Summary
Bisphosphonates distributed to bone exert toxic effects specifically towards osteoclasts. On the other hand, intravenous administration of a nitrogen‐containing bisphosphonate (N‐BP) such as zoledronate induces acute‐phase reactions (APRs), including influenza‐like fever 1 day later, indicating an interaction with immunocompetent cells circulating blood. Although it has been reported that activation of γδ T cells is pivotal to induce an APR following treatment with zoledronate, downstream events, including the production of inflammatory cytokines after activation of γδ T cells, remain obscure. We investigated the effects of zoledronate on inflammatory cytokine expression in human peripheral blood mononuclear cells (PBMCs) in vitro. While zoledronate induced mRNA expressions of tumour necrosis factor‐α (TNF‐α), interleukin (IL)‐1β, IL‐6 and interferon‐γ (IFN‐γ) in PBMC, depletion of γδ T cells abolished that zoledronate‐induced expression of those cytokines, indicating the necessity of γδ T cells for expression induction by zoledronate. However, which types of cells were responsible for the production of those cytokines in blood remained unclear. As it is generally accepted that monocytes and macrophages are primary sources of inflammatory cytokines, CD14+ cells from PBMC were exposed to zoledronate in the presence of PBMC, which resulted in induced expression of mRNAs for IL‐1β, IL‐6 and IFN‐γ, but not for TNF‐α. These results indicate that CD14+ cells are responsible, at least in part, for the production of IL‐1β, IL‐6 and IFN‐γ in blood exposed to zoledronate. This suggests that CD14+ cells play an essential role in the occurrence of APRs following N‐BP administration.
Keywords: acute‐phase reaction, bisphosphonate, inflammatory cytokines, monocytes, zoledronate, γδ T cells
Intravenous administration of a nitrogen‐containing bisphosphonate such as zoledronate induces acute‐phase reactions (APRs), including influenza‐like fever 1 day later. While the activation of γδ T cells is essential for increased production of inflammatory cytokines required for induction of the APR, the types of cells that produce cytokines remained unclear. In this study, we found that CD14‐positive monocytes are responsible, at least in part, for the production of inflammatory cytokines after exposure of peripheral blood mononuclear cells to zoledronate.

Abbreviations
- APR
acute‐phase reaction
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- FPP
farnesyl pyrophosphate
- IFN‐γ
interferon‐γ
- IL
interleukin
- IPP
isopentenyl pyrophosphate
- N‐BP
nitrogen‐containing bisphosphonate
- PBMC
peripheral blood mononuclear cell
- PGE2
prostaglandin E2
- TNF‐α
tumour necrosis factor‐α
INTRODUCTION
Bisphosphonates (BPs), specific, potent inhibitors of bone resorption, are used to treat bone diseases including osteoporosis 1 and bone metastasis, 2 and others such as Paget's disease. 3 As BP phosphate groups have a high affinity towards hydroxyapatite, BPs are selectively distributed to calcified tissues such as bone following administration. 4 , 5 Bone‐resorbing osteoclasts secrete H+ towards the surface of the bone and dissolve hydroxyapatite. Acidification of the space between osteoclasts and the bone surface causes protonation of BP phosphate groups, facilitating osteoclasts’ BP uptake. 6 , 7 It is known that nitrogen‐containing BPs (N‐BPs) inhibit farnesyl pyrophosphate synthase in the mevalonate pathway, which leads to suppression of bone‐resorbing activity and osteoclast apoptosis. 7 , 8
One of the well‐known clinical problems is that intravenous administration of an N‐BP causes an acute‐phase reaction (APR) with transient influenza‐like symptoms, including fever, fatigue, myalgia and malaise, which develop within 24–36 h after the initial administration. 9 , 10 , 11 Such APRs are frequently associated with increased blood levels of proinflammatory cytokines, such as interferon‐γ (IFN‐γ), tumour necrosis factor‐α (TNF‐α) and interleukin (IL)‐6. 12 It is also known that these proinflammatory cytokines stimulate endothelial cells in the brain to produce prostaglandin E2 (PGE2). Furthermore, activation of the EP3 receptor on neurons in the preoptic area by PGE2 induces hyperactivation of sympathetic nerves to elevate body temperature. 13 For understanding APRs that occur after intravenous N‐PB administration, it is crucial to clarify the mechanism by which N‐PBs induce enhanced production of inflammatory cytokines in the blood.
Isopentenyl pyrophosphate (IPP) is a potent activator of human γδ T cells. Its accumulation due to inhibition of farnesyl pyrophosphate synthase by an N‐BP is regarded as an essential step in the increased production of inflammatory cytokines in the blood. 14 , 15 , 16 , 17 In humans, peripheral blood γδ T cells constitute between 1% and 5% of circulating T cells and predominantly express TCRs composed of Vγ9/Vδ2. 18 Several reports have indicated that monocyte lineage cells are responsible for the production and presentation of IPP to activate γδ T cells as augmented production of inflammatory cytokines in peripheral blood mononuclear cells (PBMCs) treated with N‐PBs. 19 , 20 , 21
Nevertheless, what type of cells functions as effector cells to produce inflammatory cytokines in the blood remains unclear. In the present study, we investigated the roles of γδ T cells and monocytes in the production of proinflammatory cytokines in PBMCs following treatment with zoledronate, one of the most effective N‐PBs for suppression of bone resorption.
