Abstract
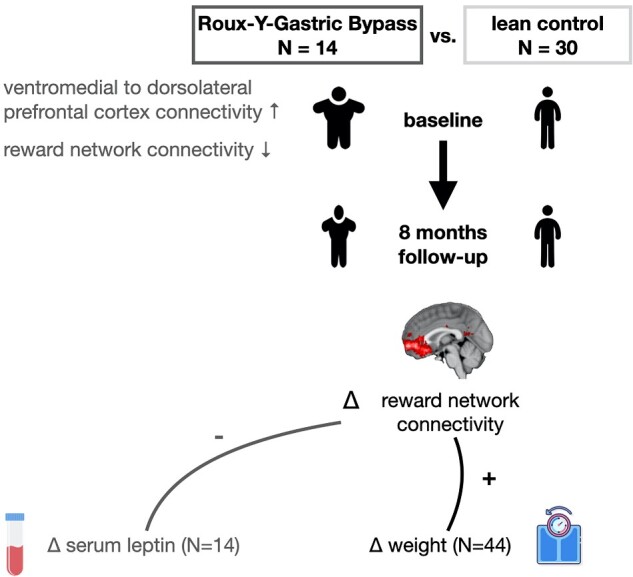
Weight gain is often associated with the pleasure of eating food rich in calories. This idea is based on the findings that people with obesity showed increased neural activity in the reward and motivation systems of the brain in response to food cues. Such correlations, however, overlook the possibility that obesity may be associated with a metabolic state that impacts the functioning of reward and motivation systems, which in turn could be linked to reactivity to food and eating behaviour and weight gain. In a study involving 44 female participants [14 patients with obesity, aged 20–63 years (mean: 42, SEM: 3.2 years), and 30 matched lean controls, aged 22–60 years (mean: 37, SEM: 1.8 years)], we investigated how ventromedial prefrontal cortex seed-to-voxel resting-state connectivity distinguished between lean and obese participants at baseline. We used the results of this first step of our analyses to examine whether changes in ventromedial prefrontal cortex resting-state connectivity over 8 months could formally predict weight gain or loss. It is important to note that participants with obesity underwent bariatric surgery at the beginning of our investigation period. We found that ventromedial prefrontal cortex–ventral striatum resting-state connectivity and ventromedial–dorsolateral prefrontal cortex resting-state connectivity were sensitive to obesity at baseline. However, only the ventromedial prefrontal cortex–ventral striatum resting-state connectivity predicted weight changes over time using cross-validation, out-of-sample prediction analysis. Such an out-of-sample prediction analysis uses the data of all participants of a training set to predict the actually observed data in one independent participant in the hold-out validation sample and is then repeated for all participants. In seeking to explain the reason why ventromedial pre-frontal cortex–ventral striatum resting-state connectivity as the central hub of the brain’s reward and motivational system may predict weight change over time, we linked weight loss surgery-induced changes in ventromedial prefrontal cortex–ventral striatum resting-state connectivity to surgery-induced changes in homeostatic hormone regulation. More specifically, we focussed on changes in fasting state systemic leptin, a homeostatic hormone signalling satiety, and inhibiting reward-related dopamine signalling. We found that the surgery-induced increase in ventromedial prefrontal cortex–ventral striatum resting-state connectivity was correlated with a decrease in fasting-state systemic leptin. These findings establish the first link between individual differences in brain connectivity in reward circuits in a more tonic state at rest, weight change over time and homeostatic hormone regulation.
Keywords: brain valuation system, leptin, obesity, resting-state connectivity, weight change
Schmidt et al. report that connectivity at rest within the brain’s reward system predicted changes in weight, differentiated between lean and participants with obesity, increased after gastric bypass surgery and correlated to circulating levels of the satiety hormone leptin. These findings show neural and hormonal determinants of weight change.
Graphical Abstract
Graphical Abstract.
Introduction
Despite the high prevalence of obesity worldwide, its neurobiological underpinnings and whether they can be used to formally predict weight change remain poorly understood. Research in cognitive neuroscience tends to focus on differences in task-based activity between obese and lean participants using functional magnetic resonance imaging (fMRI), finding that exposure to high-calorie foods alters activity in brain regions involved in reward and motivation processing (Rothemund et al., 2007; Stice et al., 2008; Volkow et al., 2008; Stoeckel et al., 2009; Scholtz et al., 2014), taste processing (Dagher, 2012; Scharmuller et al., 2012) and cognitive control (Brooks et al., 2013; Pursey et al., 2014).
Another stream of research has investigated tonic differences in the brain activity of participants with obesity and lean participants by capturing resting-state connectivity (RSC) among large-scale brain networks. Resting-state fMRI measures brain activity at rest, when participants are instructed to do nothing in the MRI scanner. This measure was first discovered in the late 90s when Biswal et al. (1997) observed that activity between brain regions of the sensorimotor system was fluctuating synchronously, suggesting an intrinsic functional organization that was active in the absence of any explicit task. This finding has since been replicated for many other brain regions, providing meaningful insights into the intrinsic anatomical and functional organization of the brain and how disease can alter this organization. These studies investigating differences in RSC between lean participants and those with obesity found differences in the salience, reward, default mode, prefrontal and temporal lobe networks (Kullmann et al., 2012; Coveleskie et al., 2015; García-García et al., 2015; Wijngaarden et al., 2015; Doornweerd et al., 2017). RSC in these brain systems and others was shown to be altered by bariatric surgery-based weight-loss interventions (Frank et al., 2014; Olivo et al., 2017; Wiemerslage et al., 2017; Li et al., 2018). It is important to note that all of these studies have not applied a formal, out-of-sample predictive analysis, but are using simple correlation, regression or analysis of variance within sample analysis.
Taken together, the above-cited studies show the importance of the pre-frontal brain circuits involved in decision making and reward processing, yet their role in predicting out-of-sample weight status [body mass index (BMI), weight, etc.] has never been tested. Work most closely related to ours, looking at formal predictions of BMI using resting-state fMRI [in combination with Diffusion-Tensor-Imaging (DTI), Park et al., 2015] and taking a whole-brain approach, found most prominently the ventromedial prefrontal cortex (vmPFC) structure-to-function connectivity together with other regions to predict BMI. However, the specific role of vmPFC RSC in predicting weight loss has never been investigated.
Investigating the specific role of vmPFC RSC is an important contribution to the literature because prior studies using fMRI have suggested that the vmPFC is a key region of the brain’s reward-related valuation system that encodes both expected and experienced value (Bartra et al., 2013). More specifically related to food decision making, the vmPFC is involved in dietary decision making (Plassmann et al., 2007, 2010) and self-control (Hare et al., 2009, 2011; Hutcherson et al., 2012). Individual differences in vmPFC anatomy are a marker of dietary regulatory success during dietary self-control (Schmidt et al., 2018). However, no research, to date, has tested whether RSC with the vmPFC as seed is sensitive to obesity and can predict weight change, which was the first goal of this study.
