Abstract
Ever since the flavonoids and other antioxidants in rice were demonstrated with immense health benefits, much interest has been diverted to study the native indigenous rice landraces. In the present investigation, three pigmented rare Indian rice landraces and two non-pigmented rice varieties were analyzed for their phytoconstituents like total phenolic content (TPC), total flavonoid content (TFC), total anthocyanin content and antioxidant potential using 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity. The phytochemical profile was compared between seed, seedling, stem, leaf and root tissues of the different varieties. Pigmented rice cultivars demonstrated higher levels of bioactive compounds as compared to the non-pigmented cultivars exhibiting a strong correlation between the antioxidant activity of the grain and their phenolic and flavonoid content. Among the five different rice studied, black navara was found to be superior in terms of its phytochemical composition. Further, the expression levels of flavonoid genes among pigmented and non-pigmented rice was studied. Higher gene expression profiles were observed in the 8 flavonoid genes studied in pigmented rice varieties as compared to non-pigmented varieties. The colorlessness of non-pigmented rice and its lower levels of phytoconstituents correlated with the low transcript levels of flavonoid genes recorded in them. This study provides a basis for understanding the health-promoting properties of pigmented rice over non-pigmented rice at the biochemical and molecular levels.
Keywords: Flavonoids, Gene expression, Oryza sativa L., Pigmented rice
Introduction
Half the world’s population is dependent on rice (Oryza sativa L.) as the primary staple food (Chakuton et al. 2012) and the Asian region produces and consumes over 90% of the world’s rice. The commonly consumed white rice is the highly refined version of raw rice, which is hulled and milled. The processing and milling results in the removal of essential parts of the grain—bran and germ which are rich in dietary fiber and nutrients that are beneficial for human health. In addition to common white rice varieties, there are pigmented varieties such as the colored black, red, brown, or purple ones (Pengkumsri et al. 2015). Colored rice is traditionally known to have health benefits and is particularly valued in local markets (Saikia et al. 2012). The color bearing compounds in plants include polyphenols, anthocyanins, carotenoids, and chlorophylls (Zinoviadou et al. 2015). Pigmentation in rice is attributed to the presence of flavonoids and the accumulation of significant quantities of anthocyanin pigments. Anthocyanins are the coloring pigments that give foods many of their deep rich red, blue, and purple color and have notable antioxidant and anti-inflammatory properties (Hou et al. 2013). Anthocyanins are also implemented in the food industry as natural alternatives to replace synthetic food colorants (Barba et al. 2015).
Epidemiological studies suggest that there is a direct correlation between low incidences of certain chronic diseases with that of the antioxidant contents in rice, especially in the rice consuming regions of the world (Hudson et al. 2000). The antioxidant activities of pigmented rice varieties could well be attributed to the presence of several compounds including phenolic acids, flavonoids, anthocyanins, tocopherols, tocotrienols, γ-oryzanol, proanthocyanidins and phytic acid (Goufo and Trindade 2014). Deng et al. (2013) reported that high levels of anthocyanins, proanthocyanidins, and phenolic acids are found in pigmented rice compared to white varieties. Low molecular weight phenolics are found in grains with light brown pericarp color whereas grains with red and black pericarp color contain higher molecular weight phenolics (Goffman and Bergman 2004). Cell cultures studies have shown that phenolic compounds in the rice may also be associated with anti-carcinogenic, anti-mutagenic, and anti-metastasis activities, due to their ability to directly protect DNA damage and affect cell proliferation (Hu et al. 2003). Phenolic compounds in rice are effective against cardiovascular problems since they play a vital role in reducing the total cholesterol concentrations in the blood, reduction in the oxidation of LDL cholesterol (Xia et al. 2003), and reduction in the ratio between LDL and HDL cholesterol (Ling et al. 2001). The ability of phenols to inhibit the oxidation of human lipoproteins of low density is associated with atherosclerosis (Rahmanian et al. 2014). Natural phenols also promise wide application in industrial sectors as natural food or beverage preservatives owing to their strong antioxidant nature by which they can extend the product shelf-life by delaying off-flavors and rancidity formation (Galanakis 2012). Many studies indicate that consumption of colored rice has also been linked to other beneficial properties including antioxidants (Hu et al. 2003), antidiabetic (Morimitsu et al. 2002), anti-inflammatory, anti-carcinogenic, α-glucosidase inhibitory and hepatoprotective effects (Shao et al. 2014). Hence colored rice promises potential as a nutraceutical, which indicates foodstuff providing health benefits (Galanakis 2013). Since flavonoids result in different colorations of the rice grain, our goal was to compare the flavonoid content and composition of colored and non-colored rice grains.
