Abstract
Purpose
To evaluate whether endometrial compaction using sequential transvaginal ultrasound is associated with improved live birth rates in medicated single euploid frozen embryo transfer (FET) cycles.
Methods
Prospective observational cohort study at a private fertility clinic. Patients who underwent FETs between January and December 2018 were assessed for inclusion. The change in endometrial thickness between the end of the estrogen phase and the day before embryo transfer, measured by sequential transvaginal ultrasound, was used to categorize cycles with compaction (≥ 5%), no change, or expansion (≥ 5%). FET cycle outcomes were then compared between groups. The primary outcome was live birth. Secondary outcomes include clinical pregnancy rate and rate of spontaneous abortion.
Results
Of the 259 single euploid medicated FETs performed during the study period, only 43/259 (16.6%) of the cycles demonstrated ≥ 5% compaction, whereas 152/259 (58.7%) expanded and 64/259 (24.7%) were unchanged. Live birth rates did not differ between cycles with compaction (58.1%), no change (54.7%), or expansion (58.6%), p = 0.96. Clinical pregnancy and spontaneous abortion rates were also similar between groups.
Conclusion
The vast majority of cycles did not demonstrate endometrial compaction. Endometrial compaction is not associated with live birth rate or spontaneous abortion rate in medicated single euploid FETs in this cohort.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-020-02043-7.
Keywords: Endometrial compaction, Endometrial thickness, Frozen embryo transfer, Endometrial receptivity
Introduction
Endometrial receptivity encompasses specific alterations in the surface epithelium, underlying vascular network, and expression of surface glycoproteins, integrins, receptors, and chemokines that occur in concert to create a permissive environment for embryo implantation. These conditions are present for a limited period of time, from approximately day 20–24 of the idealized 28-day cycle, coined “the window of implantation” (WOI) [1]. The displacement or absence of this WOI is thought to contribute significantly to infertility, especially in patients with endometriosis, recurrent pregnancy loss, and PCOS. Numerous different assays and modalities have been investigated to assess optimal endometrial receptivity.
The tools currently available for the assessment and optimization of endometrial receptivity include technologies analyzing endometrial tissue and non-invasive modalities, such as transvaginal ultrasound. Some of the more commonly used endometrial tissue assays include the endometrial receptivity array (ERA), which assesses optimal gene expression, and ReceptivaDx which uses immunohistochemistry to identify BCL6, a marker of inflammation associated with endometriosis. Traditional histopathologic examination evaluating characteristic changes in the endometrial glands and stroma to date the endometrium has also been used. Investigation into the clinical application and utility of these tests are ongoing. Use of transvaginal ultrasound for endometrial assessment, on the other hand, has been a staple of fertility care and endometrial assessment. Although there is some conflicting evidence, it is generally accepted that an endometrial thickness, as well as pattern, at the end of the proliferative phase is predictive of embryo transfer (ET) outcomes [2–5]. A recent study of 24,363 fresh and 20,114 frozen-thaw ET cycles found reduced clinical pregnancy rates (CPR) and live birth rates (LBR) in fresh and frozen ET cycles with endometrial thickness < 8 mm and < 7 mm, respectively [6]. Others have found an upper limit of endometrial thickness beyond which ET outcomes also decline, greater than 13 mm in one study, suggesting that there is an optimal range rather than simply a minimal threshold endometrial thickness [7]. However, studies examining the relationship between endometrial thickness after initiation of progesterone and clinical outcomes following ET failed to demonstrate an association [8, 9].
Recent studies have assessed whether endometrial compaction, defined as the change in endometrial thickness between the end of the estrogen-only phase and the day of embryo transfer, predicts outcomes following FET. In concept, compaction of the endometrium after progesterone initiation indicates that the endometrium is responsive to progesterone and could therefore be used as a proxy for endometrial receptivity. Three cohort studies published on the relationship between endometrial compaction and clinical outcomes following ET have shown conflicting results [10–12]. Two studies from the same group found a positive correlation between endometrial compaction and pregnancy rates. These investigators compared ongoing pregnancy rates between cycles that achieved varying degrees of compaction (5%, 10%, 15%, or 20%) to those cycles with a lesser degree of compaction or expansion. Increased ongoing pregnancy rates were seen in FET cycles that demonstrated endometrial compaction at all levels compared with cycles in which the endometrium did not compact or expanded. This was demonstrated in an analysis of 271 FETs in their first study and in 225 single euploid FETs in their follow-up study [10, 12]. In contrast, Bu et al. assessed 1757 medicated and 1334 natural cycle blastocyst FETs and found a higher CPR in cycles in which the endometrial lining expanded after progesterone initiation compared with cycles in which the lining either compacted or did not change, both in medicated and natural cycle FETs. All embryos in this study were high-quality, untested blastocysts.