Roux-en-Y gastric bypass (RYGB) offers a unique and effective theoretical model for the question of this study because it reveals whether the relationship between the RSC with vmPFC as seed is merely correlated with obesity or whether it also changes as a function of it. In other words, it allows us to investigate whether people who gained weight and became obese exhibit higher RSC in the vmPFC than people with normal corpulence, or whether weight change induced through RYGB surgery might alter such RSC. The latter finding would suggest that obesity is not merely the result of a stable pre-disposition to being more responsive to rewards at rest captured by RSC in reward-processing regions such as the vmPFC (as often assumed), but may also be explained by an alternative hypothesis, i.e., that obesity—and the metabolic profile linked to it—is related to reward sensitivity captured by vmPFC RSC, which could contribute to explaining the reason why vmPFC RSC might predict weight loss in our study.
Thus, the second goal of our article was to shed first light on a potential role of the homeostatic hormone leptin to drive the changes in vmPFC RSC. To explore one potential mechanism, we focussed our investigations on leptin because it directly regulates hunger and food intake via both homeostatic and reward-related pathways: a high leptin level in the blood signals to the hypothalamus to stop food intake and the accumulation of energy through the white adipose tissue cells that secrete leptin into the blood. Leptin is therefore necessary to maintain energy accumulation at equilibrium (Montague et al., 1997; Dhillon et al., 2006). In addition, leptin has been shown to inhibit dopamine receptors in the nucleus accumbens, suggesting it plays an inhibitory so-called hedonic aspects of hunger and food intake (for a review, see Palmiter, 2007). In addition to leptin’s role in hormonal food intake regulation via the inhibition of reward signalling, previous research has also shown that before RYGB surgery most individuals with obesity have very high levels of leptin when in a fasted state, yet its ability to signal satiety is impaired (Myers et al., 2010). After RYGB surgery, the level of leptin drops rapidly and its ability to signal satiety improves (Faraj et al., 2003). Thus, a second contribution of this article is to explore whether the extent to which RYGB surgery reduces vmPFC RSC is correlated with individual differences in surgery-induced changes in fasting-state serum leptin.
Materials and methods
Ethical considerations and open science statement
This project is formed from amendments to two pre-existing clinical trials, Microbaria (NCT01454232) and Leaky Gut (NCT02292121), both of which received ethical approval from Assistance Publique–Hôpitaux de Paris (AP-HP). We amended the AP-HP ethical protocols to include a separate brain imaging session with the patients before and on average 8 months after the RYGB surgery, where leptin was again sampled, given our hypotheses on the potential involvement of leptin. Bariatric surgery approval was acquired during multidisciplinary meeting and after an optimal surgery preparation as required by French and international recommendations. For the brain scanning of the lean control participants, ethical approval was given by the Institut National de la Santé et de la Recherche Médicale (INSERM). The study was conducted in accordance with the Declaration of Helsinki. Participants gave informed consent. Code and anonymized data sets analysed in the study are available from the corresponding authors on request, in accordance with European GDPR regulations.
Experimental set-up
The resting-state data presented in this article were acquired as part of a multi-study project including different experimental tasks such as task-based fMRI and analysis of blood and faecal samples for metabolic and gut microbiome profiling. The results of those measures are presented elsewhere (e.g. Aron-Wisnewsky et al., 2019); the focus of this article is whether changes in RSC before and after bariatric surgery can predict changes in weight over time. The scanning session consisted of a brief introduction and training, two task-based fMRI sessions, a structural fMRI scan, and the resting-state fMRI scan presented in this article.
Data were collected at two time points (T0 and T8) separated by at least 6 months. The participants with severe obesity underwent RYGB surgery on average 17 weeks (±4 weeks) after the T0 fMRI scans. The post-surgery assessments were conducted on average 8 months (±0.6 months) after RYGB surgery. For the lean participants, assessments were separated on average by 8 months (±0.3 months).
Patients with obesity were followed in the department of nutrition at the Specialized Obesity Centre for Obesity and Obesity Surgery at Pitié-Salpêtrière Hospital in Paris. fMRI data were collected at the Centre for Neuroimaging (CENIR) at the Paris Brain Institute (ICM) at Pitié-Salpêtrière Hospital in Paris. The lean participants’ brains were also scanned twice at the same facilities of the CENIR to capture changes over time.
Participants
A total of 64 female participants were enrolled at T0, including 45 lean participants and 19 with severe obesity. We recruited only female participants in an attempt to keep gender influences constant (Rolls et al., 1991). Additional standard fMRI inclusion criteria were right-handedness, normal to corrected-to-normal vision, no history of substance abuse or any neurological or psychiatric disorder and no medication or metallic devices that could interfere with performance of fMRI. The lean controls and participants with obesity were recruited based on their BMI, which was on average 22 ± 0.3 kg/m2 for the lean participants and 45 ± 1 kg/m2 for the candidates for bariatric surgery, in accordance with international guidelines (for more details on clinical characteristics and body composition, see Table 1). Lean participants were recruited by public advertisement in the Paris area. Participants with obesity were recruited by two co-authors of the study who were care providers of the patients following them through their pre- and post-surgery treatment course.
Table 1.
Participant characteristics
| Group | Age (s.e.m.) (years) | Education (s.e.m.) (years) | Weight (s.e.m.) (kg) | BMI (s.e.m.) (kg/m) | Body fat (s.e.m.) (%) | Body fat (s.e.m.) (kg) | Leptin (s.e.m.) (ng/ml) | Leptin/body fat (s.e.m.) (ng/ml/kg) | glycaemia (s.e.m.) (mmol/l) | Insulin (s.e.m.) (mUl/l) |
|---|---|---|---|---|---|---|---|---|---|---|
| Lean N = 40 | 37 (2), n.s. | 6.5 (0.2) | 62 (1) | 22 (0.3) | 27 (1) | 17 (1) | 9 (1) | 0.5 (1) | 4 (0.1) | 4 (1) |
|
obese T0 N = 16 |
42 (3), n.s. | 5 (0.4) | 119 (3) | 45 (1) | 51 (1) | 62 (2.5) | 70 (7) | 1 (0.1) | 6 (0.4) | 28 (5) |
|
obese T8 N = 14 |
85 (4) | 34 (1) | 45 (1) | 42 (2) | 25 (3) | 1 (1) | 5 (2) | 10 (1) |
Mean participant socio-demographic, bodily and systemic characteristics with standard error of the mean in brackets: Patients differed significantly in all measures, except age, before (T0) and after (T8) surgery and compared to lean participants, respectively (P < 0.05, two-sampled, two-tailed t-test). Education is described as years of education after high school. Glycaemia was measured with chemiluminescent technology (Cobas®, Roche, Switzerland). Serum insulin was measured with immunoassay technology (LiaisonXL®, Diasorin, France). Serum leptin was determined using radioimmunoassay kits (Linco Research, St. Louis, MO, USA).
Of the 64 individuals recruited for the study, 20 participants were excluded before starting our analyses due to the following pre-defined exclusion criteria: two lean and three participants with obesity were excluded because of extensive head motion (≥3.5 mm), 10 lean participants were excluded because they did not return for their 8-month follow-up fMRI evaluation and three lean and two participants with obesity had incomplete rsfMRI data. Hence, a total of 44 (30 lean and 14 obese) participants were included in all analyses concerning within-participant time effects (i.e. T0 versus T8) and group by time interactions. [Note: We were able to perform analyses concerning between-participant group effects (i.e. obese versus lean) at baseline (T0) for 56 participants (40 lean, 16 obese) who had available data at T0.]