MATERIALS AND METHODS
Bisphosphonates
Disodium etidronate and disodium pamidronate were purchased from Tokyo Kasei Kogyo Co. Ltd. Disodium zoledronate was purchased from Novartis Pharmaceuticals.
Isolation of PBMCs
Peripheral blood was drawn from healthy volunteers using a heparinized apparatus and injected into BD Vacutainer® CPT™ mononuclear cell preparation tubes containing sodium heparin (BD Biosciences). After centrifugation for 20 min at 1800 g, a fraction enriched with mononuclear cells was collected, washed with and resuspended in PBS, and used as PBMC in the following experiments.
Flow cytometry
The proportions of γδ T cells, CD14+ cells and butyrophilin (BTN) 3A1/CD277+ cells in PBMCs were determined using flow cytometry (FACSVerse™; BD Biosciences). For analysis of γδ T cells, PBMCs were incubated with an anti‐TCR γ/δ hapten antibody and anti‐hapten microbeads conjugated with fluorescein isothiocyanate (FITC), as well as a Cy3‐labelled anti‐CD3 antibody (Beckman Coulter Inc.). CD14+ cells and BTN3A1/CD277+ cells were labelled with FITC‐conjugated mouse anti‐human CD14 antibody (Clone Tk4; Miltenyi Biotec) and PE‐conjugated mouse anti‐human BTN3/CD277 antibody (BD Biosciences), respectively.
Removal of γδ T cells and CD14+ cells from PBMC
Peripheral blood mononuclear cells (1 × 107 cells) were incubated with an anti‐TCR γ/δ hapten antibody (Miltenyi Biotech), followed by incubation with anti‐hapten microbeads conjugated with FITC (Miltenyi Biotech). γδ T cells were removed from PBMC by magnetic antibody cell sorting using an autoMACS® Pro Separator (Miltenyi Biotech) according to the manufacturer's instruction. PBMCs (1 × 107 cells) were incubated for 15 min at 4°C in the dark with FITC‐labelled microbeads conjugated with a mouse anti‐human CD14 antibody (Miltenyi Biotech). CD14+ cells were removed from PBMC by magnetic antibody cell sorting using an autoMACS® Pro Separator (Miltenyi Biotech). The proportions of γδ T cells and CD14+ cells among the original PBMC and those among γδ T‐cell‐ and CD14+ cell‐depleted PBMC were determined using flow cytometry, as described above.
Isolation of γδ T cells and CD14+ cells from PBMCs
Peripheral blood mononuclear cells (1 × 107 cells) were incubated for 15 min at 4°C in the dark with the anti‐TCR γ/δ hapten antibody (Miltenyi Biotech) and FITC‐labeled microbeads conjugated with anti‐hapten antibody or FITC‐labeled microbeads conjugated with a mouse anti‐human CD14 antibody (Miltenyi Biotech). After washing by centrifugation, the cells were subjected to magnetic sorting. The proportion of γδ T cells and that of CD14+ cells were determined using flow cytometry.
Quantitative analysis of the expression of cytokine genes after treatment of cells with zoledronate
Peripheral blood mononuclear cells (5 × 106 cells/well) with or without γδ T cells were incubated for 8 h at 37°C with 5 μm of zoledronate in RPMI‐1640 medium (Life Technologies) supplemented with 10% fetal bovine serum (FBS). Transwell™ 6‐well plates (Corning Inc.) were used to examine the expression of genes for inflammatory cytokines in CD14+ cells. CD14+ cells (1 × 106 cells/well) were placed on the bottom of the plates and PBMC (5 × 106 cells/well) in the inserts. Incubation was performed for 8 h at 37°C with 5 μm of zoledronate in RPMI‐1640 medium plus 10% FBS. Total RNA was extracted from the cells using TRIzol Reagent (Life Technologies), and then, each sample was subjected to a reverse transcription reaction using SuperScript III (Life Technologies). Gene expression was analysed using real‐time PCR (Step One Plus; Applied Biosystems) with the following TaqMan probes (Thermo Fisher Scientific): IL6 (Hs00174131_m1), IL1B (Hs01555410_m1), TNFA (Hs00174128_m1), IFNG (Hs00989291_m1) and GAPDH (glyceraldehyde 3‐phosphate dehydrogenase: Hs02786624_g1).
Quantitative analyses of cytokines in culture supernatants
Peripheral blood mononuclear cells (1 × 106 cells/800 µL/well) were incubated for 24 h at 37°C with zoledronate, pamidronate and etidronate at 0, 5, 25 and 125 µm. The amounts of TNF‐α, IL‐6, IFN‐γ and IL‐1β in the culture supernatants were determined by ELISA using DuoSet ELISA kits for the corresponding cytokines (R&D Systems).
Statistical analysis
Data are expressed as the mean ± SD. Student's t‐test was used for statistical analyses, with P < 0.05 considered to be significant.
