Abstract
The aim of this study was to investigate the effects of the sequence of different thermal and mechanical treatments on the physicochemical parameters and microstructure of garlic paste, in order to improve the quality of the product. The total organosulfur compounds (OSCs) decreased steadily in blended-heated garlic, whereas OSCs decreased sharply after 2 min at 75 °C or 5 min at 85 and 95 °C in heated-blended garlic. After blanching for 5 min, allicin could maintain over 4.0 mg/g only at 75 °C; and OSCs of heated-blended garlic paste were found to drop by 29.56%, 90.63% and 94.79% at 75, 85 and 95 °C, respectively. In blended-heated garlic, the color values of L* (lightness) and a* (redness) decreased (P < 0.05), while the b* (yellowness) and C* (chroma) increased (P < 0.05), obtaining green discoloration garlic paste. The total color differences of blended-heated samples were greater than 12.08, which were 2–6 folds higher compared with heated-blended garlic. Total phenolic content and antioxidant activity decreased (P < 0.05) in all thermal treatments, thermal treatment of heated-blended garlic less than 5 min maintained over 30% of antiradical activity. The sequence of unit operations determined the pattern of garlic microstructure disruption, resulting in various enzymic and non-enzymic reactions. Our results indicated that use of heat treatment prior to blend processing is an effective and feasible method to inhibit garlic discoloration and retain high content of bioactive OSCs. It is recommended that garlic paste be prepared using heated-blended processing, with thermal processing limited to 75 °C for less than 5 min.
Keywords: Thermal processing, Garlic paste, Physicochemical characteristics, Antioxidant activity, Microstructure
Introduction
As consumers are increasingly interested in the fresh ready-to-eat vegetables, more and more pre-peeled garlic and chopped garlic products that require minimal handling appear in the marketplace (Hong and Kim 2001; Lee et al. 2007). As a ready-to-eat garlic preparation, garlic paste is not only widely used as a food or a seasoning, but also shows huge health benefits, such as anti-inflammatory, anti-atherosclerotic, and carcinogenesis prevention effects (Hitchcock et al. 2014; Salehi et al. 2019; Sobenin et al. 2016). These pharmacological benefits are mainly attributed to organosulfur compounds (OSCs, including allicin and its degradation products), which are commonly characterized as “garlic flavor”, with a pungent and sulfur-containing sensory perceptions (Block 1992; Rana et al. 2011; Yagdi et al. 2016).
Allicin is hardly present in fresh intact garlic and it is mainly derived from the reaction between substrate alliin (S-allyl-l-cysteine sulfoxide) and alliinase (EC 4.4.1.4), after garlic is crushed or chopped (Lanzotti 2006). However, allicin is unstable and readily rearranges into various volatile sulfur-containing organosulfides, such as diallyl sulfide (DAS), diallyl disulfide (DADS), diallyl trisulfide (DATS), (E/Z)-ajoene, and vinyl dithiins (VDTs) (Zhang et al. 2020). The processing conditions have a huge impact on organosulfur profiles, which is closely related to the flavor deterioration and the loss of bioactivity.
The occurrence of greening caused by mechanical disruption during garlic processing indicates the deterioration of the product and reduces its marketability and commercial use. Previous studies generally agreed that allicin was involved in pigment generation (Kubec et al. 2017). Although some measures have been taken to reduce garlic discoloration, such as high-pressure treatments (Kim et al. 2014), the addition of chemical additives (Shin and Kyung 2014), and pH adjustment (Hong and Kim 2001), the effects are unsatisfactory. Furthermore, the garlic paste obtained by optimized processing technology using thermal treatment, high pressure processing and chemical additives usually loses characteristic flavor and biological functionalities (Ahmed and Shivhare 2001, 2002; Eroman Unni et al. 2014; Kim et al. 2014). Patricia et al. (2015) reported that different processing parameters (such as temperature), and/or unit operation sequences (such as thermal treatment and blending) had a significant impact on the phytochemicals in the broccoli, tomato and carrot purees. Morover, based on headspace GC–MS fingerprinting, Koutidou et al. (2017) indicated different combinations of unit operations affected the level of off-flavour volatiles, which was closely related to different microstructures and various enzymatic reactions. Howover, there is no literature study to evaluate the effect of the sequence of unit operations on the quality of garlic paste.
Previous studies on the organosulfur profiles were mainly performed by gas chromatography-mass spectrometry (GC–MS) (Kim et al. 2011; Tocmo et al. 2014). However, GC is not recommended for the analysis of allicin, because it can be completely decomposed under high-temperature analysis conditions (Block 1992; Tocmo et al. 2017). Therefore, high-performance liquid chromatography (HPLC) was used in this study to systematically investigate the effect of the sequence of unit operations on the organosulfur profiles in garlic paste. Moreover, the relationship between physicochemical changes (organosulfur profiles and color appearance) with alliinase activity and microstructure was analyzed. The results provided a reference for the process optimization of garlic paste to improve the appearance and flavor quality of the product, which contributed to the development of garlic industry as a seasoning with high sensory acceptance and bioactivities.
Materials and methods
Materials and reagents
Garlic (Allium sativum L.) was purchased from Laiwu (Shandong, China) and stored at − 2.5 °C. DADS and DATS (≥ 98% purity by HPLC) were purchased from Sigma-Aldrich Co. Ltd. (Shanghai, China). Chromatography-grade acetonitrile and methanol were purchased from Oceanpak Alexative Chemical., Ltd. (Sweden). DADS (85% purity) was purchased from Aladdin (China). Analytical-grade hexane, methanol, dichloromethane, acetone, hydrogen peroxide (30%), glacial acetic acid, and anhydrous sodium sulfate (Na2SO4) were purchased from Kaitong Chemical Technology (Tianjin, China).
Thermo-mechanical processing conditions
A multifactorial experimental design with three factors was used in this study. The sequence of unit operations was used as the first factor with two levels: blended-heated garlic and heated-blended garlic, and the temperature and the treatment time of thermal processing were used as the other two factors. The raw garlic paste prepared by blending garlic cloves (100 g) with deionized water (50 mL) was used as the control group.