Participants were in a fasted state during the blood-sampling session to extract baseline fasting-state parameters, but not in a fasted state during the brain scanning session. Perceived hunger, measured before the brain scanning session, did not vary between groups (βgroup = 0.48, se = 0.95, P = 0.61) or time points (βtime = 0.5, se = 0.8, P = 0.5), nor was there a significant interaction group by time point (βgroup × time = −0.6, se = 0.6, P = 0.3).
Roux-en-Y gastric bypass surgery
RYGB surgery is reserved for the most severe forms of obesity (BMI, ≥40 kg/m2 or BMI ≥35 kg/m2 with obesity-related comorbidities) (Fried et al., 2014). The surgeon creates a small gastric pouch directly linked to the distal small intestine with a gastro-jejunal anastomosis. The remaining part of the stomach and the proximal small intestine are bypassed, creating a Y-Roux limb (for details, see Aron-Wisnewsky et al., 2012). Ingested food thus goes directly from the newly created gastric pouch to the rest of the small intestine, which reduces the nutrients and calories absorbed from food.
In our study, the RYGB surgery was performed laparoscopically. All participants were clinically assessed before and at 1, 3 and on average 8 months post-surgery, as recommended by international guidelines (Fried et al., 2014). The clinical assessments included obesity-related diseases and anthropometric measures estimated by whole-body fan beam dual-energy X-ray absorptiometry (Hologic Discovery W, software v12.6, 2; Hologic, Bedford, MA, USA), as detailed in Ciangura et al. (2010). Variables included weight, BMI and total body fat in kilogram and percentage (Table 1). Patients were also clinically assessed based on the following criteria: depression, using the Beck Depression Inventory; alcohol abuse, using the Alcohol Use Disorders Identification Test; nicotine consumption, using the Fagerstrom test; dietary restraint, using the Three-Factor Eating Questionnaire and diabetes (clinical assessment). Glycaemia was assessed by measuring blood glucose levels before (fasting condition) and after a standardized meal test. (Test results are shown in Supplementary Table 1.) These clinical assessments were not done for the lean participants.
Blood hormone sampling
Blood samples were collected once from the lean participants (at T0), and twice for participants with obesity, before (T0) and 8 months after RYGB (T8). Venous blood samples were collected in the fasting state (12-h fasting) for the determination of glycaemia, insulinemia and leptin. Glycaemia was measured with chemiluminescent technology (Cobas®, Roche, Switzerland). Serum insulin was measured with immunoassay technology (LiaisonXL®, Diasorin, France). Serum leptin was determined using radioimmunoassay kits (Linco Research, St. Louis, MO, USA). The Leaky Gut and Microbaria clinical trials also included the measurement of GLP1; unfortunately, for the patients included in this study, these measurements were not reliably done due to technical issues.
Brain imaging data
Image acquisition
Resting-state fMRI scanning was conducted during a 10-min scanning sequence after participants took part in several task-based fMRI sessions. Resting-state activation was assessed after task-evoked brain activation for all participants and at all time points, which was crucial for group and time point comparisons. Implementing this design allowed us to keep influences of task-evoked brain activation on resting-state brain activity constant across these comparisons. Moreover, for data-quality reasons, total scanning time (including task-based and resting-state fMRI) was limited to an hour to reduce the discomfort of obese participants that has been linked to the fMRI environment, which is an important factor to consider when combining behavioural testing with fMRI.
Participants were instructed to keep their eyes closed and relax, but not to fall asleep.
T2*-weighted echo planar images with blood–oxygen-level-dependent contrast were acquired using a 3T Siemens Verio scanner. An eight-channel phased array coil was used to assess whole-brain resting-state activity with the following ascending interleaved sequence: Each volume comprised 40 axial slices, TR = 2 s, TE = 24 ms, 3-mm slice thickness; 0.3-mm inter-slice gap corresponding to 10% of the voxel size; field of view = 204 mm; flip angle = 78°. For each participant, a total of 304 volumes were obtained. The first five volumes of the resting-state scan session were discarded to allow for T1 equilibrium effects. A single high-resolution T1-weighted structural image (magnetization-prepared rapid gradient-echo) was acquired, co-registered with the mean echo planar image, segmented and normalized to a standard T1 template. Normalized T1 structural scans were averaged across lean and obese participants, respectively, to allow group-level anatomical localization.
Preprocessing
Data were analysed using Statistical Parametric Mapping software (SPM12; Wellcome Department of Imaging Neuroscience) along with the Functional Connectivity toolbox (CONN toolbox: www.nitrc.org/projects/conn, RRID: SCR_009550). Pre-processing in SPM included spatial realignment to estimate head motion parameters. This preprocessing step was done prior to slice-time correction, because slice-time correction can lead to systematic under-estimates of motion when it is performed as a first pre-processing step (Drysdale et al., 2017). After re-alignment, pre-processing included the standard steps: slice-time correction, co-registration, normalization using the same transformation as structural images, spatial smoothing using a Gaussian kernel with full width at half maximum of 8 mm and temporal band pass filtering between 0.01 and 0.1 Hz.
Nuisance signal removal
Nuisance signal removal was performed on the pre-processed time-series data with the CONNv16 toolbox; it included linear and quadratic de-trending to adjust for scanner drift, to remove nuisance signals related to head motion and to physiological variables by means of regression analyses. More specifically, the nuisance regression included 18 head motion parameters calculated during spatial re-alignment (roll, pitch, yaw and translation in three dimensions, plus their first and second derivatives), non-neuronal signals from eroded white matter and cerebrospinal fluid masks, and regressors for outlier volumes. Individual white-matter and cerebrospinal fluid masks were obtained by segmentation of each participant’s structural magnetization-prepared rapid gradient-echo image into tissue probability maps using SPM12. The white-matter and cerebrospinal fluid masks were further eroded to reduce partial volume effects. We used CONNv16’s ART-based function to identify outlier volumes with a global signal Z-value threshold of 3 and an inframe displacement threshold of ≥0.5 mm, corresponding to the most conservative setting in the CONNv16 toolbox (95th percentile in normative sample). This more conservative setting is important in light of recent first findings that head movement might be linked to weight loss (Beyer et al., 2020). It should be noted that the nuisance signal regression and band-pass filtering were performed simultaneously, only on volumes that survived head motion censoring. We used a rather lenient head motion threshold of ≥3.5 mm in order to not exclude too many of our participants with obesity, who moved significantly more than lean participants. After pre-processing, the smoothed residual time-series data, co-registered to Montreal-Neurological Institute (MNI) space, were used for the subsequent statistical analysis steps.
Statistical analyses
We first focussed on a theory-driven, seed-to-voxel connectivity analysis with the vmPFC as a seed, and compared how vmPFC-to-voxel RSC differed between lean and obese participants at baseline. We then extracted the RSC patterns from regions that displayed a difference between groups at baseline to predict weight change over time in the main analyses.