RESULTS
Zoledronate upregulated expression of inflammatory cytokines in PBMC
To clarify the role of peripheral blood mononuclear cells in the induction of APRs, including high fever following intravenous injection of an N‐BP such as zoledronate, we performed comprehensive gene expression analysis using PBMC incubated for 8 h in the presence and absence of zoledronate (5 µm). Zoledronate increased the mRNA expressions of several inflammatory cytokines, including IFN‐γ and TNF‐α, known to be involved in fever development.
Acute‐phase reactions develop following the administration of N‐BPs, that is second‐ and third‐generation BPs, but not after the administration of first‐generation BPs, which are lacking a nitrogen atom in their side chains. Hence, we analysed the mRNA expressions of inflammatory cytokines, including those noted above in PBMC treated for 8 h with the representative first‐, second‐ and third‐generation BPs etidronate, pamidronate and zoledronate, respectively, at the same concentration (5 µm). Etidronate did not affect mRNA expressions of TNF‐α, IL‐1β, IL‐6 or IFN‐γ, whereas both pamidronate and zoledronate significantly induced the expression of their genes. Furthermore, zoledronate, a third‐generation bisphosphonate, showed particularly potent activity to induce the expression of mRNAs for TNF‐α, IL‐6, IFN‐γ and IL‐1β in PBMC (Figure 1A‐D).
Figure 1.
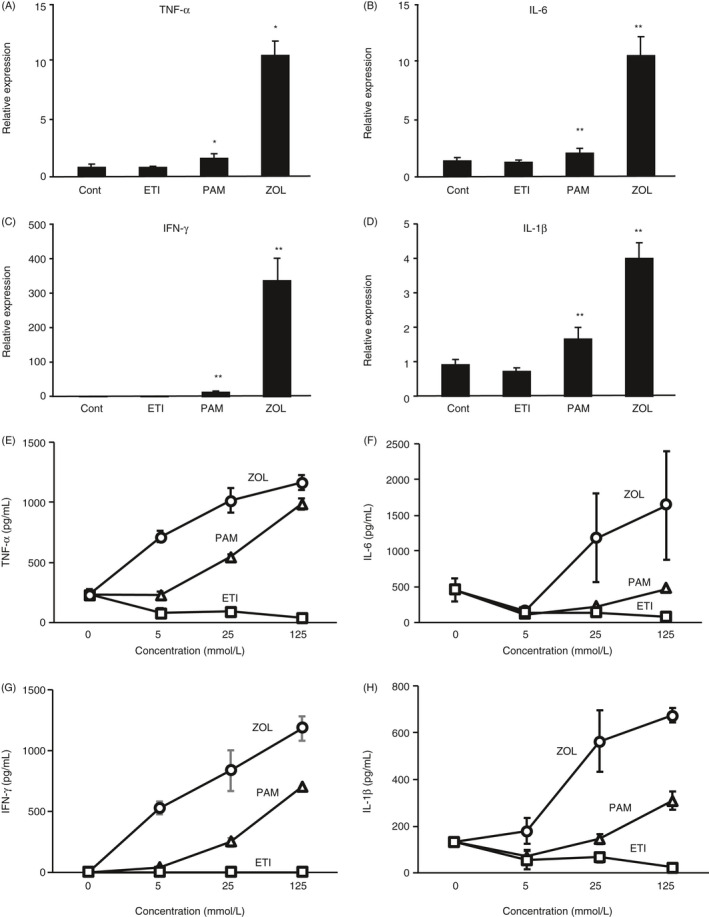
Increased expression of inflammatory cytokines in PBMCs following exposure to nitrogen‐containing bisphosphonates. (A‐D) Human PBMCs were incubated for 8 h in the absence (Cont) or presence of 5 µm of etidronate (ETI), pamidronate (PAM) or zoledronate (ZOL). Bisphosphonates classified as second‐ and third‐generation agents contain nitrogen atoms. Expression of mRNAs for TNF‐α (A), IL‐1β (B), IL‐6 (C) and IFN‐γ (D) was normalized against that of GAPDH and expressed as a value relative to that obtained in cells without treatment with bisphosphonates (Cont). **P < 0.01, *P < 0.05. (E‐H) Human PBMCs were incubated for 24 h in the absence or the presence of etidronate (square, ETI), pamidronate (triangle, PAM) or zoledronate (circle, ZOL) at the concentrations of 5, 25 or 125 mm. TNF‐α (E), IL‐6 (F), IFN‐γ (G) and IL‐1β (H) in the culture supernatants were determined by ELISA.
We assessed the concentration‐dependent effects of etidronate, pamidronate and zoledronate on the production of TNF‐α, IL‐6, IFN‐γ and IL‐1β proteins by PBMC treated for 24 h with these BPs. Etidronate did not affect these cytokines’ production. Both pamidronate and zoledronate augmented the production of all the cytokines tested in concentration‐dependent manners. The activity of zoledronate was higher than that of pamidronate (Figure 1E‐H). These results support those obtained in the mRNA expression of the cytokines after treatment with etidronate, pamidronate and zoledronate (Figure 1A‐D).
