Abstract
The auxin IAA is a vital plant hormone in controlling growth and development, but our knowledge about its complicated biosynthetic pathways and molecular regulation are still limited and fragmentary. cytokinin induced root waving 2 (ckrw2) was isolated as one of the auxin-deficient mutants in a large-scale forward genetic screen aiming to find more genes functioning in auxin homeostasis and/or its regulation. Here we show that CKRW2 is identical to Histone Monoubiquitination 1 (HUB1), a gene encoding an E3 ligase required for histone H2B monoubiquitination (H2Bub1) in Arabidopsis. In addition to pleiotropic defects in growth and development, loss of CKRW2/HUB1 function also led to typical auxin-deficient phenotypes in roots, which was associated with significantly lower expression levels of several functional auxin synthetic genes, namely TRP2/TSB1, WEI7/ASB1, YUC7 and AMI1. Corresponding defects in H2Bub1 were detected in the coding regions of these genes by chromatin immunoprecipitation (ChIP) analysis, indicating the involvement of H2Bub1 in regulating auxin biosynthesis. Importantly, application of exogenous cytokinin (CK) could stimulate CKRW2/HUB1 expression, providing an epigenetic avenue for CK to regulate the auxin homeostasis. Our results reveal a previously unknown mechanism for regulating auxin biosynthesis via HUB1/2-mediated H2Bub1 at the chromatin level.
Subject terms: Plant hormones, Histone post-translational modifications
Li Zhang et al. characterize ckrw2, cytokinin-induced root waving 2, as a mutant form of HUB1 in Arabidopsis, the gene required for histone H2B monoubiquitination. This study implicates the involvement of H2Bub1 in regulating auxin biosynthesis.
Introduction
Auxin is one of the most important plant hormones regulating plant growth and development, such as cell division, cell differentiation, apical dominance, flowering, senescence, and tropism1–4. Plants maintain auxin homeostasis by regulating its synthesis, metabolism, and polar transport5,6.
Plants are believed to have multiple and highly interconnected pathways for auxin biosynthesis, including several tryptophan (Trp)-dependent (TD) and -independent (TI) pathways7. In these pathways, Trp is synthesized from chorismate via six critical linear steps in the chloroplast8. The WEI2/ASA1 and WEI7/ASB1 genes encode the α- and β-subunit, respectively, of the anthranilate synthetase complex, which catalyzes the rate-limiting step in the conversion of chorismate to anthranilate. Under the catalysis of PAT1 and PAI, anthranilate is converted to CdRP9,10. Subsequently, the indole glycerol phosphate synthetase catalyzes the conversion of CdRP to IGP, which is the branch point of TI and TD pathways. IGP can form Trp through Trp synthetase complex, which is composed of Trp synthase ɑ (TSA1) and β (TSB1 and TSB2)11,12. According to the intermediate metabolites from Trp, the TD pathway can be divided into four branch pathways: the indole-3-pyruvic acid (IPyA), indole-3-acetamide (IAM), indole-3-acetaldoxime (IAOx), and tryptamine (TAM) pathways10,13,14. So far, only the two-step IPyA pathway has been completely elucidated at both the biochemical and the genetic level, producing IAA from Trp via IPyA under the catalysis by tryptophan aminotransferases (TAA1/TARs) and YUCCA (YUC) flavin-dependent monooxygenases, and is likely the main pathway for auxin synthesis in Arabidopsis15–22.
The synthesis of auxin in plants is subject to intricate regulations1,6,23. Nutritional signals such as glucose and nitrate induce the production of auxin by regulating the transcription of YUC2/8/9 and TAA1/TAR2, respectively24–27. Environmental stress, such as aluminum, regulates the level of auxin by regulating the transcription of TAA128. The plant hormone cytokinin (CK) regulates the level of auxin by regulating the transcription level of YUC1/4/8 and TAA118,20,29–31. These findings indicate that the transcriptional regulation of auxin synthase plays an important role in auxin homeostasis.
The epigenetic state of chromatin associated with histone modifications can profoundly influence gene expression in eukaryotes. Histone H2B monoubiquitination (H2Bub1) is a form of post-translational modification that is linked to active gene transcription32,33. In Arabidopsis, H2Bub1 normally occurs on K143 or K14534 by the heterodimeric HISTONE MONOUBIQUITINATION1/2 (HUB1/2) E3 ubiquitin ligase, a homolog of the budding yeast Bre1 protein35,36. For instance, HUB1/2-mediated H2Bub1 regulates the expression levels of FLOWERING LOCUS C (FLC) and some circadian clock genes such as CCA1 and TOC1 by stimulating the H3K4me3 modification on their chromatin37–39. In plant immune responses, H2Bub1 modulates the expression of the R gene SNC140. H2Bub1 is reported to be associated with H3K4me3 at the GhDREB locus, which triggers more rapid responses to drought stress41. Such genome-wide regulation on gene expression makes HUB1/2 to be required for multiple developmental processes in plants, including seed dormancy35, leaf, and root growth36, flowering37,42,43, photomorphogenesis, and circadian rhythms38,44, defense and immune responses40,45–47, and their loss of function mutation can produce a wide variety of defective phenotypes in growth and development.
Here, on characterizing a previously isolated auxin-deficient mutant48 of ckrw2, we reveal that CKRW2 is identical to HUB1, and HUB1/2-mediated H2Bub1 is positively associated with the transcription of several auxin synthetic genes for maintaining normal auxin homeostasis. By up-regulating HUB1/2 gene expression, CK can use this kind of histone modification as one of its effective ways to regulate auxin homeostasis in plants.
