Abstract
Microwave-assisted extraction (MAE) and solvent-free microwave extraction and Soxhlet extraction were applied to Ray Ruby grapefruit leaves (Citrus paradisi Macf.) to compare extract efficiency. Face centered composite designs were constructed via response surface methodology. Effects of factors of MAE were investigated on total phenolic content (TPC) and naringin content (NC). The optimized conditions were established as 1.4 kWL−1 for microwave power density, 20.00 gL−1 for solid/solvent ratio, 218.180 s for extraction time, while responses were calculated as 14.210 mg of gallic acid equivalent per g of the dried leaf (mg GAE g−1DL) and 13.198 mg of naringin per g of dried leaf (mg Ng−1DL) for TPC and NC, respectively. SFME and classical Soxhlet methods were also conducted for comparison reasons.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04632-x) contains supplementary material, which is available to authorized users.
Keywords: Optimization, Microwave-assisted techniques, Grapefruit leaves, Naringin, Response surface methodology
Introduction
Rapidly decreasing food supplies lead researchers to search for recovery and reuse of crop residues and biowastes as nutrition additives. Nowadays, a smaller part of these biowastes have been used as animal feed, but a large part of them have been left in the environment causing ecological problems (Baldán et al. 2020). On the other hand, many of those biomasses include a wide variety of polyphenols, which have been reported to be strong role against oxidation and microbial growth by various studies (Şahin et al. 2018; Şahin and Elhussein 2018; Ozturk et al. 2018). Therefore, those polyphenolic ingredients find application in many cosmetical, pharmaceutical and food formulations due to their bioactive properties (Bakirtzi et al. 2016). Consequently, valorization of these biowaste might occur by application of a proper extraction procedure in order to convert the concerned residue into high-added value compounds. Regarding increasing energy prices, researchers endeavored to improve green techniques to get high-added value compounds from the cheapest biowaste with higher efficiency, high rate, and lower cost. These objectives result in consideration of novel “green” extraction techniques consuming lower solvent and lower energy, such as ultrasound and microwave (Chemat and Cravotto 2013). Microwave-assisted extraction (MAE) is one of the hot-spot technique using microwave energy to make heating more efficient and enhance energy transfer (Li et al. 2013). Thus, increasing diffusion allowing the solvent mixture to stay in contact with the plant matrix (Mandal et al. 2007). Solvent-free microwave extraction (SFME) is the most eco-friendly technique without adding any solvent with numerous profits such as short extraction time (in minutes in opposition to hours), low energy consumption, high purity extract (Lucchesi et al. 2014).
In the current study, Ray Ruby grapefruit (Citrus paradisi Macf.) leaves have been investigated for the recovery of its naringin. Naringin is a well-known essential nutrient with protective effects for human health due to their antioxidants, anticancer, anticholesterol activities (Wu et al. 2007). Moreover, naringin has a crucial protective role in the neuronal system (Gopinath and Sudhandiran 2012). Lee et al. pointed out the anti-atherogenic properties of the citrus flavonoids such as naringin, and naringenin (Lee et al. 2001). Another research revealed that these flavonoids could be used as useful therapeutical agents due to the inhibition of Alzheimer's disease (Wang et al. 2013). Finally, it was observed that the oral intake of the high dose of naringin (400 mg/kg) might serve as an effective antiulcer treatment (Martin et al. 1994).
Another significant issue in the recovery processes has been emerged in optimization since 70% of the energy consumption of the overall process is spent on recovery (Farhat et al. 2001). Many extraction factors such as types of solvent, temperature, extraction time, plant type, and solid/solvent ratio also affect the extraction efficiency and feasibility of the process. For scale-up applications of the extraction method, optimal operating conditions should be examined. Response surface methodology comprises the synergy effect between the output and the independent variables (Baroutaji et al. 2015).
As a result, there exists no optimization and comparative studies on MAE and SFME techniques by means of various performance criteria, for beneficiating naringin and total phenols contents of Ray Ruby grapefruit leaves. For this purpose, microwave irradiation power density, solid/solvent ratio and extraction time were selected as process factors. Besides, solvent-free microwave extraction (SFME) and classical Soxhlet extraction methods were conducted at the same optimal conditions for comparison purposes. Moreover, the effects of these extraction techniques on the grapefruit leaf surface structure were analysed through scanning electron microscopy (SEM) analysis. The optimal findings of this study related to naringin content might be employable for pharmaceutical applications such as respiratory drug usage (Sansone et al. 2009) and gastro resistant tablet (Lauro et al. 2007). The purpose of the current study is to develop an alternative downstream process to recover high-added value components from a biowaste in a relatively more simple, rapid and cost-effective way.
Materials and methods
Plant material
During the harvesting time (October, 2015), Ray Ruby grapefruit leaves collected and were supplied from Batı Akdeniz Agricultural Research Institute (BATEM) located in Antalya, Turkey. A vacuum oven was used to dry leaves at 35 ºC for 12 h. Before being used, leaves were ground by a grinder and were screened through a 710–1000 µm sieve.
