Abstract
Purpose
To study whether the application of femtosecond laser pulses for zona pellucida (ZP) drilling of blastocysts at the embryonic or abembryonic poles can promote hatching to start immediately through the hole formed and ensure high hatching rates and embryo viability.
Methods
Mouse blastocyst (E3.5) ZP were microdissected with femtosecond laser pulses (514-nm wavelength, 280-fs pulse duration, 2.5-kHz repetition rate) close to the trophoblast or inner cell mass (ICM). The sizes of the holes formed were in the range of 4.5–8.5 μm. Additional longitudinal incisions (5–7-μm long) on either side of the hole were created to determine whether hatching had started at the correct position. Embryos post-laser-assisted ZP drilling and intact embryos were cultured under standard conditions for 2 days; embryo quality was assessed twice daily. The hatching rates and in vitro and in vivo implantation rates (only for embryos with ZP dissected close to the ICM) were estimated.
Results
Femtosecond laser–assisted ZP drilling at the early blastocyst stage facilitated embryo hatching to start at the artificial opening with probability approaching 100%. Despite the artificial opening’s small size, no embryo trapping during hatching was observed. Both experimental groups had higher hatching rates than the control groups (93.3–94.7% vs. 83.3–85.7%, respectively). The in vitro implantation rate was comparable with that of the control group (92.3% vs. 95.4%). No statistically significant differences were obtained in the in vivo implantation rates between the experimental and control groups.
Conclusions
Blastocyst-stage femtosecond laser microsurgery of ZP is fast and delicate and enables the hatching process to be initiated in a controlled manner through a relatively small opening, with no embryo trapping.
Keywords: Laser-assisted hatching, Femtosecond laser microsurgery, Zona pellucida drilling, Blastocyst, Trophectoderm cells, Inner cell mass
Introduction
Lasers are now being widely used as diagnostic or therapeutic tools in assisted reproductive technologies for both oocyte and spermatozoa treatment and manipulations [1–3]. Infrared (1.48 μm) diode lasers with milli- to microsecond pulse durations are the most popular lasers applied in assisted reproductive technologies (ART) nowadays, for either laser-induced sperm immobilization [4, 5] or embryo treatment [6]. Microsurgery of the outer envelope of the zygote or embryo is an essential part of various micromanipulation techniques, such as laser-assisted ICSI (intracytoplasmic sperm injection) [7–9], embryo biopsy [10, 11], and laser-assisted hatching (LAH) [12, 13]. Moreover, artificial holes in the zona pellucida (ZP) of the oocyte are usually created to perform enucleation of the oocyte and nuclear transfer as well as to remove necrotic blastomeres [14].
Due to the widespread application of diode laser systems in ART, numerous attempts have been aimed at increasing their safety and minimizing possible adverse effects. While the majority of authors have noted the relative safety of these laser systems [15–17], other authors have suggested that the heat produced during laser irradiation may have negative effects on embryonic development [18–20]. To avoid possible laser-related thermal risks, strong recommendations regarding the optimum regimens of embryo exposure have been developed, including requirements for minimizing the pulse lengths in laser devices intended for use in clinical practice or maintaining safe distances between the laser firing position and the nearest blastomere [19, 21]. Accordingly, most ZP microsurgical procedures are performed at the early stages of preimplantation development, when there is sufficient perivitelline space between embryo cells and the envelope to ensure laser microsurgery at a safe distance.
At the same time, several studies have reported that embryo microsurgery at the late stages of preimplantation development has some advantages over early-stage embryo microsurgery. ZP drilling might have a detrimental effect on the number of cells in the blastocyst when it is performed too early during the cleavage stage [22, 23]. Premature contact of cleavage-stage embryos with the external environment is supposed to be harmful to their subsequent development.
ZP drilling at the late stages of preimplantation development may also be beneficial if blastocyst biopsy is required. Blastocyst biopsy enables the collection of multiple trophectoderm (TE) cells, thus allowing for more reliable results and causing no harm to the cells forming the embryo proper. Studies comparing blastocyst biopsy to polar body/blastomere biopsy have reported the superiority of the former over the latter [1, 24]. The creation of an artificial opening in the ZP at the late preimplantation stages (when the inner cell mass (ICM) and TE are well distinguishable) has several advantages. Apart from the fact that the embryo is left undisturbed up to day 5, 6, or even 7 (in humans), ZP drilling can be performed in the preferred site. For example, it is expected that ZP drilling close to the TE cells would stimulate embryo hatching in a “trophoblast first” manner, thus making the process of blastocyst biopsy easier. At the same time, when blastocyst biopsy is not required, ZP drilling close to the ICM could result in a developmental advantage due to the acceleration of contact with the endometrium [25]. However, the technique applied for such drilling should be safe for the embryo.
In the present paper, we propose an effective technique for ZP drilling at the early blastocyst stage based on femtosecond (fs) laser microsurgery. Femtosecond technology is a very useful tool for high-precision embryo microsurgery. Lasers with ultrashort pulse duration (~ 100–300 fs) allow the delivery of nanojoules (nJ) of energy per pulse during laser exposure, thus minimizing the possible risks of thermal damage to embryos. Femtosecond lasers have been successfully applied for fully noncontact optical microinjection and trapping of developing embryos [26], studying embryo morphogenesis [27], blastomere fusion [28, 29], oocyte enucleation [30], TE cell dissection during embryo biopsy [31], and even for laser-assisted alphanumerical code engraving in the ZP for embryo identification during its preimplantation development [32, 33].
Here, we apply the technique of femtosecond laser–assisted ZP drilling in two ways: at a point close to the TE cells and at a point close to the ICM. Data on hatching start-point as well as on embryo viability and preimplantation development in both experimental groups have been collected. To demonstrate the absence of negative effects of ZP femtosecond microsurgery in the vicinity of the ICM, we estimated the potential of embryo implantation following laser-assisted ZP drilling in vitro and in vivo.
Materials and methods
Animals
CBA mice purchased from Federal Medical-Biological Agency, Branch “Andreevka” (Russian Federation) were used for the embryo collection. C57BL/6 × CBA F1 mice (female, weight 12–13 g and male, weight 18–20 g) and CD1 mice (weight 24–25 g) were obtained from “Stolbovaya” (Russian Federation). C57BL/6 × CBA F1 mice were used for embryo collection, and CD1 mice were used as recipients. The animals were maintained under controlled room conditions (22–24 °C and 14:10 light:dark photoperiod) and had ad libitum access to food and water. All manipulations with animals were performed according to the European Convention for the Protection of Vertebrate Animals, Strasbourg, 18.III.1986, Directive 2010/63/EU, 22 September 2010 (annexes III, IV), the Order No. 755 of the USSR Ministry of Health, 12.08.1977, and the Laboratory Practice Rules in the RF and were approved by the Bioethics Commission of Institute of Gene Biology, Russian Academy of Sciences.
Embryo collection, culture, and monitoring
The mice were killed by cervical dislocation 3.5 days after copulation, when embryos usually reach the blastocyst stage. The oviducts and uterus were removed and placed on previously warmed at 37 °C HEPES-containing medium “Ooclean” (PanEco Ltd., Moscow, Russian Federation).
Then, the oviducts were dissected from the uterus under a stereomicroscope, and each horn of the uterus was flushed with 500 μl of medium according to a standard protocol [34]. Blastocyst- and morula-stage embryos were collected under a stereomicroscope with the Cook Flexipet Adjustable Handle (Cook Medical, Bloomington, IA, USA). In the case of slow rates of embryo development, the collected morulas were cultured for one more day in the cleavage medium (PanEco Ltd.) in a CO2 incubator. The development of these embryos was evaluated two times a day (every morning and evening) until the blastocyst stage. A total of 191 embryos were collected and divided into five groups:
Experimental group A: a total of 30 blastocysts with ZP to be drilled at the abembryonic pole (close to the TE)
Control group B: a total of 35 fully intact embryos stored in a CO2 incubator at 37 °C
Control group C: a total of 60 embryos kept out of the incubator for the same duration as the experimental embryos, but not exposed to laser radiation
Experimental group D: a total of 39 embryos with ZP to be drilled at the embryonic pole (close to the ICM)
Control group E: a total of 65 fully intact embryos stored in a CO2 incubator at 37 °C and transferred (normally, on day 5.5 post-coitum) to in vitro culture medium (see subsection “Modelling embryo implantation”) to evaluate the implantation potential of intact embryos in vitro.
