day, i.e., approximately 45 days. Daily-dose-record of melatonin was self-reported by the patient. The intake of oral melatonin dose was selected in the light of previous studies [5, 43, 50]. This study was approved by the Institutional Review Board (IRB) in accordance with the Declaration of Helsinki. All patients provided written consent to participate.
Inclusion criteria
Infertile women who had failed to conceive after three intra-uterine inseminations (IUI) cycles and underwent not more than three IVF cycles by utilizing high-quality embryo, presented with a complete previous case history, have normal gynecological health with normal pelvic and abdominal ultrasonography, and exhibited normal blood endocrine hormones were included in this study. Moreover, male partners who had no history of genitourinary infection presented with normal semen culture and semen parameters (in accordance with the World Health Organization (WHO) criteria) were also included in this study [51].
The patients with unexplained infertility had gone through several diagnostic procedures to rule out the various infertility causes before participating in the study, and they declared as unexplained infertile patients, including hysterosalpingography, hysteroscopy, paternal and maternal karyotypes, cervical cultures for chlamydia, ureaplasma, mycoplasma, and complete endocrine status. Similarly, many blood tests were also conducted to diagnose the viral, bacterial, fungal, and parasitic infections, including cytomegalovirus, human immunodeficiency virus, hepatitis (B and C), rubeola, brucellosis, listeriosis, toxoplasmosis, syphilis, and vaginal candidiasis.
Exclusion criteria
The exclusion criteria included the presence of hereditary or acquired thrombophilic disorders, body mass index (BMI) ≥ 35 kg/m2, hyperandrogenemia, liver or kidney diseases, heart diseases, previous history of irregular menses, sexual dysfunction, anemia, tubal pathology, metabolic disorders, hypersensitivity to melatonin, endometriosis, elevated levels of CA-125, and autoimmune diseases. Moreover, luteal phase defects and endocrine hormones’ disorders were also excluded from the study.
Assessment of clinical parameters
BMI was calculated based on height and weight. While baseline hormones such as follicular stimulating hormone (FSH), luteinizing hormone (LH), 17β-estradiol (E2), thyroid-stimulating hormone (TSH), and anti-Mullerian duct hormone (AMH) were assessed on the second day of the menstrual cycle through electrochemiluminescence immunoassay, according to the manufacturer’s instructions (Elecsys® Roche Diagnostics, Indianapolis, USA). The antral follicle count (AFC) was assessed using transvaginal ultrasonography (TVS) on the second or third day of the menstrual cycle.
Therapeutic regimen
To minimize the possible confounding bias by varied controlled ovarian stimulation (COS) procedures, we only include patients in which ovarian stimulation was done through long GnRH agonist (decapeptyl®: ATCO pharma) administered in the middle of the luteal phase of the previous cycle. Ovarian stimulation with rFSH was evaluated by TVS and by quantifying serum 17β-estradiol level. A single dose of human chorionic gonadotrophin (6500–10,000 IU: Merk Serono, Lyon, Spain) was injected when more than two follicles reached a mean diameter of 18 mm or more.
Follicular fluid collection and estimation of melatonin and E2 concentration
Automated volume-count-sonography was used to evaluate the average volume (cm3) as well as the average diameter (mm) of each ovarian follicle, measured from three orthogonal planes (X, Y, Z). We assessed oocyte maturation based on measurements of follicular volume [52]. Mature follicles with a diameter of ≥ 18 mm and a follicular volume of 2.00–3.5 cm3 were included in this study [53]. However, FF with blood content was excluded from further analysis. After 36 h of hCG treatment, oocytes were retrieved by preventing flushing. FF samples of mature MII-oocytes were centrifuged separately at 1500×g for 20 min at 4 °C by preventing high-intensity bright light exposure. The supernatant was filtered through a 0.85-μL filter to remove cellular debris and stored immediately as aliquots of 500 μL × 2 at − 80 °C. Each mature oocyte, its related embryo, and FF sample were handled separately in the IVF laboratory. Finally, mature oocytes were subject to intracytoplasmic sperm injection (ICSI) procedure. Melatonin and E2 concentrations were evaluated by diluting FF samples 1:100 through radioimmunoassay kits (MP® diagnostics, Santa Ana, CA, USA). Intra-assay variations for melatonin and E2 were < 10%.
Assessment of embryo quality
Fertilization check was done 18–24 h after ICSI, and the embryo quality was determined through morphological grading, using standard criteria based on cytoplasmic appearance, the extent of fragmentation, number, and regularity in the symmetry of blastomeres by experienced embryologist [54].
RNA extraction from follicular fluid and relative expression analysis by RT-qPCR
A total of 500-μL frozen aliquots were thawed on ice, and cell debris was removed by centrifugation for 20 min at 3000×g at 4 °C. RNA was extracted from individual follicles using the Silica-based membrane purification technique (miRNAeasy kit, Qiagen, USA) following the given instructions, except that we diluted the sample at a 3:1 ratio with XBP buffer to optimize its use with the FF. Total RNA was dissolved in 30 μL of RNAs free water, and its concentration was measured through a Nanodrop-ND-1000 spectrophotometer (NanoDrop Technologies, USA). Expression-based digital gel electrophoresis (Bio-Rad, USA) was also used to confirm the total RNA concentration. MicroRNA profiling and data normalization were achieved as narrated by (Mestdagh et al. 2009). Complementary DNA (cDNA) was generated using the TaqMan MicroRNA reverse transcription kit (Life Technologies, USA) in combination with RNA specific stem-loop Megaplex primers (Applied Biosystems). A total of 15 μL reaction mixture contains 5 μL of a sample (10 ng miRNA), 0.5 μL of dNTP (100 mM), 1.5 μL RT buffer (10X), 1 μL/50 IU of multiScribe RT enzyme, 0.19 μL of RNase inhibitor, 3 μL of stem-loop RT primers, and 4.16 μL of nuclease-free water. Reverse transcription was performed in pulsating RT reaction, 40 cycles of 16 °C for 2 min, 42 °C for 60 s, and 50 °C for 60 s. Inactivation of reverse transcriptase was done at 85 °C for 5 min and hold step at 4 °C. Amplification was done with the following conditions: enzyme inactivation at 95 °C for 10 min and 40 cycles of two thermal amplification steps of 95 °C for 15 s, 60 s for 1 min and a hold step at 4 °C. Q-PCR was duplicated for each sample using a CFX-96® touch RT-PCR detection system (Bio-Rad, Life Sciences, USA). We used the Allele ID software® to design primers and probs. For quantification of follicular fluid miRNA expression levels, PCR reaction was performed in a total volume of 20 μL, having 3 μL of cDNA, 10 μL of TaqMan Universal PCR MasterMix (Applied Biosystems), 0.8 μL of each primer, and 5.4 μL double-distilled water. Amplification was carried out in a 96-well plate, and thermocycling conditions were10 min at 95 °C for enzyme activation, followed by 45 cycles of 95 °C for 20 s, 60 °C for 60 s. MiRNA expression levels were normalized against the expression of miR-16, which was used as an internal control because of its constant expression in FF samples. The relative expression of the five miRNAs such as miR-320a, miR766-3p, miR-132-3p, miR-16-5p, and miR-663b was calculated using the equation 2−∆Ct, while ∆Ct = Ct target miRNA − Ct miR-16. To calculate the fold change (FC), we estimated the relative expression levels between high-quality and impaired-quality embryo on day 3 using the 2−∆∆Crt formula [10].
Extraction and assessment of follicular fluid cell-free DNA
Cell-free DNA was quantified, as previously described [55]. For cfDNA extraction, each FF sample was diluted with an equal volume of buffer solution (Tween-20, Tris-50 mmol/L, EDTA-1 mmol) and incubated with proteinase K (Qiagen) at 55 °C for at least 30 min, followed by inactivation at 98 °C for 10 min. After denaturation, each FF sample was centrifuged at 3000 rpm for 15 min and then immediately stored at − 80 °C until quantification. The cfDNA concentration in each follicle with mature oocyte was estimated relative to the corresponding amplification of β-globin and GAPDH measured by the real-time PCR-SYBR green detection method as previously described [56].
Antioxidant status and oxidative stress marker measurements in follicular fluid samples
The frozen (− 80 °C) FF samples were thawed and evaluated for oxidative status. Average values of triplicate measurements were carried out from each FF sample to avoid interassay variations. ROS levels were measured by chemiluminescence assay using luminol (5-amino-2,3-dihydro-1,4-phthalazinedione) as a prob [57]. The total antioxidant capacity (TAC) was assessed using the colorimetric assay based on the manufacturer’s instructions (BioVision, Inc., CA, USA). Lipid peroxidation was evaluated by calculating the concentration of thiobarbituric acid reactive substances (TBARS) [58] while 8-hydroxy-2′-deoxyguanosine (8-OHdG) was measured using a kit based on the manufacturer’s instructions (BioVision, Inc., CA, USA). Both TBARS and 8-OHdG values were expressed as micromolar/liter and nanogram/milliliter, respectively.
Pathway analysis
We performed in silico analysis to predict miRNA targets using the web-based bioinformatics tool DIANA miRPath-v3 available on http://snf-515788.vm.okeanos.grnet.gr. Pathways were identified in both the regression analysis and fold change. The results were demonstrated as a heat map. The more intense red color directed an increased probability that a specific miRNA targets a unique pathway supplemented with target genes.