Seed region of interest: vmPFC
We leveraged Neurosynth’s meta-analysis tool (Yarkoni et al., 2011) and created an ROI map for the term ‘vmPFC’. Specifically, the seed ROI was defined by the neurosynth website using the ‘reverse inference’ map for ‘vmPFC’. The mask was thresholded at P < 0.0001 uncorrected, after smoothing the Z-map with a 6-mm FWHM kernel and averaging Z-scores across the left and right hemispheres to create a symmetrical map. We further re-sliced each mask to the lean controls’ and obese patients’ normalized mean echo planar images to make sure that all voxels were within the vmPFC in our participant sample.
Determining RSC networks of interest
In order to choose the regions exhibiting RSC that would predict out-of-sample weight loss, in the first step of our analyses we looked for regions that showed a significant difference in RSC between lean and obese participants at T0 by means of a multiple regression analysis. The latter correlated the averaged blood–oxygen-level-dependent signal from the vmPFC seed ROI to the blood–oxygen-level-dependent signal in each voxel of the brain for each participant. The Pearson’s r for each voxel was then transformed into a Z-score using Fisher r-to-z transformations to obtain normally distributed functional connectivity coefficient maps. Individual functional connectivity coefficient maps were subjected to second-level random-effects factorial analysis of variance (2 × 2 ANOVA) crossing participant group (obese versus lean participants) and time point (T0 versus T8). We considered a false-discovery rate (FDR)-corrected significance threshold of PFDR < 0.05 at the cluster level and further explored results at an uncorrected voxel-wise threshold of P < 0.001 to report the full extent of the effects (Poldrack et al., 2008). The full results of the ANOVA are summarized in Table 2 for the sake of completeness. Planned contrasts were examined between groups at baseline (T0) using cluster-corrected PFDR < 0.05 significance thresholds.
Table 2.
Main effect of group on vmPFC resting-state connectivity
| Region | BA | Size | x | y | z | Peak Z-score |
|---|---|---|---|---|---|---|
| Obese > lean participants at T0 | ||||||
| Cerebellum | 729 | −20 | −80 | −44 | 5.30 | |
| 848 | 14 | −78 | −44 | 4.95 | ||
| dlPFC | 47 | 166 | −34 | 10 | 46 | 4.26 |
| Obese < lean participants at T0 | ||||||
| Ventral striatum | 172 | −10 | 10 | −4 | 4.51 | |
| Hippocampus | 283 | 22 | −48 | 8 | 4.44 | |
| Obese > lean participants at T8 | ||||||
| IFG | 45/46/47 | 243 | −54 | 38 | 12 | 4.74 |
| vlPFC | 47/11 | 191 | 44 | 42 | −12 | 4.15 |
| 10/11 | 228 | 30 | 60 | 0 | 4.08 | |
The peak co-ordinates and Z-score values are listed for lean participants compared to obese patients before at T0 and on average 8 months after bariatric surgery at T8. All peaks surpassed a voxel-wise threshold of PFDR < 0.05 FDR corrected on the cluster level. The xyz coordinates correspond to the Montreal Neurological Institute (MNI) space.
Abbreviations: dlPFC, dorsolateral prefrontal cortex; IFG, inferior frontal gyrus; vlPFC, ventrolateral prefrontal cortex.
Against this background, we used RSC between the vmPFC-vStr and vmPFC–dorsolateral pre-frontal cortex (dlPFC) for our main analysis since these were the only ROIs that satisfied our criterion determining sensitivity to obesity.
Main analysis: out-of-sample prediction of weight change over time by vmPFC-to-voxel RSC
To test whether weight change could be predicted from vmPFC-to-voxel RSC, we conducted the following leave-one-participant-out predictive analysis. First, Z-values of vmPFC-to-voxel RSC were extracted for each participant and averaged across the voxels from the two ROIs that displayed a significant difference between obese and lean participants at baseline: the vStr (MNI = [−10, 10, −4]) and the dlPFC (MNI = [−34, 10, 46]). The average Z-values for each ROI were then, respectively, used to conduct 44 linear regressions that determined independent weights of the vmPFC-to-voxel connectivity on weight loss (kilogram at T8 minus kilogram at T0) for more than 43 participants (leaving out the 44th participant) following equation (1):
| (1) |
Each time, the weight () of the vmPFC-ROI connectivity on weight loss obtained from 43 participants together with the Z-value for vmPFC-ROI connectivity extracted from the ROI in the left-out participant was regressed to predict weight loss for the left-out participant ) using the glmval function in matlab as in equation (2):
| (2) |
Last, we quantified the association between the predicted and the observed levels of weight loss by using Pearson’s correlation, which was tested for significance by using both parametric one-sampled t-tests and non-parametric permutation tests (10 000 permutations).
Hormone correlation analysis
We conducted correlation analyses to explore whether the changes due to the weight loss intervention in vmPFC-vStr RSC co-varied with the changes in leptin per kilogram body fat lost after surgery as a hormonal marker of homeostatic control of food intake. To this aim, Pearson’s correlation coefficient ρ was calculated as in equation (3):
| (3) |
with cov corresponding to the covariance of x and y and σ corresponding to the standard deviation of x and y. Specifically, x corresponded to the change in raw serum leptin per kilogram body fat lost after surgery according to equation (4):
| (4) |
Because leptin is produced by white adipose tissue cells, the change in ng/ml leptin after surgery co-varies significantly with kilogram body fat lost after surgery (Pearson’s ρ = 0.68, P = 0.007). We therefore considered the ratio as a measure of interest in order to account for the dependency between body fat and leptin. The ratio of the changes in leptin per kilogram body fat lost from T0 to T8 reflects the change of serum leptin levels per kilogram body fat lost after bariatric surgery. This ratio x was correlated to the change in vmPFC-vStr connectivity after surgery y. Y was computed by following equation (5):
| (5) |
Mean connectivity values (zvmPFC-vStr) were extracted for each obese participant at T0 and T8 from the vStr cluster that displayed a significant connectivity to the vmPFC seed ROI for the group (obese > lean) by time point (T8 > T0) interaction (MNI coordinates = [−10 6 −2], P < 0.001 uncorrected, extend threshold 50 voxels). We used the interaction ROI in the vStr to rule out non-specific changes in vmPFC-vStr RSC over time that are not related to bariatric surgery.
The significance of Pearson’s correlation coefficients was tested by conducting both parametric one-sampled t-tests and non-parametric permutation tests, which are less sensitive to individual outliers, and estimated the 95% confidence intervals (CI) for correlations due to chance based on 10 000 permutations of the observed data. In more detail, the permutation shuffled 10 000 times the observed true values in order to calculate 10 000 Pearson’s correlation coefficients. The distribution of these correlation coefficients and hence the 95% CI revealed how much otherwise uncorrelated random variables will be correlated negatively and positively. Any observed, true positive or negative correlation should, if significant, lay outside the 95% CI for permuted chance correlations within a sample of n participants.
Data availability
Data and software/code for analysis are available upon reasonable request from the corresponding author.