γδ T cells required for induction of inflammatory cytokines in PBMC after treatment with zoledronate
It has been shown that γδ T cells are involved in inflammatory responses, including the production of inflammatory cytokines following N‐BP administration. 12 We examined the expression of TNF‐α, IL‐6, IFN‐γ and IL‐1β genes in PBMC after removal of γδ T cells, performed using microbeads conjugated with an anti‐TCR γ/δ hapten antibody, and treatment with zoledronate. γδ T‐cell removal was confirmed using a flow cytometry technique (Figure 2A,B), which resulted in a change in the ratios of γδ T cells and CD3‐ and γδ‐TCR‐positive cells to the whole PBMC sample from 6.71% to 0.42%. Zoledronate did not induce mRNA expression of TNF‐α, IL‐6, IFN‐γ and IL‐1β in PBMC following removal of the majority of γδ T cells from PBMC (Figure 2C‐F), demonstrating that γδ T cells are required for induction of these cytokines by zoledronate. On the other hand, γδ T cells isolated from PBMC did not produce a detectable amount of either TNF‐α, IL‐6 or IL‐1β after incubation for 24 h with 25 µm zoledronate. While we detected a low level of IFN‐γ in the culture supernatant of γδ T cells, zoledronate did not affect the production of IFN‐γ by γδ T cells (Figure S1A‐D). Hence, it is suggested that γδ T cells participate in the production of these cytokines by stimulating other types of cells that produce the cytokines.
Figure 2.
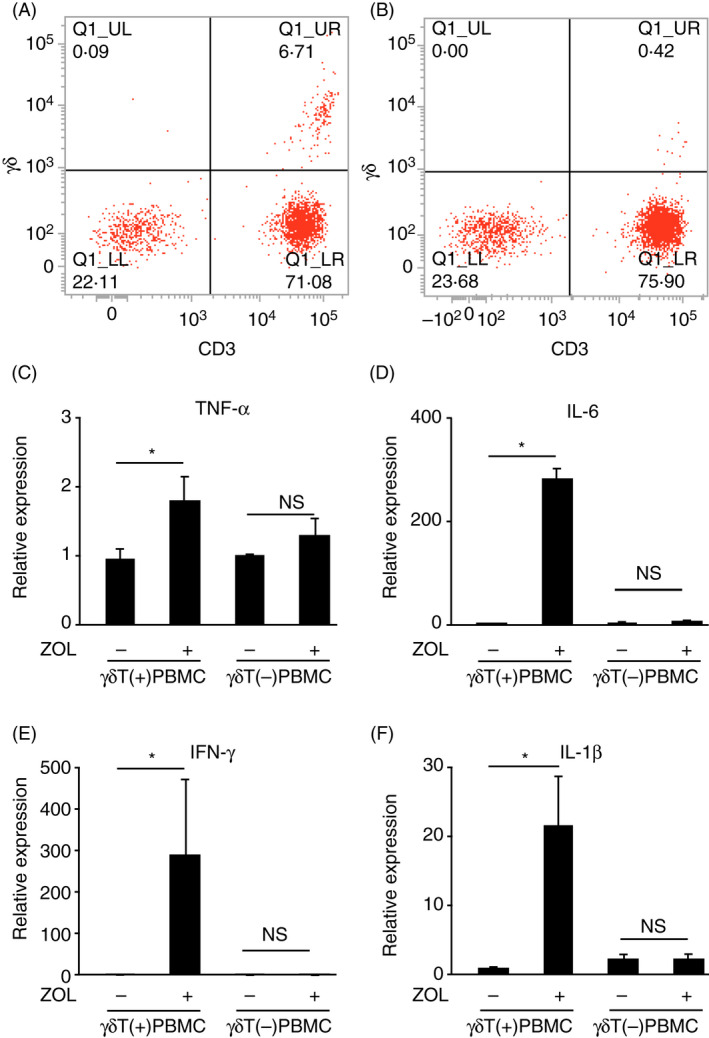
The requirement of γδ T cells for induced expression of mRNAs for inflammatory cytokines in PBMCs. γδ T cells were removed from human PBMCs using magnetic beads coupled with the antibody against γδ TCR. (A, B) Expressions of γδ TCR and CD3 by PBMCs before (A) and after (B) removal of γδ T cells. Cells expressing both γδ TCR and CD3 were considered to be γδT cells. (C‐F) PBMCs before [γδ T(+)PBMC] and after [γδ T(−)PBMC] removal of γδ T cells were incubated for 8 h in the absence (−) or presence (+) of 5 µm of zoledronate (ZOL). The expression of mRNAs for TNF‐α (C), IL‐6 (D), IFN‐γ (E) and IL‐1β (F) was normalized against that of GAPDH and expressed as a value relative to that obtained with γδ T(+)PBMC without treatment with zoledronate (far left column in each panel). *P < 0.05 [Colour figure can be viewed at wileyonlinelibrary.com]
Zoledronate‐induced expression of mRNAs for IL‐1β, IL‐6 and TNF‐α in CD14+ in the presence of PBMC
While it is generally accepted that monocytes are significant sources of inflammatory cytokines, their contribution to APR development after administration of an N‐BP has not been elucidated. Hence, we examined whether CD14+ monocytes induce production of TNF‐α, IL‐6, IFN‐γ and IL‐1β in PBMC following treatment with zoledronate. By isolating CD14+ cells from PBMC using the insert membrane, we could confirm whether CD14+ cells express the cytokines. Also, we could know whether humoral factors involve in the induced expression of cytokine genes by zoledronate. The abundance ratio of CD14+ cells isolated from PBMCs was 98.5% (Figure 3A). Zoledronate augmented expression of mRNAs for IL‐6, IFN‐γ and IL‐1β in CD14+ cells in the presence of PBMC, whereas it did not induce the expression of TNF‐α mRNA in CD14+ cells (Figure 3B‐E). These results suggest that CD14+ cells are responsible, at least in part, for the production of IL‐6, IFN‐γ and IL‐1β in PBMC exposed to zoledronate, whereas TNF‐α is produced by cells other than CD14+ cells in PBMC.