Statistical analysis
Baseline characteristics are presented as means ± SD, number percentage [n%], and median with 95% population limits as applicable. We used the Kruskal-Wallis/two-tailed test to explore the outcome differences in parameter levels between patients with low (≤ 30 pg/mL), intermediate (> 70 to ≤ 110 pg/mL), and high intrafollicular fluid (IFF) melatonin concentration (> 111 to ≤ 385 pg/mL). Based on evaluating the normality of the distribution by the Kolmogorov-Smirnov test and the Shapiro-Wilk test, we used the Mann-Whitney U test to determine the pairwise comparison between different groups. The χ2 test was used to address the categorical variables. The Spearman rank test was used to determine the correlation between IFF melatonin levels and other parameters. Receiving operating characteristic (ROC) curves were used to calculate AUC with a 95% confidence interval (Cl). The sensitivity and specificity for optimal cut-off were calculated using the XLSTAT 2020 software. SPSS (version 27; SPSS Inc., Chicago, IL, USA) was used for further statistical analysis. p < 0.05 was considered statistically significant.
Results
Baseline clinical characteristics of participants
The demographic parameters of participants are listed in Supplementary Table 1. Patients were divided into three groups based on IFF melatonin concentration: group A: ≤ 30 pg/mL; group B: > 70 to ≤ 110 pg/mL; and group C: > 111 to ≤ 385 pg/mL. Of the 425 oocytes with associated FF samples, 55 oocytes did not fertilize nor reach the 1PN stage. From the results of our study, it was evident that increased concentration of cfDNA is found in FF samples of group A (median, 95% Cl 2.01 (1.66; 3.42)) compared to groups B (median, 95% Cl 1.03 (0.41; 1.98)) and C (median, 95% Cl 0.86 (0.11; 1.03; p < 0.001)). Subsequently, ROS, TBARS, and 8-OHdG were significantly higher in group A than in group B and C patients (p < 0.001). Similarly, we observed that patients exhibiting decreased IFF melatonin concentration (≤ 30 pg/mL) have a significantly lower total antioxidant capacity [median, 95% Cl 238 (198; 305); Kruskal-Wallis test, p < 0.001] (Table 1). However, we could not find any significant association among subgroups of patients regarding BMI, endometrial eco (mm), and baseline endocrine parameters such as FSH, AMH, LH, and TSH levels. Additionally, we also screened for five selected microRNAs and detected miR-663b in 266/295 samples, miR-320a (284/295 samples), miR-766-3p (279/295 samples), miR-132-3p (268/295), and miR-16-5p in 273 out of 295 samples.
Table 1.
Relationship between different concentrations of melatonin and intrafollicular parameters
| Characteristics | Group A (intrafollicular melatonin concentration ≤ 30 pg/mL) | Group B (intrafollicular melatonin concentration > 70 to ≤ 110 pg/mL) | Group C (intrafollicular melatonin concentration > 111 to ≤ 385 pg/mL) | Kruskal-Wallis test (p value*) | p value** |
|---|---|---|---|---|---|
| Number of women [n (%)] | 97 (32.8) | 145 (49.1) | 53 (17.9) | – | – |
| Age (years) (mean ± SD) | 31.85 ± 2.11 | 32.10 ± 3.03 | 30.14 ± 4.41 | 0.032 | – |
| BMI (kg/m2) (mean ± SD) [median (95% population limit)] |
27.52 ± 0.99 22.33 (19.8; 23.8) |
26.29 ± 1.77 21.9 (20.8; 23.8) |
25.89 ± 2.25 22.01 (19.75; 23.13) |
0.455 | – |
| Intrafollicular parameters | |||||
| Intrafollicular E2 level (ng/mL) (mean ± SD) [median (95% population limit)] |
411.2 ± 113.69 389.3 (295.7; 524.2) |
598.15 ± 258.74 566.1 (488.6; 830.1) |
745.36 ± 288.98 698.3 (497; 915.3) |
< 0.001 | < 0.001a, b, 0.002c |
| Intrafollicular cfDNA (ng/μL) (mean ± SD) [median (95% population limit)] |
2.97 ± 1.36 2.01 (1.66; 3.42) |
0.97 ± 0.85 1.03 (0.41; 1.98) |
0.81 ± 0.52 0.86 (0.11; 1.03) |
< 0.001 | < 0.001a, b |
| Intrafollicular antioxidant status and oxidative stress markers [median (95% population limit)] | |||||
| ROS (cpm) | 38.98 (25.8; 77.9) | 8.98 (10.98; 15.09) | 6.89 (3.09; 10.99) | < 0.001 | < 0.001a, b |
| TAC (μM/L) | 238 (198; 305) | 965 (698; 1067) | 1275 (883; 1765) | < 0.001 | < 0.001a, b, 0.003c |
| TBARS (μM/L) | 1.98 (1.45; 2.87) | 0.95 (0.60; 1.02) | 0.65 (0.45; 0.87) | < 0.001 | < 0.001a, b |
| 8-OHdG (ng/mL) | 1.68 (0.89; 2.12) | 0.97 (0.76; 1.09) | 0.71 (0.43; 0.91) | < 0.001 | < 0.001a, b |
| Nd [n (%)] | |||||
| miR-663b | 74 (76.2) | 134 (92.4) | 47 (88.6) | – | – |
| miR-320a | 83 (85.5) | 141 (97.2) | 51 (96.2) | – | – |
| miR-766-3p | 69 (71.1) | 129 (88.9) | 49 (92.4) | – | – |
| miR-132-3p | 62 (63.9) | 135 (93.1) | 50 (94.3) | – | – |
| miR-16-5p | 52 (53.6) | 127 (87.5) | 48 (90.5) | – | – |
Intrafollicular data for patients are also subjected to statistical differences
E2 17β-estradiol, ROS reactive oxygen species, TAC total antioxidant capacity, LPO lipid peroxidation, TBARS thiobarbituric acid reactive substances, 8-OHdG 8-hydroxy-2-deoxyguanosine
p* Kruskal-Wallis test, p** pairwise comparisons between subgroups, and p < 0.05 considered statistically significant
aGroup A/C
bGroup A/B
cGroup C/B
dNumber of samples in which each miRNA was detected out of a total number of samples analyzed
Intrafollicular melatonin concentration can predict IVF outcome parameters
The characterization of IVF outcome parameters based on a pairwise comparison test between the groups is shown in Table 2. Patients with ≤ 30 pg/mL IFF melatonin levels have significantly decreased IVF outcome parameters, particularly they have a low fertilization rate, a reduced number of mature oocytes, and high-quality day 3 embryos compared to other subgroups. Our results showed that IVF outcome parameters were better in group B and achieved a maximum in group C (Kruskal-Wallis test, p < 0.001) with the same rising tendency as the IFF melatonin levels between the groups (Kruskal-Wallis test, p < 0.001). Correlation of IFF melatonin concentration on IVF outcome parameters was given in Supplementary Table 2, which shows that melatonin levels have a positive correlation with the number of MII- oocytes (rs = 0.712; p < 0.001), normal fertilized oocytes (rs = 0.731; p < 0.002), early cleaved zygotes (rs = 0.697; p < 0.001), blastomeres (6–8 cells) with regular symmetry (rs = 0.641; p < 0.001), and high-quality day 3 embryos (rs = 0.745; p < 0.003) and have a negative correlation with fragmentation rate (rs = − 0.812; < 0.001).
Table 2.