Chemicals and reagents
All chemicals were purchased from Sigma Aldrich. For all sample analyses, ultrapure water (18 mΩ) was used.
Microwave-assisted extraction and solvent-free microwave assisted extraction
Microwave-assisted extraction and SFME were performed in a microwave laboratory oven (Milestone, NEOS-GR Bergamo, Italy) operating with microwave frequency of 2.45 GHz at atmospheric pressure and maximum delivered the power of 900 W. Temperature, time and power were controlled by the installed program. In a typical MAE run, dried leaves weighted in the range of 2–5 g according to selected solid/solvent ratio (8–20 gL−1) was soaked in 250 mL of water, then microwave irradiation power (0.2–1.4 kW L−1) was turned on and system was operated during a specified time (30–240 s). For SFME, the leaves were soaked in bidistillated water in a few minutes in order to moisten. Then, the leaves were poured into a perforated Pyrex disc without the addition of water. SFME procedure was performed similarly to MAE in order to compare. A condenser was used in two methods to cool the microwave irradiation cavity down to room temperature. The extracts removed from the MW oven by earth gravity and filtered through 0.45 μm regenerated cellulose filter for further analysis. Experimental set-ups were depicted in Figs. S1(a)–S1(b), for MAE and SFME respectively.
Soxhlet extraction
As it known, Soxhlet extraction method is a very slow procedure in a high temperature. 10 g of ground leaves put into a cellulose extraction thimble and extracted with 300 mL of water for 24 h in a 500 mL Soxhlet apparatus (~ 1 cycle/4 h). After the extraction, the solution was stored at −20 °C until the analysis.
Calibration of microwave power output
The microwave power calibration test was applied using the standard method (IEC/EN 60,705), an empty glass balloon was weighed and filled with 900 g of bidistillated water at the initial temperature of 10 ± 0.5 °C and then weighed again. It inserted into the microwave oven cavity. Microwave power was adjusted to 900 W for 60 s. After 60 s, the final temperature of the water was measured by a thermocouple. The power output is calculated by using Eq. (1) as follows;
| 1 |
where mw is the mass of water (g), mc is the mass of the container, cpw is the specific heat capacity of water, 4.187 (J/g °C). cpc is the specific heat capacity of the container; 0.55 (J/g °C). T0 is the ambient temperature (25 °C), T1 is the initial temperature of water (°C). T2 is the final temperature of water; t is the heating time (s). The microwave power calibration test was performed with a range of 50–900 mL of water. The applied nominal power (Pnom) was selected so that the water's final temperature was adjacent to the ambient temperature to minimize heat loss and to apply Eq. (1) without any heat losses term for the calculation of the test power P (Temur-Ergan and Bayramglu 2013). A correction factor (p) was calculated as in Eq. (2).
| 2 |
p was calculated as 0.991 for the 250 g water load of the glass balloon used in the conducted extraction procedure.
Determination of total phenolic content
Total phenolic content (TPC) of extracts was measured by Folin–Ciocalteu reagent described as (Boukroufa et al. 2015) with slight modification. Briefly, 20 µL of the extract was mixed with 180 µL of distillated water, and added to 4 mL of plastic UV-cuvette. 1 mL of diluted (1:10 v) Folin–Ciocalteu reagent was taken, and 800 µL of sodium carbonate (7.5%) was added. During 30 min, samples were kept in the dark. By using Uv–Vis spectrophotometer, the sample of absorbance was read at 760 nm. The calibration curve was done according to gallic acid.
Determination of naringin by HPLC
Naringin concentration was determined, according to John et al. validation method (Publication Part Number: 5990-6237EN, 2010). Briefly, two mobile phases (A: Water + % 0.1 formic acid (v/v), B: Acetonitrile + % 0.1 formic acid (v/v)) were prepared. The gradient method was set utilized Agilent Eclipse Plus C18 RRHD column at 40 °C. 1 mL/min of a flow rate was entered to program. The gradient program was adjusted as 0.0–7 min (100% A), 7–7.1 min (60% A), 7.1–8.6 min (100% B), 8.6–8.7 min (100% A). The injection volume was 20µL. The wavelenght of UV detector was set as 276 nm.
Scanning electron microscopy
In order to examine the effects of different extraction methods on the grapefruit leaf of surface microstructure, scanning electron microscope (Quanta FEG 450, FEI, Oregon, USA) was used. Leaves were dried in a vacuum-oven at 40 °C after extraction methods. The leaves coated with a 5 nm thick gold film. SEM device was operated at a beam voltage of 20 kV under a high vacuum.