The goal of the present study was to determine if endometrial compaction was associated with increased LBR in medicated single euploid frozen embryo transfer cycles with use of transvaginal ultrasound for all endometrial measurements.
Materials and methods
Study design and population
This was a prospective observational cohort study at a single private fertility center. All medicated single euploid FET cycles between January and December 2018 were screened for inclusion. Natural cycle and minimal-stimulation FETs as well as gestational carrier cycles were excluded. Patients were also excluded if their endometrial thickness was < 7 mm at the end of the estrogen-only phase prior to initiation of progesterone. If a patient had multiple transfers during the study period, only the first embryo transfer was included in the analysis. Stimulation protocols were at the discretion of the physician, but were primarily antagonist protocol. Oocyte retrieval was performed 36 h after triggering final oocyte maturation with either hCG, GnRH agonist, or co-trigger with a combination of the two. Intracytoplasmic sperm injection was routinely used to fertilize all metaphase II oocytes and assisted hatching was routinely performed on day 3 of embryo development. All embryos were vitrified at the blastocyst stage after trophectoderm biopsy and preimplantation genetic testing for aneuploidy (PGT-A) with next-generation sequencing (NGS) was performed.
Hormone replacement for endometrial preparation for FET involved administration of oral micronized estradiol on cycle day 2 after suppression with oral contraceptive pills with or without GnRH agonist. Oral estradiol 2 mg × 3 days followed by 4 mg × 3 days and finally 6 mg per day was administered. Transvaginal ultrasound was performed to assess the endometrium after 12–14 days of estradiol. Once the endometrial lining was ≥ 7 mm, a combination of progesterone in oil (50 mg IM QD) and vaginal progesterone (100 mg PV TID) was started the following morning. The day of progesterone initiation was designated P+0. Patients returned the day prior to embryo transfer, on the fifth day of progesterone administration or (P+4) for reassessment of the endometrial lining using transvaginal ultrasound. Ultrasounds were performed by a uniform group of experienced ultrasound technicians. Embryo transfers were performed on the morning of the sixth day of progesterone (P+5) under transabdominal ultrasound guidance. The primary outcome was LBR. Secondary outcomes included CPR and spontaneous abortion (SAB) rate. CPR was defined as the presence of fetal cardiac activity on ultrasound.
Cycles were grouped by percentage of endometrial compaction, defined as the difference in endometrial thickness at the end of the estrogen-only phase and the day before embryo transfer after 5 days of progesterone exposure, divided by the thickness at the end of estrogen-only phase. Compaction was defined as a decrease in endometrial thickness of ≥ 5% and expansion was defined as a ≥ 5% increase in endometrial thickness. Cycles in which the percent compaction was less than ± 5% were considered unchanged.
Statistical analysis
Statistical analysis was performed using chi-square for categorical variables and ANOVA for continuous variables. Logistic regression was used for the multivariate analysis. Significance was set at p < 0.05. Statistical analyses were performed with GraphPad Prism.
Ethical approval
The study was approved by the institutional review board at the University of California, Los Angeles (IRB# 18-000559).
Results
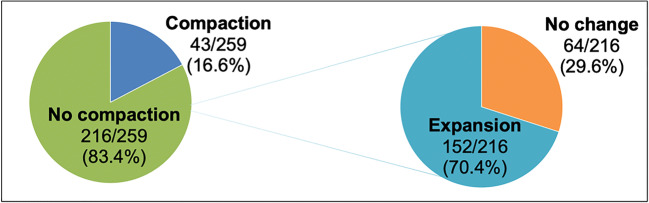
A total of 259 medicated single euploid FET cycles were performed during the study period and met inclusion criteria. Only 16.6% (43/259) of the cycles demonstrated ≥ 5% compaction, whereas 83.4% (216/259) either expanded or were unchanged. Of the cycles that did not compact, 24.7% (64/216) had no change in compaction and 58.7% (152/216) expanded ≥ 5% (Fig. 1). Overall LBR for the cohort at large was 57.5% (149/259), with a CPR of 68% (176/158) and SAB rate of 6.9% (12/176). Average endometrial thickness was 8.8 ± 1.2 mm with a range of 7.0–14.0 mm.