Association between intrafollicular melatonin levels and IVF outcome parameters
| Characteristics | Group A (intrafollicular melatonin concentration ≤ 30 pg/mL) | Group B (intrafollicular melatonin concentration > 70 to ≤ 110 pg/mL) | Group C (intrafollicular melatonin concentration > 111 to ≤ 385 pg/mL) | Kruskal-Wallis test (p value*) | p value** |
|---|---|---|---|---|---|
| No. of follicles with > 18-mm size (mean ± SD) [median (95% population limit)] |
15.91 ± 2.43 13.2 (12.11 ± 17.12) |
16.18 ± 2.12 13.5 (8.9; 16.21) |
17.31 ± 1.08 14.1 (9; 17) |
0.165 | – |
| No. of retrieved oocytes (mean ± SD) [median (95% population limit)] |
13.34 ± 3.88 11.6 (2; 14) |
14.09 ± 2.90 12 (9; 15) |
15.4 ± 2.09 14 (11; 16) |
0.543 | – |
| No. of MII- oocytes (mean ± SD) [median (95% population limit)] |
4.41 ± 0.94 4.11 (1–4.5) |
10.12 ± 1.11 9.83 (6.89; 11.11) |
13.31 ± 2.11 12.12 (10.21; 15.12) |
< 0.001 | < 0.001ab |
| No. of fertilized oocytes (mean ± SD) [median (95% population limit)] |
3.99 ± 0.95 3.21 (1; 3.5) |
9.98 ± 2.12 8.11 (6.98; 10.87) |
12.98 ± 2.01 11.91 (9.07; 14.41) |
< 0.001 | < 0.001a, 0.003b |
| No. of normal fertilized oocytes (mean ± SD) [median (95% population limit)] |
3.16 ± 0.34 2.98 (1; 3) |
9.65 ± 1.98 8.69 (6.32; 9.21) |
12.39 ± 1.88 11.01 (9.10; 14.13) |
< 0.001 | < 0.001abc |
| No. of early cleaved zygotes (mean ± SD) [median (95% population limit)] |
2.10 ± 0.54 1.89 (1; 2.8) |
8.91 ± 1.09 8.21 (7.09; 9.01) |
11.59 ± 1.87 10.61 (8.14; 12.58) |
< 0.001 | < 0.001abc |
| No. of blastomeres (6–8 cells) with regular symmetry at day 3 (mean ± SD) [median (95% population limit)] |
2.01 ± 0.12 1.62 (1; 2) |
7.26 ± 1.01 7.15 (6.09; 8.81) |
10.84 ± 1.88 10.2 (9.18; 11.86) |
< 0.001 | < 0.001abc |
| No. of high-quality day 3 embryos (mean ± SD) [median (95% population limit)] |
1.06 ± 0.76 1 (0; 2) |
6.32 ± 1.43 6.21 (5.31; 6.81) |
9.65 ± 1.92 7.94 (6.98; 9.87) |
< 0.001 | < 0.001a, 0.004b |
| No. of embryos suitable for transfer (mean ± SD) [median (95% population limit)] |
1.03 ± 0.65 1.01 (1–2.5) |
6.22 ± 0.98 6.18 (4.87; 6.58) |
9.53 ± 1.68 7.68 (7.09; 9.13) |
< 0.001 | < 0.001abc |
| Fragmentation rate (%) | 20–25 | 8–15 | ≤ 10 |
Intrafollicular data for patients are also subjected to statistical differences
p* Kruskal-Wallis test, p** pairwise comparisons between subgroups, and p < 0.05 considered statistically significant
aGroup A/C
bGroup A/B
cGroup C/B
Intrafollicular cfDNA content and IVF outcome parameters among idiopathic patients
The significant impact of IFF melatonin concentration on cfDNA content is given in Table 3, which shows that the higher IFF melatonin level significantly reduces the cfDNA content between the groups. Among the analyzed idiopathic patients, no statistically significant differences have existed for the association of cfDNA concentration and the number of retrieved oocytes, normally fertilized oocytes, and early cleaved zygotes. However, when we compared the IVF outcome parameters such as the number of MII- oocytes, blastomeres with a regular symmetry, high-quality day 3 embryos, and fragmentation rate, we observed that cfDNA content was significantly higher in group A than groups B and C (p < 0.001) (Table 3). Studying the correlation between cfDNA levels and IVF outcome parameters shows that the highest cfDNA levels are related to the lowest IVF outcome parameters and decreased melatonin concentration (Supplementary Table 3 and Fig. 1S).
Table 3.
Association between cfDNA concentration in follicular fluid of mature follicles and IVF laboratory parameters
| Characteristics | Intrafollicular cfDNA concentration (ng/μL) in group A | Intrafollicular cfDNA concentration (ng/μL) in group B | Intrafollicular cfDNA concentration (ng/μL) in group C | Kruskal-Wallis test (p value*) | p value** |
|---|---|---|---|---|---|
| Retrieved oocytes (mean ± SD) [(95% population limit)] | 2.91 ± 0.78 [1.5; 2.9] | 2.53 ± 0.43 [1.02; 2.78] | 2.21 ± 0.13 [1.01; 2.5] | 0.421 | – |
| MII- oocytes (mean ± SD) [(95% population limit)] | 2.00 ± 0.86 [0.98; 2.18] | 1.64 ± 0.97 [0.78; 1.93] | 1.53 ± 0.32 [0.98; 1.82] | < 0.001 | < 0.001a |
| Fertilized oocytes (mean ± SD) [(95% population limit)] | 1.86 ± 0.98 [1.21; 1.98] | 1.51 ± 0.65 [0.98; 1.80] | 1.43 ± 0.14 [0.91; 1.73] | 0.214 | – |
| Normal fertilized oocytes (mean ± SD) [(95% population limit)] | 1.81 ± 0.71 [1.14; 1.86] | 1.48 ± 0.88 [0.92; 1.91] | 1.40 ± 0.77 [0.81; 1.69] | 0.143 | – |
| Early cleaved zygote (mean ± SD) [(95% population limit)] | 1.78 ± 0.65 [1.08; 1.81] | 1.32 ± 0.54 [0.87; 1.53] | 1.01 ± 0.62 [0.93; 1.32] | 0.145 | – |
| Blastomeres (6–8 cells) with regular symmetry at day 3 (mean ± SD) [(95% population limit)] | 1.98 ± 0.54 [1.02; 2.31] | 0.98 ± 0.76 [0.19; 1.09] | 0.51 ± 0.98 [0.16; 0.99] | < 0.001 | < 0.001a, 0.002b |
| High-quality day 3 embryos (mean ± SD) [median (95% population limit)] | 1.59 ± 0.97 [1.04; 1.94] | 0.88 ± 0.12 [0.15; 1.13] | 0.43 ± 0.31 [0.49; 0.99] | < 0.001 | < 0.001a, 0.01bc |
| Embryos suitable for transfer (mean ± SD) [(95% population limit)] | 1.81 ± 56 [1.12; 1.99] | 1.46 ± 0.49 [0.56; 1.01] | 0.91 ± 0.64 [0.71; 0.88] | 0.214 | – |
| Fragmentation rate (≤ 25%) | 1.91 ± 0.76 [1.03; 1.96] | 0.92 ± 0.97 [0.17; 0.88] | 0.49 ± 0.29 [0.17; 0.53] | < 0.001 | < 0.001a, 0.003bc |
Group A (intrafollicular melatonin concentration ≤ 30 pg/mL), group B (intrafollicular melatonin concentration > 70 to ≤ 110 pg/mL), group C (intrafollicular melatonin concentration > 111 to ≤ 385 pg/mL). Intrafollicular data for patients are also subjected to statistical differences
p* Kruskal-Wallis test, p** pairwise comparisons between subgroups, and p < 0.05 considered statistically significant
aGroup A/C
bGroup A/B,
cGroup C/B
Effect of melatonin concentration on intrafollicular oxidative balance and day 3 embryo quality
The association of IFF melatonin levels and oxidative stress markers is shown in Supplementary Table 4. The comparison between different subgroups shows significant differences. Besides the expected differences regarding oxidative status, patients with lower melatonin concentration (≤ 30 pg/mL) have higher levels of oxidative stress markers and exhibited a higher number of impaired-quality day 3 embryos than patients with moderate to high levels of melatonin. Of note, TAC levels have markedly increased in superior-quality day 3 embryos [median (95% Cl 1164.3 (877.45; 1467.75); p < 0.001)] developed from oocytes containing higher levels of IFF melatonin (> 111 pg/mL). Likewise, they distinctively exhibit a positive correlation (R2 = 0.870; p < 0.001) (Fig. 2S).
Differential expression profile of miRNAs related to oocyte maturation and embryo quality between the groups
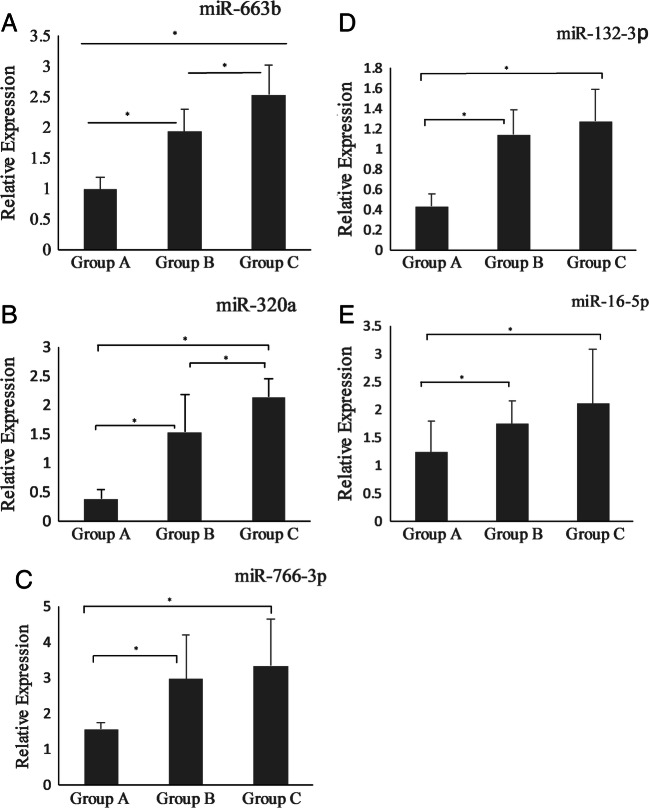
MiRNAs have shown different expression patterns that vary from groups A to C with the same trend as the melatonin levels differ among the groups (see Fig. 1). As shown in Table 4, the relative expression levels of miR-663b, miR-320a, miR-766-3p, miR-132-3p, and miR-16-5p were significantly upregulated in groups B and C (Raw Ct < 30) compared to group A (Raw Ct > 30). In group A, the relative expression levels of miR-766-3p and miR-132-3p were significantly reduced (p < 0.002) in comparison to groups B and C, while the relative expression of miR-766-3p and miR-132-3p in FF of groups B and C was slightly upregulated but did not reach the significant threshold (see Fig. 1c, d). To examine the possible correlation between miRNAs in FF and day 3 embryo quality, we compared cases of high-quality embryos to impaired-quality embryos. The correlation analysis results were summed up in Supplementary Table 5, which shows a significant positive correlation between miRNAs’ relative expression and day 3 embryo quality. In all studied subjects, when we compared the fold change (2−∆∆Crt) of miRNAs in FF samples between high-quality and impaired-quality embryos on day 3, we observed that miR-663b (FC = 1.97, p = 0.02), miR-320a (FC = 2.01, p = 0.01), miR-766-3p (FC = 2.52, p = 0.03), miR-132-3p (FC = 2.41, p = 0.04), and miR-16-5p (FC = 1.89, p = 0.05) exhibited significantly different expression levels respectively (see Table S5).