The different thermo-mechanical treatments were conducted according to Koutidou et al. (2017) with some modifications. Peeled garlic was cut into standardized slices with 2-mm thick and then divided into two batches (Batch 1 and Batch 2, respectively). The samples in Batch 1 were homogenized with deionized water (2:1, w/v) using a commercial homogenizer, followed by shaker treatment at 25 °C for 30 min. After the homogenate was divided into 100-mL glass beakers (50 g per beaker), thermal processing was conducted in a water bath at 75, 85, or 95 °C for 2, 5, 10, 15, or 25 min with stirring. A needle-type thermocouple (NZ95-8G, Nengzhao Technology, China) was used to measure the center temperature of the sample. The samples in Batch 2 were first subjected to thermal processing at different conditions. Then, the samples were cooled to room temperature and homogenized with deionized water (2:1, w/v). The OSCs of obtained products were extracted by solvent extraction. The processed samples were labeled as blended-heated processing (BH, batch 1) and sliced-heated processing (SH, batch 2). All sample preparations were performed in triplicate.
OSC extraction
OSCs were extracted using the method described by Tocmo et al (2017) with some modifications. Specifically, 10 g of garlic homogenate was extracted with hexane (3 × 10 mL). The organic phases of each extract were combined and evaporated to dryness in an air stream at room temperature. Each residue was reconstituted in acetonitrile, filtered, and analyzed by HPLC. The moisture contents of garlic samples were calculated by freeze-drying, and all organosulfur compounds data were expressed based on dry weight.
Synthesis and isolation of allicin and its derivatives
To obtain allicin, DADS (85% purity) was dissolved in glacial acetic acid and 30% hydrogen peroxide was slowly added dropwise with stirring, according to the reported method (Iberl et al. 1990). (E/Z)-Ajoene was obtained by refluxing the synthesized allicin in acetone/water (60:40, v/v) for 4.5 h (Iberl et al. 1990). 2-vinyl-[4H]-1,3-dithiin (2-VDT) was synthesized by refluxing allicin in acetone/methanol (60:40, v/v) for 2.5 h. After synthesis, the OSC products were subjected to purification by a Shimadzu semi-preparative chromatographic system (Kyoto, Japan). The isolated compounds were identified by liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry (LC-APCI-MS/MS). An Acquity LC and a Vanquish F-TSQ Quantis Triple Quadrupole equipped with an electrospray ion source (Thermo Scientific, Waltham, USA) were used in this study. The LC separation conditions were similar to those described in Sect. 2.5. The MS analysis used multi-reaction monitoring mode with positive and negative ion exchange mode.
HPLC analysis of OSCs
OSCs were analyzed according to the reported method (Tocmo et al. 2017). The HPLC system (LC-20AT; Shimadzu, Kyoto, Japan) was equipped with an InertSustain C18 column (4.6 × 250 mm, 5 μm) for separation. The HPLC conditions were: mobile phase, acetonitrile/water/methanol (50:41:9, v/v/v); flow rate, 1.0 mL/min; column temperature, 25 °C; injection volume, 10 μL; detector wavelength, 254 nm. Peak identification and quantification of individual compounds were conducted using OSC reference standards.
Color measurement
The color of garlic paste was determined using a CM-700d colorimeter (Konica Minolta, Tokyo, Japan). Garlic samples were packed into plastic bags and six scans per sample were recorded immediately. The CIE parameters of lightness (L*), redness (a*), and yellowness (b*), were determined to calculate chroma (C*) and total color difference (ΔE), as follows:
| 1 |
| 2 |
where ΔL*, Δa*, and Δb* represent the differences between the color parameter values of the processed sample and fresh garlic.
Determination of alliinase activity
The extraction of alliinase was performed at 4 °C according to the methodology of Wang et al. (2011) with some modifications. Garlic homogenate (20 g) was mixed with phosphate buffer (20 mL, 50 mmol/L, pH 7.0) containing 10% glycerin, 5% NaCl, and 5 mmol/L ethylene diamine tetraacetic acid. Then, the mixture was centrifuged at 5500 g for 10 min and filtered through a 0.8-μm filter to obtain the crude alliinase.
Alliinase activity was determined according to Yoo and Pike (2001) with some modifications. Crude alliinase (0.5 mL) was added into the alliin standard reaction mixture (1 mL, 0.7 mmol/L) containing phosphate buffer (50 mmol/L, pH 6.5) and pyridoxal 5′-phosphate (20 μmol) as the substrate. The enzymatic reaction system was incubated in a water bath at 25 °C for 5 min and then terminated by 1.5 mL of 10% trichloroacetic acid solution. An aliquot of the reaction mixture (0.5 mL) was mixed with 0.5 mL of dinitrophenyl hydrazine solution (5 mmol/L, dissolved in 0.37 mol/L H2SO4) and 5 mL of NaOH solution (2.5 mol/L). After the mixture was allowed to stand at 25 °C for 10 min, the absorbance at 520 nm was measured using a T6 spectrophotometer (Purkinje Analysis General Instrument, Beijing, China). A pyruvic acid standard curve was prepared to calculate the pyruvate concentration. One unit of alliinase activity was the amount of enzyme that catalyzed the conversion of 1 mmol of pyruvic acid per minute.
Total phenolic content and antioxidant activity
According to the method of Qiu et al. (2010), garlic homogenate (5.0 g) was extracted three times with 80% methanol (1:5, w/v) for 30 min, with an ultrasonic treatment at room temperature. After centrifuged at 5500 g for 10 min, the supernatant was concentrated by rotary evaporation at 35 °C. Total phenolic content (TPC) was determined by Folin-Ciocalteu assay (Ismail et al. 2004) with slight modifications. Briefly, the reaction mixture containing 100 μL of aqueous extract sample, 0.8 mL of Folin-Ciocalteu reagent and 0.8 mL sodium carbonate solution was kept in darkness under ambient conditions for 90 min. The absorbance of the reaction mixture was measured at 725 nm using a UV–Vis spectrophotometer. The TPC was expressed as gallic acid equivalents (mg GAE/g−1 fresh weight), using a calibration curve based on gallic acid.