Figure 3.
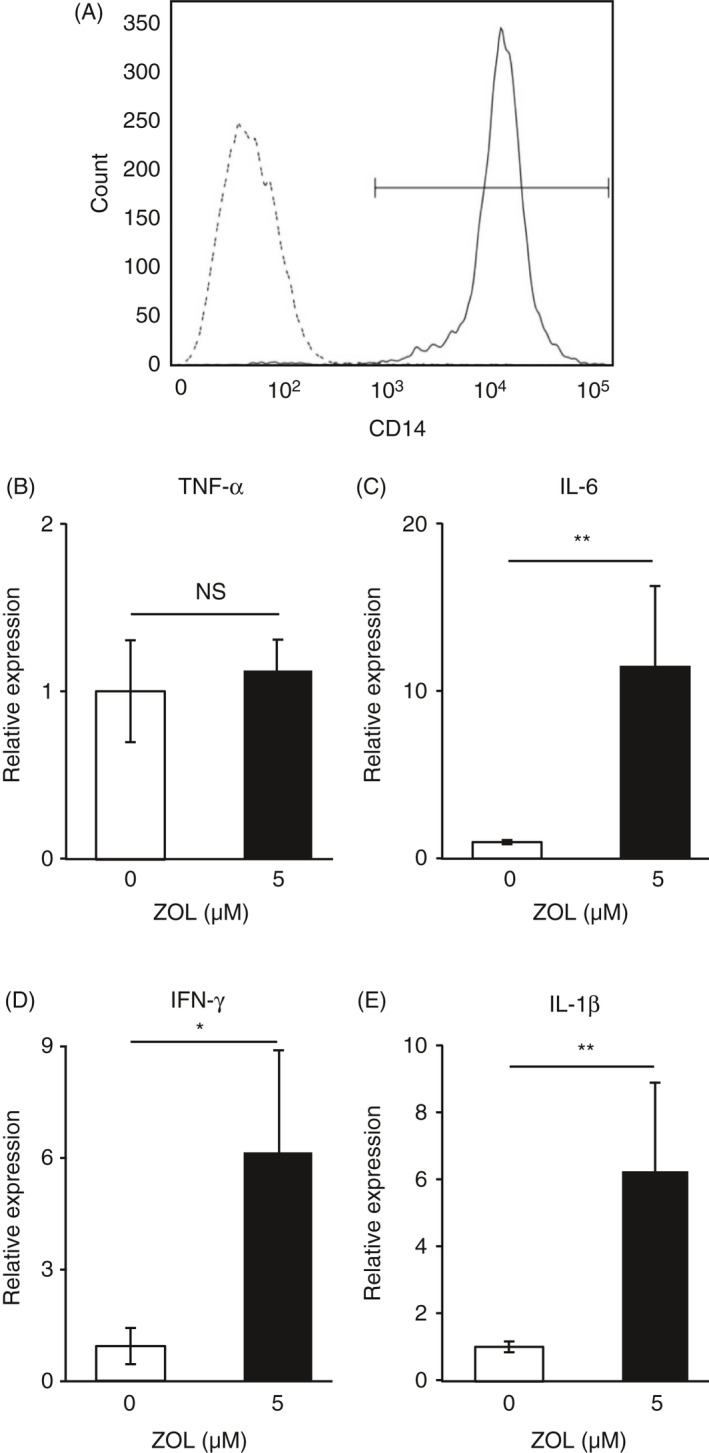
Increased expression of mRNAs for inflammatory cytokines in CD14+ cells by zoledronate in the presence of PBMCs. CD14+ cells were separated from human PBMCs using magnetic beads coupled with an antibody against CD14. (A) Levels of expression of CD14 by separated CD14+ cells (solid line) and the remaining PBMCs (dotted line) are shown. (B‐E) CD14+ cells and PBMCs were separately cultured in Transwell® Permeable Support (Corning Inc.) dishes. CD14+ cells were placed in the lower plates and PBMCs in the upper inserts, then incubated for 8 h in the absence (−) or presence (+) of 5 µm of zoledronate (ZOL), after which total RNA was isolated from CD14+ cells cultured in the lower plates. The expression of mRNAs for TNF‐α (B), IL‐6 (C), IFN‐γ (D) and IL‐1β (E) was normalized against that of GAPDH and expressed as a value relative to that obtained in CD14+ cells without treatment with zoledronate (unfilled columns). **P < 0.01, *P < 0.05.