Fig. 1.
Distribution of change in endometrial lining thickness
Differences in baseline characteristics between the compaction, no change, and expansion groups were assessed. Cycles that demonstrated endometrial compaction had significantly thicker endometrial stripes at the end of the estrogen-only phase compared with those that either did not change or expanded (9.3 mm vs 8.7 mm vs 8.7 mm, p < 0.02). There were no differences in patient age, body mass index (BMI), gravidity/parity, baseline FSH, or blastocyst day or quality between the groups (Table 1, Supplemental Table 4).
Table 1.
Baseline characteristics of cycles with any compaction, no change, or any expansion
| Any compactiona (n = 43) | No changeb (n = 64) | Any expansionc (n = 152) | p value | |
|---|---|---|---|---|
| Age at retrieval (mean ± SD) | 35.1 ± 5.2 | 34.9 ± 4.6 | 34.3 ± 5.0 | 0.65d |
| BMI (mean ± SD) | 21.7 ± 2.8 | 22.8 ± 4.9 | 21.7 ± 3.4 | 0.14d |
| Gravity, n (%) | 19/43 (41.9) | 25/64 (39.1) | 57/152 (37.5) | 0.73e |
| Parity, n (%) | 10/43 (23.3) | 17/64 (26.6) | 35/152 (23) | 0.85e |
| FSH (mean ± SD) | 8.4 ± 3.7 | 7.2 ± 2.4 | 8.1 ± 3.4 | 0.23d |
| Endometrial thickness end of E2 (mean ± SD) | 9.3 ± 1.6 | 8.7 ± 1.0 | 8.7 ± 1.1 | 0.02d |
aCycles with ≥ 5% decrease in endometrial thickness between the end of the estrogen phase and the day before embryo transfer
bCycles with < 5% change in endometrial thickness between the end of the estrogen phase and the day before embryo transfer
cCycles with ≥ 5% increase in endometrial thickness between the end of the estrogen phase and the day before embryo transfer
dANOVA
eChi-squared
Clinical outcomes were then analyzed between the three groups. When comparing cycles with compaction, no change, or expansion, no difference was seen in LBR, CPR, or SAB rate (Table 2). In a logistic regression model adjusting for endometrial lining thickness at the end of the estrogen-only phase, compaction was not associated with live birth, OR: 0.92 95% CI (0.69, 1.23), p = 0.59. Further analysis was performed including only cycles that demonstrated endometrial compaction or that had no change in endometrial thickness, to evaluate whether higher percent compaction was associated with clinical outcomes. When cycles demonstrating compaction were compared based on degree of compaction (5–9% vs 10–14% vs ≥ 15%), no difference was seen in LBR or SAB rate with increasing degrees of compaction. Interestingly, there was a higher CPR in those cycles which either had no change or compacted between 5-9% compared with cycles with a higher degree of compaction (Table 3). These findings, however, should be interpreted cautiously given the small number of cycles that achieved compaction > 5%.
Table 2.
Live birth, clinical pregnancy, and SAB rates: any compaction vs. no change vs. any expansion
| Any compactionb (n = 43) | No changec (n = 64) | Any expansiond (n = 152) | p valuef | |
|---|---|---|---|---|
| LBR, n (%) | 25/43 (58.1) | 35/64 (54.7) | 89/152 (56.6) | 0.96 |
| CPR, n (%) | 27/43 (62.8) | 46/64 (71.9) | 103/152 (67.8) | 0.61 |
| SAB ratea, n (%) | 1/27 (3.7) | 8/46 (17.4) | 9/101e (8.9) | 0.13 |
LBR live birth rate, CPR clinical pregnancy rate, SAB spontaneous abortion
aDenominator is number of clinical pregnancies in respective group
bCycles with ≥ 5% decrease in endometrial thickness between the end of the estrogen phase and the day before embryo transfer
cCycles with ≥ 5% increase in endometrial thickness between the end of the estrogen phase and the day before embryo transfer
dCycles with < 5% change in endometrial thickness between the end of the estrogen phase and the day before embryo transfer
eTwo clinical pregnancies in the “Any Expansion” group were electively terminated. These were not omitted from the denominator for calculation of the SAB rate in this group
fChi-squared
Table 3.