Fig. 1.
a–e Relative expression levels of miR-663b, miR-320a, miR-766-3p, miR-132-3p, and miR-16-5p among different groups. Asterisk symbol means that values indicate significant differences within the expression level of each miRNAs (p < 0.001)
Table 4.
Expression of miRNAs between the three groups
| Group A (intrafollicular melatonin concentration ≤ 30 pg/mL) | Group B (intrafollicular melatonin concentration > 70 to ≤ 110 pg/mL) | Group C (intrafollicular melatonin concentration > 111 to ≤ 385 pg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Raw Ct | ∆Ct | RQ | Raw Ct | ∆Ct | RQ | Raw Ct | ∆Ct | RQ | |
| miR-663b | 31.986 | 12.765 | 0.231 | 25.976 | 6.143 | 13.987 | 23.927 | 4.345 | 49.432 |
| miR-320a | 32.098 | 12.987 | 0.227 | 24.987 | 4.823 | 38.945 | 22.987 | 3.545 | 88.766 |
| miR-766-3p | 33.987 | 13.098 | 0.113 | 25.156 | 5.980 | 10.765 | 24.183 | 4.839 | 38.098 |
| miR-132-3p | 33.997 | 13.154 | 0.143 | 24.432 | 4.909 | 42.981 | 23.546 | 4.198 | 44.456 |
| miR-16-5p | 29.688 | 10.213 | 0.9754 | 26.098 | 7.654 | 5.443 | 25.872 | 5.587 | 24.098 |
Internal reference miR 16. ∆Ct = Raw Ct target miRNA – Raw Ct miR-16. Inclusion criteria: high expression-level miRNAs with (Raw Ct < 30 and ∆Ct (mRNA) < 10). Low expression-level miRNAs with (Raw Ct > 30 and ∆Ct (mRNA) > 10)
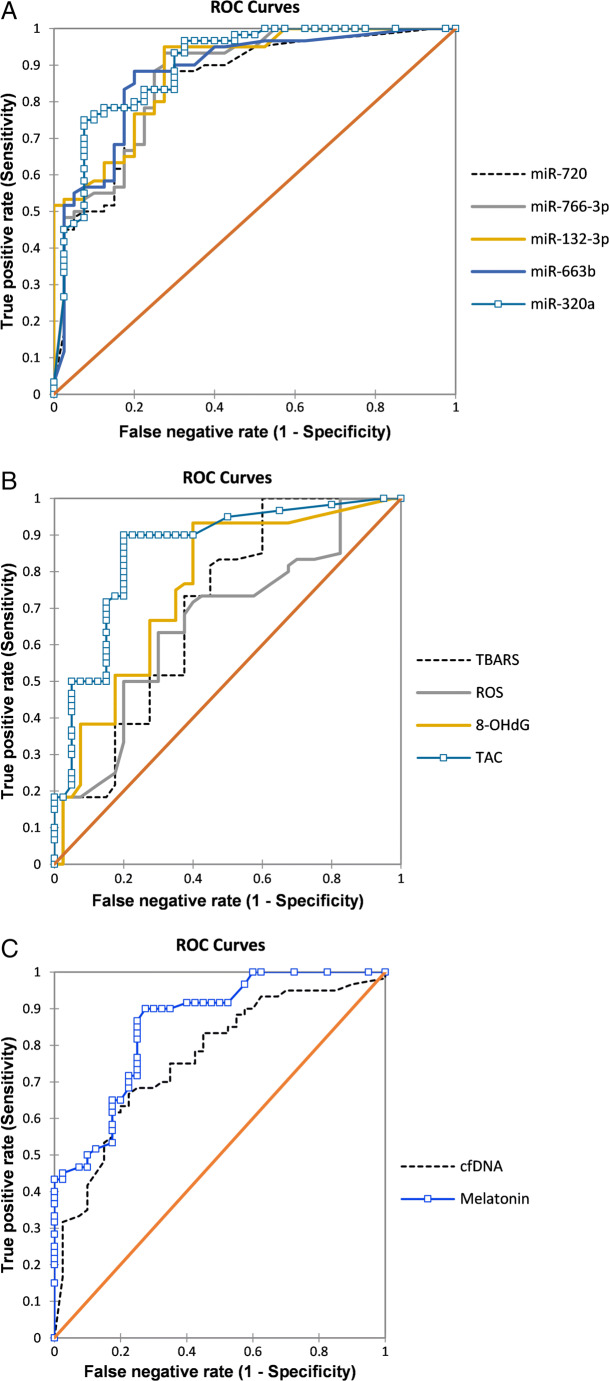
Predictive model for high-quality embryo selection
A predictive model for high-quality embryos is depicted in Fig. 2. Accordingly, the largest areas under the ROC were 0.89 [95% Cl: 0.83; 0.96] (p = 0.001) at cut-off values of ≥ 0.79 along with 75% sensitivity and 92.5% specificity for miR-320a and 0.88 (0.82; 0.94), with 95% sensitivity and 72.5% specificity (p = 0.003, at cut-off value ≥ 0.59) for miR-132-3p (see Fig. 2a). These results showed that miR-320a predicts IVF outcome parameters better than miR-132-3p (see Table 5). Likewise, the combination of all miRNAs did not improve the AUC of miR-320a (0.89) but enhance the sensitivity to 87% and slightly decrease the specificity to 83%, which may offer a potent non-invasive diagnostic tool in the selection of high-quality day 3 embryos (see Table 5). ROC analysis for oxidative stress markers showed that TAC have the highest AUC value 0.85 [95% CI 0.77; 0.93] with cut-off value of 0.89 and showed 90.2% sensitivity and 80.3% specificity (p = 0.001). On the other hand, ROS have the lowest AUC value 0.65 [95% Cl 0.54; 0.76] at cut-off value of 1.88 exhibited 63.3% sensitivity and 70% specificity (p = 0.021) (see Fig. 2b). Furthermore, the AUC value for combining all evaluated stress markers was 0.78, with a sensitivity of 83.2% and specificity of 78.3% (p = 0.001) as given in Table 5). For melatonin, AUC value was 0.85 [cut-off points, 95% Cl 0.80, 0.78; 0.92] with sensitivity of 90.7% and specificity of 72.6% whereas cfDNA have AUC value of 0.76 [cut-off points, 95% Cl 0.61, 0.67; 0.85] along with 82.4% sensitivity and 66.7% specificity (see Fig. 2c).
Fig. 2.
A predictive model for high-quality embryo selection based on melatonin concentration, cfDNA levels, oxidative stress markers, and expression patterns of miRNAs in FF samples of unexplained infertile patients. a ROC curve analysis to estimate the discriminative significance of FF concentrations of miR-663b, miR-320a, miR-766-3p, miR-132-3p, and miR-16-5p for prediction of good-quality embryos. b ROC curve analysis to evaluate oxidative stress markers and predictive values for good-quality embryo selection. c ROC curve analysis assesses the predictive values of melatonin concentration and cfDNA levels for good-quality embryo selection
Table 5.