Flavonoids are derived from the general phenylpropanoid pathway and the biosynthetic pathway is comprised of various biosynthetic branches resulting in the production of flavonoids, anthocyanins (Vogt 2010), lignin and stilbenes (Qiu et al. 2013). The majority of the genes and enzymes involved in the flavonoid biosynthetic pathway have been studied in the model plants (Allan et al. 2008). The complexity of the biosynthetic pathway diverges depending on the type of plant species, the developmental stage of a tissue, environmental signals such as stress factors (temperature, drought, high-intensity light, UV light, nutrient deficiency, pathogen infection, and wounding), hormones and sugar (Chalker-Scott 1999; Shan et al. 2009). The activity and expression pattern of the flavonoid structural genes are controlled by the expression patterns of regulatory genes (Koes et al. 2005). The expression levels are found to vary according to the tissue, gene, and colored rice genotype (Chen et al. 2013). Considering these facts, total phenolic content (TPC), total flavonoid content (TFC), total anthocyanin content (TAC), antioxidant activities using 2,2diphenyl-1 picrylhydrazyl (DPPH) radical scavenging activity of the pigmented and non-pigmented rice extracts were assessed. Further, the regulation of some flavonoid biosynthesis genes in different tissues like seedling, stem, root, and leaf of these distinctly colored rice was analyzed. There is an increasing demand for health-oriented functional products with natural antioxidants, high fiber, and low-calorie content because of their beneficial health effects (Kovacevic et al. 2018). The results will highlight the preliminary information that is worth considering for future studies involving the use of native rice varieties in the food industry, health products, pharmaceutical and medicinal applications (Chakuton et al. 2012).
Materials and methods
Non-pigmented IR64 (IR) and Pusa basmati (PB) varieties were procured from Paddy Breeding Station, Tamilnadu Agricultural University, Coimbatore, India and the pigmented red chittaini (RC), red kuruva (RK) and black navara (BN) from farmers in Kerala, India. The samples were cleaned, packed in polyethylene bags and placed inside cloth bags, and stored at 4 °C until the experiments were carried out. The samples were dehusked manually and the kernels were then ground to a fine powder using mortar and pestle and stored at 4 °C.
Sample extraction
Seed
The sample extraction was carried out according to Shen et al. (2009) with slight modification. Powdered rice (1 g) was extracted with 12.5 mL of 1% HCl methanol for 24 h at 24 °C. The procedure was repeated twice. The methanolic extract was centrifuged at 4000 g for 15 min and the supernatant was pooled and stored at 4 °C. The supernatant was then stored at − 20 °C until further analysis. For DPPH and anthocyanins estimation 75:25 v/v, acetone: water was used instead of 1% HCl methanol and the same extraction method was followed.
Seedling, root, stem, and leaf
Fifteen-day old seedlings of all the 5 samples were washed in running tap water and dried. Each sample (1 g) was ground to a fine powder using liquid nitrogen and extracted with 12.5 mL of 1% HCl methanol for 24 h at 24 °C. The procedure was repeated twice and the methanolic extract was centrifuged at 950 g for 15 min and the supernatant was pooled and stored at 4 °C (Saikia et al. 2012 with slight modification). For DPPH and anthocyanin estimation 75:25 v/v, acetone: water was used instead of 1% HCl methanol and the same extraction method was followed. Leaf, stem, and root samples from 30-day old plants of the five rice varieties were excised with sterile scissors and extracted using the same protocol described above.
Determination of total phenolic content
Total phenolic content was determined using the Folin-Ciocalteu method (Bao et al. 2005). The rice extract (0.2 mL) was mixed with Folin–Ciocalteu reagent (0.8 mL) and deionized water (5 mL). After incubation at room temperature for 2 min, 15% (3 mL) sodium carbonate was added, left for 0.5 min and the final volume was made up to 10.0 mL with water. The absorbance was measured at 755 nm after incubation for 2 h. Gallic acid was used as the standard of calibration (R2 = 0.975). The TPC was expressed as mg Gallic Acid Equivalent (GAE) per 100 g of rice (mg GAE/100 g).
Determination of total flavonoid contents
The total flavonoid content of samples was determined using the colorimetric method according to Bao et al. (2005). An aliquot (2 mL) of the extract was placed in a 10 mL volumetric flask containing deionized water (5 mL). To this 5% sodium nitrite (0.5 mL) was added, after 5 min, 10% aluminum chloride (0.5 mL) was added. After 6 min, 1 mol/L sodium hydroxide (2 mL) was added and the absorbance was measured immediately at 510 nm. The content of total flavonoids was calculated from the calibration curve of the catechin standard. Measurements were calibrated (R2 = 0.962) to a standard curve of prepared catechin solution and were expressed as mg Catechin Equivalent (CE) per 100 g rice (mg CE/100 g).
Determination of DPPH activity
The DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activity of the extracts was measured according to the method of Brand-Williams et al. (1995). The Extract (100 μL) was added to 1.4 mL DPPH radical methanolic solution (10–4 M) and the absorbance at 517 nm was measured after 30 min against blank (100 μL methanol in 1.4 mL of DPPH radical solution). The results were expressed in terms of radical scavenging activity using the following equation:
where Ao is the absorbance of control blank, and As is the absorbance of sample extract.
Determination of total anthocyanin content
The total anthocyanin content of the rice samples was determined by the pH differential method (Hosseinian et al. 2008) with some modifications. The rice extract was mixed thoroughly with 0.025 M potassium chloride buffer, pH 1.0, and allowed to stand for 30 min in dark. The absorbance was then measured at 520 and 700 nm respectively. The extract was also mixed similarly with 0.4 M sodium acetate buffer, pH 4.5, and absorbance was measured at the same wavelength after standing for 30 min. The difference in absorbance between pH values and wavelengths was calculated as,
where A (absorbance) = [(A515–A700) pH 1.0 – (A515–A700) at pH 4.5];
MW is equal to 449.2 (molecular weight of cyanidin- 3-glucoside); DF is the dilution factor of the sample; ε is the molar absorptivity of cyanidin-3-glucoside, equal to 26,900; C is the concentration of the buffer in mg/mL.