Live birth rate by percent compaction
| No changea | 5–9% | 10–14% | ≥ 15% | p valueb | |
|---|---|---|---|---|---|
| LBR, n (%) | 35/64 (54.7) | 10/17 (58.8) | 8/14 (57.1) | 7/12 (58.3) | 0.93 |
| CPR, n (%) | 46/64 (71.9) | 12/17 (70.6) | 8/14 (57.1) | 7/12 (58.3) | 0.04 |
| SAB rate, n (%) | 8/46 (17.4) | 1/12 (8.3) | 0/8 (0) | 0/7 (0) | 0.34 |
LBR live birth rate, CPR clinical pregnancy rate, SAB spontaneous abortion
aCycles with < 5% change in endometrial thickness between the end of the estrogen phase and the day before embryo transfer
bChi-squared
In order to determine if absolute endometrial thickness at the end of the estrogen phase was associated with likelihood of compaction, the rate of endometrial compaction was compared based on endometrial thickness at the end of the estrogen phase. No difference was found in the rate of compaction when comparing cycles in which endometrial thickness was < 8 mm vs 8–10 mm vs > 10 mm at the end of the estrogen phase, 7/54 (13%) vs 28/175 (16%) vs 8/31 (25.8%), p = 0.29, respectively.
We performed several additional analyses to determine if the difference in our findings from those that found a positive association of endometrial compaction with ET cycle outcomes was related to differences in cycle categorization. A sub-analysis was performed including only cycles with thickness of ≥ 8 mm at the end of the estrogen phase (n = 205). When comparing cycles with compaction, no change, or expansion, no difference was found in LBR 22/36 (61.1%) vs 30/48 (62.5%) vs 75/121 (62%), p = 0.99, CPR 24/36 (66.7%) vs 35/48 (72.9%) vs 84/121 (69.4%), p = 0.82, or SAB rates 1/24 (4.2%) vs 4/35 (11.4%) vs 5/84 (6%), p = 0.47. Finally, we redefined our three groups of compaction, no change or expansion into two groups, compaction or no compaction and compared LBR at varying levels of compaction (5%, 10%, 15%, and 20%) similar to Haas et al. and Zilberberg et al. [10, 12]. No difference was seen in LBR at any level of compaction (Supplemental Table 5).
Discussion
In this observational cohort study of 259 single euploid medicated FETs, endometrial compaction assessed using sequential transvaginal ultrasound occurred in a small fraction of cycles, and compaction was not predictive of live birth. To our knowledge, only three prior studies have evaluated the impact of endometrial compaction on FET outcomes [10–12], none of which evaluated the outcome of live birth. The first from Haas el al. found higher ongoing pregnancy rates in FET cycles with endometrial compaction, which increased significantly with increasing percent compaction. Notably, the rate of compaction in this cohort was 42.4% (with compaction defined as ≥ 5% reduction in endometrial thickness), which was significantly higher than that seen in our study of 16.6% [10]. A subsequent study from the same group reported a higher ongoing pregnancy rate with increasing degree of endometrial compaction in 225 single euploid medicated FET cycles [12]. The rate of compaction ≥ 5% in this cohort was similar to that seen in their first, at 43.1%. In contrast to these two studies, Bu et al. assessed 1757 medicated and 1334 natural cycle blastocyst FETs and found a higher CPR in cycles in which the endometrial lining expanded after progesterone initiation, both in medicated and natural cycle FETs. Assessment of endometrial thickness prior to FET was performed on the morning of transfer via transvaginal ultrasound. In this study, the medicated and natural cycle cohorts compacted at a rate of 19.6% and 26.2%, respectively [11].