Results
Differences in RSC of the vmPFC in participants with obesity compared to lean participants at baseline
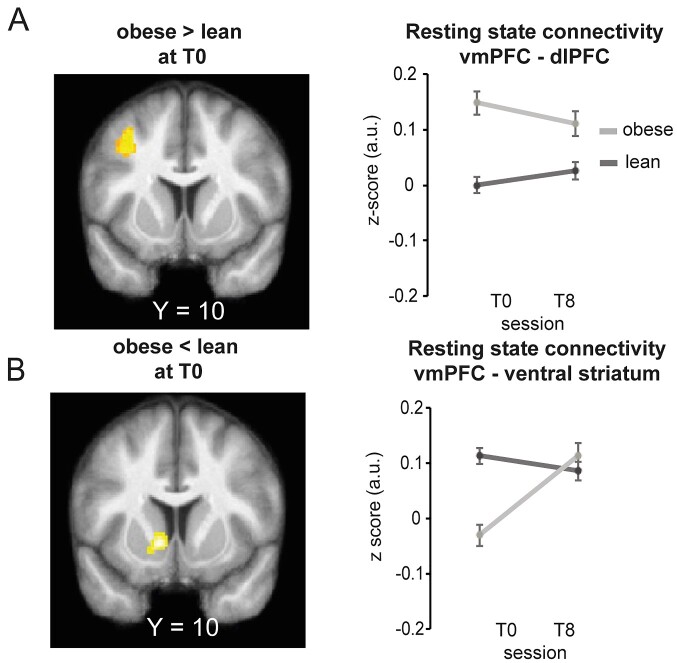
We first investigated differences in RSC in the brain’s valuation system with the vmPFC as seed between the obese and the lean participants at T0. Post hoc comparisons between groups revealed that at baseline (T0), severely obese patients compared to lean participants displayed stronger vmPFC connectivity to cognitive regulation nodes such as the dlPFC (cluster-corrected PFDR < 0.05; Fig. 1a and Table 2). We also found weaker vmPFC connectivity to motivational nodes such as the vSTR (cluster-corrected PFDR < 0.05; Fig. 1b and Table 2) at baseline (T0). Thus, we used these two regions that were sensitive to obesity at baseline as regions of interest for our out-of-sample prediction.
Figure 1.
Comparisons of vmPFC-to-brain RSC in lean participants and those with obesity before and after bariatric surgery. SPMs of the seed-to-voxel RSC between the vmPFC seed ROI and the rest of the brain, at baseline (T0) (n = 56) in A obese > lean participants and B obese < lean participants. Significant voxels are displayed for visualization purposes in orange at P < 0.001 uncorrected, with an extent threshold k = 166 and k = 172 voxels, corresponding to a FDR corrected threshold of PFDR < 0.05 at the cluster level for each contrast, respectively. SPMs are superimposed on the average structural image obtained from the lean participants. The [x, y, z] co-ordinates correspond to MNI coordinates and are taken at maxima of interest. The line graphs on the right of each SPM show average correlation coefficients between RSC of the seed region, the vmPFC and the A dlPFC and B vStr at baseline (T0) in lean (dark grey) and obese (light grey) participants.
Out-of-sample prediction of weight change over time across all participants
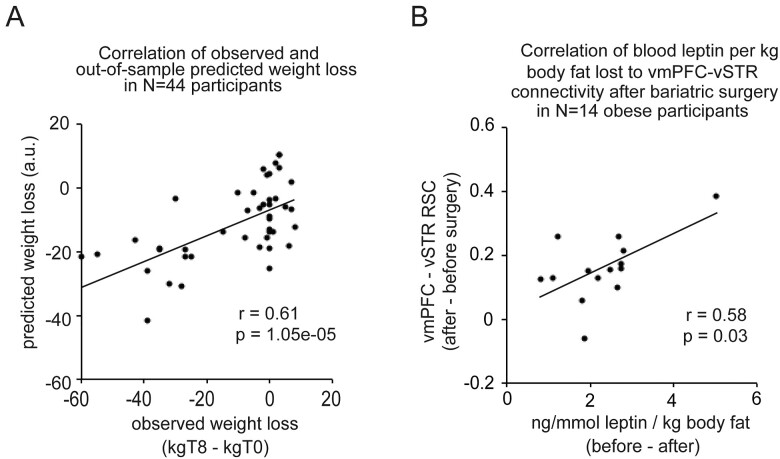
We investigated whether changes in vmPFC-to-voxel RSC could predict changes in participants’ weight between two time points using a leave-one-sample-out predictive analysis. When we based the prediction of weight change on information about the vmPFC-vStr RSC, there was a significant positive association between the predicted and the observed weight change (r = 0.61, P = 1.05e − 05, 95% CI due to chance: −0.24 to 0.25; Fig. 2a).
Figure 2.
Out-of-sample prediction of weight change, and correlations to leptin levels of vmPFC-vSTR RSC. (A) Scatterplots depict in all participants (N = 44) the correlation between observed weight loss (kilogram body weight at T8 minus kilogram body weight at T0) and predicted weight loss obtained from an out-of-sample cross-validation of the association between weight loss and vmPFC-vStr RSC. Dots correspond to obese participants. (B) Scatterplots show, in obese participants (n = 14), the change observed after, compared to before, bariatric surgery in vmPFC-vStr RSC (average correlation coefficients) as a function of ng/mmol leptin per kilogram body fat lost. Dots correspond to obese participants.
However, when using information about vmPFC-dlPFC RSC out-of-sample prediction of weight change was non-significant (Pearson’s r = 0.15, P = 0.3, 95% CI due to chance: −0.25 to 0.25). These findings indicate a specific role of vmPFC to vStr RSC for weight change.
Individual differences in relative fasting-state leptin correlate with changes in vmPFC-vStr RSC
We explored how much the change in vmPFC-vStr RSC after RYGB surgery was correlated with changes in serum leptin, taking into account the reduction of body fat. Secreted by adipose tissue, the hormone leptin is known to contribute to signalling satiety and to stop food intake via inhibition dopamine receptors in the ventral tegmental area and melanocortin (i.e. MC4) receptors in the hypothalamus. As expected, leptin and body fat were elevated in participants with obesity before surgery, and decreased significantly post-surgery [% body fat: t(13) = 9.9, P < 0.001; kilogram body fat: t(13) = 13.7, P < 0.001; ng/ml leptin: t(13) = 5.6, P < 0.001; two-tailed, paired t-test; Table 1]. Examining the link between the decrease in leptin per kilogram of body fat loss after RYGB surgery to the increase in vmPFC-vStr RSC after surgery, we found a significant positive correlation (Pearson’s ρ = 0.58, P = 0.03, 95% CI due to chance: −0.46 to 0.46; Fig. 2b). In other words, obese participants who lost more circulating leptin per unit of fat mass post-RYGB were also those who had the most important increase in vmPFC-vStr RSC post-RYGB surgery.
Discussion
Our study provides first evidence—using an out-of-sample prediction across all participants—that changes in RSC between the vmPFC and the vStr predict how much weight participants will lose over a period of 8 months. These are two key regions within the brain’s valuation system that are involved in processing reward and motivation (Knutson et al., 2005; Rangel et al., 2008). To the best of our knowledge, our study is the first to uncover an association between weight change over time and the connectivity of neural hubs at rest within the brain’s valuation and reward system.