Flavonoid biosynthetic gene transcript levels in different tissues
Total RNA was extracted from different tissues of pigmented and non-pigmented rice varieties using the Trizol method (Invitrogen, CA, USA). RNA samples were subjected to DNase-I treatment and reversed transcribed using Primescript 1st strand cDNA synthesis kit (Takara, Japan). Gene-specific primers were designed for eight different rice flavonoid structural genes from the 3′-UTR regions (Table 1). Amplification of target cDNA was performed in Applied Biosystems 7500 Real-Time PCR system (USA) with SYBR Premix Ex Taq II, Rox Plus (Takara, Japan) using the following program: 95 °C (3 min); 30 cycles of 95 °C (30 s), 60 °C (30 s), 72 °C (30 s); 72 °C (7 min).
Table 1.
List of primers used for the qRT- PCR experiments in the study
| S. No | Gene | Primer | Sequence (5′–3′) |
|---|---|---|---|
| 1 | Phenyl ammonia-lyase (PAL) | PAL F | AGCTCCGTCAAGAACTGCGTC |
| PAL R | CGATGGCGGTGAGGAGGT | ||
| 2 | 4-Coumarate:CoA ligase (CL) | CL F | CCTTCTCAACACCCATCCAA |
| CL R | CATCTTCACTCAGCTCACTCC | ||
| 3 | Chalcone synthase (CHS) | CHS F | GTTCACACCGCTGGGGATCT |
| CHS R | TCCTCTCCTTGTCCAGCCCA | ||
| 4 | Chalcone flavone isomerase (CHI) | CHI F | AGAAGTTCACGAGGGTGACG |
| CHI R | GAGTGGGTGAAGAGGATGGA | ||
| 5 | Flavanone 3-hydroxylase (F3H) | F3H F | AGCACAGAAGCCCAAGTCTC |
| F3H R | CTTCGATTTTCGACGGAAGA | ||
| 6 | Dihydroflavonol 4-reductase (DFR) | DFR F | GACATCGACTTCTGTCGCCG |
| DFR R | TGACGCTGATGAGGTCCAGC | ||
| 7 | Leucoanthocyanidin reductase (LAR) | LAR F | CTTCATCTGCTGCAACTCCA |
| LAR R | GTGCACGATCTTGTTGATGC | ||
| 8 | Anthocyanin Synthase (ANS) | ANS F | AGCTGCTCGCCATCCTCTCC |
| ANS R | GCTGACGTCGGTGTGTGCC |
Statistical analysis
All the values were expressed as means of triplicate analysis of the samples (n = 3) ± SD. The data was further analyzed using One-way analysis of variance (ANOVA) followed by Duncan's multiple range test (p < 0.05) with the aid of SPSS, statistical package program (USA). p < 0.05 was considered as indicative of significance compared to either control or between the varieties.
Results and discussion
Biochemical evaluation
Total phenolic content
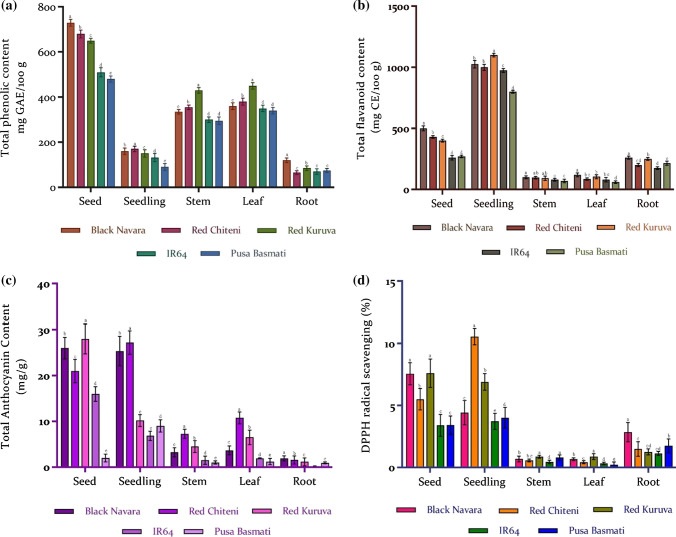
Phenolic compounds are potent antioxidants with immense potential in reducing the risk of many diseases. Apart from their dietary benefits, the antioxidant ability of phenols to reduce oil peroxidation has been applied in the preservation and fortification of different products such as vegetable oils, bakery products, milk beverages, and meat products (Galanakis 2018). Galankis et al. (2018) also reported their application as UV boosters in cosmetics. TPC of seeds, seedling, stem, leaf, and root of pigmented and non-pigmented rice varieties were evaluated. Among the five rice samples, the highest phenolic content was recorded in the seeds of black rice (730 mg GAE/100 g) followed by the red variety in the range of 650 to 680 mg GAE/100 g (Fig. 1a). Similar results have been reported by Murdifin et al. (2015) where black rice (292.74–746.25 mg GAE/100 g) recorded higher TPC than red rice (12.52–64.52 mg GAE/100). The TPC in white rice seeds was lower compared to pigmented rice, recording 480 to 510 mg GAE/100 g. Similarly, Chakuton et al. (2012), in their study, found that pigmented rice ranked higher in their TPC compared to non-pigmented rice. Phenolic contents in rice have been positively related to the pericarp color of the grains (Walter and Marchesan 2011). This is also evident from our current results, where higher phenolic contents in the pigmented genotypes were observed. Phenolic content in leaf and stem were almost similar in the range of 330 to 450 mg GAE/100 g in pigmented rice, with the highest value in red kuruva (RK) in both leaf and stem. The non-pigmented varieties did not significantly vary in the phenolic content, but values were higher in leaf samples compared to stem tissues (340 to 350 mg GAE/100 g). TPC of seedlings recorded a value of 150 to 170 mg GAE/100 g in pigmented rice compared to non-pigmented rice (90 to 130 GAE/100 g). TPC of seedlings recorded a value of 150 to 170 GAE/100 g in colored rice compared to a value of 90 to 130 mg GAE/ 100 g in non-colored rice. Among all the five tissues studied, lowest TPC was observed in roots, in which the phenolic contents were higher in black followed by red and white rice in the range of 120, 75, and 72.5 mg GAE/100 g. Similar to our results, Jain et al. (2015) in their study reported higher phenolic content in the seeds of Abrus precatorius compared to other parts of the plant. The phenolic content recorded by them was in the order Seed > Leaf > Root > Stem.