Results and discussion
ckrw2 is an auxin-deficient mutant
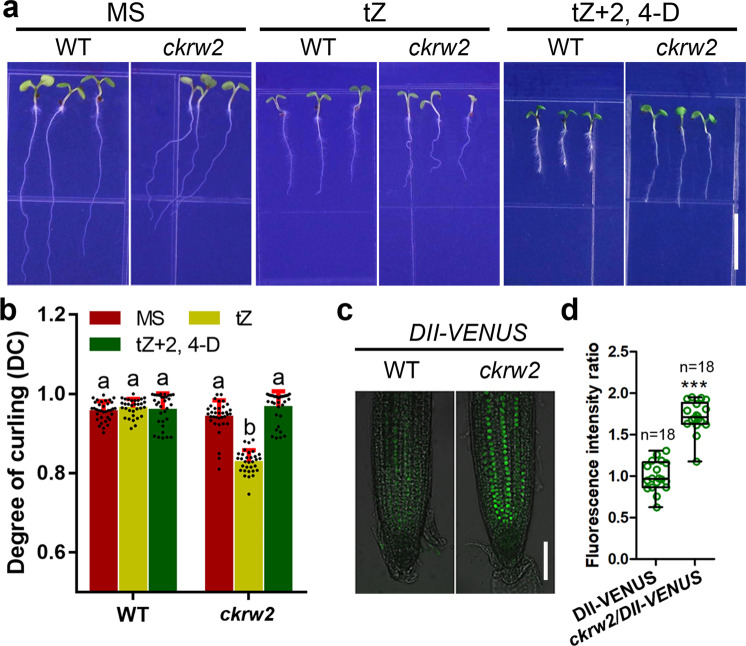
To uncover more genes functioning in auxin biosynthesis or homeostasis, we previously established an effective genetic screening protocol for isolating auxin-deficient mutants by using CK-induced root curling (ckrc) or root waving (ckrw) as a phenotypic marker, in which the ckrw2 mutant was isolated as one of the so-called group III ckrw mutants48. When grown on the medium containing 0.01 μM trans-zeatin (tZ), ckrw2 mutant displayed a root waving phenotype and had a significantly reduced endogenous IAA level48. In addition to a number of pleiotropic abnormalities in leaves, seeds, root hair, apical hook, cutin, petals, and flowering time (Supplementary Fig. 1 a–l), typical low-auxin phenotypes, such as the reduced root length, smaller meristematic zone, shorter mature epidermal cell length (Supplementary Fig. 1m–p) and weaker gravitropic response, were observed, which could be rescued by exogenous auxins48 (Fig. 1a, b). In line with these, the mutant had weaker Dr5:GUS/GFP expression49 or brighter DII-VENUS50 fluorescence (Fig. 1c, d, Supplementary Fig. 2) in the transgenic root tips, indicating a lower auxin activity that was most likely caused by the endogenous auxin deficiency.
Fig. 1. Auxin-deficient phenotypes in ckrw2 mutant.
a Auxin-deficient phenotypes in ckrw2 mutant, showing its waving roots after 7 days of vertical culture on MS with 0.01 μM tZ, which can be rescued by 0.01 μM 2, 4-D. Bar = 5 mm. b Results of statistical analysis on root curling/waving degrees (DC). Data are presented as mean ± SD (n = 30–35), three biologically independent experiments, the letters indicate a significant difference at P < 0.05 according to ANOVA followed by Tukey’s multiple comparison tests. c, d Auxin activity in root tips revealed by the expressions of DII-VENUS. The seedlings grown on MS medium for 7 days were used for GUS staining and fluorescence intensity observation (approximately to the first 200 µm from the root tip). Each circle represents the measurement from an individual root. Boxplots span the first to the third quartiles of the data. Whiskers represent the minimum and maximum values. A line in the box represents the mean. “n” represents the number of roots used in this experiment. The student’s t test, was used for statistical analyses. ***P < 0.001. Bars = 50 µm in (d).
CKRW2 gene encodes a functional E3 ubiquitin ligase for histone H2Bub1
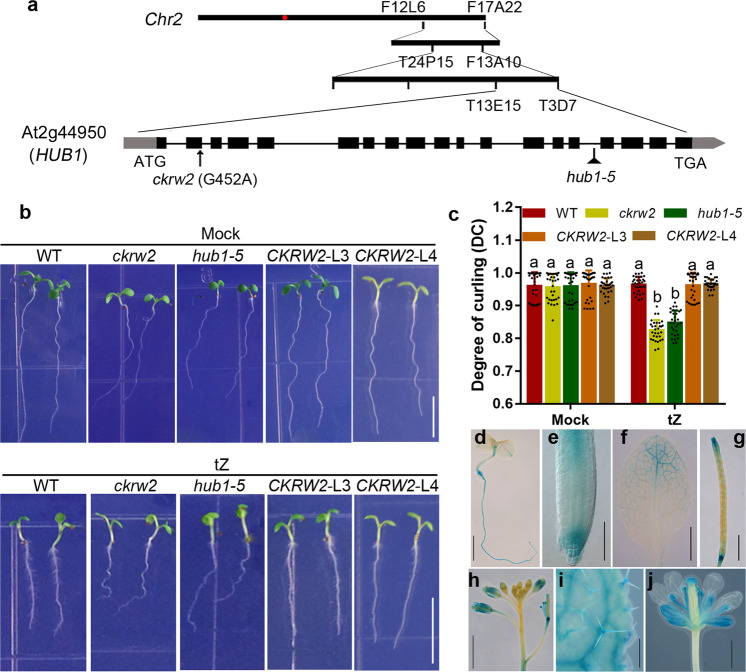
As ckrw2 was isolated from a mutant pool generated by T-DNA tagging51 we initially did Tail-polymerase chain reaction (PCR) amplification, finding a T-DNA flanking sequence located between AT5G25425-AT5G25430, which showed no genetic linkage to ckrw2 mutation48. However, map-based cloning combined with whole-genome resequencing (WGRS) identified a G > A substitution in the coding region of AT2G44950/HUB1, altering the tryptophan (aa 91) codon (TGG) to a stop codon (TAG) (Fig. 2a) in this gene. Both genetic allelic analysis (Fig. 2b) and the full rescue of the defective ckrw2 phenotypes (Fig. 2c; Supplementary Fig. 3) by the fused HUB1::YFP-HUB1 confirmed the At2g44950/HUB1 identity of CKRW2 gene.