The antioxidant activities of garlic samples were measured by 2,2-diphenyl-1-picrylhydrazyl (DPPH) method. An aliquot of extract was mixed with 2 mL of DPPH solution. After 30 min of reaction at room temperature, the absorbance at 517 nm was measured. Antiradical activity (ARA) was expressed as a percentage, as shown in Eq. (3), where Absblank is the absorbance of the solution with the same chemical substance but without samples:
| 3 |
Microstructure scanning
An IX73 microscope (Olympus, Tokyo, Japan) equipped with an Olympus digital camera was used to record micrographs. Garlic paste was diluted ten-fold with deionized water and gently mixed. One drop of the sample was placed on a microscope slide, covered with a cover glass, and observed using phase contrast mode at 10 × and 20 × magnifications. At least five images were obtained for each sample.
Statistical analysis
All analyses were performed in triplicate. The Mixed procedure was used for thermo-mechanical processing experiments (SAS, Version 9.0). Heating temperature, heating time, the sequence of mechanical–thermal treatment, and their interactions (temperature × time, temperature × sequence, time × sequence and temperature × time × sequence) were used as fixed factors. Type-3 tests of fixed factors were performed. Least square means were calculated from interactions of temperature × time × sequence of mechanical–thermal treatment, and differences were considered significant at P < 0.05.
Results and discussion
Organosulfur profiles in garlic
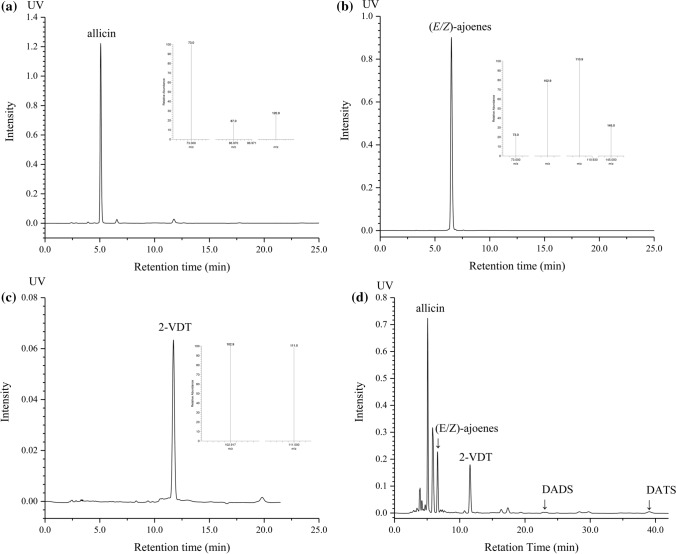
The representative HPLC chromatograms of three isolated compounds and OSCs in raw garlic were shown in Fig. 1, where the isolated compounds were identified by LC-APCI-MS/MS according to previous reports (Calvey et al. 1997; Tocmo et al. 2017). In raw garlic, the quantitative profiles of individual OSC showed that allicin (5.59 ± 0.26 mg/g) was the predominant compound. (E/Z)-Ajoene (1.68 ± 0.05 mg/g) and 2-VDT (0.54 ± 0.01 mg/g) were the main degradation products of allicin in the fresh garlic, while DADS (0.14 ± 0.01 mg/g) and DATS (0.22 ± 0.02 mg/g) had the lowest levels among the OSCs. Significant variations in the content of allicin degradation products were observed compared with previous reports (Locatelli et al. 2015; Tocmo et al. 2017). Two factors might explain this phenomenon. One was the use of different extraction solvent (dichloromethane), which facilitated the decomposition of allicin into other OSCs. Moreover, the different garlic genotypes that were used in the experiments were the other reason (Iberl et al. 1990; Martins et al. 2016; Liu et al. 2020).
Fig. 1.
Representative HPLC chromatogram and mass spectrum of three isolated sulfur-containing compounds, a Allicin,92.0% purity; b (E/Z)-ajoene, 99.7% purity; c 2-VDT, 90.35% purity, d organosulfur profiles in fresh garlic
Quantitative analysis of organosulfur profiles under different combinations of mechanical and thermal treatments
The change of OSCs contents was shown in Table 1. The OSCs contents were significantly affected by the interaction of temperature × time × sequence of mechanical–thermal treatment (P < 0.001). In BH garlic samples, the allicin content decreased steadily, and this reduction showed positive correlation with heating time and temperature. As the degradation of allicin progressed, the contents of linear polysulfides (DADS and DATS) increased significantly (P < 0.05). Heating at 95 °C could generate more polysulfides than those heating at 75 and 85 °C. The contents of the main OSCs, (E/Z)-ajoene and 2-VDT, constantly decreased during thermal processing. (E/Z)-Ajoene might undergo further reactions with allyl mercaptan to generate DATS or diallyl tetrasulfide (Block 1992). Therefore, the observed decrease in (E/Z)-ajoene appeared to be related to DATS generation. The cyclic sulfide 2-VDT was formed by the Diels–Alder reaction of thioacrolein dimer, a product of allicin degradation. The lowest 2-VDT content of 0.11 mg/g was observed after heating at 95 °C for 25 min. It was reported that 2-VDT was a main transformation product in GC analysis (Tocmo et al. 2017), indicating that it was very stable at high temperatures. Therefore, the noticeable decrease in 2-VDT observed in our study might be caused by internal interactions.
Table 1.