Fig. 1.
Biochemical composition of different parts of pigmented and non-pigmented rice a Total phenolic content; b Total flavonoid content; c Total anthocyanin content d DPPH radical scavenging activity. Values represent the mean ± SD of three replicates. Significance shown are within the different parts and between varieties of rice. Means following the same letter are not significantly different according to Duncan’s multiple range test (p < 0.05)
Total flavonoid content
Flavonoids are antioxidants with a considerable effect on the health of humans. TFC values demonstrated that seeds of black rice had higher flavonoid content (500 mg QE/100 g) than red rice (400 mg QE/100) (Fig. 1b) and TFC values were least in seeds of white rice (260 to 270 mg QE/100 g). Vichapong et al. (2010) had also demonstrated that pigmented and brown rice had higher phenolics and flavonoids compared to non-pigmented rice. Similarly, Shen et al. (2009) also reported lower flavonoid content in white rice than red and black rice. As observed in the TPC assay, the TFC values in leaf and stem were similar recording a range of 850–1200 mg QE/100 g in leaf samples and 950–1000 mg QE/100 g in stem samples in pigmented rice with the highest value in black rice. TFC in leaf and stem of white rice were lower compared to pigmented rice. Seedlings recorded the highest flavonoid content compared to seeds, stem, leaf, and root. The total flavonoid content in seedlings was maximum in black, followed by red rice and white rice. TFC in roots was also higher in pigmented rice than white rice. Among different plant parts of Abrus precatorius, the highest flavonoid content was detected in the leaves with no significant variations in the seed, stem, and root (Jain et al. 2015). Similarly, Rebaya et al. (2014) detected higher flavonoid content in leaves compared to flowers of Cistaceae. del Valle et al. (2015) suggested that flavonoid production in each organ is regulated independently.
Determination of anthocyanin content
Anthocyanins are synthesized by a conserved route of the flavonoid pathway. They are responsible for the various colors seen in plants. The anthocyanin content was higher in pigmented rice compared to non-pigmented rice. In leaf samples, the highest anthocyanin content was detected in red rice (6.6–10.7 mg/g), followed by black and white rice. Cyanidin-3-glucoside and peonidin-3-glucoside are the prominent anthocyanins in pigmented rice (Saikia et al. 2012). Similar results were recorded for shoot tissues where red rice ranked first followed by black and white. The values in white rice were seven-fold lower compared to red rice and three-fold lower compared to black rice. Seedlings recorded the highest anthocyanin content with red rice ranking first with a value ranging from 25 to 27 mg/g. The anthocyanin content in seedlings of black rice was two-fold lesser compared to red rice and the values were least in white rice seedlings (Fig. 1c). The anthocyanin concentration in white rice was almost nine times lower compared to pigmented rice. In root samples, anthocyanin content in both black and red rice were similar in the range of 1.2–1.9 mg/g, which was higher than non-pigmented rice. The values were 11 fold higher compared to white rice roots. A differential regulation pattern in anthocyanin production in different tissues and organs is followed in plants and they can also differ between cells of the same tissue. Maier et al. (2013) reported light-dependent transcription factors responsible for controlling anthocyanin accumulation in vegetative tissues. A positive correlation in anthocyanin production in response to UV-B radiation and temperature and a negative correlation with precipitation has been suggested by Chalker-Scott. (1999).
Determination of DPPH activity
Natural antioxidants have both nutritional and functional properties in terms of oxidative stress reduction, prevention of arteriosclerosis, cancer, aging processes, and preservative of vegetable oils and emulsions (Galanakis 2015). The antioxidant potential in different tissues of all the five rice varieties was measured by the DPPH radical scavenging assay and red rice seedlings recorded the highest values in the range of 7–10.6 µg/g, followed by black rice and least in white (Fig. 1d). The DPPH results showed that seeds of black navara (BN) and red rice were similar in the range of 7.57–7.61 µg/g but red chittaini (RC) was comparatively lower. The values were least in white rice. Previous work by Chakuton et al. (2012) also reported higher DPPH activity in pigmented rice seeds compared to the non-pigmented counterpart. Saikia et al. (2012) also reported higher DPPH activity in pigmented red rice compared to non-pigmented rice. This may be due to the higher phenolic contents in pigmented grains as phenolic contents have been positively correlated with the antioxidant capacity in rice grains (Shen et al. 2009). Condensed tannins or oligomeric proanthocyanins in pigmented rice could also be a contributing factor for higher antioxidant potential in pigmented rice. The DPPH scavenging activity in leaves was found to be higher in red rice followed by black, and the scavenging activity was lowest in the leaves of white rice. A similar trend was observed in stem samples with red rice ranking the highest followed by black rice and least in white rice. Among all the five tissue samples, scavenging activity was highest in seedlings, followed by seed and root. Red rice seedlings recorded the highest scavenging activity, followed by black and white rice. In root samples, values were elevated in red rice followed by black and least in white rice. Similar to our results, Jain et al. (2015) reported higher antioxidant potential in seeds of Abrus precatorius followed by root > leaf > stem. Rebaya et al. (2014) reported higher scavenging activity by DPPH and FRAP (ferric reducing antioxidant power assay) in the leaves of Cistaceae compared to flowers.