Fig. 2. Gene cloning and the expression pattern of CKRW2 gene.
a Map-based cloning, showing the position of the G452A point mutation causing premature termination in the AT2G44950 gene coding region. b, c Similar phenotypes of ckrw2 and hub1-5 (b), and molecular complementation (c). Seedlings were grown on MS with or without 0.01 μM tZ. Bars = 5 mm. Data are presented as mean ± SD (n = 30–35), three independent experiments, the letters indicate a significant difference at P < 0.05, according to ANOVA followed by Tukey’s multiple comparison tests. d–j GUS activity was detected in various organs at different developmental stages of pCKRW2: GUS transgenic plant. 4-day-old seedlings (d–g) or 4-week-old plants (h–j). Scale bars = 5 mm in (d) and (j); 100 µm in (e); 20 mm in (f) and (h); 10 mm in (g).
In Arabidopsis HUB1 and its paralog HUB2 are E3 ubiquitin ligases to act non-redundantly in a conserved heterotetrameric complex to catalyze H2Bub1 in chromatin, activating a variety of genes functioning in diverse biological processes of growth, development, stress, and immunity response33,35–38,40,44–47,52–57, some of which were also observed in ckrw2 mutant (Supplementary Figs. 1 and 3). In planta, CKRW2/HUB1 had a constitutive or wide expression pattern at the organ/tissue levels, as revealed by the pCKRW2:GUS reporter transgene expression (Fig. 2d–j)35,37. It was highly active in the meristematic and vascular tissues of the primary root, hypocotyl, stem, cotyledon, and leaves (Fig. 2d–j). Significantly, the expression of CKRW2 at the apical root overlapped some of the auxin synthetic genes (Fig. 2e)10,18,20,58. As expected, the global defect in H2Bub1 was confirmed by western blot analysis using the anti-H2Bub1 antibody in ckrw2 mutant (Supplementary Figs. 4 and 5)37.
CKRW2/HUB1 activates the transcription of TSB1, WEI7, AMI1, and YUC7 through H2Bub1
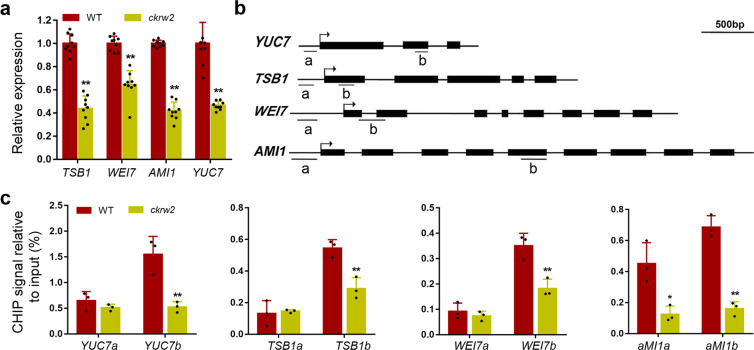
To investigate how ckrw2 mutation affected auxin homeostasis, we measured the expression of a number of known auxin biosynthesis genes2 by qRT-PCR (Fig. 3a and Supplementary Fig. 6), detecting significant reductions in the expression levels of TRP2/TSB1, WEI7/ASB1, YUC7, and AMI1 (Fig. 3a), which functioning at distinct steps in the complex tryptophan/auxin biosynthetic pathways, either upstream of L-Trp biosynthesis (ASB1/WEI7 and TSB1/TRP2)10,13, or downstream of it in the IPA pathway (YUC7) or the proposed IAM pathway (AMI1)2,59–62. Subsequent ChIP analysis detected a significantly lower amount of H2Bub1-associated DNA fragment in the coding but not 5′ upstream promoter or untranscribed regions of the four affected genes in ckrw2 mutant (Fig. 3b, c), which is a prominent feature of histone H2Bub1 modification in affecting gene activity in the process of transcriptional elongation. These data demonstrate that YUC7, TSB1, WEI7, and AMI1 in the auxin biosynthesis pathways are targeted by HUB1-mediated H2Bub1.
Fig. 3. Reduced expression of auxin synthesis genes in ckrw2 and analysis of H2Bub1 at these loci.
a Relative transcription levels of TSB1, WEI7, AMI1, and YUC7 genes in roots. ACTIN8 was used as an internal control. b Diagram representing the genomic structure and regions analyzed by ChIP assays, arrows indicate ATG start codon sites, and bars labeled “a” or “b” represent regions amplified by RT-qPCR in ChIP analysis. c H2Bub1 deposition at specific loci. LCRa and FLCP4 were used as a negative and positive control, respectively (shown in Supplementary Fig. 7). Data are presented as mean ± SD, three independent biological experiments, the asterisk indicates a significant difference based on Student’s t test with **P < 0.01, 0.01 < *P < 0.05.
To clarify the functional roles of each of the four affected genes in HUB1/2-mediated regulation on auxin homeostasis, we did the mutant analysis. Among their loss of function mutants, tsb1 and wei7 had slightly more obvious auxin-deficient phenotypes of ckrw2-like curling/waving primary roots with a reduced length, but yuc7 and ami1 had not (Supplementary Fig. 8a–c), suggesting that WEI7 and TSB1 are the two major functional genes in H2Bub1-mediated regulation on auxin biosynthesis. These two genes, encoding the β-subunit of anthranilate synthetase (WEI7/ASB1) complex and tryptophan synthase β (TSB1), respectively, are required for L-Trp biosynthesis8, and their roles in auxin biosynthesis10,63 and/or root waving64 have been confirmed. Consisting with that, ckrw2 tsb1 double mutant displayed very similar or the same phenotype of tsb1 single mutant (Supplementary Fig. 8a–c). Moreover, like wei7 and tsb1, L-TRP can rescue ckrw2, but not ckrc1 in Dr5:GUS expression and plant growth analyses (Fig. 4c; Supplementary Fig. 9), indicating that CKRW2 affects auxin homeostasis through regulating WEI7/ASB1 and TSB1 for L-Trp biosynthesis.