Each blastocyst from experimental groups A and D had clearly identified ICM and TE.
Blastocyst-stage embryos (normally on day 3.5 post-coitum or day 4.5 for embryos with low developmental rates) from the experimental groups and control group C were transferred to fresh drops (up to five embryos per drop) of embryo manipulation medium in Petri dishes under mineral oil balanced with the medium. Each dish contained two drops: the first with experimental embryos and the second with control group C embryos. Then, the ZP of the experimental group embryos underwent femtosecond laser drilling. After the procedure, embryos were placed into 4-well plates (five embryos per well; experimental and control embryos were placed in separate wells), returned to the incubator, and cultured in blastocyst medium (PanEco Ltd.) until they hatched along with the intact control group B embryos. Embryonic development following laser-assisted ZP drilling was assessed twice a day under an AxioObserver Z1 microscope (Zeiss, Oberkochen, Germany) to ascertain whether hatching had occurred.
Modelling embryo implantation
We used 6-well Nunc Nunclon Delta Surface plates (Thermo Fisher Scientific, Waltham, MA, USA) with highly adhesive surfaces for modelling embryo implantation in vitro. Intact group E embryos and experimental group D embryos were transferred to in vitro culture medium on day 4.5 or day 5.5 post-coitum. The composition of the freshly prepared IVC1 in vitro medium was as follows: Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium without glutamine (1:1) (C 470 p, PanEco Ltd.), 20% Fetal Clone III (SH30109.03, HyClone Laboratories Inc., Logan, UT, USA), 2 mM l-glutamine (F032, PanEco Ltd.), 25 mg/ml penicillin/streptomycin (A65, PanEco Ltd.), 2% insulin-transferrin-selenite (50×) (F065, PanEco Ltd.), 8 nM beta-estradiol (E2758, Sigma-Aldrich, St. Louis, MO, USA), and 200 ng/ml progesterone (P8783, Sigma-Aldrich). The embryos were cultured in the medium under standard conditions (5% CO2, 37 °C) up to embryonic day 8.5 and were assessed two times a day during that period.
Embryo staining and imaging during in vitro implantation modelling
Implanted blastocysts were differentially stained using propidium iodide (PI, P4170, Sigma-Aldrich) to detect dead cells, with calcein acetoxymethyl ester (calcein AM) (C3099, Invitrogen, Carlsbad, CA, USA) for detecting live cells and with Hoechst 33342 (Sigma-Aldrich) for nuclei labelling. The samples were incubated in freshly prepared DMEM/F12 medium (1:1) containing 0.15 mg/ml Hoechst 33342, 0.001 mg/ml PI, and 0.1% calcein AM 0.1% for 1 h at 5% CO2 and 37 °C. Then, the embryos were washed three times for 5 min in HEPES-containing (15 mM) DMEM/F12 medium. The labelled samples were analyzed using an Olympus FluoView 10i confocal microscope (Tokyo, Japan). The images were processed using Photoshop CS6.
Immunofluorescence staining
The differential staining of ICM in the in vitro implanted blastocysts was made with the anti-Oct4 antibodies (Ab181557, Abcam, Cambridge, UK) according to the manufacturer’s instructions with slight modifications. Briefly, embryos were fixed in 4% paraformaldehyde for 30 min at room temperature. Then, they were washed three times for 15 min in 0.1% PBST (1× PBS plus 0.1% Triton X-100) to remove the residual paraformaldehyde and for permeabilization of the membranes. After permeabilization, embryos were incubated in the blocking solution (10% serum and 0.1% PBST) for 1 h at room temperature. Then blastocysts were incubated for 3 h at + 37 °C in the primary anti-Oct4 antibodies produced in rabbit (1:250). After washing four times in blocking solution (for 15 min each time), embryos were incubated for 2 h at room temperature in secondary antibodies (1:200, produced in goat, FITC conjugated, 111-095-003, Jackson ImmunoResearch Europe Ltd, Cambridge, UK). Then, the nuclei of the cells were stained with Hoechst 33342 (4 μg/ml) for 30 min at room temperature. After 4 washes, samples were examined under the confocal microscope Olympus Fluoview FV10i. Cells that were positive for OCT4 staining were identified as ICMs.
Blastocyst transfers post-laser-assisted hatching at the embryonic pole
To better understand how laser-assisted ZP drilling at blastocyst stage affects the implantation potential and further embryo development and survival, the transfer of the blastocysts post-LAH as well as control blastocysts into the uterus of the recipient females has been performed.
Pre-implantation embryos were obtained by mating super-ovulated (according to the standard protocol) C57BL/6 × CBA F1 female mice (12-13g weight) with C57BL/6 × CBA F1 males (18-20g weight). Pre-implantation embryos were flushed at E0.5 from oviducts of donor C57BL/6 × CBA F1 females and were cultured in Cleavage medium (PanEco Ltd.) microdrops covered in mineral oil (PanEco Ltd.) under standard conditions (5% CO2, 37 °C) up to the early blastocyst stage. A total of 85 early-stage blastocysts were laser-processed and artificial openings in the ZP at the embryonic pole were created (experimental group). A total of 72 embryos were kept out of the incubator for the same duration as the experimental embryos, but not exposed to laser radiation (control group). Then, embryos at E3.5 were transferred by injection into the uteri of pseudopregnant CD1 recipients 2.5 days after mating them with vasectomized C57Bl/6 males (3–7 embryos per each uteri horn). Transferred blastocysts were allowed to implant and implantation sites were dissected away from the uterine myometrium at E13.5 in cold 1× phosphate buffered saline. A total number of implantation sites as well as number of developed and failed to develop embryos were calculated and analyzed.
Experimental setup
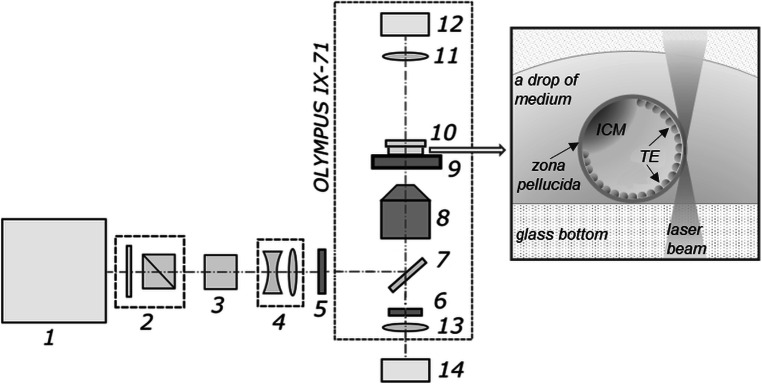
Figure 1 shows the schematic of the process of femtosecond laser–assisted ZP drilling. The laser system used in this study was a TETA femtosecond ytterbium laser (Avesta LLC, Moscow, Russian Federation), which generates ultrashort pulses of 280 fs at a repetition rate of 2.5 kHz. We employed second harmonic radiation (wavelength λ = 514 nm) to perform the ZP drilling. The laser beam is guided via an electromechanical shutter and an attenuator to an inverted microscope IX-71 (Olympus) and focused on the sample with a 20×, numerical aperture (NA) 0.5 objective (UPLFLN, Olympus). The focal spot diameter was measured to be about 2 μm full width at half maximum. The pulse energies were optimized to be slightly above the ablation threshold to create thin, well-defined cuts in the ZP volume while preventing (minimizing) the formation of multiple large cavitation bubbles.
Fig. 1.