Flavonoid biosynthetic gene transcript levels in different tissues
The expression of eight flavonoid biosynthetic genes in various tissues of pigmented and non-pigmented rice varieties were analyzed and the results demonstrated higher gene expression in pigmented varieties as compared to non-pigmented varieties. The transcript levels of flavonoid biosynthetic genes varied based on the selected gene, the tissue, and the rice type.
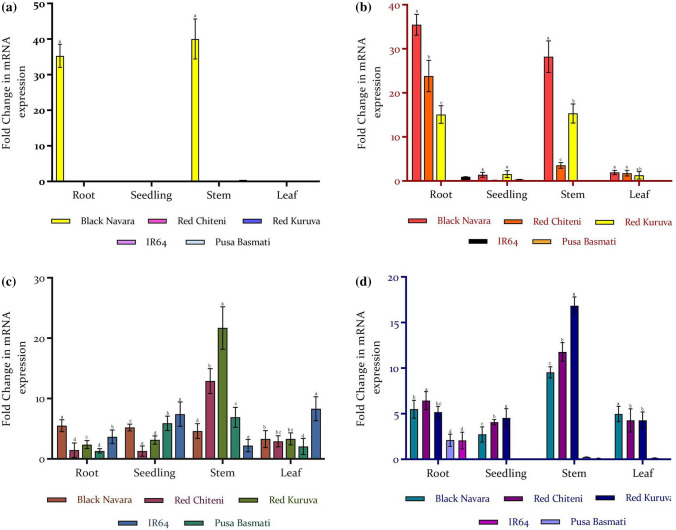
Transcript levels of the PAL gene, which catalyzes the first step of the phenylpropanoid pathway was found to vary depending on the tissue and rice type. Higher transcript levels were recorded in the stem tissues followed by roots when compared to leaf and seedlings (Fig. 2a). The highest expression was recorded in the stems of black rice, which was 40 fold higher as compared to red rice. Transcript levels of CHS gene, which catalyzes the first committed step towards flavonoid production was found to be highest in root tissues of all rice varieties followed by the stem when compared to leaf and seedlings (Fig. 2b). The expression was higher in black rice followed by red, and least in non-pigmented rice. CHI gene expression was higher in roots and seedling of black rice with no significant variation, followed by stem and leaf. However, in red rice, higher expression was recorded in stem tissues followed by leaf. Roots and seedlings recorded similar transcript levels for red rice. However, non-pigmented varieties also recorded relatively significant transcript levels in all the tissues compared to pigmented varieties. In the phenylpropanoid pathway, CHI gene acts immediately downstream of CHS to produce naringenin from naringenin chalcone, the product of CHI. However, in the present study, higher CHS expression was observed in roots and stems of red rice, whereas CHI expression was least in roots of red rice (Fig. 2c).
Fig. 2.
Relative gene expression of flavonoid genes in different parts of pigmented and non-pigmented rice varieties a PAL; b CHS; c CHI; d CL. Values represent the mean ± SD of three replicates. Significance shown are within the different parts and between varieties of rice. Means following the same letter are not significantly different according to Duncan’s multiple range test (p < 0.05)
Similarly, Chen et al. (2013) reported higher CHS expression in roots of red rice than green, but on the contrary CHI expression was lower in red rice than green rice. They reported the highest expression of CHS gene by 20fold in root tissues of rice when compared with sheath, tillering leaf, and flag leaf. Transcript levels were almost nil in tillering leaf compared to other tissues. The same authors reported higher CHS gene expression in red rice followed by green, black, and white varieties. A similar pattern of expression was reported for CHI gene by the same group with maximum expression in the root tissue of green rice followed by red, black, and white rice. Similar to CHS expression, the tillering leaf of green rice recorded nil transcript levels. This postulates that the gene expression pattern does not co-relate with downstream genes. Moreover, flavonoid biosynthetic genes are regulated independently of each other.