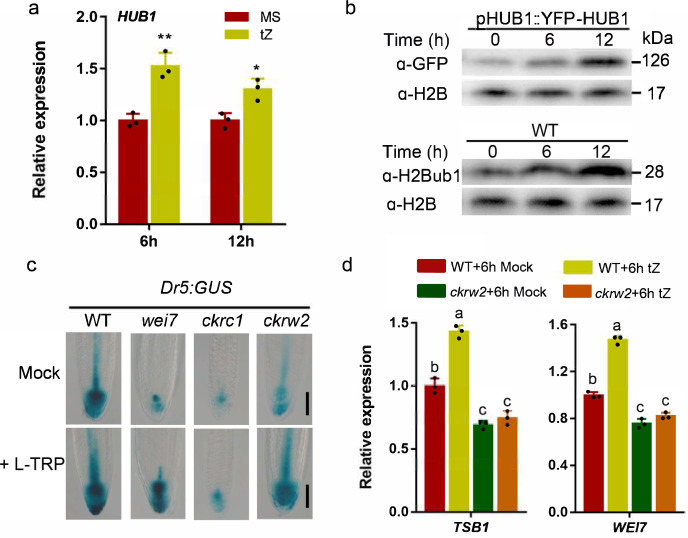
Fig. 4. Evidences supporting the role of HUB1/2-mediated H2Bub1 in mediating regulation on auxin biosynthesis by CK in Arabidopsis.
a, b Effects of 1 µM tZ on CKRW2/HUB1 expression and on the protein levels of YFP-HUB1 and H2Bub1. H2B was used as a loading control. c DR5:GUS activity showing the rescuing effect of 3 μM L-TRP on wei7 and ckrw2, but bot on ckrc1. Bar = 50 µm. d Comparison of relative expression levels of TSB1 and WEI7 genes between WT and ckrw2 mutant after tZ treatment. Data are presented as mean ± SD, three independent experiments, the asterisk b indicates a significant difference based on Student’s t test (**P < 0.01, 0.05 > *P > 0.01), and the letters d indicate a significant difference at P < 0.05, according to ANOVA followed by Tukey’s multiple comparison tests. ACTIN8 was used as an internal control (a, d).
The expression of CKRW2 are induced by CK
The above results promoted us to study how the regulation of auxin biosynthesis by CK was related to H2Bub1. Some mechanisms have been revealed for CK-mediated regulation on auxin production, mostly via transcriptional factors31,65,66. The qRT-PCR results (Fig. 4a) and both of GUS staining to detect the pCKRW2:GUS expression (Supplementary Fig. 8d) and YFP fluorescence intensity to detect the pHUB1::YFP-HUB1 expression (Supplementary Fig. 8e) showed that tZ treatment can stimulate HUB1/CKRW2 expression and increase the HUB1 protein level (Fig. 4b and Supplementary Fig. 10), leading to an increase of H2Bub1 activity. Consequently, the expression of TSB1 and WEI7 were significantly upregulated, which was not observed in ckrw2 mutant (Fig. 4d), revealing that this upregulation depends on CKRW2/HUB1 function.
In summary, our present studies reveal a mechanism at the chromatin level via H2Bub1 to control transcription of auxin biosynthesis genes. In this process, H2B proteins in the chromatin wrapped by the DNAs of auxin biosynthesis genes of WEI7 and TSB1 can be monoubiquitinated by HUB1/2 heterotetramer after the recruitment of UBC1/237, activating the transcriptional elongation of these genes33. Significantly, such an epigenetic mechanism via H2Bub1 can be used by CK as an effective way to regulate auxin biosynthesis through up-regulating HUB1/2 expression.
Methods
Plant material and growth conditions
The conditions of germination and growth, as previously described48, at 25 °C with a 16 h light/8 h dark photoperiod. For growth analyses, seedlings were grown on vertical MS (Murashige and Skoog) plates with 1.1% w/v agar supplemented with 10 g/L sucrose for 7 days.
Arabidopsis accession Col was used as WT. The hub1-5 (SALK_044415; AT2G44950), hub2-2 (SALK_071289; AT1G55250), and hub1-5 hub2-2 mutants were kindly provided by Prof. Ligen Ma (Capital Normal University) and Dr. Baohong Zou (Nanjing Agricultural University), and ami1 (N660737, AT1G08980) by Yan Guo (China Agriculture University). The Dr5: GUS/ Dr5: GFP/DII-VENUS marker line49,50, tsb1 (N8327; AT5G54810), wei7 (wei7 Dr5: GUS) (N16436; AT1G25220), yuc7 (N659416; AT2G33230) were purchased from the Nottingham Arabidopsis Stock Centre (NASC).
To generate ckrw2/DR5: GUS and ckrw2 tsb1 mutants, the ckrw2 mutant was crossed with DR5: GUS and tsb1 mutant, respectively, and double homozygous mutants were obtained from the F2 generation.
Phenotype characterization
The degree of root curling/waving (DC) was calculated by dividing the distance between the two ends of seedlings’ roots (L0) by the length of their roots (L)48. Primary root length was measured after grown vertically on MS plates for 7 days18,48.
For biochemical complementation experiments, seedlings were grown on a medium containing 0.01 μM tZ with auxin (0.01 μM 2, 4-D), and phenotypic observation and statistics were performed after 7 days of vertical cultivation.