Schematic setup of the system for femtosecond laser–assisted ZP drilling. 1, Femtosecond ytterbium laser; 2, beam attenuator; 3, second-harmonic generator; 4, telescope; 5, electro-mechanical shutter; 6, notch filter; 7, dichroic mirror; 8, microscope objective; 9, X-Y motorized stage; 10, Petri dish; 11, condenser lens; 12, microscope lamp; 13, tube lens; 14, CMOS camera. Inset: schematic presentation of glass-bottom Petri dish containing a blastocyst (in a fresh drop of embryo manipulation medium) during the process of laser-assisted ZP drilling in close proximity to TE cells
The embryos were placed in 170-μm-thick glass-bottom plastic Petri dishes. The dishes were mounted on a motorized X-Y stage (Märzhäuser Wetzlar, Germany). Laser-assisted manipulations with embryos were recorded using a DFK 72AUC02 CMOS camera (The Imaging Source, Bremen, Germany). Custom-built software was developed for imaging and controlling the laser exposure parameters. After visual assessment of the embryo, a primitive element (such as lines or curves) was drawn in the software right above the live image from the camera to mark the area where the artificial opening in the ZP was to be formed. Each drawn element was converted to a sequence of commands to the motorized stage. The location of the motorized stage during ZP drilling as well as synchronous laser shutter opening was automatically controlled by the software. As the ZP is transparent to laser radiation at the chosen wavelength, the beam propagates freely within. When focusing, the laser beam diameter decreases along the beam axis, leading to laser intensity growth and reaching its maximum in the beam waist. Laser-assisted ZP drilling occurs as soon as the incoming laser intensity exceeds the threshold intensity.
Statistical analysis
Statistical analyses were performed using Statistica 7.0 software (Dell Inc., USA). To analyze the preimplantation embryo development in the experimental group A (with ZP dissected close to the TE) and experimental group D (with ZP dissected close to the ICM), the number of successfully hatched embryos and nonhatched embryos in vitro was used for the analysis by 2 × 2 contingency tables and chi-square test. In vivo embryo development post-laser-assisted hatching and embryo transfer in comparison with control embryos development was also analyzed. For this purpose, the number of successfully developed embryos as well as the number of embryos failed to develop in the uterus post-embryo transfer were used for the analysis by 2 × 2 contingency tables and chi-square test. P < 0.05 was considered significant.
Results
Femtosecond laser–assisted ZP drilling at the abembryonic pole (close to the TE)
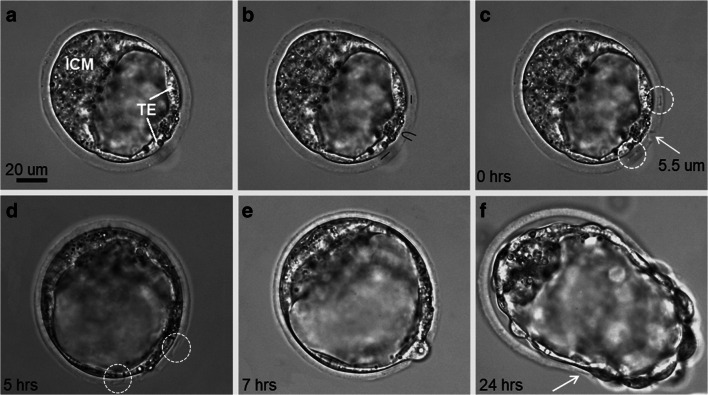
Thirty blastocysts from group A underwent ZP drilling at a position close to the TE. Figure 2 illustrates the successive steps of femtosecond laser–assisted ZP microsurgery in the vicinity of the TE. Figure 2a shows an early blastocyst with a well-defined ICM and TE. Figure 2b and c show a trajectory for laser processing and the corresponding opening in the ZP at the abembryonic pole created by the application of femtosecond laser pulses with 28.4 ± 1.5 nJ energy along the set trajectory, respectively. Taking into account that the blastocyst hatching may start at night and end prior to the morning assessment, we decided to create additional linear marks on either side of the hole in the ZP (Fig. 2c). As demonstrated in Fig. 2d, the marks created on the ZP may be blurred due to blastocyst rotation during expansion and hatching. These marks nevertheless remained recognizable during the hatching process and even after its completion (as demonstrated in the next section), thus proving the fact that the embryo exited the ZP through the artificially created opening. The initial herniation of the TE cells through the artificial opening was observed within 7 h after ZP drilling (Fig. 2e). At 24 h after ZP drilling, the blastocyst continued to hatch, and one of the laser-created marks became tiny, but remained visible (the incision is marked by the white arrow, Fig. 2f).
Fig. 2.
Femtosecond laser–assisted ZP drilling at the abembryonic pole. a Bright-field image of blastocyst with the ICM and TE clearly distinguishable. b The creation of primitive elements in the program interface at a position close to the TE cells. c Femtosecond laser–assisted ZP drilling (formation of an artificial opening in the ZP and two additional incisions [white circles]). d, e TE cells start herniating through the artificial opening in the ZP (5 h and 7 h post-ZP drilling, respectively); incisions on the ZP are blurred but nevertheless can be distinguished. f Embryo continues to hatch out of its shell at 24 h post-ZP drilling
The femtosecond laser–assisted drilling (the formation of the hole and two additional cuts) usually took 5–10 s, while the entire process, including the creation of the trajectory for the ZP laser processing, could be performed within 40–60 s, depending on the quality of the operator. The size of the hole created and the length of the additional cuts were in the range of 4.5–8.5 μm and 5–7 μm, respectively.
Table 1 summarizes the results for embryo development in experimental group A (with ZP dissected close to the TE) and untreated control groups B and C. The number of hatching embryos and the number of successfully hatched embryos at the time of their assessment on day 5–5.5 are presented. As far as we believe that such a technique should facilitate embryo hatching to start at a prescribed site, the number of embryos in the experimental group that hatched through an artificial opening was calculated. Twenty-eight of 30 embryos (93.3%) from experimental group A hatched through the artificial opening, while only one embryo hatched through another site.
Table 1.
Development of embryos at E5-5.5 following ZP drilling at a position close to the TE (group A) and the ICM (group D) in comparison with untreated embryos (groups B, C, and E)
| Group | Embryos (no.) | Expanded blastocysts or blastocysts at an early stage of hatching (no.) | Hatched embryos (no.) | In vitro implanted embryos (no.) | |
|---|---|---|---|---|---|
| Embryos hatched through the hole formed (no.) | Embryos hatched at another site (no.) | ||||
| Experimental group A (ZP dissection close to the TE) | 30 | 1 (3.4%) | 28 (93.3%) | 1 (3.4%) | – |
| Control group B | 35 | 5 (14.3%) | 30 (85.7%) | – | |
| Control group С | 60 | 10 (16.7%) | 50 (83.3%) | – | |
| Experimental group D (ZP dissection close to the ICM) | 39 | 1 (2.6%) | 38 (97.4%) | 0 (0%) | 36 (92.3%) |
| Control group E | 65 | – | – | 62 (95.4%) | |
| Total no. of embryos | 229 | ||||
Our data show that the femtosecond laser–assisted hatching did not compromise embryo viability. Moreover, the hatching rate in the experimental group was higher than that in both control groups (experimental group A total hatching rate: 96.6% vs. 85.7% in the intact control group B and 83.3% in control group C).
Femtosecond laser–assisted ZP drilling at the embryonic pole (close to the ICM)
Provided that the femtosecond laser–assisted ZP drilling at the abembryonic pole did not impair embryonic development and caused no adverse effects on hatching rate, we decided to perform ZP drilling at the embryonic pole. All necessary safety precautions were taken to prevent damage to the ICM as far as it would form the entire embryo. Apart from Montag and van der Ven [35], who reported immediate collapse of blastocysts (and the subsequent delay in the timing of hatching) as a result of ZP drilling, we observed no blastocyst collapse at all during (or immediately after) the femtosecond laser–assisted ZP drilling, which might serve as indirect confirmation of the safety of the proposed technique.