The expression of CL gene was higher in the stem tissues followed by root tissues in pigmented rice when compared with leaf and seedlings (Fig. 2d). On the contrary, in non-pigmented varieties, transcript levels were higher in root followed by stem tissues. The highest expression was recorded in stems of red rice, followed by stem tissues of black rice. F3H gene expression highly varied based on the tissues among pigmented rice itself. In black rice the pattern was root > seedling > stem > leaf. However, in red rice the pattern varied as stem > leaf > seedling > root (Fig. 3a). In non-pigmented varieties, expression was maximum in root tissues. Higher transcript levels of the DFR gene were recorded by Chen et al. (2013) in the root and sheath of green rice and roots of red rice when compared with sheath, tillering leaf, and flag leaf. The root-specific expression was in the order of green > red > black > white rice and in the sheath it followed the order green > black > white > red rice. In our study, DFR expression was found only in tissues of black rice in the order root > stem > leaf > seedling. In red and white rice, DFR transcript levels were shallow with no variation among different tissues (Fig. 3b). LAR and ANS expression followed a similar pattern, where high expression was observed in root tissues in the order red > black > white for LAR and black > red > white for ANS gene respectively (Fig. 3c, d). LAR and ANS transcripts were barely detected in stem, leaf, and seedlings. According to Chen et al. (2013), maximum transcript levels of LAR were recorded in roots followed by the sheath and flag leaf in the order green > black > white > red rice. Expression was barely detected in the tillering leaf of green and red rice. For the ANS gene, they reported the highest expression in root tissues of red and black rice when compared with sheath, tillering leaf, and flag leaf of red, black green, and white rice.
Fig. 3.
Relative gene expression of flavonoid genes in different parts of pigmented and non-pigmented rice varieties a F3H; b DFR; c LAR; d ANS. Values represent the mean ± SD of three replicates. Significance shown are within the different parts and between the varieties of rice. Means following the same letter are not significantly different according to Duncan’s multiple range test (p < 0.05)
Lower transcript levels were observed for the eight flavonoid biosynthetic genes in all the non-pigmented rice tissues demonstrating minimal variations. Similar to our results Chen et al. (2013) reported lower transcript levels of CHI, CHS, DFR, LAR, and ANS in white rice with minimal variation compared to green, black, and red. Thus we can relate the colorlessness of white rice to the lower expression of flavonoid biosynthetic genes. Pigmentation in grains can be related to their phenolic contents. Hence higher expression of flavonoid genes in pigmented rice could be the reason for the higher level of phenolics and flavonoids in them, in addition to their colored hue. However, among the pigmented varieties, there was a significant difference in the expression level of flavonoid pathway genes. In our study, LAR and ANS expression were higher in root tissues. However, LAR transcript levels were maximum in red rice roots whereas ANS was maximum in roots of black rice. ANS expression concurs with the results of Chen et al. (2012), where they reported rich anthocyanin deposits in black rice due to higher ANS expression. LAR expression levels in the present study concurs with the results of Furukawa et al. (2007), which reported higher DFR expression to be responsible for the red pericarp in red rice.
Lo and Nicholoson (1998) reported the independent expression of flavonoid biosynthetic genes in sorghum. Higher gene expression of flavonoid biosynthetic genes in pigmented varieties suggests the possibility of a strong promoter or other associated factors capable of inducing up-stream transcription of flavonoid biosynthetic genes in these rice varieties under study. Transcription factors like PIF3 (phytochrome-interacting transcription factor) and LH5 (long hypocotyl l5) have been reported to bind to gene promoters of Arabidopsis flavonoid genes namely CHI, CHS, DFR, F3H and LDOX (Lee et al. 2007). Enhanced phenolic and flavonoid contents were reported in Arabidopsis on sucrose treatment due to the up-regulation of flavonoid biosynthetic genes (Solfanelli et al. 2006). All the above factors could act as a positive trigger, but further research is required to illustrate the facts behind the actual triggers for enhanced expression of flavonoid genes transcription in rice.
Conclusion
Analysis in different tissues of pigmented and non-pigmented rice varieties revealed the presence of higher levels of phytochemicals in seeds and seedlings followed by root, stem, and leaves. Gene expression studies involving different rice varieties and tissues showed higher transcript levels of flavonoid biosynthetic genes in pigmented rice as compared to non-pigmented. Hence higher levels of phenolics, flavonoids, anthocyanins, and antioxidant potential of pigmented rice can be correlated to the higher expression of flavonoid biosynthetic genes in them. This also provides a functional elucidation for expression differences among different pigmented rice varieties and the colorlessness in non-pigmented endosperms. Expression patterns varied with individual genes, tissue, and rice variety. PAL gene expression was higher in stem followed by root, but for CHS, the gene downstream to PAL expression was higher in roots followed by stem. A uniform expression pattern on a tissue level could not be drawn in the present investigation, but transcript levels were always higher in pigmented rice tissues as compared to non-pigmented tissues. Thus it is concluded that flavonoid genes are regulated independently of each other, and further research in this area would facilitate more insights into the actual mechanism governing the expression pattern of flavonoid biosynthetic genes.