Experimental design
The effects of binary interaction of factors on responses were evaluated using face-centered central composite design (FCDD). Microwave irradiation power density (0.2–1.4 kW L−1), solid/solvent ratio (8–20 g L−1) and heating time (30–240 s) were selected as independent factors with three levels. Total phenolic content (TPC), naringin content (NC) were chosen as a dependent factor, called as responses. Experiments were applied with six replication at center points to define pure error sum of squares by using Design Expert 12.0 software (trial version). Responses were evaluated through the Response Surface Methodology of the statistical analysis system and fitted to the polynomial model. The quadratic equation suggested is presented as follows in Eq. (3);
| 3 |
Y is the predicted value, β0 is the intercept; βi is the first-order model coefficient; βii is the squared coefficient for the factor i; and βij is the binary interaction between factors i and j; ε is the error. Experimental design with three levels as shown in Table 1.
Table 1.
Experimental design matrix with numerical factors consisting of 20 experiments and the effects of factors on responses
| Run | Microwave power density (X1) (kWL−1) | Solid/Solvent ratio (X2) (gL−1) | Extraction Time (X3) (s) | TPC (mg GAEg−1DL) | NC (mg Ng−1DL) |
|---|---|---|---|---|---|
| 5 | 0.2 | 8 | 30 | 8.484 | 4.10 |
| 2 | 1.4 | 8 | 30 | 14.379 | 3.45 |
| 4 | 0.2 | 20 | 30 | 4.219 | 4.55 |
| 8 | 1.4 | 20 | 30 | 4.211 | 5.66 |
| 1 | 0.2 | 8 | 240 | 11.679 | 5.33 |
| 16 | 1.4 | 8 | 240 | 22.863 | 7.40 |
| 17 | 0.2 | 20 | 240 | 6.822 | 10.05 |
| 7 | 1.4 | 20 | 240 | 14.994 | 13.12 |
| 19 | 0.2 | 14 | 135 | 7.381 | 9.35 |
| 14 | 1.4 | 14 | 135 | 15.047 | 9.89 |
| 18 | 0.8 | 8 | 135 | 20.214 | 7.40 |
| 11 | 0.8 | 20 | 135 | 10.066 | 10.60 |
| 3 | 0.8 | 14 | 30 | 8.005 | 5.01 |
| 10 | 0.8 | 14 | 240 | 11.996 | 8.89 |
| 15 | 0.8 | 14 | 135 | 11.850 | 9.65 |
| 12 | 0.8 | 14 | 135 | 13.057 | 9.33 |
| 20 | 0.8 | 14 | 135 | 12.757 | 9.25 |
| 9 | 0.8 | 14 | 135 | 12.854 | 9.85 |
| 13 | 0.8 | 14 | 135 | 13.143 | 9.57 |
| 6 | 0.8 | 14 | 135 | 13.579 | 9.69 |
Statistical analysis
To evaluate the synergic effects of factors on responses, Design Expert Trial Version 10.0.0 (Stat Ease, USA) was used. Statistical indicators such as correlation factors were determined by ANOVA analysis.
Results and discussions
Development of regression model equation
Table 1 depicted the effects of factors on responses according to FCCD. The packaged software recommended the quadratic models with regression for all responses. The adequacies of the models were confirmed using variance analysis (ANOVA) for each response. Table 2 summarized ANOVA analysis for TPC and NC, respectively. In addition, Table 2 depicted the values of correlation coefficient (R2, R2adj and, R2 pred), coefficient of variation and adequate precision. Correlation coefficients between predictive models and actual data are higher than 0.95 so predictive values fit experimental data well.
Table 2.
ANOVA for the quadratic equations of Design Expert 12.0.1 for MAE of TPC and NC in grapefruit leaf and statistical indicators for each response
| Source | Sum of squares | df | Mean Square | F value | P-valueProb > F |
|---|---|---|---|---|---|
| Model (TPC) | 406.78 | 9 | 45.2 | 44.84 | < 0.0001 |
| X1-Microwave power density | 108.30 | 1 | 108.30 | 107.44 | < 0.0001 |
| X2-Solid/solvent ratio | 139.18 | 1 | 139.18 | 138.06 | < 0.0001 |
| X3-Extraction time | 84.43 | 1 | 84.43 | 83.76 | < 0.0001 |
| X1X2 | 9.93 | 1 | 9.93 | 9.86 | 0.0105 |
| X1X3 | 22.68 | 1 | 22.68 | 22.50 | 0.0008 |
| X12 | 6.46 | 1 | 6.46 | 6.41 | 0.0298 |