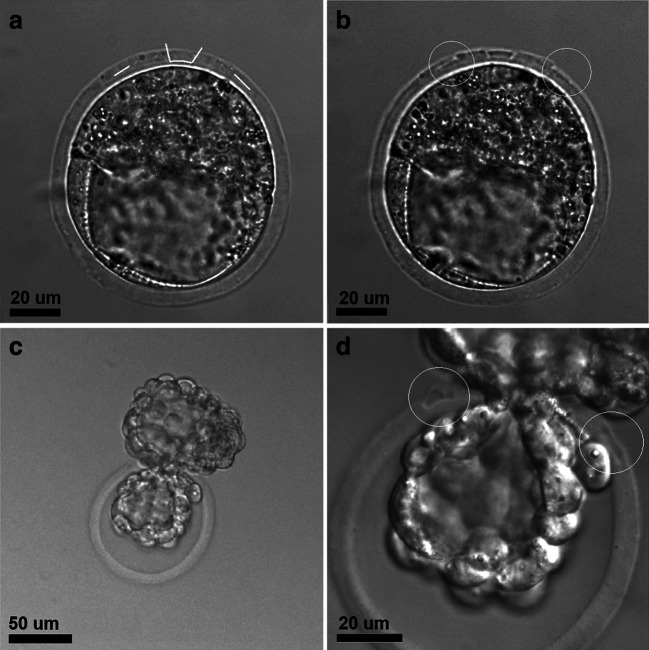
To evaluate whether the laser-assisted ZP drilling might have negative effects on further embryo development, we assessed the implantation potential of the embryos after laser-assisted ZP drilling in vitro. In the absence of the possibility of studying the implantation processes in vivo, several experimental model systems have recently been developed that simulate various events taking place in vitro during the peri-implantation period of embryo development. The study of the implantation processes in a solid-phase, cell-free system became possible due to the development of a specialized, highly adhesive plastic with a modified surface for cultivating cells with low adhesion ability. Mammalian blastocysts can attach to such a surface and continue to develop for several days [36]. Figure 3 demonstrates an example of embryo implantation to the bottom of an adhesive plate. The embryos attached to the bottom of the high-adhesive plastic dish and started to grow on its surface. The size of the nuclei of cells spread out on the bottom significantly exceeded that of the cells in the central parts of the embryo (Fig. 3c, d). As the TE cells in mouse embryos are characterized by the presence of polyploidy (“giant nuclei of the trophectoderm”), we defined the cells spread out on the bottom surface as TE cells, and the array of cells not spread out on the bottom was defined as the ICM. We also confirmed this assumption by immunofluorescence staining of ICM cells in the in vitro implanted blastocysts with anti-Oct4 antibodies (Fig. 3b, d). The ICM grew in a plane perpendicular to the bottom of the plastic dish and towered above the layer of TE cells. The embryos were also stained with calcein AM to visualize the cytoplasm and live cells, Hoechst 33342 to visualize the nuclei, and PI to detect dead cells. Maximum TE growth was observed at 2 days after landing on the platform (Fig. 3f). The embryos remained viable for 2 days after landing on plastic dish, and then, the percentage of PI-positive cells increased and most of the cells were dead on day 4 of cultivation (for comparison, see Fig. 3g and h).
Fig. 3.
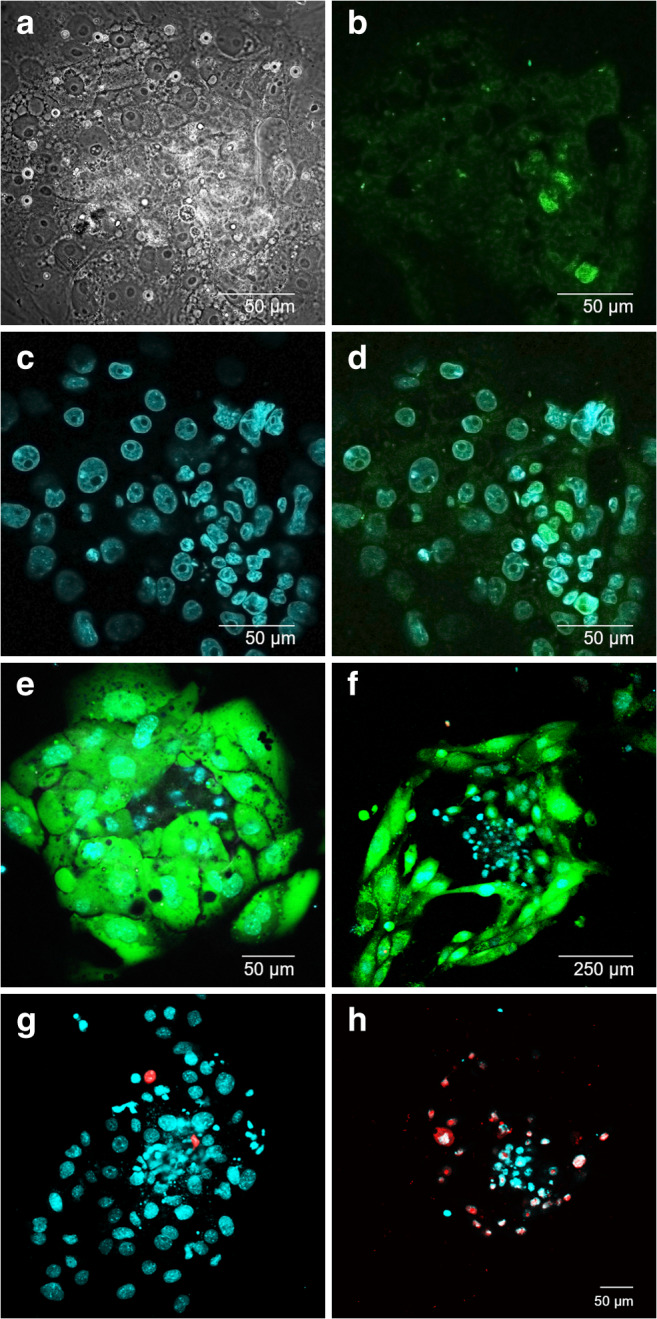
Peri-implantation mouse embryos cultured on adhesive platform. а Bright-field image of mouse embryo on day 2 (6.5 days post-fertilization) of cultivation on the adhesive platform. b Immunofluorescence staining of embryo on day 2 (6.5 days post-fertilization) with otc4 for ICM visualization. c Embryo staining on day 2 (6.5 days post-fertilization) with Hoechst 33342 for nuclei detection and d merge. e, f Embryos on day 1 (5.5 days post-fertilization) and day 4 (8.5 days post-fertilization) of cultivation stained with calcein AM and Hoechst 33342; extensive TE growth is shown. g, h Embryos on day 1 (5.5 days post-fertilization) and day 4 (8.5 days post-fertilization) of cultivation stained with PI and Hoechst 33342
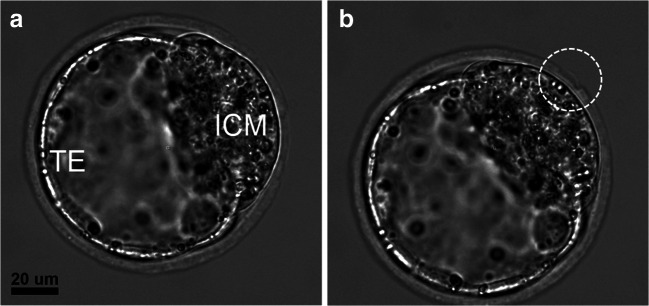
A total of 39 early-stage blastocysts (experimental group D, embryonic day 3.5 post-coitum) were laser-processed, and artificial openings in the ZP at the embryonic pole were created. Figure 4 demonstrates the process of femtosecond laser–assisted ZP drilling close to the ICM as well as further embryo development, its hatching, and in vitro implantation. Additional microdissections on either side of the hole in the ZP were created (Fig. 4b) and remained visible during the embryo hatching process (Fig. 4d). The hatching rate was as high as 97.4% (Table 1). Thirty-eight of 39 embryos from experimental group D hatched through the artificial opening, while only one embryo had not started to hatch at the time of the embryo assessment on day 5. The results for the in vitro embryo implantation are also shown in Table 1. The implantation rates of embryos from experimental group D were slightly lower than that of control group E (95.4%) but were nevertheless as high as 92.3%. Thus, our results show that there was an increase in the hatching rate in experimental group D as compared with the intact control groups B and C (97.4% vs. 85.7% and 83.3%, respectively) and a nonsignificant decrease in in vitro implantation rates as compared with intact control group E.
