Abstract
Background
Acute gastroenteritis (AGE) is a common reason for children to receive medical care. However, the viral etiology of AGE illness is not well described in the post–rotavirus vaccine era, particularly in the outpatient (OP) setting.
Methods
Between 2012 and 2015, children 15 days through 17 years old presenting to Vanderbilt Children’s Hospital, Nashville, Tennessee, with AGE were enrolled prospectively from the inpatient, emergency department, and OP settings, and stool specimens were collected. Healthy controls (HCs) were enrolled and frequency matched for period, age group, race, and ethnicity. Stool specimens were tested by means of reverse-transcription real-time quantitative polymerase chain reaction for norovirus, sapovirus, and astrovirus RNA and by Rotaclone enzyme immunoassay for rotavirus antigen, followed by polymerase chain reaction verification of antigen detection.
Results
A total of 3705 AGE case patients and 1563 HCs were enrolled, among whom 2885 case patients (78%) and 1110 HCs (71%) provided stool specimens that were tested. All 4 viruses were more frequently detected in AGE case patients than in HCs (norovirus, 22% vs 8%, respectively; rotavirus, 10% vs 1%; sapovirus, 10% vs 5%; and astrovirus, 5% vs 2%; P < .001 for each virus). In the OP setting, rates of AGE due to norovirus were higher than rate for the other 3 viruses. Children <5 years old had higher OP AGE rates than older children for all viruses.
Conclusions
Norovirus remains the most common virus detected in all settings, occurring nearly twice as frequently as the next most common pathogens, sapovirus and rotavirus. Combined, norovirus, sapovirus, rotavirus, and astrovirus were associated with almost half of all AGE visits and therefore are an important reason for children to receive medical care.
Keywords: acute gastroenteritis, viral detection, outpatient, rotavirus, healthy controls
Our study documented a decline in rotavirus post–rotavirus vaccine implementation and noted that norovirus has emerged to be the leading cause of pediatric acute gastroenteritis. Enteric viruses accounted for nearly half of the cases.
Acute gastroenteritis (AGE) is the second leading cause of death worldwide in children <5 years old, outside the neonatal period [1]. Specifically, rotavirus infection is the leading cause of diarrhea-associated disease and death in young children worldwide [2]; however, the implementation of rotavirus vaccination has decreased rotavirus-associated mortality rates markedly [2]. Moreover, rotavirus vaccination has contributed to the overall decrease of all causes of AGE [3]. In the United States, the New Vaccine Surveillance Network (NVSN) documented norovirus as the leading cause of AGE hospitalization and emergency department (ED) visits in children <5 years old after rotavirus vaccine introduction [4]. Specifically, norovirus-attributable AGE was estimated to contribute to 14 000 hospitalizations, 281 000 ED visits, and 627 000 outpatient (OP) visits per year in children <5 years old in the United States [4].
In the United States, other studies found evidence of a decreased overall incidence of rotavirus-associated disease in the post–rotavirus vaccine era, with a novel biennial pattern of rotavirus incidence and an increased median age of rotavirus case patients [5]. Despite the increasing age of children with rotavirus, the pathogenic causes of viral AGE in older children are not well characterized. Moreover, less is known about OP rates for other common AGE viruses (eg, sapovirus and astrovirus) owing to a lack of prospective AGE OP surveillance studies.
Gastroenteritis multiplex molecular assays have been adopted for use mainly in inpatient (IP) hospital settings [6, 7]; these molecular assays are very sensitive, and it is sometimes unclear whether multiple detection of pathogens represents disease, carriage, or remote infection. In addition, most clinicians do not order stool sample testing for children with mild-to-moderate AGE illness and typically advise parents to treat these children with supportive care; therefore, the true OP burden of viral AGE is probably underestimated. Furthermore, the role of sapovirus and astrovirus in the disease burden of pediatric AGE hospitalizations, ED, and OP visits had not been well described. Therefore, we sought to elucidate potential differences in rates of viral AGE due to rotavirus, norovirus, astrovirus, and sapovirus from children enrolled prospectively from IP, ED, and OP settings who presented with vomiting and/or diarrhea. Because asymptomatic carriage is also not well defined for these viruses, we compared viral detection in AGE case patients with that in healthy controls (HCs).
METHODS
Study Design
Active, population-based viral AGE surveillance in children who presented to IP, ED, and OP settings was conducted in Nashville, between 1 December
2012 and 30 November 2015 as a site-specific activity in the NVSN [8–10]. During these 3 surveillance years, HCs were also enrolled and frequency matched by period, age group, race, and ethnicity.
Study Population
AGE Case Patients
AGE case patients who presented to Vanderbilt University Medical Center Children’s Hospital within 10 days after symptom onset and who resided in Davidson County were eligible. Hospitalized children were approached if they were between the ages of 15 days to 10 years, and children seen in the ED or OP clinic if they were between the ages of 15 days and 17 years. AGE illness was defined as having diarrhea (≥3 episodes of loose stools within 24 hours) and/or vomiting (≥1 episode within 24 hours).