On the other hand, we did not observe a significant change in the production of any of those cytokines in CD14+ cells in the absence of PBMC (Figure S1E‐H), indicating that the production of these cytokines by CD14+ cells requires stimulation by other types of cells in PBMC. The removal of CD14+ cells reduced the cytokines’ production by PBMC to negligible levels (Figure S1I‐L). Hence, CD14+ cells are essential for cytokine production by PBMC.
It is reported that BTN3A/CD277 plays a vital role in activating γδ T cells by phosphor‐antigens such as IPP. 22 , 23 Hence, we analysed the proportion of BTN3A/CD277+ cells in PBMC and found that they account for 89.6% (56.58% + 33.05%) of the total PBMC. Also, 33.05% of PBMC expressed both BTN3A/CD277 and CD14, and almost all CD14+ cells expressed BTN3A/CD277 (Figure S2A). An antibody for BTN3/CD277 (BD Biosciences) did not affect the zoledronate‐induced production of TNF‐α by PBMC (Figure S2B). The anti‐BTN3A/CD277 antibody enhanced the production of IL‐1β and IL‐6 by PBMC in the absence of zoledronate, while it did not affect the production of these cytokines in the presence of zoledronate. Besides, the anti‐BTN3A/CD277 antibody augmented the effect of zoledronate on the production of IFN‐γ by PBMC (Figure S2D‐F).
DISCUSSION
To determine cells responsible for producing inflammatory cytokines following intravenous administration of zoledronate, we examined the effects of zoledronate on inflammatory cytokine expression in PBMC in vitro. It was previously reported that an infusion of zoledronate induced an increase in the population of CD14+ monocytes and concentrations of IFN‐γ and IL‐6 in peripheral blood of affected patients. 24 The present findings revealed that CD14+ monocytes in PBMC express mRNAs for IL‐6, IFN‐γ and IL‐1β following treatment with zoledronate. Additionally, after exposure to zoledronate, PBMC expressed the TNF‐α gene, whereas zoledronate did not alter the TNF‐α gene expression level in CD14+ monocytes. These findings suggested that CD14+ cells are not responsible for the production of TNF‐α after zoledronate treatment. Furthermore, crosstalk between CD14+ monocytes and γδ T cells via cell–cell contact might be necessary for zoledronate‐induced expression of TNF‐α in CD14+ cells.
Bisphosphonates are antiresorptive agents widely used for the treatment of various bone diseases, including osteoporosis. First‐generation BPs form ATP analogs in osteoclasts and show cytotoxic effects, whereas the second‐ and third‐generation BPs, or N‐BPs, inhibit bone resorption by osteoclasts and induce their apoptosis. N‐BPs are known to inhibit farnesyl pyrophosphate synthase in the mevalonate pathway to prevent protein prenylation of GTP‐binding proteins necessary for osteoclast function. 20 , 25 The specific action of BPs towards osteoclasts is based on their selective distribution to the bone.
The half‐life of zoledronate, a representative third‐generation bisphosphonate, in the blood following intravenous infusion (5 mg), was reported to be 74.7 h in patients with primary osteoporosis, 26 indicating the possibility that the drug affects various types of cells in circulating blood. Zoledronate, as well as other N‐BPs, is known to have several adverse effects, including APRs after intravenous administration. Thus, the production of inflammatory cytokines by cells in the circulation is vital for the induction of an APR, including high fever. The mechanism of APRs caused by zoledronate has been partially elucidated and shown to be associated with the release of inflammatory cytokines including TNF‐α and IL‐6. 10 , 11 Inhibition of farnesyl pyrophosphate synthase in the mevalonate pathway by zoledronate results in accumulation of its upstream compounds, that is IPP and geranyl pyrophosphate, both of which stimulate γδ T cells. 26 It has also been reported that IPP and geranyl pyrophosphate activate γδ TCR via BTN3A1/CD277, a ubiquitously expressed molecule in antigen‐presenting cells. 27 As expected, we found that etidronate, a bisphosphonate that does not contain nitrogen, did not affect the expression of inflammatory cytokines in PBMC (Figure 1), indicating a possible involvement of γδ T cells in induced expression of inflammatory cytokines, at least under our experimental conditions. Furthermore, removal of γδ T cells from PBMC abrogated the zoledronate‐induced expression of TNF‐α, IL‐1β, IL‐6 and LFN‐γ (Figure 2), clearly showing the requirement of γδ T cells for the production of these cytokines during that process.
It is generally accepted that monocytes and macrophages are cellular sources of inflammatory cytokines in response to various stimulation types. 28 , 29 IL‐1β, IL‐6 and TNF‐α, three important cytokines produced by monocytes and macrophages, mediate numerous immune functions. 29 These cytokines are known to induce fever and modulate the production of acute‐phase proteins by hepatocytes, such as C‐reactive protein and serum amyloid A. 30 On the other hand, the previously reported lack of changes in the level of IL‐1 in plasma after administration of zoledronate indicates that monocyte/macrophage lineage cells are not a significant source of those cytokines. 28 The present findings clearly showed that CD14+ monocytes in PBMC express IL‐1β, as well as IL‐6 and IFN‐γ, in response to treatment with zoledronate (Figure 3). Hence, it is reasonable to assume that CD14+ monocytes are responsible, at least in part, to produce these cytokines in blood following treatment with zoledronate.