Acknowledgements
Safia N thanks University Grants Commission-Basic Science Research, New Delhi for fellowship support (UGC BSR No. F.25.1/2014–15). Author Sathish. S acknowledges Indian Council for Medical Research (ICMR), New Delhi (No.3/1/2/102/2018-Nut.) for fellowship support. Author Sree Preethy Kuppuraj thanks Department of Science and Technology-Promotion of University Research and Scientific Excellence (DST-PURSE) Phase II (Award letter No.BU/DST PURSE(II)/Appointment/01) for fellowship support. We would also like to thank, University Grants Commission-Special Assistance Programme (UGC-SAP) and Department of Science & Technology-Fund for Improvement of S&T Infrastructure (DST-FIST) for the financial support to carry out this research.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Allan AC, Hellens RP, Laing WA. MYB transcription factors that colour our fruit. Trends Plant Sci. 2008;13:99–102. doi: 10.1016/j.tplants.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Bao J, Cai Y, Sun M, Wang G, Corke H. Anthocyanins, flavonols, and free radical scavenging activity of Chinese bayberry (Myricarubra) extracts and their color properties and stability. J Agr Food Chem. 2005;53:2327–2332. doi: 10.1021/jf048312z. [DOI] [PubMed] [Google Scholar]
- Barba FJ, Galanakis CM, Esteve MJ, Frigola A, Vorobiev E. Potential use of pulsed electric technologies and ultrasounds to improve the recovery of high-added value compounds from blackberries. J Food Eng. 2015;167:38–44. [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset CL. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28:25–30. [Google Scholar]
- Chakuton K, Puangpronpitag D, Nakornriab M. Phytochemical content and antioxidant activity of colored and non-colored Thai rice cultivars. Asian J Plant Sci. 2012;11:285. [Google Scholar]
- Chalker-Scott L. Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol. 1999;70:1–9. [Google Scholar]
- Chen X, Itani T, Wu X, Chikawa Y, Irifune K. Physiological factors affecting transcription of genes involved in the flavonoid biosynthetic pathway in different rice varieties. Plant Signal Behav. 2013;8:27555. doi: 10.4161/psb.27555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XQ, Nagao N, Itani T, Irifune K. Anti-oxidative analysis, and identification and quantification of anthocyanin pigments in different coloured rice. Food Chem. 2012;135:2783–2788. doi: 10.1016/j.foodchem.2012.06.098. [DOI] [PubMed] [Google Scholar]
- del Valle JC, Buide ML, Casimiro-Soriguer I, Whittall JB, Narbona E. On flavonoid accumulation in different plant parts: variation patterns among individuals and populations in the shore campion (Silene littorea) Front Plant Sci. 2015;6:939. doi: 10.3389/fpls.2015.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng GF, Xu XR, Zhang Y, Li D, Gan RY, Li HB. Phenolic compounds and bioactivities of pigmented rice. Crit Rev Food Sci. 2013;53:296–306. doi: 10.1080/10408398.2010.529624. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Maekawa M, Oki T, Suda I, Iida S, Shimada H, Kadowaki KI. The Rc and Rd genes are involved in proanthocyanidin synthesis in rice pericarp. Plant J. 2007;49:91–102. doi: 10.1111/j.1365-313X.2006.02958.x. [DOI] [PubMed] [Google Scholar]
- Galanakis CM. Recovery of high added-value components from food wastes: conventional, emerging technologies and commercialized applications. Trends Food Sci Technol. 2012;26:68–87. [Google Scholar]
- Galanakis CM. Emerging technologies for the production of nutraceuticals from agricultural by-products: a viewpoint of opportunities and challenges. Food Bioprod Process. 2013;91:575–579. [Google Scholar]
- Galanakis CM. Separation of functional macromolecules and micromolecules: from ultrafiltration to the border of nanofiltration. Trends Food Sci Technol. 2015;42:44–63. [Google Scholar]
- Galanakis CM. Phenols recovered from olive mill wastewater as additives in meat products. Trends Food Sci Technol. 2018;79:98–105. [Google Scholar]
- Galanakis CM, Tsatalas P, Galanakis IM. Implementation of phenols recovered from olive mill wastewater as UV booster in cosmetics. Ind Crops Prod. 2018;111:30–37. [Google Scholar]
- Goffman FD, Bergman CJ. Rice kernel phenolic content and its relationship with antiradical efficiency. J Sci. 2004;84:1235–1240. [Google Scholar]
- Goufo P, Trindade H. Rice antioxidants: phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci Nutr. 2014;2:75–104. doi: 10.1002/fsn3.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinian FS, Li W, Beta T. Measurement of anthocyanins and other phytochemicals in purple wheat. Food Chem. 2008;109:916–924. doi: 10.1016/j.foodchem.2007.12.083. [DOI] [PubMed] [Google Scholar]
- Hou Z, Qin P, Zhang Y, Cui S, Ren G. Identification of anthocyanins isolated from black rice (Oryza sativa L.) and their degradation kinetics. Food Res. 2013;50:691–697. [Google Scholar]
- Hu C, Zawistowski J, Ling W, Kitts DD. Black rice (Oryza sativa L. indica) pigmented fraction suppresses both reactive oxygen species and nitric oxide in chemical and biological model systems. J Agric Food Chem. 2003;51:5271–5277. doi: 10.1021/jf034466n. [DOI] [PubMed] [Google Scholar]
- Hudson EA, Dinh PA, Kokubun T, Simmonds MS, Gescher A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol Biomarkers Prev. 2000;9:1163–1170. [PubMed] [Google Scholar]