Predictive values of sensitivity and specificity estimates for the probability of obtaining the best quality embryo develops from mature oocytes
| AUC (95% population limit) | S.E.M | p value* | Cut-off point** | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| miR-663b | 0.87 (0.80; 0.94) | 0.036 | 0.002 | ≥ 0.64 | 88.3 | 80 |
| miR-320a | 0.89 (0.83; 0.96) | 0.032 | 0.001 | ≥ 0.79 | 75.0 | 92.5 |
| miR-766-3p | 0.86 (0.79; 0.94) | 0.037 | 0.001 | ≥ 0.57 | 93.3 | 72.5 |
| miR-132-3p | 0.88 (0.82; 0.94) | 0.032 | 0.003 | ≥ 0.59 | 95 | 72.5 |
| miR-16-5p | 0.85 (0.78; 0.93) | 0.039 | 0.001 | ≥ 0.68 | 88.8 | 80.5 |
| Combination of ff miR-663, 320a, 766-3p, 132-3p, and 16-5p | 0.89 (0.71; 0.93) | 0.034 | 0.001 | – | 87 | 83 |
| ROS | 0.65 (0.54; 0.76) | 0.055 | 0.021 | 1.88 | 63.3 | 70 |
| TAC | 0.85 (0.77; 0.93) | 0.049 | 0.001 | 0.89 | 90.2 | 80.3 |
| LPO | 0.83 (0.75; 0.91) | 0.042 | 0.001 | 0.78 | 91.7 | 72.5 |
| TBARS | 0.69 (0.58; 0.80) | 0.052 | 0.003 | 0.71 | 80 | 40 |
| 8-OHdG | 0.75 (0.65; 0.85) | 0.058 | 0.001 | 0.75 | 93.3 | 60.1 |
| Combination of ff evaluated oxidative stress markers | 0.78 (0.69; 0.85) | 0.045 | 0.001 | – | 83.2 | 78.36 |
| cfDNA | 0.76 (0.67; 0.85) | 0.047 | 0.001 | 0.61 | 82.4 | 66.7 |
| Melatonin | 0.85 (0.78; 0.92) | 0.037 | 0.001 | 0.80 | 90.7 | 72.6 |
*The null hypothesis was true area = 0.5, after the adjustment of a number of attempts and the number of embryos. p values in italics considered statistically significant p < 0.05
**Estimated cut points that maximize sensitivity and specificity for observed range predictors
Multitargeting activity of upregulated miRNAs
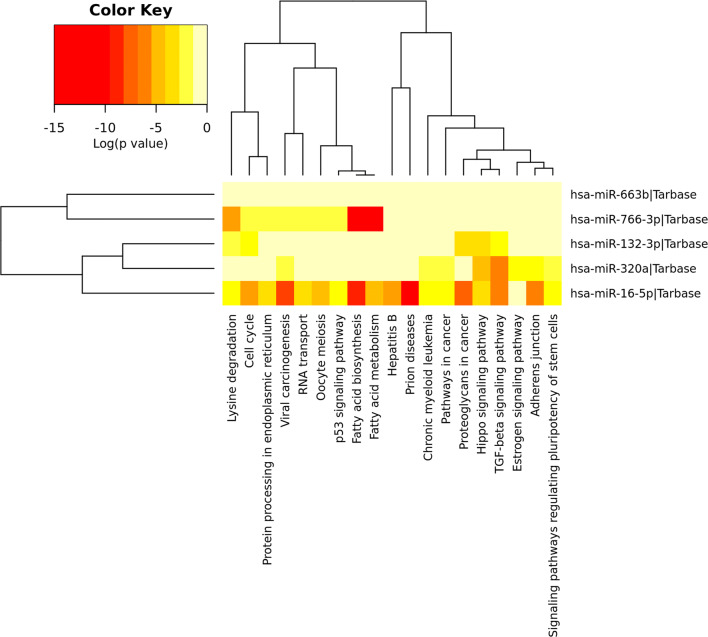
MicroRNAs are the genetic switches that fine-tune essential cellular responses and are required to streamline the signal transductions in several cell types. They are depicted as multivalent with single miRNA able to target numerous genes, thus regulating molecules’ structural and functional expression within a pathway. In the present study, we identified several pathways responsible for embryo development, which involves at least one of the studied miRNAs, including TGF-beta signaling, MAPK signaling, Wnt signaling, PI3K-Akt pathway, Notch signaling, Estrogen signaling, and Hippo signaling pathways (see Figs. 3 and 3S).
Fig. 3.
MiRNAs and predictive pathway heat map. Red color indicates high expression and lower p values. Yellow color indicates intermediate expression
Discussion
The follicular microenvironment is a highly complex and critical indicator of an individual oocyte’s developmental capability to be fertilized and mature into a good-quality embryo. The antioxidant activity of FF is required for normal fertilization as well as embryo development. Our study revealed that IFF melatonin concentration increased by two doses of melatonin supplementation (3 mg/day or 6 mg/day) in unexplained infertile women, reducing oxidative stress markers and decreasing DNA damage response, and enhances oocyte maturation along with embryo quality. Moreover, our results indicate that the relative expression of five miRNAs (miR-663b, miR-320a, miR-766-3p, miR-132-3p, miR-16-5p) is related to oocyte maturation and embryo-developing potential, are markedly different between high-quality and impaired-quality embryos, and their relative expression differs between the three groups.
Our results demonstrated that patients with higher melatonin concentration were associated with elevated 17β-estradiol levels in their FF samples. Estradiol (E2) is a key player in the final steps of oocyte’s nuclear and cytoplasmic maturation, and deviation from the normal levels may lead to deterioration in the oocyte quality [59]. A non-human primate study demonstrated that IFF melatonin can improve oocytes’ developmental competence during in vitro maturation (IVM) [60]. Our study showed that patients with lower IFF melatonin concentration exhibited a considerable oxidative imbalance (e.g., lower TAC levels and higher ROS, 8-OHdG, and TBARS levels) jeopardize the quality of oocytes and, thus, hampers the oocyte’s maturation along with limiting IVF outcome parameters. These findings follow much earlier research reporting that an imbalance between lipid peroxidation marker (TBARS) and antioxidant system plays a significant role in the pathogenesis of unexplained infertility [61]. Our study’s outcome agrees with Jana et al., which revealed a direct relationship between low TAC levels and poor embryo quality and a sharp decline in fertilization rate [3]. Recent studies also validate that decreased melatonin concentration is responsible for reduced TAC levels in the FF samples [5, 62]. These studies suggest that elevated follicular lipid peroxidation and lower TAC levels negatively impact the outcomes of in vitro fertilization.
Over the past decade, advancement in scientific knowledge have established that depreciation in IFF melatonin assets resulted in excessive ROS production, responsible for single- or double-strand DNA breaks, thus introducing mutations in nuclear DNA and reducing mitochondrial function [63]. Together with this reference, our study tends to confirm that ROS concentration raised high in those patients who had lower levels of melatonin (≤ 30 pg/mL) than those who had moderate to higher concentrations in their FF samples. ROS production might be a result of the suboptimal IFF microenvironment or impaired metabolism of the developing oocyte. Increasing evidence highlights that melatonin is responsible for the upregulation of specific messenger RNAs (mRNAs), which control antioxidative enzymes’ expression, more likely by acting as a potent free radical scavenger [64]. Similarly, another group reported that melatonin significantly upregulates the expression of genes and the formation of proteins responsible for synthesizing antioxidant enzymes. Therefore, compromised antioxidant capacity might result from oxidative stress and flare-up apoptotic events [65].
One of the significant obstacles for IVF is oxidative damage to nuclear DNA that can be estimated by assessing IFF cfDNA concentration and levels of 8-OHdG. The increased concentrations of cfDNA and 8-OHdG levels also indicate the embryo’s inappropriate quality and often have a low pregnancy rate [33, 66]. In the same context, our results showed that cfDNA concentration and 8-OHdG levels were significantly raised in good-quality embryos of those patients who have decreased IFF melatonin concentration (≤ 30 pg/mL). Alternatively, those patients who exhibited a higher concentration of melatonin (> 111 pg/mL) have decreased cfDNA concentration and levels of 8-OHdG in their FF samples, which is in agreement with previous observations [67, 68]. Furthermore, in the present study, higher melatonin concentration resulted in an enrichment of high-quality day 3 embryos leading to an increase in the availability of embryos suitable for transfer, ultimately resulting in an augmented future pregnancy rate per embryo transfer [42].
Our exploratory analysis exhibited that miR-320a, miR766-3p, miR-132-3p, miR-16-5p, and miR-663b significantly decreased their relative expression in FF samples with a lower concentration of IFF melatonin (≤ 30 pg/mL) and yielded a smaller number of high-quality embryos on day 3. Similarly, these miRNAs were significantly downregulated in the impaired-quality embryos than high-quality embryos. In agreement with our study, Feng et al. compared miRNAs from the FF that generate poor-quality and top-quality embryos. They found that miR-132-3p and miR-16-5p were downregulated in the FF containing mature oocytes that produce a higher number of poor-quality embryos than top-quality embryos and vice versa [17, 69]. However, the findings were statistically insignificant. Bioinformatic analysis of studied miRNAs reveals a fundamental role in mediating genes that regulate dynamic aspects of multiple biological functions. They mediated cell-to-cell communication and target genes associated with follicular development, growth, and oocyte maturation, further indicating that these endogenous messenger molecules have a critical role in oogenesis [70]. Subsequently, the molecular signature based on these miRNAs’ differential levels enabled them to participate in the cell junction assembly, TGF-beta signaling pathway, MAPK signaling pathway, Wnt signaling pathway, PI3K-Akt pathway, Notch signaling pathway, Estrogen signaling pathway, and Hippo signaling pathway [71, 72].
Our study showed that miR-320 was among the highest expressed miRNA in FF samples with melatonin concentration > 111 pg/mL and exhibited a positive correlation with day 3 embryo quality. Diez-frail et al. observed that miR-320 was over the top 10 highest expressed miRNA in FF samples of good-quality embryos [73]. Furthermore, it was reported that knockdown of miR-320a expression in mouse metaphase-II oocytes resulted in embryos arrested at first cleavage or very few develop into top-quality embryos, indicating that the miR-320a has a potential role in modulating gene expression and regulating embryonic development [74]. Intriguingly, another investigation did not find miR-320 in human FF [12]. This inconsistency in the results might be explained by genetic heterogeneity due to the population’s different ethnic origins, resulting in varied gene expression in body fluids. Moreover, different types of stimulation protocols may be responsible for a varied expression of genes or miRNAs. Similarly, studies focused on the relationship between miR-320a relative expression levels in FF and embryonic development provide additional support to the physiological and molecular mechanisms underlying in vivo and in vitro fertilization [69, 75, 76]. Evidence from another investigation has indicated that melatonin exerts its antioxidant activities by coordinating crosstalk between miRNAs and interrelated pathways [17].