Differences in endometrial preparation protocols, the mode and timing of the post-progesterone ultrasound assessment, and definitions of endometrial compaction may have contributed to the conflicting results in these studies [11–13]. The studies by Haas et al. and Zilberberg et al. which reported a higher rate of endometrial compaction used transvaginal ultrasound for endometrial assessment at the end of the estrogen phase and transabdominal ultrasound for assessment prior to transfer [10, 12]. This may explain the significantly higher compaction rate seen in those studies compared with the current study and that of Bu et al., both of which used transvaginal ultrasound for all measurements and found compaction in a minority of cycles [11]. The timing of post-progesterone endometrial assessment may also play a role in these differences. The studies by Haas et al., Zilberberg et al., and Bu et al. performed the second endometrial assessment on the day of FET, as opposed to the day prior to FET as was done in our study [10–12]. However, given the conflicting results of these studies despite having similar duration of progesterone exposure at the time of endometrial assessment, this is unlikely to explain the discrepant results. Additionally, unlike the other studies, we made the decision to assign cycles with ≤ 5% change in endometrial thickness, equivalent to 0.4 mm difference in the average 8.8 mm endometrium, as “no change”. This was done to provide a small margin of error and to maximize the chance that findings were clinically relevant and reproducible. Notably, re-analysis of our data eliminating the no change group demonstrates that differences in categorization of cycles do not change our results and do not explain differences in outcomes between these studies.
It is biologically plausible that endometrial compaction may have validity as an indicator of endometrial receptivity. Follicular phase endometrial proliferation ends roughly 3 days after ovulation, related to the influence of rising serum progesterone [13]. Classically, endometrial height is considered to be fixed at this point, with further growth of glands and blood vessels contributing to increased density rather than volume. This is what gives the secretory endometrium its homogenous, hyperechoic appearance on ultrasound. Continued endometrial proliferation in the secretory phase may be an indicator of progesterone resistance, which would portend a suboptimal environment for embryo implantation. Given the limited and conflicting data in prior studies, further investigation of this topic is warranted.
Ours is the largest study evaluating the association of endometrial compaction with FET outcomes that is composed solely of single euploid medicated FETs, effectively eliminating embryo quality as a confounding variable. Additionally, endometrial thickness monitoring was done exclusively with transvaginal ultrasound and not abdominal ultrasound. Furthermore, prior studies investigated clinical and ongoing pregnancy as their primary outcomes, whereas our primary outcome was live birth. Our study is limited by its sample size, the relatively small number of FET cycles that demonstrated compaction, and lack of fertility diagnosis information. While only the first FET was included for patients who had multiple cycles during the study period, patients may have had prior successful or failed FETs and this is an important potential confounder. Additionally, although all ultrasounds were performed by a small number of highly experienced ultrasound technicians, variability in measurement, particularly when investigating differences of sub-millimeters, is realistically unavoidable.
The current study adds to the limited body of literature on the clinical relevance of endometrial compaction. In the current study, no relationship was seen between endometrial compaction and LBR or SAB rates. Unexpectedly, cycles with no change or the lowest level of compaction (5–9%) were found to have a higher CPR than those cycles in which a higher degree of compaction was achieved. This should be interpreted cautiously, however, given the small number of cycles that attained compaction ≥ 10% in our cohort 26/259 (10%). Given that the addition of one ultrasound the day of or morning prior to FET is non-invasive and low-cost, future prospective studies are warranted to further evaluate utility of endometrial compaction as a predictor of FET success. Assessment of endometrial compaction in natural cycle FETs with euploid embryo transfers would be an interesting follow-up study to see if utility is demonstrated in that setting. Currently, based on the available data, alteration or cancellation of FET cycle plan would not be justified based solely on compaction results.
In conclusion, endometrial compaction is not associated with LBR in single euploid FETs in our cohort. The use of transvaginal ultrasound for endometrial assessment and inclusion of only single euploid FETs may have contributed to the difference in our findings compared with prior studies. Prospective investigation of this question with increased power would be beneficial.
Supplementary Information
(DOCX 14 kb)
Authors’ contributions
C.R., L.K, and A.A. participated in study design. C.R. performed the data collection. C.R., M.Q., L.K., and A.A. participated in data analysis. C.R., M.Q., L.K., A.A., S.G., H.D., and M.S. participated in manuscript drafting and critical discussion.
Data availability
Coded data pertaining to this study are stored on a password- and firewall-protected server and can be made available if needed.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was approved by the Institutional Review Board of the University of California, Los Angeles (IRB# 18-000559).
Consent to participate
Because of the retrospective nature of this study, the UCLA IRB deemed the study to have minimal risk and waived the requirement for informed consent.