| X22 | 15.75 | 1 | 15.75 | 15.63 | 0.0027 |
| X32 | 20.74 | 1 | 20.74 | 20.57 | 0.0011 |
| Residual | 10.08 | 10 | 1.01 | ||
| Lack of fit | 8.41 | 5 | 1.68 | 5.05 | 0.0500 |
| Pure error | 1.67 | 5 | 0.3331 | ||
| Cor total | 416.86 | 19 | |||
| Model (NC) | 127.18 | 9 | 14.13 | 170.78 | < 0.0001 |
| X1-Microwave power density | 3.77 | 1 | 3.77 | 45.56 | < 0.0001 |
| X2-Solid/solvent ratio | 26.57 | 1 | 26.57 | 321.08 | < 0.0001 |
| X3-Extraction time | 48.49 | 1 | 48.49 | 585.97 | < 0.0001 |
| X1X2 | 0.95 | 1 | 0.9522 | 11.51 | 0.0069 |
| X1X3 | 2.74 | 1 | 2.74 | 33.09 | 0.0002 |
| X2X3 | 7.57 | 1 | 7.57 | 91.43 | < 0.0001 |
| X12 | 0.0657 | 1 | 0.0657 | 0.7937 | 0.3939 |
| X22 | 0.5958 | 1 | 0.5958 | 7.20 | 0.0230 |
| X32 | 17.40 | 1 | 17.40 | 210.28 | < 0.0001 |
| Residual | 0.8275 | 10 | 0.0827 | ||
| Lack of Fit | 0.5964 | 5 | 0.1139 | 2.21 | 0.2028 |
| Pure Error | 0.2581 | 5 | 0.0516 | ||
| Cor Total | 128.01 | 19 |
| Responses | Standart deviation | R2 | Adj R2 | Pred R2 | Variance coefficient | Adeq. precisior |
|---|---|---|---|---|---|---|
| TPC (mg GAEg−1-DL) | 1.00 | 0.9758 | 0.9541 | 0.7995 | 8.45 | 27.97 |
| NC (mg Ng−1-DL) | 0.28 | 0.9935 | 0.9877 | 0.9548 | 3.55 | 46.82 |
The difference between R2adj and R2pred should be almost 0.2. In design experiments, the coefficient of variation (CV) is an important term. For confirming the repeatability of the model, it is desirable to have a CV less than 10 (Beg et.al. 2003).
The quadratic equation of TPC is represented in Eq. (4)
| 4 |
F value (44.84) of TPC model represents the model is significant. Values of If Prob > F-value is lower than 0.05, model terms are significant. If the Eq. (4) is examined, the terms of X1, X2, X3, X1.X2, X1.X3, X12, X22, X32 are significant (Table 2). Equation (4) shows that the crucial factor is microwave power density for TPC due to the higher coefficient. The quadratic equation of NC is represented in Eq. (5)
| 5 |
The NC model of F-value is 170.78 implying the model is significant. Values of Prob > F-value less than 0.05 indicate that model terms are significant. If the Eq. (5) is investigated, the terms of X1, X2, X3, X1.X2, X1.X3, X2.X3, X22, X32 are significant (Table 4). Equation (5) shows that the crucial factor is microwave power density for NC.
Design optimization results and effects of factors on responses
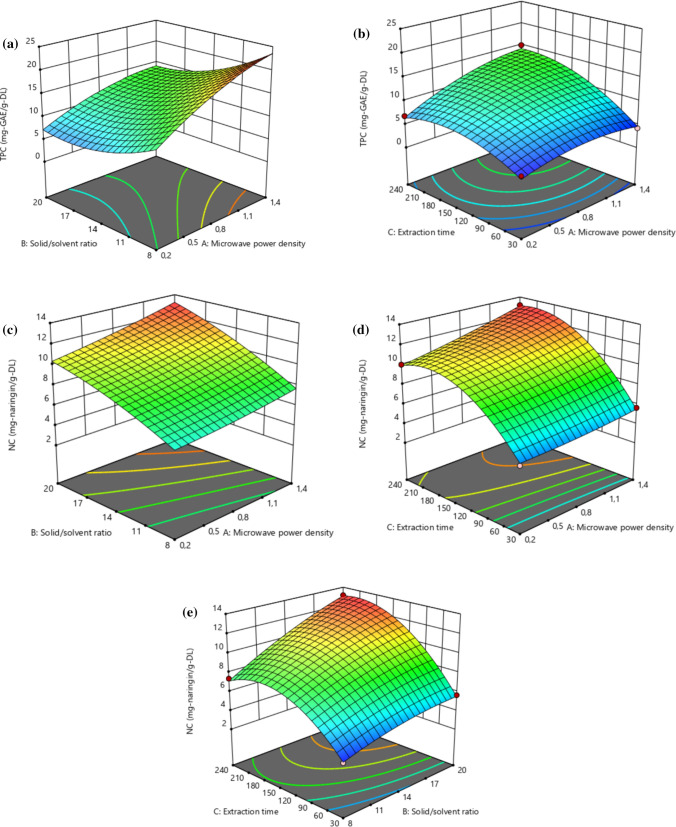
The optimized conditions for multi-optimization responses were established as 1.4 kWL−1 for microwave power density, 20.00 gL−1 for solid/solvent ratio, 218.180 s for extraction time, while responses were calculated as 14.210 mg of gallic acid equivalent per g of the dried leaf (mg GAE g−1DL) and 13.198 mg of naringin per g of dried leaf (mg Ng−1DL) for TPC and NC, respectively. The higher the microwave power density is, the more extraction yields occur. It can be said that increasing microwave power density results in increasing extraction temperature, causing to enhance the extraction rate (Fig. 1a–d). Our results are in agreement with those of Wang et al. (2011) and Xiao et al. (2008). It was stated that higher microwave output power helped to break the cell wall more easily, and released a bioactive compound into the surrounding solvent in their papers. Higher power density leads to increasing solvent temperature. The hotter solvent can solubilize more bioactive compounds because of its lower viscosity and surface tension (Kwon et al. 2003). If the effect of extraction time on extract yields is examined, a positive influence is seen. Extraction yields show a sharp increasing tendency up to a certain time, then increase slightly by increasing time due to the overexposure of leaf to microwave