Effects of heating temperature, heating time and sequence of unit operations on OSC profiles of garlic paste
| T | t | Allicin | (E/Z)-ajoene | 2-VDT | DADS | DATS | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| °C | min | BH | SH | BH | SH | BH | SH | BH | SH | BH | SH |
| 75 | 0 | 5.59ax | 5.28ax | 1.68ax | 1.74bx | 0.54abx | 0.40ay | 0.14bx | 0.08by | 0.22ex | 0.18ax |
| 2 | 5.28aix | 4.86aix | 0.86bjx | 1.93aix | 0.58aix | 0.43ajy | 0.08cjy | 0.12bjx | 0.24ejy | 0.17aky | |
| 5 | 4.91abix | 4.40bix | 0.78bcjx | 0.51ciy | 0.57aix | 0.15biy | 0.12bjx | 0.27aix | 0.32dkx | 0.08biy | |
| 10 | 4.41bix | 0.98ciy | 0.65cjx | 0.07diy | 0.53abix | 0.00ciy | 0.19ajx | 0.08biy | 0.46ckx | 0.03biy | |
| 15 | 3.76bix | 0.77ciy | 0.76bcix | 0.06diy | 0.48bix | 0.00ciy | 0.28akx | 0.12biy | 0.61bkx | 0.04biy | |
| 25 | 2.78cix | 0.55ciy | 0.57cix | 0.08diy | 0.30cix | 0.00ciy | 0.21akx | 0.10biy | 0.76akx | 0.04biy | |
| 85 | 0 | 5.59ax | 5.28ax | 1.68cx | 1.74ax | 0.54ax | 0.40by | 0.14cx | 0.08by | 0.22fx | 0.18bx |
| 2 | 5.46aix | 4.29bijy | 0.77ajx | 0.99bjx | 0.36bjy | 0.50ajx | 0.13cix | 0.20aix | 0.30eijx | 0.26ajx | |
| 5 | 4.76bix | 0.63cjy | 0.86bjy | 0.02cjy | 0.32bjx | 0.00ciy | 0.19biy | 0.04bcjy | 0.39djx | 0.03ciy | |
| 10 | 3.37cjx | 0.45ciy | 0.60bjx | 0.01ciy | 0.23cjx | 0.00ciy | 0.26aix | 0.04cjy | 0.63cjx | 0.03ciy | |
| 15 | 2.74cjx | 0.36ciy | 0.62cjx | 0.00ciy | 0.16djx | 0.00ciy | 0.28ajx | 0.04cjy | 0.77bjx | 0.04ciy | |
| 25 | 1.48djx | 0.23ciy | 0.42cjx | 0.00aix | 0.11djx | 0.00ciy | 0.25ajx | 0.03cjy | 1.02ajx | 0.04ciy | |
| 95 | 0 | 5.59ax | 5.28ax | 1.68ax | 1.74by | 0.54ax | 0.40by | 0.14dx | 0.08cy | 0.22fx | 0.18bx |
| 2 | 4.96aix | 3.95bjy | 1.70aix | 1.10bjy | 0.41bjy | 1.33aix | 0.10dijy | 0.20bix | 0.33eix | 0.33aix | |
| 5 | 4.28bjx | 0.31cjy | 1.62aix | 0.00cjx | 0.35bcjx | 0.00ciy | 0.18cix | 0.04ajy | 0.49dix | 0.05ciy | |
| 10 | 2.39ckx | 0.27ciy | 0.92bix | 0.00ciy | 0.29cjx | 0.00ciy | 0.27bix | 0.03djy | 0.83cix | 0.04ciy | |
| 15 | 1.66dkx | 0.21ciy | 0.57cjx | 0.00ciy | 0.18djx | 0.00ciy | 0.36aix | 0.02djy | 1.05bix | 0.04ciy | |
| 25 | 0.52ekx | 0.18cix | 0.35djx | 0.00ciy | 0.11djx | 0.00ciy | 0.33aix | 0.02djy | 1.28aix | 0.04ciy | |
Standard error of the least square mean (SE): allicin (0.26); (E/Z)-ajoene (0.05); 2-VDT (0.01); DADS (0.01); DATS (0.02)
BH = blended-heated processing, SH = sliced-heated processing
a−fMeans within the columns at the same temperature with different letters indicate a significant difference (P < 0.05)
i−kMeans within the columns at the same processing time with different letters indicate a significant difference (P < 0.05)
x−yMeans within the rows for the same OSC with different letters indicate a significant difference (P < 0.05)
The pattern of OSCs contents in the SH group was very different from that in the BH group. A high level of allicin was maintained for the first 5 min, 2 min and 2 min at 75 °C, 85 °C and 95 °C, respectively. Afterwards, allicin decreased sharply (P < 0.05). (E/Z)-ajoene and 2-VDT had similar tracks with allicin. The content of DADS and DATS increased first and then sharply decreased (P < 0.05). It was found that the total OSCs dropped by 29.56%, 90.63% and 94.79% after blanching 5 min at 75, 85 and 95 °C, respectively. When the heating treatment continued to 10 min, the total OSCs contents decreased by 84.90%, 93.10% and 95.57% at 75, 85 and 95 °C, respectively. In addition, the each OSC content of the samples in the SH group was significantly lower than those in the BH group (P < 0.05), except for (E/Z)-ajoene.
In general, a large difference in sulfur compounds was observed between the samples obtained by two thermal processing sequences. Garlic paste treated with blended-heated processing led to a significant higher level of allyl polysulfide content, which was in agreement with a previous study (Tocmo et al. 2017). Heating time was critical for the OSCs contents in garlic paste, especially in the SH group. In SH garlic paste, longer heating times (≥ 5 min) resulted in removal of all the OSCs. Mild temperature (75 °C) was recommended for maintaining OSCs.
Color analysis for different combinations of mechanical and thermal treatment
The changes in the lightness (L*), green (a*), yellow (b*), chroma and the total color difference (ΔE) of garlic paste were shown in Table 2. The color indicators were significantly affected by the interaction of temperature × time × sequence of mechanical–thermal treatment (P < 0.001). The L* values of BH garlic paste were in the range of 52.62–63.56, and they decreased significantly (P < 0.05) as the heating time prolonged. A similar trend of L* was also observed in SH garlic paste. However, the lightness of SH garlic paste (L*, 70.23–76.30) was significantly higher than that of BH garlic paste, and even close to that of fresh garlic (L*, 75.49). The a* values of BH garlic paste decreased rapidly (P < 0.05) at the initial heating processing. With the extension of heating time, the a* values decreased steadily at 75 °C, while an obvious increase happened at higher temperature (85 and 95 °C). For b* and C* (chroma) values, the samples treated at three heating temperatures (75, 85 and 95 °C) showed a trend of decreasing first and then increasing. In addition, the ΔE values in BH garlic paste were from 12.08 to 24.75. The results indicated that BH garlic paste turned a vivid green color at first, which gradually faded and turned yellow at higher temperature (85 and 95 °C). In contrast, although there were some changes in the color indicators in SH group, not significant differences indicated that color changes were slight (P > 0.05) over time. Moreover, the ΔE values of SH garlic paste were less than 8.42. The ΔE values of BH garlic paste were 2–6 folds higher than SH garlic paste. The appearance of the SH garlic paste produced by heating and then blending was basically unchanged, compared with fresh garlic.
Table 2.