Consent for publication
Consent to publish was not required as the UCLA IRB deemed this study of minimal risk.
Code availability
Code for the data can be made available if required.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Carrie Riestenberg, Email: criestenberg@reproductivepartners.com.
Molly Quinn, Email: mquinn@mednet.ucla.edu.
Alin Akopians, Email: aakopians@scrcivf.com.
Lindsay Kroener, Email: lkroener@mednet.ucla.edu.
References
- 1.Bergh PA, Navot D. The impact of embryonic development and endometrial maturity on the timing of implantation. Fertil Steril. 1992;58:537–42. doi: 10.1016/S0015-0282(16)55259-5. [DOI] [PubMed] [Google Scholar]
- 2.Kovacs P, Matyas S, Boda K, Kaali SG. The effect of endometrial thickness on IVF/ICSI outcome. Hum Reprod. 2003;18:2337–2341. doi: 10.1093/humrep/deg461. [DOI] [PubMed] [Google Scholar]
- 3.Kumbak B, Erden HF, Tosun S, Akbas H, Ulug U, Bahçeci M. Outcome of assisted reproduction treatment in patients with endometrial thickness less than 7 mm. Reprod BioMed Online. 2009;18:79–84. doi: 10.1016/S1472-6483(10)60428-2. [DOI] [PubMed] [Google Scholar]
- 4.Yuan X, Saravelos SH, Wang Q, Xu Y, Li TC, Zhou C. Endometrial thickness as a predictor of pregnancy outcomes in 10787 fresh IVF–ICSI cycles. Reprod BioMed Online. 2016;33:197–205. doi: 10.1016/j.rbmo.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Zhao J, Zhang Q, Wang Y, Li Y. Endometrial pattern, thickness and growth in predicting pregnancy outcome following 3319 IVF cycle. Reprod BioMed Online. 2014;29:291–298. doi: 10.1016/j.rbmo.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Liu KE, Hartman M, Hartman A, Luo ZC, Mahutte N. The impact of a thin endometrial lining on fresh and frozen-thaw IVF outcomes: an analysis of over 40 000 embryo transfers. Hum Reprod. 2018;33:1883–1888. doi: 10.1093/humrep/dey281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Toukhy T, Coomarasamy A, Khairy M, Sunkara K, Seed P, Khalaf Y, et al. The relationship between endometrial thickness and outcome of medicated frozen embryo replacement cycles. Fertil Steril. 2008;89(4):832–839. doi: 10.1016/j.fertnstert.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 8.Barker MA, Boehnlein LM, Kovacs P, Lindheim SR. Follicular and luteal phase endometrial thickness and echogenic pattern and pregnancy outcome in oocyte donation cycles. J Assist Reprod Genet. 2009;26:243–249. doi: 10.1007/s10815-009-9312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griesinger G, Trevisan S, Cometti B. Endometrial thickness on the day of embryo transfer is a poor predictor of IVF treatment outcome. Hum Reprod Open. 2018;(1)1–8. [DOI] [PMC free article] [PubMed]
- 10.Haas J, Smith R, Zilberberg E, Nayot D, Meriano J, Barzilay E, Casper RF. Endometrial compaction (decreased thickness) in response to progesterone results in optimal pregnancy outcome in frozen-thawed embryo transfers. Fertil Steril. 2019;112:503–509. doi: 10.1016/j.fertnstert.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Bu Z, Yang X, Song L, Kang B, Sun Y. The impact of endometrial thickness change after progesterone administration on pregnancy outcome in patients transferred with single frozen-thawed blastocyst. Reprod Biol Endocrinol. 2019;17(1):99. doi: 10.1186/s12958-019-0545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zilberberg E, Smith R, Nayot D, Haas J, Meriano J, Barzilay E, Casper RF. Endometrial compaction before frozen euploid embryo transfer improves ongoing pregnancy rates. Fertil Steril. 2020;113:990–995. doi: 10.1016/j.fertnstert.2019.12.030. [DOI] [PubMed] [Google Scholar]
- 13.Tabibzadeh S. Proliferative activity of lymphoid cells in human endometrium throughout the menstrual cycle. J Clin Endocrinol Metab. 1990;70:437–443. doi: 10.1210/jcem-70-2-437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 14 kb)
Data Availability Statement
Coded data pertaining to this study are stored on a password- and firewall-protected server and can be made available if needed.