For the L-TRP experiment, the 7-day-old seedlings were transferred to MS plates with or without 0.25 mM L-TRP and cultured for 2 weeks, and then the phenotype was observed67.
Gene cloning
For map-based cloning, ckrw2 plants from the F2 population of a cross between the ckrw2 and Ler and Simple sequence length polymorphism (SSLP) markers (Supplementary Data 2) were used to locate the chromosomal position of ckrw2 mutation, and the mutated gene was identified by WGRS (Hangzhou Guhe Information and Technology Co., Ltd., China. http://www.guheinfo.com/).
Molecular complementation and GUS transgenic plants
A 2 kb promoter sequence (2000 bp upstream of ATG) was amplified using pCKRW2-GUS-F (BamHI)/pCKRW2-GUS-R (NcoI) primers (Supplementary Data 2) and subcloned into a modified pCAMBIA1301 binary vector harboring a GUS gene to generate a pCKRW2:GUS reporter gene construct. For molecular complementation, the native CKRW2 promoter (2000 bp upstream of ATG, Supplementary Data 2) and CDS were amplified by PCR, and placed in a pCAMBIA1300 vector. All amplified DNA fragments were verified by sequencing, then transformed into WT plants (for pCKRW2:GUS) or ckrw2 mutant plants (for pCKRW2:CKRW2) by the floral dip method using Agrobacterium tumefaciens (GV3101)68. The seeds of the transformants were stratified in 4 °C for 3 days, sterilized with 0.1% mercuric chloride, washed with sterilized water, and then isolated on MS medium containing 25 mg/L hygromycin B. The seedlings were transferred to the soil until maturity.
Microscopic analysis
For the GUS staining assay, 7-day-old seedlings were placed in the centrifugal tube, fixed with pre-cooled acetone for 20 min, washed twice with GUS base solution (50 mM NaH2PO4·2H2O, 50 mM Na2HPO4·2H2O, 10 mM EDTA·2Na, 0.1% Triton100, 0.5 mM K3[Fe(CN)6], 0.5 mM K4Fe(CN)6·3H2O), and incubated at 37 °C with 1 mM X-gluc (5-bromo-4-chloro-3-indolyl-β-d-glucuronide acid), and visualized under a microscope (Axio Imager.Z2, Zeiss, Germany).
For confocal microscopic analyses, 7-day-old seedlings were treated in propidium iodide (PI) solution (10 μg/mL) for 5 min (time can be adjusted according to the pre-experiment), then washed three times with ddH2O, and visualized at 600–640 nm for PI and 500–560 nm for green fluorescent protein (GFP)/VENUS on a confocal microscope (TCS SP8, Leica, Germany). The DR5:GUS /DR5:GFP/DII-VENUS signal intensity of the root tip containing the GUS/GFP/VENUS signal (approximately to the first 200 µm from the root tip) was quantified by measuring the mean gray value with ImageJ69.
For detecting the effect of CK on HUB1, seedlings were grown on MS medium for 7 days and then transferred to a liquid medium containing 1 µM tZ for 6 h. And then GUS staining and fluorescence observation were performed.
RNA extraction and quantitative real-time PCR
RNA was isolated using Trizol (No. B511321, Sangon Biotech) and reverse-transcribed using a reverse transcription kit (RR047, TAKARA). Quantitative RT-PCR was performed in a Real-time System (Bio-RAD CFX96, America) using TB Green (RR820A, Takara), with primers listed in Supplementary Data 2. The auxin synthesis gene expression analysis was carried out using the primary roots of the seedling grown on the MS medium for 7 days.
Protein extraction and immunoblot analysis
In order to detect the protein levels of HUB1 and H2Bub1, 7-day-old seedlings of pHUB1::YFP-HUB1 and WT were treated with tZ for a different time, respectively. For protein extraction and immunoblot analysis, a previously used experimental procedure was followed70. H2B was used as a loading control. The immunoblot analysis was carried out using an anti-H2B antibody (ab1790, Abcam) at a concentration of 0.1 μg/mL, anti-H2Bub1 antibody (MM-0029, Medimabs) at a concentration of 3–5 μg per sample, and an anti-GFP antibody (M20004, Abmart) at a concentration of 0.2 μg/mL. The signal was detected by a chemiluminescent horseradish peroxidase substrate system (No. C500044, Sangon).
Chromatin immunoprecipitation (ChIP) assays
ChIP assays were performed as previously described71 using 7-day-old seedlings, which were grown on MS medium. In brief, the seedlings were vacuum cross-linked in 1% formaldehyde for 10 min, then 0.125 M glycine was added to the vacuum for 5 min to stop the cross-linking. To obtain 200–1000 bp DNA fragments, sonicate chromatin solution 5 times (5 s on, 15 s off in each time) by 50% power. Chromatin was immunoprecipitated using a specific anti-H2Bub1 antibody (MM-0029, Medimabs) and then specific protein A-agarose (11418033001, Roche). After the IP complex was pulled down and washed, the DNA was reverse cross-linked and then extracted using the phenol/chloroform method. The ChIP experiment used an equal amount of sample and protein A-agarose without antibody as a control. The ChIP DNA was finally analyzed by qPCR with three independent biological replicates.
Statistics and reproducibility
All results are expressed as the means ± standard deviation. The numbers of samples and replicates of experiments were shown as mentioned in the figure legends. Comparisons between groups were determined using Student’s t test (significant difference at 0.01 < *P < 0.05, **P < 0.01, ***P < 0.001) or ANOVA followed by Tukey’s multiple comparison test (significant difference at P < 0.05). All data were analyzed using GraphPad Prism 7 software.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We are grateful to Professor Ligen Ma (Capital Normal University), Professor Yan Guo (China Agricultural University), and Dr. Baohong Zou (Nanjing Agricultural University) for providing seeds. We thank Mieke Van Lijsebettens (Ghent University, Belgium) for providing microarray data analysis for genes involved in auxin biosynthetic pathways. And, we thank the Core Facility of the School of Life Sciences, Lanzhou University. This study was supported by the National Natural Science Foundation of China (NSFC) (grant Nos. 31970713 and 31671458).