Effects of heating temperature, heating time and the sequence of unit operations on color stability of garlic paste
| T | t | L* | a* | b* | Chroma (C*) | ΔE | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| °C | min | BH | SH | BH | SH | BH | SH | BH | SH | BH | SH |
| 75 | 0 | 75.49a | 75.49a | −3.00a | −3.00bc | 13.65a | 13.65a | 13.97b | 13.97a | ||
| 2 | 63.04bjy | 75.83aix | −6.19biy | −2.81bix | 5.68cky | 13.39aix | 8.40fky | 13.68aix | 15.13cix | 4.96biy | |
| 5 | 59.10cjy | 76.30aix | −9.51ciy | −2.61aix | 1.91eky | 9.89bcjx | 9.70ejy | 10.23bix | 21.19bix | 3.89ciy | |
| 10 | 55.98dky | 74.02bix | −10.94diy | −2.86bix | 1.92eky | 9.79bix | 11.11dkx | 10.01biy | 24.11aix | 4.33cky | |
| 15 | 54.38eky | 72.87cix | −11.88eky | −3.09cix | 4.38dky | 9.72bix | 12.66ckx | 10.37biy | 24.72aix | 4.25cjy | |
| 25 | 52.62fky | 70.95dix | −11.30fky | −2.99cix | 9.12bky | 9.96bckx | 14.52akx | 10.40bjy | 24.75aix | 5.86ajy | |
| 85 | 0 | 75.49a | 75.49a | −3.00a | −3.00a | 13.65b | 13.65ajx | 13.97d | 13.97a | ||
| 2 | 65.56biy | 74.12bix | −6.18biy | −3.08ajx | 7.56diy | 12.09bix | 9.77ejy | 12.47bjx | 12.08djx | 2.12cky | |
| 5 | 61.03ciy | 71.54cjx | −9.11diy | −3.12ajx | 3.75ejy | 8.93djx | 9.86ejx | 10.46djx | 18.58cjx | 7.93ajy | |
| 10 | 57.51djy | 71.34cjx | −12.18fky | −3.06ajx | 8.78fjy | 9.72cix | 15.06cjx | 10.19diy | 20.82ajx | 5.72bjy | |
| 15 | 58.16djy | 71.83cjx | −9.51ejy | −3.34bjx | 18.46cjx | 9.35ciy | 20.77bjx | 10.93diy | 19.13bjx | 5.70biy | |
| 25 | 57.86djy | 70.23dix | −7.06cjy | −3.05ajx | 20.80ajx | 9.38cjy | 21.96ajx | 11.78ciy | 19.45bjx | 5.77bijy | |
| 95 | 0 | 75.49a | 75.49a | −3.00a | −3.00a | 13.65d | 13.65a | 13.97d | 13.97a | ||
| 2 | 63.82bjy | 72.94bkx | −9.09djy | −3.05ajx | 6.39fjy | 12.08bix | 11.11fiy | 12.46bjx | 15.04dix | 3.02cjy | |
| 5 | 59.70cjy | 68.28ejx | −10.34ejy | −3.27bjx | 7.19eiy | 9.36cix | 12.60eix | 9.91dijy | 18.58bjx | 8.42aiy | |
| 10 | 60.19ciy | 69.33dkx | −8.88ciy | −3.15ajx | 19.41cix | 9.73diy | 21.35cix | 9.29diy | 16.51ckx | 7.91aiy | |
| 15 | 59.47ciy | 70.83ckx | −5.83biy | −3.26bjx | 23.09bix | 9.82ciy | 23.82bix | 10.35ciy | 18.81bjx | 6.04biy | |
| 25 | 59.61ciy | 70.71cjx | −3.08aix | −3.28bkx | 25.36aix | 9.72ciy | 25.55aix | 10.26cjy | 19.73ajx | 6.20biy | |
Standard error of the least square mean (SE): L* (0.44); a* (0.07); b* (0.22); Chroma (0.19); ΔE (0.52)
BH = blended-heated processing, SH = sliced-heated processing
a−fMeans within the columns at the same temperature with different letters indicate a significant difference (P < 0.05)
i−kMeans within the columns at the same processing time with different letters indicate a significant difference (P < 0.05)
x−yMeans within the rows for the same attribute with different letters indicate a significant difference (P < 0.05)
Kubec et al. (2017) reported that greening occurred in crushed garlic after 4 h at room temperature, significantly later than the samples in this study (P < 0.05). This indicated that high temperature accelerated the reaction of garlic greening discoloration. Additionally, allicin was involved in green pigment formation (Zang et al. 2013), and it was reasonable to explain that the total OSCs contents decreased markedly in BH garlic. Therefore, the sequence of heating prior to blending contributed to the color stability of garlic paste, which might be due to lower generation of allicin after heating.
Changes in alliinase activity
The relative activity of alliinase and the temperature curve during thermal processing were shown in Fig. 2. Alliinase was linked to the generation of OSCs. The enzyme activity in raw garlic was defined as a relative activity of 100%. The alliinase activity decreased faster in SH garlic paste than in BH garlic paste, and alliinase inactivation was positively correlated with treatment temperature (Fig. 2a). Alliinase was completely inactivated after 15 min in the BH garlic (at 95 °C), which was only 10 min in the SH garlic. The results were in consistent with a previous report that alliinase failed to maintain activity at 80 °C for 15 min (Wang et al. 2011). According to the temperature curve, the samples in the SH group had a higher heating rate and a higher center temperature than those in the BH group (Fig. 2b). Therefore, the speed of alliinase inactivation might be linked to difference in the rate of temperature increase and heat distribution. Furthermore, the activity of alliinase at different thermal temperatures and different operation orders directly determined the OSCs contents of garlic paste. The faster inactivation rate and lower enzyme activity in the SH garlic led to a lower level of OSC, compared with the samples in the BH garlic.
Fig. 2.