To further quantify this somewhat reversed inference using non-task-based resting-state fMRI data, Neurosynth.org’s association test maps were leveraged. They consist of Z-scores in voxels activated more consistently and specifically in studies that mention the terms used here (e.g. ‘reward’ and ‘emotion’) than for studies that do not (Poldrack, 2010; Yarkoni et al., 2011). To demonstrate the specific link with the vmPFC and vStr for value and reward, we searched Neurosynth.org’s database for the terms ‘reward’, ‘value’, ‘cognitive control’ and ‘emotion’. We examined the positive Z-scores for our vmPFC seed ROI’s centre MNI co-ordinates (MNI = [0, 43, −8]) and our second ROI’s peak’s coordinate in the vStr that showed sensitivity to obesity (MNI = [−10, 6, −2]). Table 3 lists the Z-scores for the two MNI co-ordinates of interest. They reflect how much the vmPFC and vStr, respectively, are involved in the reward, valuation, emotion and cognitive control processes. They lend support to the role of the vmPFC and vStr in reward and valuation processes, rather than in emotion or cognitive control processes that could be seen as related.
Table 3.
Positive Z-scores in Neurosynth’s association maps for different mental processes
| Search term | vmPFC [0, 43, −8] | Ventral striatum [−10, 6, 2] | Maximum Z-score |
|---|---|---|---|
| Reward | 6.56 | 14.82 | 28.56 |
| Value | 4.01 | 5.05 | 11.68 |
| Cognitive control | 0 | 0 | 8.94 |
| Emotion | 0 | 0 | 18.93 |
Note: Values correspond to Z-scores. The maximum Z-score corresponds to brain voxels with the maximum association to the search term, and that provide a references for the Z-scores of the [x, y, z] MNI coordinates of interest located our ROIs in the vmPFC and ventral striatum.
Our findings—that vmPFC-vStr RSC was attenuated among participants with obesity (versus lean participants)—parallel others using task-based fMRI that have shown a de-sensitization of the brain’s reward circuitry in response to food rewards among obese participants (Volkow et al., 2013; Carter et al., 2016). It has led to the stigma and interpretation that people with obesity consume more palatable foods rich in calories because they have de-sensitized brain reward circuitry. However, our results show that the alternative idea cannot be ruled out: that being obese may be linked to a metabolic profile that partly also impacts reward sensitivity. If so, then an altered reward sensitivity as captured by vmPFC-vStr RSC would not make people pre-disposed to obesity in the first place. Moreover, should the same participants be found to differ in vmPFC-vStr RSC as a function of their weight status and BMI, it would provide evidence that obesity is linked to an altered reward system at rest. This was, indeed, what we found: vmPFC-vStr RSC increased after surgery in participants with obesity and was a predictor of the magnitude of weight change in the overall sample.
An interesting next question is, what is the mechanism underlying this? Such a bi-directional link might be related to improved functioning of dopaminergic projections from the mid-brain to regions of the brain’s hedonic valuation system. This idea is supported by studies using positron emission tomography of dopaminergic functioning in obese patients, which found dopamine D2 receptor availability to increase 6 weeks post-RYGB surgery (Steele et al., 2010), reaching levels similar to those observed in non-obese controls. Although we could not directly measure dopamine in our study, we could sample fasting-state serum leptin that can be indirectly linked to dopamine, as leptin acts on hypothalamic melanocortin and basal ganglia dopamine receptors to regulate energy homeostasis—notably to reduce appetite and inhibit food intake. Fasting-state leptin levels are generally high in obese patients before surgery, suggesting they may have resistance to its anorexic action (Stice et al., 2008; Crujeiras et al., 2015), and rapidly decrease after bariatric surgery (to a higher extent than surgery-induced decreases in fat mass) (Faraj et al., 2003). Strengthening the potential link between our findings and dopamine functioning as the underlying mechanism, we found that vmPFC-vStr RSC was positively correlated with the reduction of fasting-state serum leptin (taking into account fat-mass loss) after RYGB surgery. It might suggest that the one underlying mechanism explaining the reason why changes in vmPFC-vStr RSC predict weight could be linked to hormonal homeostatic control that targets hypothalamic and dopaminergic pathways in order to influence food-related behaviour and weight loss.
However, this association does not constitute causal evidence that bariatric surgery (through body fat loss) decreases leptin levels, which then act upon dopaminergic projections from mid-brain neurons to improve the RSC in the brain’s reward-related valuation system, or that improved dopamine functioning is a result of the improved vmPFC-vStr RSC and is independent of the observed decreased leptin levels. Our study opens up opportunities for future research to investigate the causal links between vmPFC-vStr RSC changes induced by bariatric surgery, leptin and dopamine functioning (e.g. by using positron emission tomography in combination with dopamine markers).
We further found that compared with those who were lean, participants with severe obesity displayed an enhanced RSC between the vmPFC and a set of lateral prefrontal cortex regions that are associated with the cognitive regulation of affective states, working memory and the cognitive control of goal-directed action selection (Ochsner et al., 2002; Wager and Smith, 2003; Charron and Koechlin, 2010). This result is in line with the findings from fMRI studies, showing an impulse control-related activation of the lateral and dorsolateral pre-frontal cortex in patients with milder obesity (Weygandt et al., 2015). However, investigating connectivity at rest in participants with severe obesity, we did not find a prominent role of vmPFC-dlPFC RSC in weight loss as reported in prior task-based fMRI studies. Possible reasons could either be driven by methodological differences between studies or differences in the extent of obesity in participants. In our study sample, we imaged participants with severe obesity who qualified for RYGB surgery and were moderately restrained in their eating behaviour (Supplemental Table 1). Other possible reasons suggested by previous study using similarly characterized participants and methods (e.g. Olivo et al., 2017) could be linked to the timing of our study. Olivo et al.’s (2017) findings suggest that RSC changes in related control regions might occur only after a longer period of time—they found RSC changes only after 12 months (but not 1 month) post-RYGB surgery.
Our study has several limitations, one being the relatively small number of participants with obesity. Studies that rely on such patients often have a smaller sample size than studies conducted in healthy participants since the recruitment of such patients is challenging, and especially difficult in longitudinal studies such as ours (which, despite a relatively low number of patients, took 3 years to conduct). However, even with relatively small samples, studies such as ours are important to advance the understanding of the mechanisms of weight change. Another aspect to consider is the estimated effect sizes of changes in RSC before and after bariatric surgery. Prior studies, even with a small sample size (e.g. Li et al., 2018, N = 22; Cerit et al., 2019, N = 14) showed that the effect of bariatric surgery on RSC is quite drastic. Given the severity of the weight loss intervention, effect sizes of β parameter estimates in ROIs vary between −0.1 pre-surgery, and 0.18 post-surgery (Li et al., 2018; Cerit et al., 2019). Our effect sizes (β = −0.05 before surgery and β = 0.11 after surgery for vmPFC-vStr RSC, corrected for multiple comparisons) are in line with the reported effect sizes in such a patient population. One could therefore argue that this patient population may show larger effect sizes and thus may require smaller samples as compared with the studies of healthy participants.