Author contributions
G.Q.G. conceived and directed the research; L.Z. performed the major part of the research; P.L. isolated the ckrw2 mutant; J.B., L.W., D.W.D., H.Q.L., J.J.L., Y.L.L., A.J.K. performed the experiments; W.L. assisted G.Q.G. to organize and coordinate the project; L.Z. wrote the draft of the paper and the final revision was accomplished by G.Q.G.; C.M.Z. and G.Q.G supervised L.Z. in her post-doctoral program. All authors discussed the data and the article.
Data availability
The nucleotide sequence of CKRW2 was submitted to GenBank, and the accession number is BankIt2414347 ckrw2 MW431056. All other source data are included in the article as supplementary data 1–2. Uncropped scans of Western blots are shown in Supplementary Information. The unique biological materials of ckrw2, ckrw2/Dr5:GUS, ckrw2/DII-VENUS are available upon request to our lab.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chang-Ming Zhao, Email: zhaochm@lzu.edu.cn.
Guang-Qin Guo, Email: gqguo@lzu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-021-01733-x.
References
- 1.Zhao Y. Auxin biosynthesis. Arabidopsis Book. 2014;12:e0173. doi: 10.1199/tab.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di D-W, Zhang C, Luo P, An C-W, Guo G-Q. The biosynthesis of auxin: how many paths truly lead to IAA? Plant Growth Regul. 2015;78:275–285. doi: 10.1007/s10725-015-0103-5. [DOI] [Google Scholar]
- 3.Kasahara H. Current aspects of auxin biosynthesis in plants. Biosci. Biotechnol. Biochem. 2016;80:34–42. doi: 10.1080/09168451.2015.1086259. [DOI] [PubMed] [Google Scholar]
- 4.Paque S, Weijers D. Q&A: auxin: the plant molecule that influences almost anything. BMC Biol. 2016;14:67. doi: 10.1186/s12915-016-0291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korasick DA, Enders TA, Strader LC. Auxin biosynthesis and storage forms. J. Exp. Bot. 2013;64:2541–2555. doi: 10.1093/jxb/ert080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ljung K. Auxin metabolism and homeostasis during plant development. Development. 2013;140:943–950. doi: 10.1242/dev.086363. [DOI] [PubMed] [Google Scholar]
- 7.Normanly J, Cohen JD, Fink GR. Arabidopsis thaliana auxotrophs reveal a tryptophan-independent biosynthetic pathway for indole-3-acetic acid. Proc. Natl Acad. Sci. USA. 1993;90:10355–10359. doi: 10.1073/pnas.90.21.10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radwanski ER, Last RL. Tryptophan biosynthesis and metabolism: biochemical and molecular genetics. Plant Cell. 1995;7:921–934. doi: 10.1105/tpc.7.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niyogi KK, Last RL, Fink GR, Keith B. Suppressors of trp1 fluorescence identify a new Arabidopsis gene, TRP4, encoding the anthranilate synthase beta subunit. Plant Cell. 1993;5:1011–1027. doi: 10.1105/tpc.5.9.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM. A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell. 2005;17:2230–2242. doi: 10.1105/tpc.105.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radwanski ER, Barczak AJ, Last RL. Characterization of tryptophan synthase alpha subunit mutants of Arabidopsis thaliana. Mol. Gen. Genet. 1996;253:353–361. doi: 10.1007/pl00008602. [DOI] [PubMed] [Google Scholar]
- 12.Last RL, Bissinger PH, Mahoney DJ, Radwanski ER, Fink GR. Tryptophan mutants in Arabidopsis: the consequences of duplicated tryptophan synthase beta genes. Plant Cell. 1991;3:345–358. doi: 10.1105/tpc.3.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiyohara S, et al. Tryptophan auxotroph mutants suppress the superroot2 phenotypes, modulating IAA biosynthesis in Arabidopsis. Plant Signal. Behav. 2011;6:1351–1355. doi: 10.4161/psb.6.9.16321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao Y, et al. Two homologous INDOLE-3-ACETAMIDE (IAM) HYDROLASE genes are required for the auxin effects of IAM in Arabidopsis. J. Genet. Genomics. 2020;47:157–165. doi: 10.1016/j.jgg.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stepanova AN, et al. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell. 2008;133:177–191. doi: 10.1016/j.cell.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 16.Tao Y, et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell. 2008;133:164–176. doi: 10.1016/j.cell.2008.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada M, Greenham K, Prigge MJ, Jensen PJ, Estelle M. The TRANSPORT INHIBITOR RESPONSE2 gene is required for auxin synthesis and diverse aspects of plant development. Plant Physiol. 2009;151:168–179. doi: 10.1104/pp.109.138859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou ZY, et al. Functional characterization of the CKRC1/TAA1 gene and dissection of hormonal actions in the Arabidopsis root. Plant J. 2011;66:516–527. doi: 10.1111/j.1365-313X.2011.04509.x. [DOI] [PubMed] [Google Scholar]
- 19.Chen Q, et al. Auxin overproduction in shoots cannot rescue auxin deficiencies in Arabidopsis roots. Plant Cell Physiol. 2014;55:1072–1079. doi: 10.1093/pcp/pcu039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di DW, et al. Functional roles of Arabidopsis CKRC2/YUCCA8 gene and the involvement of PIF4 in the regulation of auxin biosynthesis by cytokinin. Sci. Rep. 2016;6:36866. doi: 10.1038/srep36866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, et al. YUCCA-mediated auxin biogenesis is required for cell fate transition occurring during de novo root organogenesis in Arabidopsis. J. Exp. Bot. 2016;67:4273–4284. doi: 10.1093/jxb/erw213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang, Y. et al. GmYUC2a is important for mediating auxin biosynthesis during root development and nodulation in soybean. J Exp. Bot. 70, 3165–3176 (2019). [DOI] [PMC free article] [PubMed]