Effect of different combinations of mechanical and thermal treatments on a alliinase activity and b temperature change. (BH = blended-heated processing, SH = sliced-heated processing)
Changes in antioxidant activities
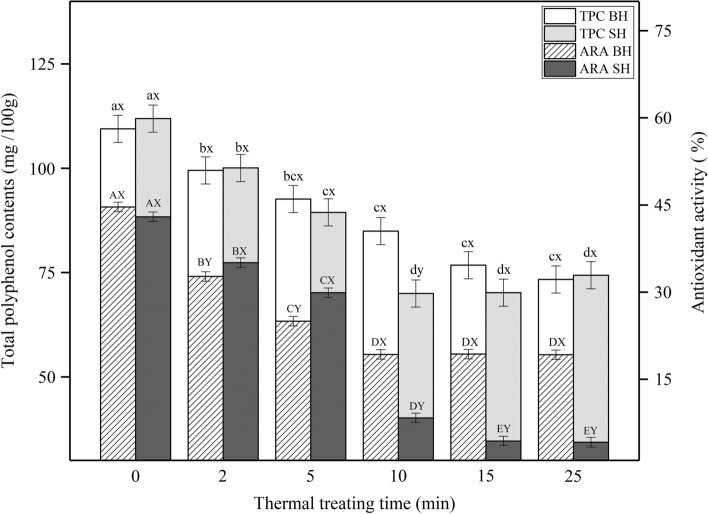
The P values for the three-factor interaction for TPC and ARA were 0.22 and 0.16, respectively, which meant there was no significant interaction of temperature × time × sequence for TPC and ARA. The Mixed procedure showed that TPC and ARA were significantly affected by the interaction of time × sequence of mechanical–thermal treatment (P < 0.001). The TPC and DPPH free-radical scavenging ability were reduced significantly in thermally treated garlic paste compared with the control group (Fig. 3). The analysis of variance showed that SH garlic lost more TPC in the first 10 min. However, after 10 min, there was no statistically significant decrease (P > 0.05) in the SH or BH garlic. It was reported that garlic paste produced at high temperature (90 °C, 5 min) had a less TPC compared with fresh garlic or the garlic treated under high pressure (200–600 MPa, 15 min) (Ormerod et al. 2004). This result was likely caused by the sensitivity of TPC to high temperature.
Fig. 3.
Changes in antioxidant activity and total polyphenolic contents of thermal processing treated garlic paste according to different sequence of unit operations and heating times. a−d and A−E Means within the same sequence of unit operations with different letters differ (P < 0.05). x, y and X, Y Means within the same heating time with different letters differ (P < 0.05). (BH = blended-heated processing, SH = sliced-heated processing)
The ARA of samples obtained by different thermal processing times and sequence of unit operation exhibited a trend of rapid decline and then stability, which was similar to the trend observed in TPC. This finding is consistent with a previous report by Xiang et al. (2019), who detected low levels of TPC corresponding to low DDPH activity. Compared with the BH garlic, the SH garlic showed a higher ARA within 5 min of thermal processing, but a significantly lower ARA at longer processing times (P < 0.05). It should be noted that a rapid decrease from 30.01% to 8.30% was observed for the SH garlic when the heating treatment increased from 5 to 10 min, while the ARA decreased steadily in the BH samples in the early stage of processing. Considering similar changes in OSC levels, the results indicated that lower allicin corresponded to lower TPC and ARA. These observations were consistent with previous reports (Locatelli et al. 2017; Vaidya et al. 2009) that the presence of allicin showed strong and positive correlations with TPC and DPPH scavenging activity. The antioxidant activity ascribed to allicin is actually due to the trapping of peroxyl radicals by 2-propenesulfenic acid. Allicin is able to form 2-propenesulfenic acid by Cope elimination reaction (Vaidya et al. 2009).
Relationship between microstructure and garlic paste quality
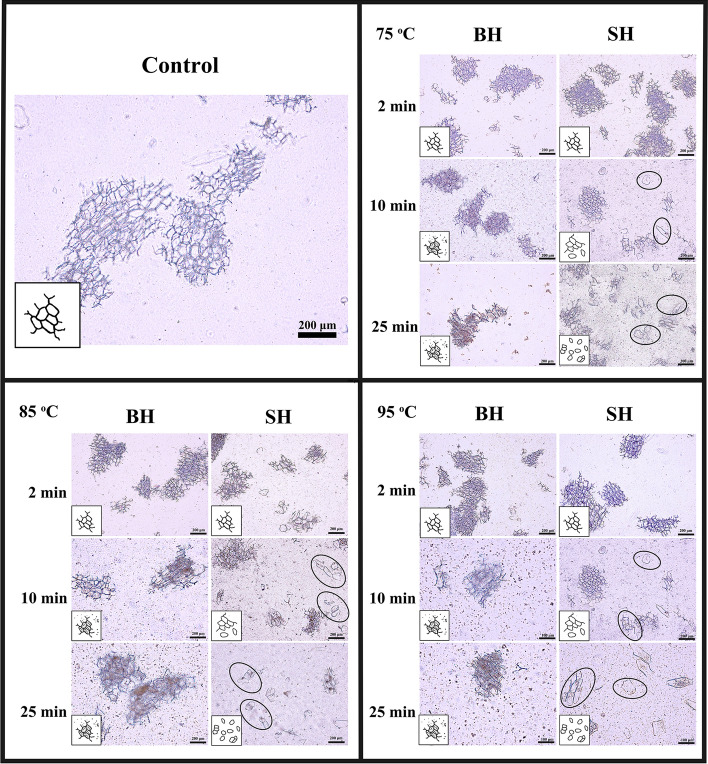
Light microscopy images of BH and SH garlic paste (after heating for 2, 10, and 25 min) were shown in Fig. 4. There were no differences in all garlic paste samples after 2 min of heating treatment, and cell clusters consisting of many cells with broken edges were clearly visible. Heating the samples for 10 min could discriminate the BH garlic and SH garlic. During blending process of the BH garlic, shearing occurred through the strong cell wall polymers resulting in large cell clusters with broken edges. Heating was unable to break the cell adhesion, and thus the cell clusters remained in the images. For SH garlic, pectin was thermally depolymerized via β-elimination during heating, resulting in reduced cell adhesion (Patricia et al. 2015). This meant that shearing was favored through the middle lamella, causing smaller cell clusters or free cells without broken edges. Similar effect of the sequence of unit operations on microstructures in other vegetable pastes has been reported (Koutidou et al. 2016, 2017). In addition, our results indicated that there were internal relationships among the differences in microstructure and change in OSCs contents between BH and SH garlic paste. Notably, green discoloration was observed in the BH garlic after thermal treatment, while SH garlic retained original color. This difference might also be linked to the microstructure. SH garlic samples contained numerous intact cells, which meant that there were insufficient levels of allicin to form pigments.
Fig. 4.