power density (Fig. 1b, d, e). The findings of Simsek et al. (2012); Pan et al. (2008), Xiao et al. (2008) and Chen et al. (2007) were in agreement with our findings. Chen et al. (2007) found that flavonoid content from Platycladus orientalis (L.) Franco increased within 4 min, then did not show any change from 4 to 10 min. Pan et al. (2008) observed similar results while studying the effect of microwave periods on total phenols from green tea leaves. Karabegovic et al. (2014) reported the microwave assisted-extraction on extract yield of cherry laurel fruits. They investigated the effect of extraction time, and indicated that increasing extraction time from 10 to 25 min increased the extract yield. Then, the extract yield was dropped. Their findings were in aggrement with our results.
Fig. 1.
a The binary interaction of solid/liquid ratio and microwave power density on TPC (218 s for extraction time). b the binary interaction of time and microwave power density on TPC (20.00 gL−1 for solid/solvent ratio). c the binary interaction of solid/solvent ratio and microwave power density on NC (218 s for extraction time). d the binary interaction of time and microwave power density on NC (20 gL−1 for solid/solvent ratio). e the binary interaction of time and solid/solvent ratio on NC (1.40 kWL−1)
Regarding the solid/solvent ratio, both TPC and NC were increased with increasing solid/solvent ratio.
According to literature survey, Rouseff et al. (1987) examined naringin content from grapefruit juices with various species. They reported naringin content levels from higher to lower as Canned, Duncan, Foster, Marsh, Ruby Red, Star Ruby. The range of naringin was determined as between 73 and 419 ppm. Yu et al. (2007) investigated naringin from grapefruit seed using the supercritical extraction method. They selected temperature, time, pressure, and ethanol concentration as independent variables via Box-Behnken design. The highest naringin content was found to be 0.2 mg g−1 at 41.4 MPa for pressure, 50 °C for extraction temperature and, 20% for ethanol concentration. Hayat et al. (2009) extracted phenolic acids from mandarin through microwave-assisted extraction method. Microwave power, extraction time, solid/liquid ratio, methanol concentration were selected as variables with Box- Behnken design. Şahin (2015) examined antioxidant activities and phenol content from mandarin leaves using solvent-free microwave extraction method. Optimization results were found as 53.15 s for extraction time, 339 W for microwave power and 2.5 g of dried leaf. The highest total phenol was obtained as 0.8610 mg GAEg−1-DL. Ateş et al. (Ateş et al. 2019) studied the total phenols content of mandarin leaf waste by using microwave-assisted extraction. They obtained the highest total phenols amount as 17.2254 mg GAE/g-DL when they applied 275 W of microwave irradiation power, 2 g of mandarin leaf and 45 s of time.
Consumed energy was also crucial in a view of the product cost, thus the extraction consumed electrical energy must be measured for industrial scale. Table 3 illustrated the compared with the consumed energy, naringin content and TPC of extracts obtained by MAE, SFME and Soxhlet methods. The highest naringin content was obtained in Soxhlet extraction, whereas the highest TPC was gained in MAE. For soxhlet, this situation may be explained by the fact that the longer contact time provides enhancing mass transfer between the target material of the plant matrix and solvent (Tan et al. 2013). Minimum extract yields were gained by SFME. This situation showed that usage of solvent was necessary for the extraction process by taking into account of SFME.
Table 3.