- Jain AM, Sinha PR, Jain AN, Vavilala SI. Estimation of flavonoid content, polyphenolic content and antioxidant potential of different parts of Abrus precatorius (L.) Int J Pharm Pharm Sci. 2015;7:157–163. [Google Scholar]
- Koes R, Verweij W, Quattrocchio F. Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005;10:236–242. doi: 10.1016/j.tplants.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Kovacevic DB, Barba FJ, Granato D, Galanakis CM, Herceg Z, Dragovic-Uzelac V, Putnik P. Pressurized hot water extraction (PHWE) for the green recovery of bioactive compounds and steviol glycosides from Stevia rebaudiana Bertoni leaves. Food chem. 2018;254:150–157. doi: 10.1016/j.foodchem.2018.01.192. [DOI] [PubMed] [Google Scholar]
- Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, Deng XW. Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell. 2007;19:731–749. doi: 10.1105/tpc.106.047688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling WH, Cheng QX, Ma J, Wang T. Red and black rice decrease atherosclerotic plaque formation and increase antioxidant status in rabbits. Nutr J. 2001;131:1421–1426. doi: 10.1093/jn/131.5.1421. [DOI] [PubMed] [Google Scholar]
- Lo SCC, Nicholson RL. Reduction of light-induced anthocyanin accumulation in inoculated sorghum mesocotyls: implications for a compensatory role in the defence response. Plant Physiol. 1998;116:979–989. doi: 10.1104/pp.116.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier A, Schrader A, Kokkelink L, Falke C, Welter B, Iniesto E, Hoecker U. Light and the E3 ubiquitin ligase COP 1/SPA control the protein stability of the MYB transcription factors PAP 1 and PAP 2 involved in anthocyanin accumulation in Arabidopsis. Plant J. 2013;74:638–651. doi: 10.1111/tpj.12153. [DOI] [PubMed] [Google Scholar]
- Morimitsu Y, Kubota K, Tashiro T, Hashizume E, Kamiya T, Osawa T. Inhibitory effect of anthocyanins and colored rice on diabetic cataract formation in the rat lenses. Int Cong. 2002;1245:503–508. [Google Scholar]
- Murdifin M, Pakki E, Rahim A, Syaiful SA, Evary YM, Bahar MA. Physicochemical properties of Indonesian pigmented rice (Oryza sativa Linn.) varieties from South Sulawesi. Asian J Plant Sci. 2015;14:59. [Google Scholar]
- Pengkumsri N, Chaiyasut C, Saenjum C, Sirilun S, Peerajan S, Suwannalert P, Sirisattha S, Sivamaruthi BS. Physicochemical and antioxidative properties of black, brown and red rice varieties of northern Thailand. Food Sci Technol (Campinas) 2015;35:331–338. [Google Scholar]
- Qiu J, Gao F, Shen G, Li C, Han X, Zhao Q, Zhao D, Hua X, Pang Y. Metabolic engineering of the phenylpropanoid pathway enhances the antioxidant capacity of Saussurea involucrata. PLoS ONE. 2013;8:70665. doi: 10.1371/journal.pone.0070665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmanian N, Jafari SM, Galanakis CM. Recovery and removal of phenolic compounds from olive mill wastewater. J Am Oil Chem Soc. 2014;91:1–18. [Google Scholar]
- Rebaya A, Belghith SI, Baghdikian B, Leddet VM, Mabrouki F, Olivier E, Ayadi MT. Total phenolic, total flavonoid, tannin content, and antioxidant capacity of Halimium halimifolium (Cistaceae) J Appl Pharm. 2014;5:52–57. [Google Scholar]
- Saikia S, Dutta H, Saikia D, Mahanta CL. Quality characterisation and estimation of phytochemicals content and antioxidant capacity of aromatic pigmented and non-pigmented rice varieties. Food Res. 2012;46:334–340. [Google Scholar]
- Shan X, Zhang Y, Peng W, Wang Z, Xie D. Molecular mechanism for jasmonate-induction of anthocyanin accumulation in Arabidopsis. J Exp Bot. 2009;60:3849–3860. doi: 10.1093/jxb/erp223. [DOI] [PubMed] [Google Scholar]
- Shao Y, Xu F, Sun X, Bao J, Beta T. Phenolic acids, anthocyanins, and antioxidant capacity in rice (Oryza sativa L.) grains at four stages of development after flowering. Food Chem. 2014;143:90–96. doi: 10.1016/j.foodchem.2013.07.042. [DOI] [PubMed] [Google Scholar]
- Shen Y, Jin L, Xiao P, Lu Y, Bao J. Total phenolics, flavonoids, antioxidant capacity in rice grain and their relations to grain color, size and weight. J Cereal Sci. 2009;49:106–111. [Google Scholar]
- Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P. Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol. 2006;140:637–646. doi: 10.1104/pp.105.072579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichapong J, Sookserm M, Srijesdaruk V, Swatsitang P, Srijaranai S. High performance liquid chromatographic analysis of phenolic compounds and their antioxidant activities in rice varieties. Food Sci Technol. 2010;43:1325–1330. [Google Scholar]
- Vogt T. Phenylpropanoid biosynthesis. Mol Plant. 2010;3:2–20. doi: 10.1093/mp/ssp106. [DOI] [PubMed] [Google Scholar]
- Walter M, Marchesan E. Phenolic compounds and antioxidant activity of rice. Braz Arch Biol Technol. 2011;54:371–377. [Google Scholar]
- Xia M, Ling WH, Ma J, Kitts DD, Zawistowski J. Supplementation of diets with the black rice pigment fraction attenuates atherosclerotic plaque formation in apolipoprotein e deficient mice. J Nutr. 2003;133:744–751. doi: 10.1093/jn/133.3.744. [DOI] [PubMed] [Google Scholar]
- Zinoviadou KG, Galanakis CM, Brncic M, Grimi N, Boussetta N, Mota MJ, Barba FJ. Fruit juice sonication: Implications on food safety and physicochemical and nutritional properties. Food Res. 2015;77:743–752. [Google Scholar]