Another limitation of our study is that we included participants with severe obesity and lean participants, populations at the two extremes of the spectrum, for our prediction analyses. The strength of our study lies in the follow-up after a successful weight loss intervention, which was the reason why we selected such extreme BMI values. To generalize our results, we call for future studies that replicate our findings using participants who are in between these extremes—that is, those who are overweight or less severely obese or severely obese waiting for surgery.
In such replications, we would also encourage researchers to make a direct, physiological assessment of whether participants are awake during resting-state scanning, for example by also taking measurements of eye movement. In our study, we asked participants to close their eyes and relied on their self-reports that they remained awake during the resting-state scan.
Another limitation concerns the relationship of our findings to other metabolic markers of obesity that (i) influence hedonic and homeostatic hunger and (ii) are changed by bariatric surgery in obese participants. Notably, insulin has been linked to weight status, resting-state activity and reward sensitivity (Heni et al., 2012; Kullmann et al., 2012; Tiedemann et al., 2017). We found that the patients with improved insulin sensitivity after surgery also displayed stronger vmPFC-vStr RSC. However, this correlation was non-significant, potentially because of the small sample size raised above. We call for more research in this field to verify these promising trends and also put them in perspective with respect to other clinical markers of obesity (e.g. changes in regional body fat across lean and obese participants that in our study we collected only for the participants with obesity) and how they relate to changes in vmPFC-vStr RSC.
Such future study would contribute to a more complete understanding of the biological and psychological underpinnings of weight loss and obesity.
Conclusion
The contribution of our study is in providing first evidence that vmPFC-vStr RSC (i) formally predicts weight loss and (2) is linked to RYGB surgery-induced changes in leptin levels (i.e. a marker of the hormonal homeostatic system of food intake control). It is also one of the first to integrate hedonic and homeostatic factors in controlling food intake (Berthoud 2006).
Supplementary Material
Supplementary material is available at Brain Communications online.
Supplementary Material
Acknowledgements
The authors thank Nicolas Manoharan for collecting the fMRI data; Valentine Lemoine (clinical research assistant, ICAN) for help in clinical investigation; Dr Florence Marchelli (NutriOmics research team) for data management; ICAN CRB members for contribution to bio-banking; Valerie Godefroy for recruiting the control participants; Anne-Dominique Lodeho for technical advice for the rsMRI sequence; Cecile Gallea and Romain Valabrèque for advice in data analysis; Michele Chabert, Armelle Leturque and Patricia Serradas for their feedback during various stages of the project; and Pierre Chandon and Etienne Koechlin for their continuous support in implementing the overall protocol.
Funding
The study was supported by the Sorbonne University Emergence Grant and Agence National de Recherche—European Research CouncilTemplin Grant (T-ERC CoG) awarded to H.P.; an Institute of Cardiometabolism and Nutrition research grant funding leaky gut research awarded to H.P., J.A.W., C.P.B. and K.C.; and P.H.R.C. (Microbaria) funding awarded to K.C.
Competing interests
The authors report no competing interests.
Glossary
- ANOVA =
analysis of variance
- AP-HP =
Assistance Publique–Hôpitaux de Paris
- BMI =
body mass index
- CENIR =
Centre for Neuroimaging
- CI =
confidence intervals
- dlPFC =
dorsolateral pre-frontal cortex
- DTI =
diffusion-tensor-imaging
- FDR =
false-discovery rate
- fMRI =
functional magnetic resonance imaging
- FWHM =
full width at half maximum
- GDPR =
general data privacy regulations
- ICM =
Paris Brain Institute
- INSERM =
Institute National de la Santé et de la Recherche Médicale
- MC =
melanocortin
- MNI =
Montreal-Neurological Institute
- ROI =
region of interest
- rsfMRI =
resting state functional magnetic resonance imaging
- RSC =
resting-state connectivity
- RYGB =
Roux-en-Y gastric bypass
- SPM =
Statistical Parametric Mapping
- TEFQ =
Three-Factor Eating Questionnaire
- vmPFC =
ventromedial prefrontal cortex
- vSTR =
ventral striatum
References
- Aron-Wisnewsky J, Doré J, Clement K.. The importance of the gut microbiota after bariatric surgery. Nat Rev Gastroenterol Hepatol 2012; 9: 590–8. [DOI] [PubMed] [Google Scholar]
- Crujeiras AB, Carreira MC, Cabia B, Andrade S, Amil M, Casanueva FF.. Leptin resistance in obesity: an epigenetic landscape. Life Sci 2015; 140: 57–63. [DOI] [PubMed] [Google Scholar]
- Dagher A.Functional brain imaging of appetite. [Review]. Trends Endocrinol Metab 2012; 23: 250–60. [DOI] [PubMed] [Google Scholar]
- Doornweerd S, van Duinkerken E, de Geus EJ, Arbab-Zadeh P, Veltman DJ, IJzerman RG.. Overweight is associated with lower resting state functional connectivity in females after eliminating genetic effects: a twin study. Hum Brain Mapp 2017; 38: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med 2017; 23: 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraj M, Havel PJ, Phélis S, Blank D, Sniderman AD, Cianflone K.. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocr Metab 2003; 88: 1594–602. [DOI] [PubMed] [Google Scholar]
- Fried M, Yumuk V, Oppert JM, Scopinaro N, Torres A, Weiner R, on behalf of International Federation for the Surgery of Obesity and Metabolic Disorders—European Chapter (IFSO-EC) and European Association for the Study of Obesity (EASO), et al. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Surg 2014; 24: 42–55. [DOI] [PubMed] [Google Scholar]
- García-García I, Jurado MA, Garolera M, Marqués-Iturria I, Horstmann A, Segura B, et al. Functional network centrality in obesity: a resting-state and task fMRI study. Psychiatry Res 2015; 233: 331–8. [DOI] [PubMed] [Google Scholar]
- Hare TA, Malmaud J, Rangel A.. Focusing attention on the health aspects of food changes value signals in vmPFC and improves dietary choice. J Neurosci 2011; 31: 11077–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcherson CA, Plassmann H, Gross JJ, Rangel A.. Cognitive regulation during decision making shifts behavioral control between ventromedial and dorsolateral prefrontal value systems. J Neurosci 2012; 32: 13543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G.. Distributed neural representation of expected value. J Neurosci 2005; 25: 4806–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, Leibel RL, Seeley RJ, Schwartz MW.. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab 2010; 21: 643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE.. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. J Cogn Neurosci 2002; 14: 1211–29. [DOI] [PubMed] [Google Scholar]
- Palmiter RD.Is dopamine a physiologically relevant mediator of feeding behavior? [Review]. Trends Neurosci 2007; 30: 35–46. [DOI] [PubMed] [Google Scholar]