Fig. 4.
Femtosecond laser–assisted ZP drilling close to the embryonic pole. a The creation of primitive elements in the program interface at a position close to the ICM. b Femtosecond laser–assisted ZP microsurgery for forming an artificial opening and two additional incisions in the ZP (white circles). c, d The embryo hatching through the artificial opening; incisions on either side of the opening are clearly visualized and marked in white circles
To further explore possible effects of laser-assisted ZP drilling at the embryonic pole on the implantation potential and embryo survival, a group of blastocysts subjected to LAH (a total of 85 blastocysts) as well as control blastocyst group (a total of 72 blastocysts) have been transferred into the oviducts of the pseudopregnant recipients. Transferred blastocysts were allowed to implant and the implantation sites were dissected at E13.5. Table 2 summarizes the results on implantation and pregnancy rates. No statistically significant (P > 0.2) differences were obtained in the number of developed embryos between both groups (33 in the experimental group vs. 35 in the control group). The implantation rate in the experimental group was slightly higher than that in the control group and indirectly confirmed the safety of the proposed technique of femtosecond laser–assisted ZP drilling. Nevertheless, as can be seen, the ratio of implanted but arrested in development embryos to the total number of transferred embryos or to the implantation sites number in the experimental group is higher than that one in the control group (36.4% vs. 23.6% and 48.4% vs. 32.7% correspondingly).
Table 2.
In vivo development of experimental embryos (with ZP dissected close to the ICM) and control embryos after embryo transfer into the uterus of the recipient females
| Group | Experimental group (post-ZP drilling close to the ICM) | Control group |
|---|---|---|
| No. of embryos transferred (T) | 85 | 72 |
| No. of implantation sites (IS) (implantation rate IS/T) | 64 (75.3%) | 52 (72.2%) |
| No. of developed embryos (L) (pregnancy rate L/IS) | 33 (51.6%) | 35 (67.3%) |
| No. of implanted, but arrested in development embryos (D) | 31 | 17 |
| No. of abnormally developed or nondeveloped embryos (T-L) | 52 | 37 |
| D/T ratio | 48.4% | 32.7% |
| D/IS ratio | 36.4% | 23.6% |
| Total no. of embryos | 157 | |
Discussion
Embryo hatching from the ZP is crucial for implantation and continued development of the embryo. While the process of embryo hatching has been extensively studied, it remains a topical issue. Understanding of the characteristics of natural embryo hatching is especially important in terms of the development of effective techniques for assisted hatching (AH).
Assisted hatching is a microsurgical procedure performed to facilitate embryo hatching and to improve ART success [12, 13, 37]. Compared with mechanical or chemical procedures of ZP breaching, laser systems provide better results and have recently become the most common technology for AH. Since its first mention in 1988 [38] and more than 30 years of application, the performance of AH in terms of efficacy is still under debate. AH has been shown to not improve outcome in women with endometriosis [39] or increase clinical pregnancy rates when performed in fresh embryos transferred to unselected or nonpoor prognosis women or to women of advanced age [40]. However, the benefits of AH in frozen-thawed blastocysts have been consistently demonstrated [37]. Performed on frozen embryos, laser-assisted hatching can improve the chances of pregnancy formation in older women by overcoming the negative effect of zona hardening [41].
The controversial results regarding the benefits of AH may be partly explained by differences in the techniques and protocols used. Insufficient artificial hole size and number of holes created may also influence embryo development and the efficiency of the AH technique [35]. Miyata et al. have hypothesized that the choice of AH site could affect the hatching process [42]. ZP drilling performed without consideration of the site (i.e., on day 2 or 3) might either promote or inhibit blastocyst hatching. Ebner et al. have also suggested that blastocyst hatching close to the ICM could result in a developmental advantage [25]. Later, Ren et al. showed that the site of assisted hatching has no influence on the implantation, pregnancy, and live birth rate in human vitrified-warmed blastocysts [43]. In contrast to that, the results presented by Ryu et al. a year later [44] confirmed that, in certain patients, AH close to the ICM of frozen-thawed blastocysts demonstrated significantly higher clinical outcomes (clinical pregnancy and implantation rates) than AH close to the TE. Rather, controversial data regarding the most preferential site of embryo hatching in humans and other mammalian species have been obtained so far. While some authors have demonstrated that blastocyst hatching in mice and rats usually begins with the protrusion of TE cells out of the ZP [45, 46], others have presented contradicting results, showing that most blastocyst hatching sites are close to the ICM [47]. Likewise, in humans, various authors have reported the formation of natural hatching sites close to the ICM [42, 48] as well as opposite to the ICM [49, 50]. For bovine embryos, nearly equal probability of hatching from the embryonic or abembryonic poles has been demonstrated [51].
However, while the issue of the possible influence of the AH site on the hatching rate, blastocyst implantation, and embryo development remains controversial [43, 46, 47] and requires further study, the proposed technique enables safe, quick, and reliable ZP drilling at both abembryonic and embryonic poles and thus seems to be very useful for improving the AH effectiveness and overall embryo development. Given recent findings [52], LAH at earlier stages of development might have a detrimental effect on the number of cells in the blastocyst. This unfavorable effect does not occur when the ZP is opened after the embryos have reached the early blastocyst stage.
In the present study, we develop a laser-based technique for ZP drilling at the early blastocyst stage and demonstrate that embryo hatching started right at the location of the artificial opening (with > 93% probability). Femtosecond laser pulses have a highly localized effect, which fades away for out-of-focus cellular structures. In combination with the relatively low pulse energy and ultrahigh intensity, these features make femtosecond lasers a unique tool for precise ZP microsurgery with minimal risk of thermal damage to the adjacent embryo cells. Femtosecond laser–assisted ZP drilling of blastocysts with an even higher degree of expansion can be successfully performed (see, for example, Figs. 2, 4, or 5). Figure 5 demonstrates such a blastocyst right after ZP drilling in the vicinity of the ICM, and the partially detached tiny flap of the ZP is also visible. Only the ZP was exposed to the femtosecond laser irradiation while leaving the embryo cells intact, and no collapse of the blastocyst in response to the laser action occurred.
Fig. 5.
Expanded blastocyst before (a) and immediately after (b) femtosecond laser–assisted ZP drilling at a position near the ICM. A tiny detached flap of the ZP is visible (white circle)
The results of ZP drilling at blastocyst stage in locations close to either the TE or ICM have recently been reported [42–44, 52–56] and are summarized in Table 3. As a rule, an infrared diode laser (λ = 1.48 μm) is used to create a hole. The application of a single laser pulse results in the formation of an approximately 10-μm hole in the ZP, and the final size of the artificial opening is usually set in the range of 20–50 μm. In the present study, we demonstrate for the first time that forming a smaller hole (4.5–8.5 μm) is also sufficient for obtaining hatching rates as high as 93.3–97.4%. In contrast to previous reports [42, 53], we did not observe embryo trapping or hatching delay through the small hole (< 10 μm). Embryo hatching through a small opening is of particular importance when blastocyst biopsy is to be performed, as the protrusion of a relatively small number of cells through a small hole occurs simplifies the process of cell removal. It should be noted that the blastocyst biopsy is considered the most promising approach for preimplantation genetics diagnosis/screening (applied for identifying chromosomal or genetic disorders) [53, 54, 57], and its implementation in ART is gradually increasing. The importance of developing techniques aimed at simplifying blastocyst biopsy has been emphasized by Capalbo et al. [54], and we believe that our results are useful for improving the overall effectiveness of the procedure.
Table 3.