Furthermore, a transcriptome analysis reveals that high-quality human preimplantation embryos secrete miR-320a that regulates the decidualized human endometrial stromal cell (hESC) migration by targeting cell adhesion and cytoskeleton organization [69]. The outcome of their study specifies the promising effect of miR-320a to boost success rates in assisted reproduction. Another evidence indicates that miR766-3p, miR-132-3p, miR-16-5p, and miR-663b have decreased relative expression in the samples, yielding impaired-quality embryos compared with the top-quality embryos on day 3 [10]; this is in line with our findings. In the same frame of reference, a recent study conducted by Ragusa et al. confirmed the hypothesis that miR766-3p fine-tunes cellular responses, especially in the cell cycle control and plays a vital role in the first phase of embryogenesis [77]. Conversely, Fu et al. demonstrated that miR-663b has a substantial negative correlation in FF of oocytes that produce viable blastocyst than those yielding poor-quality blastocyst [78]. However, the authors of the study did not mention the exact cause of infertility in the subjects. We speculated that it is potentially resulting from vast differences in the selection criteria of patients, detection methodology, and biological variability.
One of the best vantage points of this study is similar age groups, and patients were undergoing the same stimulation protocol that reduces the effect of age over confounding efficacy and power. However, this study still holds some significant limitations, as other confounding factors were not addressed. First, the oocyte and embryo quality are associated with morphological scores and not the number and composition of chromosomes; therefore, it is imperative to find the numerical chromosome euploidy by preimplantation genetic diagnosis (PGD). Second, we were not able to knockdown miRNAs to assess their actual effects on embryonic development. Third, the primary goal is embryo quality, not live birth. Further studies should be planned to find a relationship between these miRNAs’ expression in FF and chromosomal anomalies.
Conclusions
Conclusively, our study showed that melatonin concentration was significantly associated with oxidative stress markers and cfDNA concentration in the follicular ambient microenvironment on oocyte development and embryo quality. Moreover, our results reveal that the therapeutic use of melatonin produces a considerable rise in the number of mature oocytes retrieved, fertilization rate, and embryo quality that might positively impact future clinical pregnancy rate per embryo transfer. Furthermore, information compiled herein improved our understanding that miRNA signature enhances embryo selection quality and minimizes the chance of multiple gestations, thus, eventually enhancing the probability of successful IVF pregnancies and may allow us to classify them as non-invasive biomarkers to identify good-quality embryos.
Supplementary information
(DOCX 26 kb).
Relationship between cfDNA levels and melatonin concentration in individual FF samples. (PNG 194 kb).
Pearson’s correlation between IFF melatonin concentration and different biomarkers of oxidative balance. (A) Total antioxidant capacity (TAC) (B) Reactive oxygen species (ROS) (C) Thiobarbituric acid reactive substances (TBARS) and (D) 8-hydroxy-2′-deoxyguanosine (8-OHdG) concentrations. (PNG 220 kb).
(PNG 190 kb).
(PNG 229 kb).
(PNG 204 kb).
Predicted pathway analysis heat map for all miRNAs in detail. Red color indicates high expression and lower p values. Yellow color indicates intermediate expression. (PNG 974 kb).
Acknowledgments
We acknowledge the research initiative and gratefully thank Professor Dr. Rashid Latif Khan, Professor of Emeritus in Obstetrics and Gynecology, for manuscript editing. The study is conducted under the infertility research center of Hameed Latif Hospital.
Abbreviations
- IVF
in vitro fertilization
- IFF
intrafollicular fluid
- AFC
antral follicle count
- AMH
anti-Müllerian hormone
- PCOS
polycystic ovarian syndrome
- cfDNA
cell-free DNA
- FF
follicular fluid
- PGD
preimplantation genetic diagnosis
- hESCs
human endometrial stromal cells
- TAC
total antioxidant capacity
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- GSH
glutathione
- miRNA
microRNA
- ROC
receiving operating characteristics
- E2
17β-estradiol
- BMI
body mass index
- FSH
follicular stimulating hormone
- LH
luteinizing hormone
- TSH
thyroid-stimulating hormone
- TVS
transvaginal ultrasonography
- COS
controlled ovarian stimulation
- ICSI
intracytoplasmic sperm injection
- TBARS
thiobarbituric acid reactive substances
- 8-OHdG
8-hydroxy-2′-deoxyguanosine
- CI
confidence interval
Authors’ contributions
HLK and YLK: reviewing and editing, software; SB: conceptualization, methodology, software, data curation, project administration, writing—original draft preparation, supervision, writing—reviewing and editing; SA: formal analysis, validation, methodology, investigation, writing—reviewing and editing; CK: writing—reviewing and editing; SQ: data curation, writing—original draft preparation; ZH: reviewing and editing, methodology, software; NZT: writing—reviewing and editing; HHY: software, validation, methodology.
Data availability
The data set used and analyzed during the current study is available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
The study was approved by our Institutional Ethical Committee (IEC). Informed consent was obtained from all subjects before the research and publishing of the results of the investigation.
Consent for publication
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Turchi P. Prevalence, definition, and classification of infertility. In: Clinical management of male infertility, no. 1. Cham: Springer; 2015. pp. 5–11.
- 2.Gelbaya TA, Potdar N, Jeve YB, Nardo LG. Definition and epidemiology of unexplained infertility. Obstet Gynecol Surv. 2014;69(2):109–115. doi: 10.1097/OGX.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 3.Jana SK, Babu N, Chattopadhyay R, Chakravarty B, Chaudhury K. Upper control limit of reactive oxygen species in follicular fluid beyond which viable embryo formation is not favorable. Reprod Toxicol. 2010;29(4):447–451. doi: 10.1016/j.reprotox.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Oyawoye OA, Abdel-Gadir A, Garner A, Leonard AJ, Perrett C, Hardiman P. The interaction between follicular fluid total antioxidant capacity, infertility and early reproductive outcomes during in vitro fertilization. Redox Rep. 2009;14(5):205–213. doi: 10.1179/135100009X12525712409418. [DOI] [PubMed] [Google Scholar]
- 5.Espino J, Macedo M, Lozano G, Ortiz Á, Rodríguez C, Rodríguez AB, Bejarano I. Impact of melatonin supplementation in women with unexplained infertility undergoing fertility treatment. Antioxidants. 2019;8(9):338. doi: 10.3390/antiox8090338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becatti M, Fucci R, Mannucci A, Barygina V, Mugnaini M, Criscuoli L, Giachini C, Bertocci F, Picone R, Emmi G. A biochemical approach to detect oxidative stress in infertile women undergoing assisted reproductive technology procedures. Int J Mol Sci. 2018;19(2):592. doi: 10.3390/ijms19020592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cadet J, Davies KJ. Oxidative DNA damage & repair: an introduction. Free Radic Biol Med. 2017;107:2–12. doi: 10.1016/j.freeradbiomed.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pauli A, Rinn JL, Schier AF. Non-coding RNAs as regulators of embryogenesis. Nat Rev Genet. 2011;12(2):136–149. doi: 10.1038/nrg2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez RM, Liang L, Racowsky C, Dioni L, Mansur A, Adir M, Bollati V, Baccarelli AA, Hauser R, Machtinger R. Extracellular microRNAs profile in human follicular fluid and IVF outcomes. Sci Rep. 2018;8(1):1–10. doi: 10.1038/s41598-018-35379-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machtinger R, Rodosthenous RS, Adir M, Mansour A, Racowsky C, Baccarelli AA, Hauser R. Extracellular microRNAs in follicular fluid and their potential association with oocyte fertilization and embryo quality: an exploratory study. J Assist Reprod Genet. 2017;34(4):525–533. doi: 10.1007/s10815-017-0876-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scalici E, Traver S, Mullet T, Molinari N, Ferrieres A, Brunet C, Belloc S, Hamamah S. Circulating microRNAs in follicular fluid, powerful tools to explore in vitro fertilization process. Sci Rep. 2016;6(1):1–10. doi: 10.1038/srep24976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santonocito M, Vento M, Guglielmino MR, Battaglia R, Wahlgren J, Ragusa M, Barbagallo D, Borzì P, Rizzari S, Maugeri M. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil Steril. 2014;102(6):1751–1761. doi: 10.1016/j.fertnstert.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Montazerian M, Yasari F, Aghaalikhani N. Ovarian extracellular microRNAs as the potential non-invasive biomarkers: an update. Biomed Pharmacother. 2018;106:1633–1640. doi: 10.1016/j.biopha.2018.07.073. [DOI] [PubMed] [Google Scholar]