In another previous study, monocytes were suggested to play a role in the activation of γδ T cells after treatment with zoledronate through the accumulation of IPP and its isomer dimethylallyl pyrophosphate. 19 It has also been suggested that zoledronate stimulates γδ T cells directly 31 or indirectly by presenting to monocyte lineage cells. 18 As 36.9% of BTN3A/CD277+ cells in PBMC or 33.05% of the total PBMC was CD14+ cells (Figure S2A), it is possible that CD14+ cells activate γδ T cells by cell–cell contact in the presence of zoledronate, and then activated γδ T cells, in turn, stimulate CD14+ monocytes to produce inflammatory cytokines in PBMC. The anti‐BTN3A/CD277 antibody used in this study did not interfere with the cytokine production by PBMC but instead stimulated the production of IFN‐γ by PBMC in the presence of zoledronate (Figure S2D). The anti‐BTN3A/CD277 antibody augmented IL‐6 and IL‐1β production by PBMC in the absence of zoledronate (Figure S2C,E). Considering that CD14+ cells also expressed BTN3A/CD277 (Figure S2A), the antibody might stimulate CD14+ cells through their BTN3A/CD277.
On the other hand, CD14+ cells isolated from PBMC by the chamber filters expressed these cytokines (Figure 3C‐E), indicating some humoral factors involved in the activation of CD14+ cells. Further studies are required to describe CD14+ cells’ role in producing inflammatory cytokines in the blood. We might say that CD14+ cells are one of the inflammatory cytokine‐producing cells after treatment with zoledronate.
AUTHOR CONTRIBUTIONS
TS, AY and YM designed the work. RT, TS, AY, KS, KY, MK, KI, MI and MY performed the experiments. YM prepared the manuscript. TS and RK critically reviewed the manuscript.
Supporting information
Figure S1. The inability of γδ T cells and CD14+ cells to produce inflammatory cytokines and the requirement of CD14+ cells for the production of the inflammatory cytokines by PBMC.
Figure S2. The role of BTN3A/CD277+ cells in cytokine production induced by zoledronate.
ACKNOWLEDGEMENTS
This study was funded in part by Asahi Kasei Pharma Co. (Tokyo, Japan) and grants from JSPS Kakenhi (18K09511, 18K09512, 18K09513, 18K09528 and 18K19655) and the MEXT‐Supporting Program for the Strategic Research Foundation at Private Universities (S1411009), as well as by the Private University Research Branding Project from MEXT of Japan.
DATA AVAILABILITY STATEMENT
All data sets generated or analysed during this study are included in this published article and its Supplementary materials.
REFERENCES
- 1. Delmas PD. Treatment of postmenopausal osteoporosis. Lancet. 2002;359:2018–26. [DOI] [PubMed] [Google Scholar]
- 2. Neville‐Webbe HL, Holen I, Coleman RE. The anti‐tumour activity of bisphosphonates. Cancer Treat Rev. 2002;28:305–19. [DOI] [PubMed] [Google Scholar]
- 3. Ralston SH, Langston AL, Reid IR. Pathogenesis and management of Paget's disease of bone. Lancet. 2008;372:155–63. [DOI] [PubMed] [Google Scholar]
- 4. Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Mönkkönen J, et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88:2961–78. [DOI] [PubMed] [Google Scholar]
- 5. Russell RGG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–59. [DOI] [PubMed] [Google Scholar]
- 6. Rogers MJ, Crockett JC, Coxon FP, Mönkkönen J. Biochemical and molecular mechanisms of action of bisphosphonates. Bone. 2011;49:34–41. [DOI] [PubMed] [Google Scholar]
- 7. Takami M, Suda K, Sahara T, Itoh K, Nagai K, Sasaki T, et al. Involvement of vacuolar H+‐ATPase in incorporation of risedronate into osteoclasts. Bone 2003;32:341–9. [DOI] [PubMed] [Google Scholar]
- 8. Selander KS, Mönkkönen J, Karhukorpi E, Härkönen P, Hannuniemi R, Väänänen K. Characteristics of clodronate‐induced apoptosis in osteoclasts and macrophages. Mol Pharmacol. 1996;50:1127–38. [PubMed] [Google Scholar]
- 9. Adami S, Bhalla AK, Dorizzi R, Dorizzi R, Montesaniti F, Rossini S, et al. The acute‐phase response after bisphosphonates administration. Calcif Tissue Int. 1987;41:326–31. [DOI] [PubMed] [Google Scholar]
- 10. Schweitzer DH, Oostendorp‐van de Ruit M, van der Pluijm G, Löwik CWGM, Papapoulos SE. Interleukin 6 and the acute phase response during treatment of patients with Paget's disease with the nitrogen containing bisphosphonate dimethylaminohydroxypropylidene bisphosphonate. J Bone Miner Res. 1995;10:956–62. [DOI] [PubMed] [Google Scholar]
- 11. Thiébaud D, Sauty A, Burckhardt P, Leuenberger P, Sitzler L, Green JR, et al. An in vitro and in vivo study of cytokines in the acute‐phase response associated with bisphosphonates. Calcif Tissue Int. 1997;61:386–92. [DOI] [PubMed] [Google Scholar]