Naringin content, energy consumed and TPC of extract yield between Soxhlet, MAE and SMAE techniques
| ExtractionMethod | NC | TPC | Energy Consumed(kW-h) |
|---|---|---|---|
| Soxhlet | 33.780 | 11.46 | 2.614 |
| MAE | 13.198 | 14.210 | 0.033 |
| SFME | 0.032 | 0.464 | 0.033 |
NC: mg g−1-DL
Scanning electron mMicroscopy
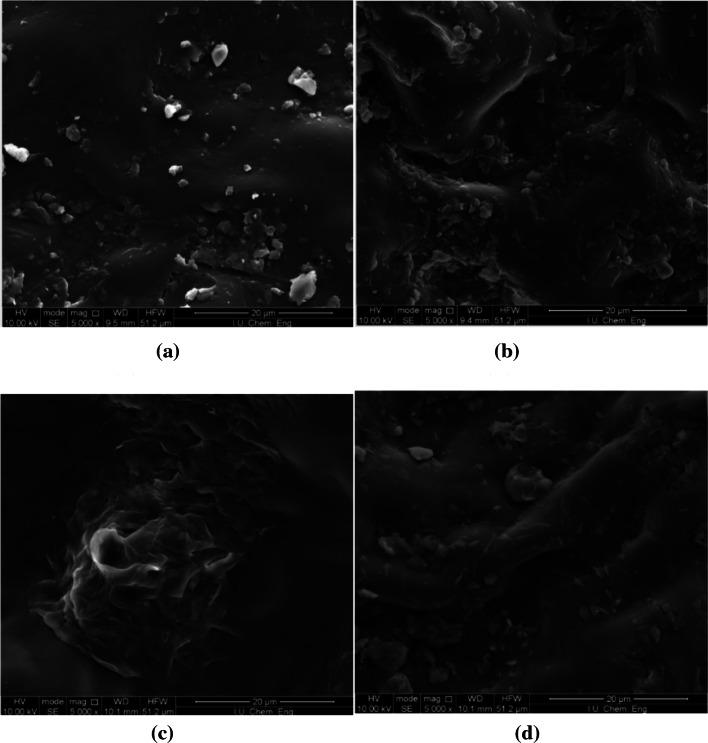
Figure 2a–d depicted the SEM images of grapefruit leaves after and before extracted by MAE, SFME and Soxhlet methods. The physical changes in plant tissues’ cell walls have effects on extraction efficiency (Fig. 2b–d). Grapefruit leaf tissues’ microstructures were monitored after extractions via SEM images. The morphology of untreated of grapefruit leaf was smooth (Fig. 2a). It is obviously seen that there are changes in the material structure after MAE, SFME and Soxhlet through the SEM. The physical characteristics of tissues’ cell walls were significantly changed by MAE and SFME, which broke the cell structure and improved capillary pores of tissues (Fig. 2b, c). In addition, the usage of electromagnetic wave signal caused molecules rotating like magnet effect. Produced energy was converted into heat, causing disruption of cell walls as seen in Fig. 2b, c. Soxhlet extraction method results in the breakage of the leaf's cell (Fig. 2d). It is seen that there is less fragmentation of cell wall than the mentioned other methods (Fig. 2d).
Fig. 2.
a SEM image of untreated grapefruit leaves, b SEM image of grapefruit leaves extracted by MAE under optimal conditions. c SEM image of grapefruit leaves extracted by SMAE. d SEM image of grapefruit leaves extracted by Soxhlet extraction method
Conclusion
Grapefruit (Citrus paradisi Macf.) leaves were extracted by MAE and SFME as novel and alternative to conventional methods because of economic and environmental issues. Soxhlet extraction was conducted in order to be a control. To sum up, the present article is the first to investigate the effects of comparison of MAE, SFME, and Soxhlet extraction methods on the recovery of TPC from grapefruit leaf as a valorization of a biowaste. Besides, electrical energy consumptions of MAE, SFME, and Soxhlet methods were also measured, where Soxhlet consumed much more energy than that of the microwave. Even though the naringin yield of Soxhlet was almost twice over that of MAE, the energy consumption of MAE was found to be 79 times less than that of Soxhlet. The findings of the present study demonstrate that MAE with severely lower extraction time and energy consumption is a highly economical process.