Light microcopy (LM) images of garlic processed by different combinations of mechanical and thermal processing. Images were taken with 10 and 20 × magnifications. (BH = blended-heated processing, SH = sliced-heated processing)
Conclusion
Our results clearly demonstrated that thermal processing decreased the contents of organosulfur compounds, including allicin, (E/Z)-ajoene, and 2-VDT. After blanching for 5 min, only mild temperature (75 °C) could maintain allicin over 4.0 mg/g. Greening discoloration occurred only in BH garlic. Heating time was critical not only for controlling the OSCs contents, but also for maintaining antioxidant activity. Thermal treatment of heated-blended garlic less than 5 min maintained ARA over 30%. In SH garlic paste, smaller cell clusters and more intact cells were observed, whereas in BH garlic paste, large cell clusters with broken edges were observed. Our results support literature (Koutidou et al. 2016) suggestions that microstructure could be linked to the observed differences in the physicochemical properties. We concluded that there are three main pathways that may affect the organosulfur profiles: (i) in BH garlic, allicin is involved in garlic discoloration during thermal processing; (ii) in SH garlic, the inactivation of alliinase causes a dramatic decrease in OSCs contents; and (iii) in BH and SH garlic, allicin rearrangement reactions occur during thermal processing. Based on our observations, garlic paste is best treated with the SH process, using mild conditions (75 °C) with heating time not exceeding 5 min. These conditions are recommended to retain the pungent flavor and color appearance, as well as to maximize total phenolic content and antioxidant activity.
Acknowledgements
This project was supported by the Special National Key Research and Development Plan (2016YFD0400204), Funds of Shandong “Double Tops” Program (SYT2017XTTD04), Key R&D Program of Yantai (Grant No. 2018ZDCX014), Major Scientific and Technological Innovation Projects of Key R&D Program of Shandong Province (2019JZZY020607).
Abbreviations
- BH
Blended-heated processing
- SH
Sliced-heated processing
- GC–MS
Gas chromatography–mass spectrometry
- HPLC
High-performance liquid chromatography
- LC–APCI–MS/MS
Liquid chromatography–atmospheric pressure chemical ionization–tandem mass spectrometry
- TPC
Total phenolic content
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
- ARA
Antiradical activity
- OSCs
Organosulfur compounds
- 2-VDT
2-Vinyl-[4H]-1,3-dithiin
- DADS
Diallyl disulfides
- DATS
Diallyl trisulfides
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmed J, Shivhare US. Thermal kinetics of color change, rheology, and storage characteristics of garlic puree/paste. J Food Sci. 2001;66:754–757. doi: 10.1111/j.1365-2621.2001.tb04633.x. [DOI] [Google Scholar]
- Ahmed J, Shivhare US. Preparation and storage studies on onion-ginger-garlic paste. J Food Sci Technol Mysore. 2002;39:566–568. doi: 10.1111/j.1365-2621.2002.tb08817.x. [DOI] [Google Scholar]
- Block E. The organosulfur chemistry of the genus Allium—implications for the organic chemistry of sulfur. Angew Chem Int Edit. 1992;31:1135–1178. doi: 10.1002/anie.199211351. [DOI] [Google Scholar]
- Calvey E, Matusik JE, White KD, DeOrazio R, Sha D, Block E. Allium chemistry: supercritical fluid extraction and synthesis of 1-propanesulfinothioic acid S-allyl ester. J Agric Food Chem. 1997 doi: 10.1021/jf970314e. [DOI] [Google Scholar]
- Eroman Unni L, Chauhan OP, Raju PS. High pressure processing of garlic paste: effect on the quality attributes. Int J Food Sci Technol. 2014;49(49):1579–1585. doi: 10.1111/ijfs.12456. [DOI] [Google Scholar]
- Hitchcock J, Schäfer G, Katz A, Kaschula CH. 979: the immunomodulating and anti-inflammatory effects of garlic organosulfur compounds in cancer prevention. Eur J Cancer. 2014;50:S238–S239. doi: 10.1016/S0959-8049(14)50868-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S-I, Kim D-M. Storage quality of chopped garlic as influenced by organic acids and high-pressure treatment. J Sci Food Agric. 2001;81:397–403. doi: 10.1002/1097-0010(200103)81:4<397::AID-JSFA831>3.0.CO;2-R. [DOI] [Google Scholar]
- Iberl B, Winkler G, Knobloch K. Products of allicin transformation: ajoenes and dithiins, characterization and their determination by HPLC*. Planta Med. 1990;56:202–211. doi: 10.1055/s-2006-960926. [DOI] [PubMed] [Google Scholar]
- Ismail A, Marjan ZM, Foong CW. Total antioxidant activity and phenolic content in selected vegetables. Food Chem. 2004;87:581–586. doi: 10.1016/j.foodchem.2004.01.010. [DOI] [Google Scholar]
- Kim NY, Park MH, Jang EY, Lee J. Volatile distribution in garlic (Allium sativum L.) by solid phase microextraction (SPME) with different processing conditions. Food Sci Biotechnol. 2011;20(20):775–782. doi: 10.1007/s10068-011-0108-4. [DOI] [Google Scholar]
- Kim KW, Kim YT, Kim M, Noh BS, Choi WS. Effect of high hydrostatic pressure (HHP) treatment on flavor, physicochemical properties and biological functionalities of garlic. LWT Food Sci Technol. 2014;55:347–354. doi: 10.1016/j.lwt.2013.08.027. [DOI] [Google Scholar]
- Koutidou M, Grauwet T, Acharya P. Effect of different combined mechanical and thermal treatments on the volatile fingerprint of a mixed tomato–carrot system. J Food Eng. 2016;168:137–147. doi: 10.1016/j.jfoodeng.2015.07.028. [DOI] [Google Scholar]