- 12. Hewitt RE, Lissina A, Green AE, Slay ES, Price DA, Sewell AK. The bisphosphonate acute phase response: rapid and copious production of proinflammatory cytokines by peripheral blood γδ T cells in response to aminobisphosphonates is inhibited by statins. Clin Exp Immunol. 2005;139:101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ushikubi F, Segi E, Sugimoto Y, Murata T, Matsuoka T, Kobayashi T, et al. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3 . Nature. 1998;395:281–4. [DOI] [PubMed] [Google Scholar]
- 14. Green AE, Lissina A, Hutchinson SL, Hewitt RE, Temple B, James D, et al. Recognition of nonpeptide antigens by human V gamma 9V delta 2 T cells requires contact with cells of human origin. Clin Exp Immunol. 2004;136:472–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Das H, Wang L, Kamath A, Bukowski JF. Vγ2Vδ2 T‐cell receptor‐mediated recognition of aminobisphosphonates. Blood. 2001;98:1616–8. [DOI] [PubMed] [Google Scholar]
- 16. Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor γ δ cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kabelitz D, He W. The multifunctionality of human Vγ9Vδ2γ δT cells: clonal plasticity or distinct subsets? Scand J Immunol. 2012;76:213–22. [DOI] [PubMed] [Google Scholar]
- 18. Miyagawa F, Tanaka Y, Yamashita S, Minato N. Essential requirement of antigen presentation by monocyte lineage cells for the activation of primary human γ δ T cells by aminobisphosphonate antigen. J Immunol. 2001;166:5508–14. [DOI] [PubMed] [Google Scholar]
- 19. Roelofs AJ, Jauhiainen M, Mönkkönen H, Rogers MJ, Mönkkönen J, Thompson K. Peripheral blood monocytes are responsible for γδ T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol. 2009;144:245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen‐containing bisphosphonates inhibit the mevalonate pathway and prevent post‐translational prenylation of GTP‐binding proteins, including Ras. J Bone Miner Res. 1998;13:581–9. [DOI] [PubMed] [Google Scholar]
- 21. Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of γδ T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–92. [PubMed] [Google Scholar]
- 22. Blazquez J‐L, Benyamine A, Pasero C, Olive D. New insights into the regulation of γδ T cells by BTN3A nd other BTN/BTNL in tumor immunity. Front Immunol. 2018;9:1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Herrmann T, Fichtner AS, Karunakaran MM. An update on the molecular basis of phosphoantigen recognition by Vγ9Vδ2 T cells. Cells. 2020;9:1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Welton JL, Morgan MP, Marti S, Stone MD, Moser B, Sewell AK, et al. Monocytes and γδ T cells control the acute‐phase response to intravenous zoledronate: insights from a phase IV safety trial. J Bone Miner Res. 2013;28:464–71. [DOI] [PubMed] [Google Scholar]
- 25. Fisher JE, Rogers MJ, Halasy JM, Luckman SP, Hughes DE, Masarachia PJ, et al. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro . Proc Natl Acad Sci USA. 1999;96:133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shiraki M, Tanaka S, Suzuki H, Ueda S, Nakamura T. Safety, pharmacokinetics, and changes in bone metabolism associated with zoledronic acid treatment in Japanese patients with primary osteoporosis. J Bone Miner Metab. 2017;35:675–84. [DOI] [PubMed] [Google Scholar]
- 27. Vavassori S, Kumar A, Wan GS, Ramanjaneyulu GS, Cavallari M, El Daker S, et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human γδ T cells. Nat Immunol. 2013;14:908–1610. [DOI] [PubMed] [Google Scholar]
- 28. Tanaka Y, Sano S, Nieves E, De Libero G, Rosa D, Modlin RL, et al. Nonpeptide ligands for human γδ T cells. Proc Natl Acad Sci USA. 1994;91:8175–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sauty A, Pecherstorfer M, Zimmer‐Roth I, Fioroni P, Jullierat L, Markert M, et al. Interleukin‐6 and tumor necrosis factor α levels after bisphosphonates treatment in vitro and in patients with malignancy. Bone. 1996;18:133–9. [DOI] [PubMed] [Google Scholar]
- 30. Beutler B, Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987;316:379–85. [DOI] [PubMed] [Google Scholar]
- 31. Dinarello CA, Cannon JG, Wolff SM, Bernheim HA, Beutler B, Cerami A, et al. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induction of interleukin 1. J Exp Med. 1986;163:1433–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The inability of γδ T cells and CD14+ cells to produce inflammatory cytokines and the requirement of CD14+ cells for the production of the inflammatory cytokines by PBMC.
Figure S2. The role of BTN3A/CD277+ cells in cytokine production induced by zoledronate.
Data Availability Statement
All data sets generated or analysed during this study are included in this published article and its Supplementary materials.