The optimized conditions were established as 1.4 kWL−1 for microwave power density, 20.00 gL−1 for solid/solvent ratio and 218.180 s for extraction time, while responses were calculated as 14.210 (mg GAE/g-DL) and 13.198 mg NC/g-DL) for TPC and NC, respectively.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Funding
This study was supported by Istanbul University Research Fund (BAP, Project No. 3426). All authors thank BAP for support.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ateş F, Şahin S, İlbay Z, Kırbaşlar Şİ. A green valorisation approach using microwaves and supercritical CO2 for high-added value ingredients from Mandarin (Citrus deliciosa Tenore) leaf waste. Waste Biomass Valorization. 2019;10(3):533–546. doi: 10.1007/s12649-017-0074-z. [DOI] [Google Scholar]
- Bakirtzi C, Triantafyllidou K, Makris DP. Novel lactic acid-based natural deep eutectic solvents: efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J Appl Res Med Aroma. 2016;3(3):120–127. [Google Scholar]
- Baldán Y, Fernandez A, Urrutia AR, Fabani MP, Rodriguez R, Mazza G. Non-isothermal drying of bio-wastes: kinetic analysis and determination of effective moisture diffusivity. J Environ Manage. 2020;262:110348. doi: 10.1016/j.jenvman.2020.110348. [DOI] [PubMed] [Google Scholar]
- Baroutaji A, Gilchrist MD, Smyth D, Olabi AG (2015) Analysis and optimization of sandwich tubes energy absorbers under lateral loading. J Impact Eng Int
- Beg Q, Sahai V, Gupta R. Statistical media optimization and alkaline protease production from Bacillus mojavensis in a bioreactor. Process Biochem. 2003;39:203. doi: 10.1016/S0032-9592(03)00064-5. [DOI] [Google Scholar]
- Boukroufa M, Boutekedjiret C, Petigny L, Rakotomanomana N, Chemat F. Bio-refinery of orange peels waste: a new concept based on integrated green and solvent free extraction processes using ultrasound and microwave techniques to obtain essential oil, polyphenols and pectin. Ultrason Sonochem. 2015;24:72–79. doi: 10.1016/j.ultsonch.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Chemat F, Cravotto G. Microwave-assisted extraction for bioactive compounds: theory and practice. New York, Heidelberg, Dordrecht, London: Springer; 2013. [Google Scholar]
- Chen L, Ding L, Yu A, Yang R, Wang X, Li J, Jin H, Zhang H. Continuous determination of total flavonoids in Platycladus orientalis (L.) Franco by dynamic microwave-assisted extraction coupled with on-line derivatization and ultraviolet–visible detection. Analytica Chimica Acta. 2007;596(1):164–170. doi: 10.1016/j.aca.2007.05.063. [DOI] [PubMed] [Google Scholar]
- Farhat A, Fabiano-Tixier A-S, El MM, Maingonnat J-F, Romdhane Chemat MF. Microwave steam diffusion for extraction of essential oil from orange peel: kinetic data, extract’s global yield and mechanism. Food Chem. 2001;125:255–261. doi: 10.1016/j.foodchem.2010.07.110. [DOI] [Google Scholar]
- Gopinath K, Sudhandiran G. Naringin modulates oxidative stress and inflammation in 3-nitropropionic acid-induced neurodegeneration through the activation of nuclear factor-erythroid 2-related factor-2 signalling pathway. Neuroscience. 2012;227:134–143. doi: 10.1016/j.neuroscience.2012.07.060. [DOI] [PubMed] [Google Scholar]
- Hayat K, Hussain S, Abbas S, Farooq U, Ding B, Xia S, Jia C, Zhang X. Optimized microwave-assisted extraction of phenolic acids from citrus mandarin peels and evaluation of antioxidant activity in vitro. Sep Purif Technol. 2009;70:63–70. doi: 10.1016/j.seppur.2009.08.012. [DOI] [Google Scholar]
- I.E.C.—International Electrotechnical Commission Household Microwave Ovens—Methods for Measuring Performance International Electrotechnical Commission (2015) Standard Nr. 60705.
- John, W.; Jr., Henderson, Judy, Berry, A.; Mack, Long, W. Agilent Technology, Rapid Gradient and Elevated Temperature UHPLC of Flavonoids in Citrus Fruit. https://www.chem.agilent.com/Library/applications/5990-6237EN.pdf.
- Karabegovic IT, Stojičević SS, Veličković DT, Nikolić NČ, Lazić ML. Optimization of microwave-assisted extraction of cherry laurel fruit. Separ Sci Technol. 2014;49:416. doi: 10.1080/01496395.2013.838967. [DOI] [Google Scholar]
- Kwon JH, Belanger JMR, Yaylayan VA. Application of the microwave-assisted process (MAP™) to the fast extraction of ginseng saponins. Food Res Int. 2003;36:491–498. doi: 10.1016/S0963-9969(02)00197-7. [DOI] [Google Scholar]
- Lauro MR, De Simone F, Sansone F, Iannelli P, Aquino RP. Preparations and release characteristics of naringin and naringenin gastro-resistant microparticles by spray-drying. J Drug Deliv Sci Technol. 2007;17:119. doi: 10.1016/S1773-2247(07)50018-3. [DOI] [Google Scholar]