ZP drilling at the blastocyst stage with infrared diode lasers (wavelength λ = 1480 nm)
| Embryo type | Laser system | Exposure parameters (power/pulse duration) | Artificial hole size | Embryos (no.) | ZP drilling position close to the TE/ICM or botha | Purpose of ZP drilling | Ref. |
|---|---|---|---|---|---|---|---|
| Human | Research Instruments, UK | No data | 10–20 μm | 956 | TE | Blastocyst biopsy | [54] |
| ZILOS-tk, Hamilton Thorne, USA | 285 mW/400 μs | Quarter ZP opening (≈ 100–150 μm) | 195b | No data | LAH | [55] | |
| No data | 20 μm |
TE group, n = 16 ICM group, n = 16 |
Bothc | LAH | [42] | ||
| No data | No data | 20–25 μm | 115 patients (128 cycles)d | Both | LAH | [44] | |
| OCTAX, Vitrolife, Sweden | No data | 50 μm |
TE group, n = 138 ICM group, n = 143 |
Both | LAH | [43] | |
| Mouse | XYClone, Hamilton Thorne, USA | 140 mW/– | 20 μm |
TE group, n = 125 ICM group, n = 125 |
Both | LAH | [52] |
| Thorlabs, USA | –/2 ms | 20 μm | 30 | No data | No hatching was observed | [56] | |
| Fertilase, MTM Medical Technologies, Switzerland | –/6 ms | No data | No data | TE | LAH | [35] | |
| Cattle | Fertilase, MTM Medical Technologies |
–/16 ms, No. of pulses: 1 or 4–5 |
Group 1: 7–15–μm wide, 40–μm long Group 2: 40 μm in diameter |
Group 1, n = 48 Group 2, n = 44 |
No data | LAH | [53] |
aTE/ICM or both means that ZP drilling was performed either close to the TE, close to the ICM or both experimental groups were in the study
bThe number of blastocysts that survived post-vitrification
cThe blastocysts were in a dehydrated state, resulting in ZP drilling at a distance from the ICM
dNo data reported regarding the total amount of embryos
In this study, we have analyzed the influence of femtosecond laser–assisted ZP drilling not only on preimplantation embryo development but also on further intrauterine development. The number of successfully developed embryos at E13.5 was nearly the same in the experimental group (post-LAH in the vicinity of the ICM) and in the control group, which confirms the safety of the proposed technique. Higher values obtained for the ratio of implanted but failed to develop embryos to the total number of transferred embryos (D/T) or to implantation sites number (D/IS) in the experimental group as compared with the control one may be explained as follows. Blastocyst hatching out of the ZP is a crucial step for embryo implantation and its further development. This step may be a serious barrier for the “weak” embryos with low viability. LAH procedure enables us to remove this natural barrier for such embryos. Potentially, these embryos may implant into the uterus with high probability of further abortion in development, resulting in high value for D/T ratio. Our current study was performed on healthy mice with initially no need for ART procedures. LAH performed on such model objects may not be as effective as it would be in patients undergoing infertility treatment. We believe that women who suffer from, for example, zona pellucida hardening may benefit from such a procedure. Therefore, further studies are required to evaluate the efficiency of femtosecond laser–assisted ZP drilling performed on human embryos.
Conclusion
In this study, a noncontact femtosecond laser-based technique for ZP microdissection at the early blastocyst stage aimed at promoting embryo hatching at the prescribed site has been proposed. A high probability of embryo hatching through the artificial opening (93.3% through the hole created close to the TE and 97.4% through the hole created close to the ICM) has been demonstrated. We have also shown that femtosecond laser–assisted ZP drilling results in higher hatching rates in both experimental groups as compared with the control groups (96.6% and 97.4% vs. 85.7% and 83.3%) and does not compromise further intrauterine embryo development. Taking into account the controversy regarding the preferred site of embryo hatching in various mammals, the proposed technique enabling safe ZP drilling at both sites (close to either the TE or the ICM) seems to be particularly useful for improving hatching rates in humans.
Another benefit of ZP drilling at the early blastocyst stage (when the TE and ICM are clearly visualized) becomes obvious when blastocyst biopsy is required. ZP microdissection close to the abembryonic pole initiates the protrusion of only TE cells (not the ICM) through an artificial hole. Moreover, we have demonstrated for the first time that even a small opening created in the ZP (4.5–8.5 μm in this study instead of 20 μm [42] or 50 μm [43]) is sufficient for successful embryo hatching at a prescribed location. The small hole size resulted in the protrusion of a small number of TE cells and could simplify the cell removal.
Thus, the main advantages of the proposed technique include postponement in breaching ZP integrity and leaving the embryo undisturbed up to the blastocyst stage, the achievement of high hatching rates, and fostering embryo hatching to start at the prescribed site. It might be of particular importance for improving the chances of successful implantation and increasing the effectiveness of the blastocyst biopsy (if required). In conclusion, we have demonstrated that femtosecond lasers can be employed as precise and effective tools for embryo microsurgery.
As far as femtosecond lasers offer several important advantages (such as high precision, minimal invasiveness, versatility) over conventional milli/microsecond lasers, we believe that further advances in ultrafast laser technology aimed at reducing the complexity, size, and high price of femtosecond lasers will help them gain popularity in the field of assisted reproduction.
Acknowledgments
The work was conducted using Unique Facility “Terawatt Femtosecond Laser Complex” in the Center for Collective Usage “Femtosecond Laser Complex” of JIHT RAS. This work was performed using the equipment of IGB RAS facilities supported by the Ministry of Science and Higher Education of the Russian Federation. The authors are immensely thankful to Diana S. Korshunova, IGB RAS, for excellent support with embryo collection, blastocyst handling prior to transfer, and further embryo analysis.
Funding
The reported study was funded by RFBR and Moscow city Government according to the research project no. 19-3270036.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All manipulations with animals were performed according to the European Convention for the Protection of Vertebrate Animals, Strasbourg, 18.III.1986 (Appendix A, Section A), Directive 2010/63/EU, 22 September 2010 (annexes III, IV), the Order No. 755 of the USSR Ministry of Health, 12.08.1977 (Appendices 2, 4), and the Laboratory Practice Rules in the RF and were approved by the Bioethics Commission of Institute of Gene Biology, Russian Academy of Sciences.
Consent to participate
Not applicable
Consent for publication
All authors consent to the publication of the manuscript in the Journal of Assisted Reproduction and Genetics
Code availability
Not applicable
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Davidson LM, Liu Y, Griffiths T, Jones C, Coward K. Laser technology in the ART laboratory: a narrative review. Reprod BioMed Online. 2019;38(5):725–739. doi: 10.1016/j.rbmo.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Bedient C, Khanna P, Desai N (2011) Laser pulse application in IVF. In: Jakubczak K (ed) Lasers - applications in science and industry. Rijeka, InTech, pp. 193–214. 10.5772/24024.