- 23.Wang Q, et al. A phosphorylation-based switch controls TAA1-mediated auxin biosynthesis in plants. Nat. Commun. 2020;11:679. doi: 10.1038/s41467-020-14395-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra BS, Singh M, Aggrawal P, Laxmi A. Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development. PLoS ONE. 2009;4:e4502. doi: 10.1371/journal.pone.0004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sairanen I, et al. Soluble carbohydrates regulate auxin biosynthesis via PIF proteins in Arabidopsis. Plant Cell. 2012;24:4907–4916. doi: 10.1105/tpc.112.104794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma W, et al. Auxin biosynthetic gene TAR2 is involved in low nitrogen-mediated reprogramming of root architecture in Arabidopsis. Plant J. 2014;78:70–79. doi: 10.1111/tpj.12448. [DOI] [PubMed] [Google Scholar]
- 27.Yu LH, et al. MADS-box transcription factor AGL21 regulates lateral root development and responds to multiple external and physiological signals. Mol. Plant. 2014;7:1653–1669. doi: 10.1093/mp/ssu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang ZB, et al. TAA1-regulated local auxin biosynthesis in the root-apex transition zone mediates the aluminum-induced inhibition of root growth in Arabidopsis. Plant Cell. 2014;26:2889–2904. doi: 10.1105/tpc.114.127993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones B, et al. Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction. Plant Cell. 2010;22:2956–2969. doi: 10.1105/tpc.110.074856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng WJ, et al. Type-B ARABIDOPSIS RESPONSE REGULATORs Specify the shoot stem cell niche by dual regulation of WUSCHEL. Plant Cell. 2017;29:1357–1372. doi: 10.1105/tpc.16.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan Z, et al. Type B response regulators act as central integrators in transcriptional control of the auxin biosynthesis enzyme TAA1. Plant Physiol. 2017;175:1438–1454. doi: 10.1104/pp.17.00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavri R, et al. Histone H2B monoubiquitination functions cooperatively with FACT to regulate elongation by RNA polymerase II. Cell. 2006;125:703–717. doi: 10.1016/j.cell.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 33.Woloszynska, M. et al. Histone 2B monoubiquitination complex integrates transcript elongation with RNA processing at circadian clock and flowering regulators. Proc. Natl Acad. Sci. USA116, 8060–8069 (2019). [DOI] [PMC free article] [PubMed]
- 34.Bergmuller E, Gehrig PM, Gruissem W. Characterization of post-translational modifications of histone H2B-variants isolated from Arabidopsis thaliana. J. Proteome Res. 2007;6:3655–3668. doi: 10.1021/pr0702159. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Koornneef M, Soppe WJ. The absence of histone H2B monoubiquitination in the Arabidopsis hub1 (rdo4) mutant reveals a role for chromatin remodeling in seed dormancy. Plant Cell. 2007;19:433–444. doi: 10.1105/tpc.106.049221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleury D, et al. The Arabidopsis thaliana homolog of yeast BRE1 has a function in cell cycle regulation during early leaf and root growth. Plant Cell. 2007;19:417–432. doi: 10.1105/tpc.106.041319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao Y, Dai Y, Cui S, Ma L. Histone H2B monoubiquitination in the chromatin of FLOWERING LOCUS C regulates flowering time in Arabidopsis. Plant Cell. 2008;20:2586–2602. doi: 10.1105/tpc.108.062760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Himanen K, et al. Histone H2B monoubiquitination is required to reach maximal transcript levels of circadian clock genes in Arabidopsis. Plant J. 2012;72:249–260. doi: 10.1111/j.1365-313X.2012.05071.x. [DOI] [PubMed] [Google Scholar]
- 39.Malapeira J, Khaitova LC, Mas P. Ordered changes in histone modifications at the core of the Arabidopsis circadian clock. Proc. Natl Acad. Sci. USA. 2012;109:21540–21545. doi: 10.1073/pnas.1217022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou B, Yang DL, Shi Z, Dong H, Hua J. Monoubiquitination of histone 2B at the disease resistance gene locus regulates its expression and impacts immune responses in Arabidopsis. Plant Physiol. 2014;165:309–318. doi: 10.1104/pp.113.227801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen, H. et al. An Arabidopsis E3 ligase HUB2 increases histone H2B monoubiquitination and enhances drought tolerance in transgenic cotton. Plant Biotechnol. J.17, 556–568 (2018). [DOI] [PMC free article] [PubMed]
- 42.Gu X, Jiang D, Wang Y, Bachmair A, He Y. Repression of the floral transition via histone H2B monoubiquitination. Plant J. 2009;57:522–533. doi: 10.1111/j.1365-313X.2008.03709.x. [DOI] [PubMed] [Google Scholar]
- 43.Xu L, et al. The E2 ubiquitin-conjugating enzymes, AtUBC1 and AtUBC2, play redundant roles and are involved in activation of FLC expression and repression of flowering in Arabidopsis thaliana. Plant J. 2009;57:279–288. doi: 10.1111/j.1365-313X.2008.03684.x. [DOI] [PubMed] [Google Scholar]