- Lee C-H, Jeong T-S, Choi Y-K, Hyun B-H, Oh G-T, Kim E-H, Kim J-R, Han J, Bok S-H. Anti-atherogenic effect of citrus flavonoids, naringin and naringenin, associated with hepatic ACAT and Aortic VCAM-1 and MCP-1 in high cholesterol-fed rabbits. Biochem Biophys Res Commun. 2001;284:681–688. doi: 10.1006/bbrc.2001.5001. [DOI] [PubMed] [Google Scholar]
- Li Y, Fabiano-Tixier AS, Vian MA, Chemat F. Solvent-free microwave extraction of bioactive compounds provides a tool for green analytical chemistry. TrAC, Trends Anal Chem. 2013;47:1–11. doi: 10.1016/j.trac.2013.02.007. [DOI] [Google Scholar]
- Lucchesi ME, Chemat F, Smadja J. An original solvent free microwave extraction of essential oils from spices. Flavour Frag J. 2014;19:134–138. doi: 10.1002/ffj.1274. [DOI] [Google Scholar]
- Mandal V, Mohan Y, Hemalatha S. Microwave assisted extraction—an innovative and promising extraction tool for medicinal plant research. Pharmacogn Rev. 2007;1(1):7–18. [Google Scholar]
- Martin MJ, Marhuenda E, Perez-Guerrero C, Franco JM. Antiulcer effect of naringin on gastric lesions induced by ethanol in rats. Pharmacology. 1994;49:144–150. doi: 10.1159/000139228. [DOI] [PubMed] [Google Scholar]
- Ozturk B, Parkinson C, Gonzalez-Miquel M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep Purif Technol. 2018;206:1–13. doi: 10.1016/j.seppur.2018.05.052. [DOI] [Google Scholar]
- Pan Y, Wang K, Huang S, Wang H, Mu X, He C, Ji Z, Huang F. Antioxidant activity of microwave-assisted extract of longan (Dimocarpus longan Lour.) peer. Food Chem. 2008;106:1264–1270. doi: 10.1016/j.foodchem.2007.07.033. [DOI] [Google Scholar]
- Rouseff RL, Martin SF, Youtsey CO. Quantitative survey of narirutin, naringin, hesperidin, and neohesperidin in citrus. J Agric Food Chem. 1987;35:1027–1030. doi: 10.1021/jf00078a040. [DOI] [Google Scholar]
- Sansone F, Aquino RP, Gaudio PD, Colombo P, Russo P. Physical characteristics and aerosol performance of naringin dry powders for pulmonary delivery prepared by spray-drying. Eur J Pharm Biopharm. 2009;72(1):206–213. doi: 10.1016/j.ejpb.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Simsek M, Sumnu G, Sahin S. Microwave assisted extraction of phenolic compounds from sour cherry pomace. Separ Sci Technol. 2012;47:1248–1252. doi: 10.1080/01496395.2011.644616. [DOI] [Google Scholar]
- Şahin S. A novel technology for extraction of phenolic antioxidants from mandarin (Citrus deliciosa Tenore) leaves: Solvent-free microwave extraction. Korean J Chem Eng. 2015;32:950–957. doi: 10.1007/s11814-014-0293-y. [DOI] [Google Scholar]
- Şahin S, İlbay Z, Kırbaşlar Şİ. Pulsed ultrasound-assisted extraction of natural antioxidants from mandarin (Citrus deliciosa Tenore) leaves: Experimental and modeling study. Chem Eng Commun. 2018;205(6):717–726. doi: 10.1080/00986445.2017.1328414. [DOI] [Google Scholar]
- Şahin S, Elhussein EAA. Assessment of sesame (Sesamum indicum L.) cake as a source of high-added value substances: from waste to health. Phytochem Rev. 2018;17(4):691–700. doi: 10.1007/s11101-018-9554-4. [DOI] [Google Scholar]
- Tan MC, Tan CP, Ho CW. Effects of extraction solvent system, time and temperature on total phenolic content of henna (Lawsonia inermis) stems. Int Food Res J. 2013;20(6):3117. [Google Scholar]
- Temur-Ergan B, Bayramoglu M. The effects of microwave power and dielectric properties on the microwave-assisted decomposition kinetics of AIBN in n-butanol. J Ind Eng Chem. 2013;19:299–304. doi: 10.1016/j.jiec.2012.08.015. [DOI] [Google Scholar]
- Wang D-M, Yang Y-J, Zhang L, Zhang X, Guan F-F, Zhang LF. Naringin enhances CaMKII activity and improves long-term memory in a mouse model of Alzheimer’s disease. Int J Mol Sci. 2013;14:5576–5586. doi: 10.3390/ijms14035576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Shang Q, Wang W, Xuejiao F. Microwave-assisted extraction and liquid chromatography/mass spectrometry analysis of flavonoids Ffrom grapefruit peel. J Food Process Eng. 2011;34:844–859. doi: 10.1111/j.1745-4530.2009.00513.x. [DOI] [Google Scholar]
- Wu T, Guan Y, Ye J. Determination of flavonoids and ascorbic acid in grapefruit peel and juice by capillary electrophoresis with electrochemical detection. Food Chem. 2007;100:1573–1579. doi: 10.1016/j.foodchem.2005.12.042. [DOI] [Google Scholar]
- Xiao W, Han L, Shi B. Microwave-assisted extraction of flavonoids from Radix Astragali. Sep Purif Technol. 2008;62(3):614–618. doi: 10.1016/j.seppur.2008.03.025. [DOI] [Google Scholar]
- Yu J, Dandekar DV, Toledo RT, Singh RK, Patil BS. Supercritical fluid extraction of limonoids and naringin from grapefruit (Citrus paradisi Macf.) seeds. Food Chem. 2007;105:1026–1031. doi: 10.1016/j.foodchem.2007.04.062. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.