- Plassmann H, O’Doherty JP, Rangel A.. Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. J Neurosci 2010; 30: 10799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann H, O’Doherty JP, Rangel A.. Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. J Neurosci 2007; 27: 9984–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Fletcher PC, Henson RN, Worsley KJ, Brett M, Nichols TE.. Guidelines for reporting an fMRI study. [Review]. Neuroimage 2008; 40: 409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharmuller W, Ubel S, Ebner F, Schienle A.. Appetite regulation during food cue exposure: a comparison of normal-weight and obese women. Neurosci Lett 2012; 518: 106–10. [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Kim J, Weller RE, Cox JE, Cook IIE, Horwitz B.. Effective connectivity of a reward network in obese women. Brain Res Bull 2009; 79: 388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedemann LJ, Schmid SM, Hettel J, Giesen K, Francke P, Büchel C, et al. Central insulin modulates food valuation via mesolimbic pathways. Nature Commun 2017; 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD.. Obesity and addiction: neurobiological overlaps. Obes Rev 2013; 14: 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE.. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci 2003; 3: 255–74. [DOI] [PubMed] [Google Scholar]
- Wiemerslage L, Zhou W, Olivo G, Stark J, Hogenkamp PS, Larsson EM, et al. A resting‐state fMRI study of obese females between pre‐ and postprandial states before and after bariatric surgery. Eur J Neurosci 2017; 45: 333–41. [DOI] [PubMed] [Google Scholar]
- Wijngaarden MA, Veer IM, Rombouts SARB, van Buchem MA, Willems van Dijk K, Pijl H, et al. Obesity is marked by distinct functional connectivity in brain networks involved in food reward and salience. Behav Brain Res 2015; 287: 127–34. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack R, Nichols T, Essen D, Wager T.. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods 2011; 8: 665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron-Wisnewsky J, Prifti E, Belda E, Ichou F, Kayser BD, Dao MC, et al. Major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut 2019; 68: 70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW.. The valuation system: a coordinate-based meta analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage 2013; 76: 412–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR.Homeostatic and non-homeostatic pathways involved in the control of food intake and energy balance. Obesity 2006; 14: 197S–200S. [DOI] [PubMed] [Google Scholar]
- Beyer F, Prehn K, Wüsten KA, Villringer A, Ordemann J, Flöel A, Witte AV.. Weight loss reduces head motion: revisiting a major confound in neuroimaging. Hum Brain Mapp 2020; 41: 2490–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Van Kylen J, Hyde JS.. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed 1997; 10: 165–70. [DOI] [PubMed] [Google Scholar]
- Brooks SJ, Cedernaes J, Schiöth HB.. Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: a meta-analysis of fMRI studies. PloS One 2013; 8: e60393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A, Hendrikse J, Lee N, Yücel M, Verdejo-Garcia A, Andrews ZB, et al. The neurobiology of “food addiction” and its implications for obesity treatment and policy. Annu Rev Nutr 2016; 36: 105–28. [DOI] [PubMed] [Google Scholar]
- Cerit H, Davidson P, Hye T, Moondra P, Haimovici F, Sogg S, et al. Resting-state brain connectivity predicts weight loss and cognitive control of eating behavior after vertical sleeve gastrectomy. Obesity 2019; 27: 1846–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron S, Koechlin E.. Divided representation of concurrent goals in the human frontal lobes. Science 2010; 328: 360–3. [DOI] [PubMed] [Google Scholar]
- Ciangura C, Bouillot J-L, Lloret-Linares C, Poitou C, Veyrie N, Basdevant A, et al. Dynamics of change in total and regional body composition after gastric bypass in obese patients. Obesity 2010; 18: 760–5. [DOI] [PubMed] [Google Scholar]
- Coveleskie K, Gupta A, Kilpatrick LA, Mayer ED, Ashe-McNalley C, Stains J, et al. Altered functional connectivity within the central reward network in overweight and obese women. Nutr Diabetes 2015; 5: e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern R, Tang V, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body weight homeostasis. Neuron 2006; 49: 191–203. [DOI] [PubMed] [Google Scholar]
- Frank S, Wilms B, Veit R, Ernst B, Thurnheer M, Kullman S, et al. Altered brain activity in severely obese women may recover after Roux-en-Y gastric bypass surgery. Int J Obes 2014; 38: 341–8. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A.. Self-control in decision-making involves modulation of the vmPFC valuation system. Science 2009; 324: 646. [DOI] [PubMed] [Google Scholar]
- Heni M, Kullman S, Ketterer C, Guthoff M, Linder K, Wagner R, et al. Nasal insulin changes peripheral insulin sensitivity simultaneously with altered activity in homeostatic and reward-related human brain regions. Diabetologia 2012; 55: 1773–82. [DOI] [PubMed] [Google Scholar]
- Kullmann S, Heni M, Veit R, Ketterer C, Schick F, Häring H-U, et al. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp 2012; 33: 1052–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Ji G, Hu Y, Xu M, Jin Q, Liu L, et al. Bariatric surgery in obese patients reduced resting connectivity of brain regions involved with self‐referential processing. Hum Brain Mapp 2018; 39: 4755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague CT, Farooqi S, Whitehead JP, Soos MA, Rau H, Wareham NJ, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 1997; 387: 903–8. [DOI] [PubMed] [Google Scholar]
- Olivo G, Zhou W, Sundbom M, Zhukovsky C, Hogenkamp P, Nikontovic L, et al. Resting-state brain connectivity changes in obese women after Roux-en-Y gastric bypass surgery: a longitudinal study. Sci Rep 2017; 7: Article 6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BY, Seo J, Yi J, Park H.. Structural and functional brain connectivity of people with obesity and prediction of body mass index using connectivity. PLoS One 2015; 10: e0141376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.Mapping mental function to brain structure: how can cognitive neuroimaging succeed? [Review]. Perspect Psychol Sci 2010; 5: 753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pursey KM, Stanwell P, Callister RJ, Brain K, Collins CE, Burrows TL.. Neural responses to visual food cues according to weight status: a systematic review of functional magnetic resonance imaging studies. Front Nutr 2014; 1: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR.. A framework for studying the neurobiology of value-based decision making. [Review]. Nat Rev Neurosci 2008; 9: 545–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls BJ, Fedoroff IC, Guthrie JF.. Gender differences in eating behavior and body weight regulation. Health Psychol 1991; 10: 133–42. [DOI] [PubMed] [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht H-C, Klingebiel R, Flor H, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage 2007; 37: 410–21. [DOI] [PubMed] [Google Scholar]
- Schmidt L, Tusche A, Manoharan N, Hutcherson C, Hare T, Plassman H.. Neuroanatomy of the vmPFC and dlPFC predicts individual differences in cognitive regulation during dietary self-control across regulation strategies. J Neurosci 2018; 38: 5799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtz S, Miras AD, Chhina N, Prechtl CG, Sleeth ML, Daud NM, et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut 2014; 63: 891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele KE, Prokopowicz GP, Schweitzer MA, Magunsuon TH, Lidor AO, Kuwabawa H, et al. Alterations of central dopamine receptors before and after gastric bypass surgery. Obes Surg 2010; 20: 369–74. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM.. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 2008; 322: 449–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F.. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Phil Trans R Soc B 2008; 363: 3191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weygandt M, Mai K, Dommes E, Ritter K, Leupelt V, Spranger J, et al. Impulse control in the dorsolateral prefrontal cortex counteracts post-diet weight regain in obesity. Neuroimage 2015; 109: 318–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and software/code for analysis are available upon reasonable request from the corresponding author.