- 14.Qasemi M, Amidi F. Extracellular microRNA profiling in human follicular fluid: new biomarkers in female reproductive potential. J Assist Reprod Genet. 2020;37:1769–80. doi: 10.1007/s10815-020-01860-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang J, Wang S, Wang Z. Role of microRNAs in embryo implantation. Reprod Biol Endocrinol. 2017;15(1):90. doi: 10.1186/s12958-017-0309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capalbo A, Ubaldi FM, Cimadomo D, Noli L, Khalaf Y, Farcomeni A, Ilic D, Rienzi L. MicroRNAs in spent blastocyst culture medium are derived from trophectoderm cells and can be explored for human embryo reproductive competence assessment. Fertil Steril. 2016;105(1):225–235. doi: 10.1016/j.fertnstert.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Feng R, Sang Q, Zhu Y, Fu W, Liu M, Xu Y, Shi H, Xu Y, Qu R, Chai R. MiRNA-320 in the human follicular fluid is associated with embryo quality in vivo and affects mouse embryonic development in vitro. Sci Rep. 2015;5:8689. doi: 10.1038/srep08689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abu-Halima M, Khaizaran ZA, Ayesh BM, Fischer U, Khaizaran SA, Al-Battah F, et al. MicroRNAs in combined spent culture media and sperm are associated with embryo quality and pregnancy outcome. Fertil Steril. 2020;133(5):970–980.e2. doi: 10.1016/j.fertnstert.2019.12.028. [DOI] [PubMed] [Google Scholar]
- 19.Kropp J, Khatib H. Characterization of microRNA in bovine in vitro culture media associated with embryo quality and development. J Dairy Sci. 2015;98(9):6552–6563. doi: 10.3168/jds.2015-9510. [DOI] [PubMed] [Google Scholar]
- 20.Sang Q, Yao Z, Wang H, Feng R, Wang H, Zhao X, Xing Q, Jin L, He L, Wu L. Identification of microRNAs in human follicular fluid: characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J Clin Endocrinol Metab. 2013;98(7):3068–3079. doi: 10.1210/jc.2013-1715. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan V, Singh J, Pandi-Perumal SR, Brown GM, Spence DW, Cardinali DP. Jet lag, circadian rhythm sleep disturbances, and depression: the role of melatonin and its analogs. Adv Ther. 2010;27(11):796–813. doi: 10.1007/s12325-010-0065-y. [DOI] [PubMed] [Google Scholar]
- 22.Cajochen C, Kräuchi K, Wirz-Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol. 2003;15(4):432–437. doi: 10.1046/j.1365-2826.2003.00989.x. [DOI] [PubMed] [Google Scholar]
- 23.Hadi A, Ghaedi E, Moradi S, Pourmasoumi M, Ghavami A, Kafeshani M. Effects of melatonin supplementation on blood pressure: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res. 2019;51(03):157–164. doi: 10.1055/a-0841-6638. [DOI] [PubMed] [Google Scholar]
- 24.Najafi M, Shirazi A, Motevaseli E, Geraily G, Norouzi F, Heidari M, Rezapoor S. The melatonin immunomodulatory actions in radiotherapy. Biophys Rev. 2017;9(2):139–148. doi: 10.1007/s12551-017-0256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaspar do Amaral F, Cipolla-Neto J. A brief review about melatonin, a pineal hormone. Arch Endocrinol Metab. 2018;62(4):472–479. doi: 10.20945/2359-3997000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez-Hidalgo M, Alarcon de la Lastra C, Carrascosa-Salmoral MP, Naranjo MC, Gomez-Corvera A, Caballero B, et al. Age-related changes in melatonin synthesis in rat extrapineal tissues. Exp Gerontol. 2009;44(5):328–34. [DOI] [PubMed]
- 27.Bodis J, Hartmann G, Tinneberg H-R, Török A, Hanf V, Papenfuss F, Schwarz H. Relationship between the monoamine, progesterone and estradiol content in follicular fluid of preovulatory graafian follicles after superovulation treatment. Gynecol Obstet Investig. 1993;35(4):232–235. doi: 10.1159/000292706. [DOI] [PubMed] [Google Scholar]
- 28.Itoh MT, Ishizuka B, Kuribayashi Y, Amemiya A, Sumi Y. Melatonin, its precursors, and synthesizing enzyme activities in the human ovary. Mol Hum Reprod. 1999;5(5):402–408. doi: 10.1093/molehr/5.5.402. [DOI] [PubMed] [Google Scholar]
- 29.Tamura H, Tanabe M, Jozaki M, Taketani T, Sugino N. Antioxidative action of melatonin and reproduction. Glycative Stress Research. 2019;6(3):192–197. [Google Scholar]
- 30.Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007;42(1):28–42. doi: 10.1111/j.1600-079X.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36(1):1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 32.Tamura H, Nakamura Y, Korkmaz A, Manchester LC, Tan D-X, Sugino N, Reiter RJ. Melatonin and the ovary: physiological and pathophysiological implications. Fertil Steril. 2009;92(1):328–343. doi: 10.1016/j.fertnstert.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 33.Khan HL, Bhatti S, Khan YL, Abbas S, Munir Z, Sherwani IARK, Suhail S, Hassan Z, Aydin HH. Cell-free nucleic acids and melatonin levels in human follicular fluid predict embryo quality in patients undergoing in-vitro fertilization treatment. J Gynecol Obstet Hum Reprod. 2020;49(1):101624. doi: 10.1016/j.jogoh.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Tanabe M, Tamura H, Taketani T, Okada M, Lee L, Tamura I, et al. Melatonin protects the integrity of granulosa cells by reducing oxidative stress in nuclei, mitochondria, and plasma membranes in mice. J Reprod Dev. 2015;61:35–41. doi: 10.1262/jrd.2014-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L, et al. Melatonin as a free radical scavenger in the ovarian follicle. Endocr J. 2013;60:1–13. doi: 10.1507/endocrj.ej12-0263. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura Y, Tamura H, Takayama H, Kato H. Increased endogenous level of melatonin in preovulatory human follicles does not directly influence progesterone production. Fertil Steril. 2003;80(4):1012–1016. doi: 10.1016/s0015-0282(03)01008-2. [DOI] [PubMed] [Google Scholar]
- 37.Ishizuka B, Kuribayashi Y, Murai K, Amemiya A, Itoh MT. The effect of melatonin on in vitro fertilization and embryo development in mice. J Pineal Res. 2000;28(1):48–51. doi: 10.1034/j.1600-079x.2000.280107.x. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez-Osorio N, Kim I, Wang H, Kaya A, Memili E. Melatonin increases cleavage rate of porcine preimplantation embryos in vitro. J Pineal Res. 2007;43(3):283–288. doi: 10.1111/j.1600-079X.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- 39.Asgari Z, Ghasemian F, Ramezani M, Bahadori MH. The effect of melatonin on the developmental potential and implantation rate of mouse embryos. Cell J. 2012;14(3):203. [PMC free article] [PubMed] [Google Scholar]
- 40.Kim MK, Park EA, Kim HJ, Choi WY, Cho JH, Lee WS, Cha KY, Kim YS, Lee DR, Yoon TK. Does supplementation of in-vitro culture medium with melatonin improve IVF outcome in PCOS? Reprod BioMed Online. 2013;26(1):22–29. doi: 10.1016/j.rbmo.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Matsunaga R, Watanabe S, Mita W, Miura M, Kobayashi Y, Yamanaka N, Kamihata M, Kuwahata A, Ochi M, Horiuchi T. Effect of melatonin on developmental competence of denuded human oocytes during in vitro maturation. Fertil Steril. 2017;108(3):e145–e146. [Google Scholar]
- 42.Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, Taketani T, Matsuoka A, Yamagata Y, Shimamura K. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44(3):280–287. doi: 10.1111/j.1600-079X.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- 43.Nishihara T, Hashimoto S, Ito K, Nakaoka Y, Matsumoto K, Hosoi Y, Morimoto Y. Oral melatonin supplementation improves oocyte and embryo quality in women undergoing in vitro fertilization-embryo transfer. Gynecol Endocrinol. 2014;30(5):359–362. doi: 10.3109/09513590.2013.879856. [DOI] [PubMed] [Google Scholar]
- 44.Tamura H, Jozaki M, Tanabe M, Shirafuta Y, Mihara Y, Shinagawa M, Tamura I, Maekawa R, Sato S, Taketani T. Importance of melatonin in assisted reproductive technology and ovarian aging. Int J Mol Sci. 2020;21(3):1135. doi: 10.3390/ijms21031135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Unfer V, Raffone E, Rizzo P, Buffo S. Effect of a supplementation with myo-inositol plus melatonin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: a prospective, longitudinal, cohort study. Gynecol Endocrinol. 2011;27(11):857–861. doi: 10.3109/09513590.2011.564687. [DOI] [PubMed] [Google Scholar]
- 46.Jahnke G, Marr M, Myers C, Wilson R, Travlos G, Price C. Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague-Dawley rats. Toxicol Sci. 1999;50(2):271–279. doi: 10.1093/toxsci/50.2.271. [DOI] [PubMed] [Google Scholar]
- 47.Khaksar M, Oryan A, Sayyari M, Rezabakhsh A, Rahbarghazi R. Protective effects of melatonin on long-term administration of fluoxetine in rats. Exp Toxicol Pathol. 2017;69(8):564–574. doi: 10.1016/j.etp.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Sugden D. Psychopharmacological effects of melatonin in mouse and rat. J Pharmacol Exp Ther. 1983;227(3):587–591. [PubMed] [Google Scholar]