- 44.Bourbousse C, et al. Histone H2B monoubiquitination facilitates the rapid modulation of gene expression during Arabidopsis photomorphogenesis. PLoS Genet. 2012;8:e1002825. doi: 10.1371/journal.pgen.1002825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhawan R, et al. HISTONE MONOUBIQUITINATION1 interacts with a subunit of the mediator complex and regulates defense against necrotrophic fungal pathogens in Arabidopsis. Plant Cell. 2009;21:1000–1019. doi: 10.1105/tpc.108.062364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu M, Pei BL, Zhang LF, Li YZ. Histone H2B monoubiquitination is involved in regulating the dynamics of microtubules during the defense response to Verticillium dahliae toxins in Arabidopsis. Plant Physiol. 2014;164:1857–1865. doi: 10.1104/pp.113.234567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao J, et al. H2Bub1 regulates RbohD-dependent hydrogen peroxide signal pathway in the defense responses to Verticillium dahliae toxins. Plant Physiol. 2020;182:640–657. doi: 10.1104/pp.19.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu L, et al. Forward genetic screen for auxin-deficient mutants by cytokinin. Sci. Rep. 2015;5:11923. doi: 10.1038/srep11923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brunoud G, et al. A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nature. 2012;482:103–106. doi: 10.1038/nature10791. [DOI] [PubMed] [Google Scholar]
- 51.Weigel D, et al. Activation tagging in Arabidopsis. Plant Physiol. 2000;122:1003–1013. doi: 10.1104/pp.122.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou S, Chen Q, Sun Y, Li Y. Histone H2B monoubiquitination regulates salt stress-induced microtubule depolymerization in Arabidopsis. Plant Cell Environ. 2017;40:1512–1530. doi: 10.1111/pce.12950. [DOI] [PubMed] [Google Scholar]
- 53.Menard R, et al. Histone H2B monoubiquitination is involved in the regulation of cutin and wax composition in Arabidopsis thaliana. Plant Cell Physiol. 2014;55:455–466. doi: 10.1093/pcp/pct182. [DOI] [PubMed] [Google Scholar]
- 54.Feng H, et al. GhHUB2, a ubiquitin ligase, is involved in cotton fiber development via the ubiquitin-26S proteasome pathway. J. Exp. Bot. 2018;69:5059–5075. doi: 10.1093/jxb/ery269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma S, et al. Reversible histone H2B monoubiquitination fine-tunes abscisic acid signaling and drought response in rice. Mol. Plant. 2019;12:263–277. doi: 10.1016/j.molp.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 56.Zhang B, et al. The chromatin-modifying protein HUB2 is involved in the regulation of lignin composition in xylem vessels. J. Exp. Bot. 2020;71:5484–5494. doi: 10.1093/jxb/eraa264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang H, et al. OsHUB1 and OsHUB2 interact with SPIN6 and form homo- and hetero-dimers in rice. Plant Signal. Behav. 2015;10:e1039212. doi: 10.1080/15592324.2015.1039212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nawy T, et al. Transcriptional profile of the Arabidopsis root quiescent center. Plant Cell. 2005;17:1908–1925. doi: 10.1105/tpc.105.031724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pollmann S, Müller A, Piotrowski M, Weiler EW. Occurrence and formation of indole-3-acetamide in Arabidopsis thaliana. Planta. 2002;216:155–161. doi: 10.1007/s00425-002-0868-4. [DOI] [PubMed] [Google Scholar]
- 60.Neu D, Lehmann T, Elleuche S, Pollmann S. Arabidopsis amidase 1, a member of the amidase signature family. FEBS J. 2007;274:3440–3451. doi: 10.1111/j.1742-4658.2007.05876.x. [DOI] [PubMed] [Google Scholar]
- 61.Mano Y, et al. The AMI1 gene family: indole-3-acetamide hydrolase functions in auxin biosynthesis in plants. J. Exp. Bot. 2010;61:25–32. doi: 10.1093/jxb/erp292. [DOI] [PubMed] [Google Scholar]
- 62.Sanchez-Parra B, et al. Characterization of four bifunctional plant IAM/PAM-amidohydrolases capable of contributing to auxin biosynthesis. Plants. 2014;3:324–347. doi: 10.3390/plants3030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nonhebel HM. Tryptophan-independent indole-3-acetic acid synthesis: critical evaluation of the evidence. Plant Physiol. 2015;169:1001–1005. doi: 10.1104/pp.15.01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rutherford R, Gallois P, Masson PH. Mutations in Arabidopsis thaliana genes involved in the tryptophan biosynthesis pathway affect root waving on tilted agar surfaces. Plant J. 1998;16:145–154. doi: 10.1046/j.1365-313x.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- 65.Wojcikowska B, et al. LEAFY COTYLEDON2 (LEC2) promotes embryogenic induction in somatic tissues of Arabidopsis, via YUCCA-mediated auxin biosynthesis. Planta. 2013;238:425–440. doi: 10.1007/s00425-013-1892-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou Y, et al. TCP transcription factors regulate shade avoidance via directly mediating the expression of both phytochrome interacting factors and auxin biosynthetic genes. Plant Physiol. 2018;176:1850–1861. doi: 10.1104/pp.17.01566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jing Y, et al. Tryptophan deficiency affects organ growth by retarding cell expansion in Arabidopsis. Plant J. 2009;57:511–521. doi: 10.1111/j.1365-313X.2008.03706.x. [DOI] [PubMed] [Google Scholar]
- 68.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, et al. Auxin-mediated statolith production for root gravitropism. N. Phytol. 2019;224:761–774. doi: 10.1111/nph.15932. [DOI] [PubMed] [Google Scholar]
- 70.Qin Q, et al. Arabidopsis DELLA protein degradation is controlled by a type-one protein phosphatase, TOPP4. PLoS Genet. 2014;10:e1004464. doi: 10.1371/journal.pgen.1004464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gendrel A-V, Martienssen R, Colot V. Profiling histone modification patterns in plants using genomic tiling microarrays. Nat. Methods. 2005;2:213–218. doi: 10.1038/nmeth0305-213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The nucleotide sequence of CKRW2 was submitted to GenBank, and the accession number is BankIt2414347 ckrw2 MW431056. All other source data are included in the article as supplementary data 1–2. Uncropped scans of Western blots are shown in Supplementary Information. The unique biological materials of ckrw2, ckrw2/Dr5:GUS, ckrw2/DII-VENUS are available upon request to our lab.