- 3.Montag MHM, Klose R, Köster M, Rösing B, van der Ven K, Rink K, van der Ven H. Application of non-contact laser technology in assisted reproduction. Med Laser Appl. 2009;24(1):57–64. doi: 10.1016/j.mla.2008.11.001. [DOI] [Google Scholar]
- 4.Schopper B, Ludwig M, Edenfeld J, Al-Hasani S, Diedrich K. Possible applications of lasers in assisted reproductive technologies. Hum Reprod. 1999;14(Suppl 1):186–193. doi: 10.1093/humrep/14.suppl_1.186. [DOI] [PubMed] [Google Scholar]
- 5.Ebner T, Yaman C, Moser M, Sommergruber M, Hartl J, Tews G. Laser assisted immobilization of spermatozoa prior to intracytoplasmic sperm injection in humans. Hum Reprod. 2001;16:2628–2631. doi: 10.1093/humrep/16.12.2628. [DOI] [PubMed] [Google Scholar]
- 6.Tadir Y. Ten years of laser-assisted gametes and embryo manipulations. In: Wyss P, Tadir Y, Tromberg BJ, Haller U, editors. Photomedicine in gynecology and reproduction. Basel: Karger; 2000. pp. 326–339. [Google Scholar]
- 7.Rienzi L, Greco E, Ubaldi F, Iacobelli M, Martinez F, Tesarik J. Laser-assisted intracytoplasmic sperm injection. Fertil Steril. 2001;76:1045–1047. doi: 10.1016/S0015-0282(01)02861-8. [DOI] [PubMed] [Google Scholar]
- 8.Demirol A, Benkhalifa M, Sari T, Gurgan T. Use of laser-assisted intracytoplasmic sperm injection (ICSI) in patients with a history of poor ICSI outcome and limited metaphase II oocytes. Fertil Steril. 2006;86(1):256–258. doi: 10.1016/j.fertnstert.2005.11.078. [DOI] [PubMed] [Google Scholar]
- 9.Choi KH, Lee JH, Yang YH, Yoon TK, Lee DR, Lee WS. Efficiency of laser-assisted intracytoplasmic sperm injection in a human assisted reproductive techniques program. Efficiency of laser-assisted intracytoplasmic sperm injection in a human assisted reproductive techniques program. Clin Exp Reprod Med. 2011;38(3):148–152. doi: 10.5653/cerm.2011.38.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veiga A, Sandalinas M, Benkhalifa M, Boada M, Carrera M, Santalo J, Barri PN, Ménézo Y. Laser blastocyst biopsy for preimplantation diagnosis in the human. Zygote. 1997;5:351–354. doi: 10.1017/s0967199400003920. [DOI] [PubMed] [Google Scholar]
- 11.McArthur SJ, Leigh D, Marshall JT, De Boer KA, Jansen RPS. Pregnancies and live births following biopsy and PGD analysis of human embryos at the blastocyst stage. Fertil Steril. 2005;84(6):1628–1636. doi: 10.1016/j.fertnstert.2005.05.063. [DOI] [PubMed] [Google Scholar]
- 12.Tadir Y, Wright WH, Vafa O, Liaw LH, Asch R, Berns MW. Micromanipulation of gametes using laser microbeams. Hum Reprod. 1991;6:1011–1016. doi: 10.1093/oxfordjournals.humrep.a137451. [DOI] [PubMed] [Google Scholar]
- 13.Rink K, Delacrétaz G, Salathé RP, Senn A, Nocera D, Germond M, Grandi PD, Fakan S. Non-contact microdrilling of mouse zona pellucida with an objective-delivered 1.48-μm diode laser. Lasers Surg Med. 1996;18:52–62. doi: 10.1002/(SICI)1096-9101(1996)18:1<52::AID-LSM7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 14.Rienzi L, Ubaldi F, Iacobelli M, Minasi MG, Romano S, Ferrero S, Sapienza F, Baroni E, Tesarik J, Greco E. Developmental potential of fully intact and partially damaged cryopreserved embryos after laser-assisted removal of necrotic blastomeres and post-thaw culture selection. Fertil Steril. 2005;84:888–894. doi: 10.1016/j.fertnstert.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 15.Germond M, Nocera D, Senn A, Rink K, Delacretaz G, Fakan S. Microdissection of mouse and human zona pellucida using a 1.48 um diode laser beam: efficacy and safety of the procedure. Fertil Steril. 1995;64:604–611. doi: 10.1016/S0015-0282(16)57800-5. [DOI] [PubMed] [Google Scholar]
- 16.Taylor TH, Gilchrist JW, Hallowell SV, Hanshew KK, Orris JJ, Glassner MJ, Wininger JD. The effects of different laser pulse lengths on the embryo biopsy procedure and embryo development to the blastocyst stage. J Assist Reprod Genet. 2010;27(11):663–667. doi: 10.1007/s10815-010-9461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsieh YY, Huang CC, Cheng TC, Chang CC, Tsai HD, Lee MS. Laser-assisted hatching of embryos is better than the chemical method for enhancing the pregnancy rate in women with advanced age. Fertil Steril. 2002;78:179–182. doi: 10.1016/s0015-0282(02)03172-2. [DOI] [PubMed] [Google Scholar]
- 18.Malter HE, Schimmel T, Cohen J. Zona dissection by infrared laser: developmental consequences in the mouse, technical considerations, and controlled clinical trial. Reprod BioMed Online. 2001;3:117–123. doi: 10.1016/s1472-6483(10)61979-7. [DOI] [PubMed] [Google Scholar]
- 19.Douglas-Hamilton DH, Conia J. Thermal effects in laser-assisted pre-embryo zona drilling. J Biomed Opt. 2001;6:205–213. doi: 10.1117/1.1353796. [DOI] [PubMed] [Google Scholar]
- 20.Tucker MJ, Ball GD. Assisted hatching as a technique for use in human in vitro fertilization and embryo transfer is long overdue for careful and appropriate study. J Clin Embryol. 2009;12:5–8. [Google Scholar]
- 21.Chatzimeletiou K, Morrison EE, Panagiotidis Y, Prapas N, Prapas Y, Rutherford AJ, Grudzinskas G, Handyside AH. Comparison of effects of zona drilling by non-contact infrared laser or acid Tyrode’s on the development of human biopsied embryos as revealed by blastomere viability, cytoskeletal analysis and molecular cytogenetics. Reprod BioMed Online. 2005;11(6):697–710. doi: 10.1016/S1472-6483(10)61688-4. [DOI] [PubMed] [Google Scholar]
- 22.Chailert C, Sanmee U, Piromlertamorn W, Samchimchom S, Vutyavanich T. Effects of partial or complete laser-assisted hatching on the hatching of mouse blastocysts and their cell numbers. Reprod Biol Endocrinol. 2013;11:21. doi: 10.1186/1477-7827-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montag M, Koll B, Holmes P, van der Ven H. Significance of the number of embryonic cells and the state of the zona pellucida for hatching of mouse blastocysts in vitro versus in vivo. Biol Reprod. 2000;62(6):1738–1744. doi: 10.1095/biolreprod62.6.1738. [DOI] [PubMed] [Google Scholar]
- 24.Aoyama N, Kato K. Trophectoderm biopsy for preimplantation genetic test and technical tips: a review. Reprod Med Biol. 2020;00:1–10. doi: 10.1002/rmb2.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebner T, Gruber I, Moser M. Location of herniation predicts implantation behaviour of hatching blastocyst. J Turkish-German Gynecol Assoc. 2007;8(2):184–188. [Google Scholar]
- 26.Torres-Mapa ML, Antkowiak M, Cizmarova H, Ferrier DEK, Dholakia K, Gunn-Moore FJ. Integrated holographic system for all-optical manipulation of developing embryos. Biomed Opt Express. 2011;2:1564–1575. doi: 10.1364/BOE.2.001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Supatto W, Débarre D, Farge E, Beaurepaire E. Femtosecond pulse-induced microprocessing of live Drosophila embryos. Med Laser Appl. 2005;20(3):207–216. doi: 10.1016/j.mla.2005.07.001. [DOI] [Google Scholar]
- 28.Ilina IV, Ovchinnikov AV, Sitnikov DS, Rakityanskiy MM, Agranat MB, Khramova YV, Semenova ML. Application of femtosecond laser pulses in biomedical cell technologies. High Temp. 2013;51:173–178. doi: 10.1134/S0018151X13020089. [DOI] [Google Scholar]
- 29.Osychenko AA, Zalessky AD, Kostrov AN, Ryabova AV, Krivokharchenko AS, Nadtochenko VA. Femtosecond laser surgery of two-cell mouse embryos: effect on viability, development, and tetraploidization. J Biomed Opt. 2017;22(12):1–9. doi: 10.1117/1.JBO.22.12.125006. [DOI] [PubMed] [Google Scholar]