- 49.Andersen LPH, Gögenur I, Rosenberg J, Reiter RJ. The safety of melatonin in humans. Clin Drug Investig. 2016;36(3):169–175. doi: 10.1007/s40261-015-0368-5. [DOI] [PubMed] [Google Scholar]
- 50.Bejarano I, Monllor F, Marchena AM, Ortiz A, Lozano G, Jiménez MI, Gaspar P, García JF, Pariente JA, Rodríguez AB. Exogenous melatonin supplementation prevents oxidative stress-evoked DNA damage in human spermatozoa. J Pineal Res. 2014;57(3):333–339. doi: 10.1111/jpi.12172. [DOI] [PubMed] [Google Scholar]
- 51.World Health Organization . WHO laboratory manual for the examination and processing of human semen. 5. Geneva: World Health Organization; 2010. pp. 1–286. [Google Scholar]
- 52.Vandekerckhove F, Vansteelandt S, Gerris J, De Sutter P. Follicle measurements using sonography-based automated volume count accurately predict the yield of mature oocytes in in vitro fertilization/intracytoplasmic sperm injection cycles. Gynecol Obstet Investig. 2013;76(2):107–112. doi: 10.1159/000353432. [DOI] [PubMed] [Google Scholar]
- 53.Hernández J, Rodríguez-Fuentes A, Puopolo M, Palumbo A. Follicular volume predicts oocyte maturity: a prospective cohort study using three-dimensional ultrasound and SonoAVC. Reprod Sci. 2016;23(12):1639–1643. doi: 10.1177/1933719116671003. [DOI] [PubMed] [Google Scholar]
- 54.Lazzaroni-Tealdi E, Barad DH, Albertini DF, Yu Y, Kushnir VA, Russell H, Wu Y-G, Gleicher N. Oocyte scoring enhances embryo-scoring in predicting pregnancy chances with IVF where it counts most. PLoS One. 2015;10(12):e0143632. doi: 10.1371/journal.pone.0143632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dimopoulou M, Anifandis G, Messini C, Dafopoulos K, Kouris S, Sotiriou S, et al. Follicular fluid oocyte/cumulusfree DNA concentrations as a potential biomolecular marker of embryo quality and IVF outcome. Biomed Res Int. 2014;2014:289306. doi: 10.1155/2014/289306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Munakata Y, Shirasuna K, Kuwayama T, Iwata H. Cell-free DNA in medium is associated with the maturation ability of in vitro cultured oocytes. J Reprod Dev. 2019;65(2):171–5. doi: 10.1262/jrd.2018-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Attaran M, Pasqualotto E, Falcone T, Goldberg JM, Miller KF, Agarwal A, Sharma RK. The effect of follicular fluid reactive oxygen species on the outcome of in vitro fertilization. Int J Fertil Womens Med. 2000;45(5):314–320. [PubMed] [Google Scholar]
- 58.Rice-Evans CA, Diplock AT, Symons MR. Techniques in free radical research. Lab Techn Biochem Mol Biol. 1991;22:1–278. [Google Scholar]
- 59.Sarhan D, El Mazny A, Taha T, Aziz A, Azmy O, Fakhry D, et al. Estradiol and luteinizing hormone concentrations in the follicular aspirate during ovum pickup as predictors of in vitro fertilization (IVF) outcome. Middle East Fertil Soc J. 2017;22(1):27–32. [Google Scholar]
- 60.Zheng P, Si W, Bavister BD, Yang J, Ding C, Ji W. 17β-estradiol and progesterone improve in-vitro cytoplasmic maturation of oocytes from unstimulated prepubertal and adult rhesus monkeys. Hum Reprod. 2003;18(10):2137–2144. doi: 10.1093/humrep/deg410. [DOI] [PubMed] [Google Scholar]
- 61.Polak G, Rola R, Gogacz M, Kozioł-Montewka M, Kotarski J. Malonyldialdehyde and total antioxidant status in the peritoneal fluid of infertile women. Ginekol Pol. 1999;70(3):135–140. [PubMed] [Google Scholar]
- 62.Fernando S, Osianlis T, Vollenhoven B, Wallace E, Rombauts L. A pilot double-blind randomised placebo-controlled dose–response trial assessing the effects of melatonin on infertility treatment (MIART): study protocol. BMJ Open. 2014;4(8). 10.1136/bmjopen-2014-005986. [DOI] [PMC free article] [PubMed]
- 63.Ménézo Y, Dale B, Cohen M. DNA damage and repair in human oocytes and embryos: a review. Zygote. 2010;18(4):357–365. doi: 10.1017/S0967199410000286. [DOI] [PubMed] [Google Scholar]
- 64.Antolín I, Rodríguez C, Sáinz RM, Mayo JC, Uría H, Kotler ML, Rodríguez-Colunga MJ, Tolivia D, Menéndez-Peláez A. Neurohormone melatonin prevents cell damage: effect on gene expression for antioxidant enzymes. FASEB J. 1996;10(8):882–890. doi: 10.1096/fasebj.10.8.8666165. [DOI] [PubMed] [Google Scholar]
- 65.Mayo J, Sainz R, Antolin I, Herrera F, Martin V, Rodriguez C. Melatonin regulation of antioxidant enzyme gene expression. Cell Mol Life Sci. 2002;59(10):1706–1713. doi: 10.1007/PL00012498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seino T, Saito H, Kaneko T, Takahashi T, Kawachiya S, Kurachi H. Eight-hydroxy-2′-deoxyguanosine in granulosa cells is correlated with the quality of oocytes and embryos in an in vitro fertilization-embryo transfer program. Fertil Steril. 2002;77(6):1184–1190. doi: 10.1016/s0015-0282(02)03103-5. [DOI] [PubMed] [Google Scholar]
- 67.Xu G, Zhao J, Liu H, Wang J, Lu W. Melatonin inhibits apoptosis and oxidative stress of mouse leydig cells via a SIRT1-dependent mechanism. Molecules. 2019;24(17):3084. doi: 10.3390/molecules24173084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu R, Fu A, Hoffman AE, Zheng T, Zhu Y. Melatonin enhances DNA repair capacity possibly by affecting genes involved in DNA damage responsive pathways. BMC Cell Biol. 2013;14(1):1–8. doi: 10.1186/1471-2121-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berkhout RP, Keijser R, Repping S, Lambalk CB, Afink GB, Mastenbroek S, et al. High-quality human preimplantation embryos stimulate endometrial stromal cell migration via secretion of microRNA hsa-miR-320a. bioRxiv. 2020;21(91):32–6. [DOI] [PMC free article] [PubMed]
- 70.Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75(1):50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang D, Luo Y, Wang G, Yang Q. Circular RNA expression profiles and bioinformatics analysis in ovarian endometriosis. Mol Genet Genomic Med. 2019;7(7):e00756. doi: 10.1002/mgg3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Su S-C, Reiter RJ, Hsiao H-Y, Chung W-H, Yang S-F. Functional interaction between melatonin signaling and noncoding RNAs. Trends Endocrinol Metab. 2018;29(6):435–445. doi: 10.1016/j.tem.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 73.Diez-Fraile A, Lammens T, Tilleman K, Witkowski W, Verhasselt B, De Sutter P, Benoit Y, Espeel M, D’Herde K. Age-associated differential microRNA levels in human follicular fluid reveal pathways potentially determining fertility and success of in vitro fertilization. Hum Fertil. 2014;17(2):90–98. doi: 10.3109/14647273.2014.897006. [DOI] [PubMed] [Google Scholar]
- 74.Salas-Huetos A, James ER, Aston KI, Jenkins TG, Carrell DT, Yeste M. The expression of miRNAs in human ovaries, oocytes, extracellular vesicles, and early embryos: a systematic review. Cells. 2019;8(12):1564. doi: 10.3390/cells8121564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liang L-F, Qi S-T, Xian Y-X, Huang L, Sun X-F, Wang W-H. Protective effect of antioxidants on the pre-maturation aging of mouse oocytes. Sci Rep. 2017;7(1):1–10. doi: 10.1038/s41598-017-01609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reza AMMT, Choi YJ, Han SG, Song H, Park C, Hong K, Kim JH. Roles of microRNAs in mammalian reproduction: from the commitment of germ cells to peri-implantation embryos. Biol Rev. 2019;94(2):415–438. doi: 10.1111/brv.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ragusa M, Barbagallo D, Chioccarelli T, Manfrevola F, Cobellis G, Di Pietro C, Brex D, Battaglia R, Fasano S, Ferraro B. CircNAPEPLD is expressed in human and murine spermatozoa and physically interacts with oocyte miRNAs. RNA Biol. 2019;16(9):1237–1248. doi: 10.1080/15476286.2019.1624469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fu J, Qu R-g, Zhang Y-j, Gu R-h, Li X, Sun Y-j, Wang L, Sang Q, Sun X-x. Screening of miRNAs in human follicular fluid reveals an inverse relationship between microRNA-663b expression and blastocyst formation. Reprod BioMed Online. 2018;37(1):25–32. doi: 10.1016/j.rbmo.2018.03.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 26 kb).
Relationship between cfDNA levels and melatonin concentration in individual FF samples. (PNG 194 kb).
Pearson’s correlation between IFF melatonin concentration and different biomarkers of oxidative balance. (A) Total antioxidant capacity (TAC) (B) Reactive oxygen species (ROS) (C) Thiobarbituric acid reactive substances (TBARS) and (D) 8-hydroxy-2′-deoxyguanosine (8-OHdG) concentrations. (PNG 220 kb).
(PNG 190 kb).
(PNG 229 kb).
(PNG 204 kb).
Predicted pathway analysis heat map for all miRNAs in detail. Red color indicates high expression and lower p values. Yellow color indicates intermediate expression. (PNG 974 kb).
Data Availability Statement
The data set used and analyzed during the current study is available from the corresponding author on reasonable request.