- Koutidou M, Grauwet T, Loey AV, Acharya P. Potential of different mechanical and thermal treatments to control off-flavour generation in broccoli puree. Food Chem. 2017;217:531–541. doi: 10.1016/j.foodchem.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Kubec R, Curko P, Urajová P, Rubert J, Hajšlová J. Allium discoloration: color compounds formed during greening of processed garlic. J Agric Food Chem. 2017;65:10615–10620. doi: 10.1021/acs.jafc.7b04609. [DOI] [PubMed] [Google Scholar]
- Lanzotti V. The analysis of onion and garlic. J Chromatogr A. 2006;1112:3–22. doi: 10.1016/j.chroma.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Lee E, Cho J-E, Kim J-H, Lee S-K. Green pigment in crushed garlic (Allium sativum L.) cloves: purification and partial characterization. Food Chem. 2007;4:1677–1686. doi: 10.1016/j.foodchem.2006.04.028. [DOI] [Google Scholar]
- Liu P, Weng R, Sheng X, Wang X, Zhang W, Qian Y, Qiu J. Profiling of organosulfur compounds and amino acids in garlic from different regions of China. Food Chem. 2020;305:125499. doi: 10.1016/j.foodchem.2019.125499. [DOI] [PubMed] [Google Scholar]
- Locatelli DA, Altamirano JC, González RE, Camargo AB. Home-cooked garlic remains a healthy food. J Funct Foods. 2015;16:1–8. doi: 10.1016/j.jff.2015.04.012. [DOI] [Google Scholar]
- Locatelli DA, Nazareno MA, Fusari CM, Camargo AB. Cooked garlic and antioxidant activity: correlation with organosulfur compound composition. Food Chem. 2017;220:219–224. doi: 10.1016/j.foodchem.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Martins N, Petropoulos S, Ferreira ICFR. Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre- and post-harvest conditions: a review. Food Chem. 2016;211:41–50. doi: 10.1016/j.foodchem.2016.05.029. [DOI] [PubMed] [Google Scholar]
- Ormerod AP, Ralfs JD, Jackson R, Milne J, Gidley MJ. The influence of tissue porosity on the material properties of model plant tissues. J Mater Sci. 2004;39:529–538. doi: 10.1023/B:JMSC.0000011508.02563.93. [DOI] [Google Scholar]
- Patricia LS, Vos RCHD, Jonker HH, Mumm R, Hall RD, Bialek L, Leenman R, Strassburg K, Vreeken R, Hankemeier T. Comprehensive metabolomics to evaluate the impact of industrial processing on the phytochemical composition of vegetable purees. Food Chem. 2015;168:348–355. doi: 10.1016/j.foodchem.2014.07.076. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Liu Q, Beta T. Antioxidant properties of commercial wild rice and analysis of soluble and insoluble phenolic acids. Food Chem. 2010;121:140–147. doi: 10.1016/j.foodchem.2009.12.021. [DOI] [Google Scholar]
- Rana SV, Pal R, Vaiphei K, Sharma SK, Ola RP. Garlic in health and disease. Nutr Res Rev. 2011;24:60–71. doi: 10.1017/s0954422410000338. [DOI] [PubMed] [Google Scholar]
- Salehi B, Zucca P, Erdogan Orhan I, Azzini E, Adetunji C, Mohammed S, Banerjee S, Sharopov F, Rigano D, Sharifi-Rad J, Armstrong L, Martorell M, Sureda A, Martins N, Selamoglu Z, Ahmad Z. Allicin and health: a comprehensive review. Trends Food Sci Technol. 2019 doi: 10.1016/j.tifs.2019.03.003. [DOI] [Google Scholar]
- Shin YK, Kyung KH. Cysteine reacts to form blue–green pigments with thiosulfinates obtained from garlic ( Allium sativum L.) Food Chem. 2014;142:217–219. doi: 10.1016/j.foodchem.2013.07.057. [DOI] [PubMed] [Google Scholar]
- Sobenin IA, Andrianova IV, Lakunin KY, Karagodin VP, Bobryshev YV, Orekhov AN. Anti-atherosclerotic effects of garlic preparation in freeze injury model of atherosclerosis in cholesterol-fed rabbits. Phytomedicine. 2016;23:1235–1239. doi: 10.1016/j.phymed.2015.10.014. [DOI] [PubMed] [Google Scholar]
- Tocmo R, Lin Y, Huang D. Effect of processing conditions on the organosulfides of shallot (Allium cepa L. Aggregatum Group) J Agric Food Chem. 2014;62:5296–5304. doi: 10.1021/jf500739n. [DOI] [PubMed] [Google Scholar]
- Tocmo R, Wu Y, Liang D, Fogliano V, Huang D. Boiling enriches the linear polysulfides and the hydrogen sulfide-releasing activity of garlic. Food Chem. 2017;221:1867–1873. doi: 10.1016/j.foodchem.2016.10.076. [DOI] [PubMed] [Google Scholar]
- Vaidya V, Ingold KU, Pratt DA. Garlic: source of the ultimate antioxidants–sulfenic acids. Angew Chem Int Ed Engl. 2009;48:157–160. doi: 10.1002/anie.200804560. [DOI] [PubMed] [Google Scholar]
- Wang J, Cao Y, Sun B, Wang C, Mo Y. Effect of ultrasound on the activity of alliinase from fresh garlic. Ultrason Sonochem. 2011;18:534–540. doi: 10.1016/j.ultsonch.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Xiang J, Zhang M, Apea-Bah FB, Beta T. Hydroxycinnamic acid amide (HCAA) derivatives, flavonoid C-glycosides, phenolic acids and antioxidant properties of foxtail millet. Food Chem. 2019;295:214–223. doi: 10.1016/j.foodchem.2019.05.058. [DOI] [PubMed] [Google Scholar]
- Yagdi E, Cerella C, Dicato M, Diederich M. Garlic-derived natural polysulfanes as hydrogen sulfide donors: Friend or foe? Food Chem Toxicol. 2016;95:219–233. doi: 10.1016/j.fct.2016.07.016. [DOI] [PubMed] [Google Scholar]
- Yoo KS, Pike LM. Determination of background pyruvic acid concentrations in onions, Allium species, and other vegetables. Sci Hortic. 2001;89:249–256. doi: 10.1016/S0304-4238(00)00196-5. [DOI] [Google Scholar]
- Zang J, Wang D, Zhao G. Mechanism of discoloration in processed garlic and onion. Trends Food Sci Technol. 2013;30:162–173. doi: 10.1016/j.tifs.2013.01.008. [DOI] [Google Scholar]
- Zhang Y, Liu X, Ruan J, Zhuang X, Zhang X, Li Z. Phytochemicals of garlic: promising candidates for cancer therapy. Biomed Pharmacother. 2020;123:109730. doi: 10.1016/j.biopha.2019.109730. [DOI] [PubMed] [Google Scholar]