- 30.Kuetemeyer K, Lucas-Hahn A, Petersen B, Lemme E, Hassel P, Niemann H, Heisterkamp A. Combined multiphoton imaging and automated functional enucleation of porcine oocytes using femtosecond laser pulses. J Biomed Opt. 2010;15:046006. doi: 10.1117/1.3463012. [DOI] [PubMed] [Google Scholar]
- 31.Ilina IV, Khramova YV, Filatov MA, Semenova ML, Sitnikov DS. Application of femtosecond laser scalpel and optical tweezers for noncontact biopsy of late preimplantation embryos. High Temp. 2015;53:804–809. doi: 10.1134/S0018151X15060103. [DOI] [Google Scholar]
- 32.Ilina IV, Khramova YV, Filatov MA, Sitnikov DS. Femtosecond laser is effective tool for zona pellucida engraving and tagging of preimplantation mammalian embryos. J Assist Reprod Genet. 2019;36(6):1251–1261. doi: 10.1007/s10815-019-01424-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ilina IV, Khramova YV, Filatov MA, Sitnikov DS. Application of femtosecond laser microsurgery in assisted reproductive technologies for preimplantation embryo tagging. Biomed Opt Express. 2019;10(6):2985–2995. doi: 10.1364/BOE.10.002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo: a laboratory manual. New York: Cold Spring Harbor Lab; 2014. [Google Scholar]
- 35.Montag M, van der Ven H. Laser-assisted hatching in assisted reproduction. Croatian Med J. 1999;40:398–403. [PubMed] [Google Scholar]
- 36.Shahbazi MN, Jedrusik A, Vuoristo S, Recher G, Hupalowska A, Bolton V, Fogarty NNM, Campbell A, Devito L, Ilic D, Khalaf Y, Niakan KK, Fishel S, Zernicka-Goetz M. Self-organization of the human embryo in the absence of maternal tissues. Nat Cell Biol. 2016;18(6):700–708. doi: 10.1038/ncb3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alteri A, Viganò P, Maizar AA, Jovine L, Giacomini E, Rubino P. Revisiting embryo assisted hatching approaches: a systematic review of the current protocols. J Assist Reprod Genet. 2018;35(3):367–391. doi: 10.1007/s10815-018-1118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen J, Malter H, Fehilly C, Wright G, Elsner C, Kort H, Massey J. Implantation of embryos after partial opening of oocyte zona pellucida to facilitate sperm penetration. Lancet. 1988;2(8603):162. doi: 10.1016/s0140-6736(88)90710-6. [DOI] [PubMed] [Google Scholar]
- 39.Ciray HN, Bener F, Karagenc L, Ulug U, Bahçeci M. Impact of assisted hatching on ART outcome in women with endometriosis. Hum Reprod. 2005;20(9):2546–2549. doi: 10.1093/humrep/dei064. [DOI] [PubMed] [Google Scholar]
- 40.Martins WP, Rocha IA, Ferriani RA, Nastri CO. Assisted hatching of human embryos: a systematic review and meta-analysis of randomized controlled trials. Hum Reprod Update. 2011;17(4):438–453. doi: 10.1093/humupd/dmr012. [DOI] [PubMed] [Google Scholar]
- 41.Kanyo K, Zeke J, Kriston R, Szücs Z, Cseh S, Somoskoi B, Konc J. The impact of laser-assisted hatching on the outcome of frozen human embryo transfer cycles. Zygote. 2016;24(5):742–747. doi: 10.1017/S0967199416000058. [DOI] [PubMed] [Google Scholar]
- 42.Miyata H, Matsubayashi H, Fukutomi N, Matsuba J, Koizumi A, Tomiyama T. Relevance of the site of assisted hatching in thawed human blastocysts: a preliminary report. Fertil Steril. 2010;94(6):2444–2447. doi: 10.1016/j.fertnstert.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 43.Ren X, Liu Q, Chen W, Zhu G, Zhang H. Effect of the site of assisted hatching on vitrified-warmed blastocyst transfer cycles: a prospective randomized study. J Assist Reprod Genet. 2013;30(5):691–697. doi: 10.1007/s10815-013-9984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryu MJ, Kim HS, Lee JH, Kim MH, Jeong HJ, Chung MK. Assisted hatching close to inner cell mass (ICM) increases clinical outcomes of frozen-thawed blastocyst transfer cycles in unexplained or refractory repeated implantation failure patients. ASRM Abstracts. 2014;102(3):565. doi: 10.1016/j.fertnstert.2014.07.1097. [DOI] [Google Scholar]
- 45.Niimura S, Fujii M. A morphological study of blastocyst hatching in the mouse and rat. J Reprod Develop. 1997;43(4):295–302. doi: 10.1262/jrd.43.295. [DOI] [Google Scholar]
- 46.Liu S, Sun J, Hao X, Han Z, Wen X, Wang XY, Zhou CJ, Lianget CG. Blastocyst hatching site is regularly distributed and does not influence foetal development in mice. Sci Rep. 2020;10:2475. doi: 10.1038/s41598-020-59424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Onodera Y, Takahashi K, Goto M, Anzai M, Ono N, Shirasawa H, Sato W, Miura H, Sato N, Sato A, Kumazawa Y, Terada Y. The location of a 8-shaped hatching influences inner cell mass formation in mouse blastocysts. PLoS One. 2017;12(4):e0175150. doi: 10.1371/journal.pone.0175150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Veeck LL, Zaninovic N. Blastocyst hatching. In: Veeck LL, Zaninovic N, editors. An atlas of human blastocysts. New York: Parthenon Publishing Group; 2003. pp. 159–171. [Google Scholar]
- 49.Sathananthan H, Menezes J, Gunasheela S. Mechanisms of human blastocyst hatching in vitro. Reprod BioMed Online. 2003;7:228–234. doi: 10.1016/s1472-6483(10)61757-9. [DOI] [PubMed] [Google Scholar]
- 50.Menezes J, Gunasheela S, Sathananthan H. Video observations on human blastocyst hatching. Reprod BioMed Online. 2003;7:217–218. doi: 10.1016/s1472-6483(10)61755-5. [DOI] [PubMed] [Google Scholar]
- 51.Negrón-Pérez VM, Hansen PJ. The bovine embryo hatches from the zona pellucida through either the embryonic or abembryonic pole. J Assist Reprod Genet. 2017;34:725–731. doi: 10.1007/s10815-017-0933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanmee U, Piromlertamorn W, Vutyavanich T. The effect of the site of laser zona opening on the complete hatching of mouse blastocysts and their cell numbers. Clin Exp Reprod Med. 2016;43(3):152–156. doi: 10.5653/cerm.2016.43.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmoll F, Schneider H, Montag M, Wimmers K, Rink K, Schellander K. Effects of different laser-drilled openings in the zona pellucida on hatching of in vitro–produced cattle blastocysts. Fertil Steril. 2010;80(Suppl. 2):714–719. doi: 10.1016/S0015-0282(03)00989-0. [DOI] [PubMed] [Google Scholar]
- 54.Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, Nagy ZP, Ubaldi FM. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. 2014;29(6):1173–1181. doi: 10.1093/humrep/deu033. [DOI] [PubMed] [Google Scholar]
- 55.Wan CY, Song C, Diao LH, Li GG, Bao ZJ, Hu XD, Zhang HZ, Zeng Y. Laser-assisted hatching improves clinical outcomes of vitrified-warmed blastocysts developed from low-grade cleavage-stage embryos: a prospective randomized study. Reprod BioMed Online. 2014;28(5):582–589. doi: 10.1016/j.rbmo.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 56.Zakharchenko EO, Zalessky AD, Osychenko AA, Krivokharchenko AS, Shakhbazyan AK, Ryabova AV, Nadtochenko VA. Effect of laser optoperforation of the zona pellucida on mouse embryo development in vitro. Biochemistry (Mosc) 2015;80(6):769–775. doi: 10.1134/S0006297915060127. [DOI] [PubMed] [Google Scholar]
- 57.Cimadomo D, Capalbo A, Ubaldi FM, Scarica C, Palagiano A, Canipari R, Rienzi L. The impact of biopsy on human embryo developmental potential during preimplantation genetic diagnosis. Biomed Res Int. 2016;2016:7193075–7193010. doi: 10.1155/2016/7193075. [DOI] [PMC free article] [PubMed] [Google Scholar]