HC Group
HCs between the ages of 15 days to 17 years and residents of Davidson County were approached during a scheduled well-child visit at the Vanderbilt University Medical Center pediatric OP clinic. HCs were frequency matched by age, race/ethnicity, and time of enrollment based on AGE case patients who provided a stool sample, with case-control ratios of 2:1 in the first 2 study years and 3:1 in the third year. HC children were deemed ineligible if they reported acute respiratory infection symptoms within 3 days of enrollment, AGE symptoms within 14 days, or clinical immunodeficiency.
After informed written consent from a parent or guardian, demographic and clinical data were collected through parent/guardian interviews, and records were reviewed to determine outcome data. Institutional review board approval was obtained from the Centers for Disease Control and Prevention, the Tennessee Department of Health, and Vanderbilt University Medical Center.
Specimen Testing
Per protocol, whole stool specimens were collected within 5 and 10 days after enrollment for HCs and AGE case patients, respectively and those collected outside the window period were excluded. (See Supplementary Material for details of testing.)
Analyses
Descriptive statistics were summarized as frequency (percentage), median (interquartile range), or mean (standard deviation), as appropriate. Across-group comparisons were performed using the Pearson × 2 test for categorical variables and a 2-sample t test allowing unequal variances or linear regression with robust standard errors for continuous variables. We used a significance level of .05 (2-tailed) for all analyses. All statistical analyses were performed using StataCorp software (version 15.0). Cycle threshold (Ct) values were calculated to estimate viral load, with lower Ct values representing higher viral loads, which are semiquantitative. Ct values in case patients versus HCs and single versus codetection were compared. OP AGE rates and OP virus-specific rates were calculated (see Supplementary Material for comprehensive description).
RESULTS
Study Population
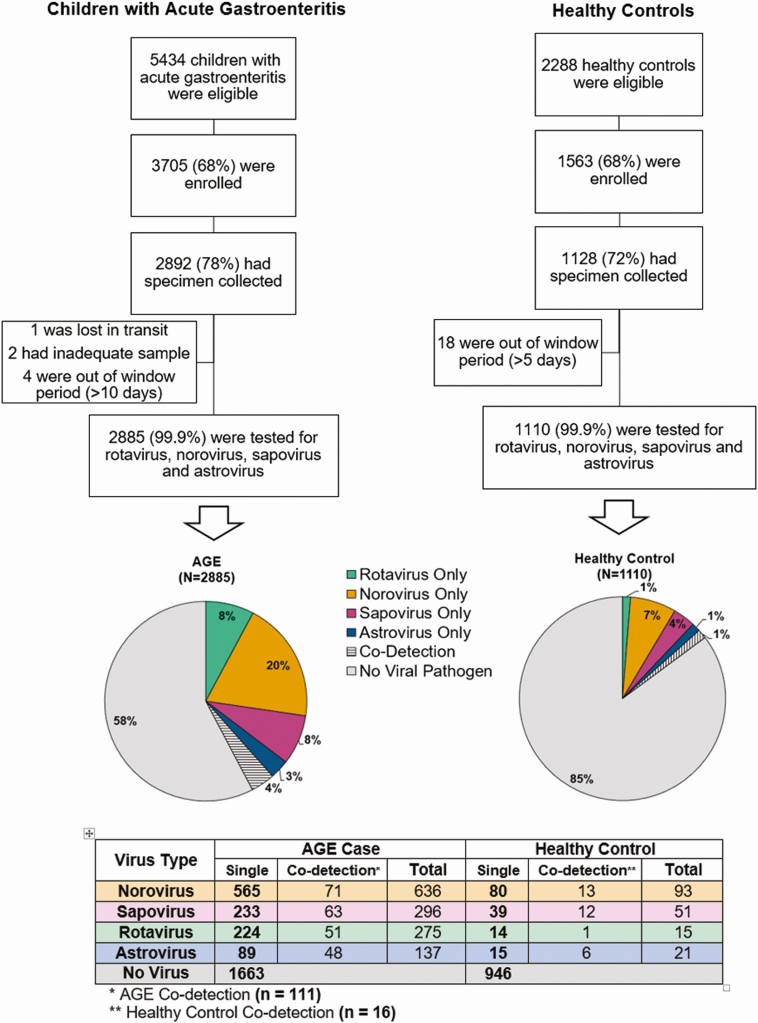
Our final cohort of 2885 AGE case patients and 1110 HCs is outlined in Figure 1. Demographic and clinical characteristics of enrolled AGE case patients who provided a stool specimen are presented in Table 1. The vast majority of children were enrolled from the ED setting, followed by the OP and IP settings. The demographic characteristics of AGE case patients and HCs were similar, except that HCs were significantly less likely than case patients to have been born prematurely, to attend preschool or school, or to have been exposed to AGE (Table 1).
Figure 1.
Study enrollment algorithm and results of viral testing. Abbreviations: AGE, acute gastroenteritis; HCs, healthy controls.
Table 1.
Demographic and Clinical Characteristics of Children Whose Stool Samples Were Tested
| Children, No. (%)a | AGE Case Patientsa | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | HCs (n = 1110) | AGE Case Patients (n = 2885) | P Valueb | IP (n = 231) | ED (n = 1657) | OP (n = 997) | P Valueb |
| Male sex | 564 (51) | 1510 (52) | .39 | 140 (61) | 863 (52) | 507 (51) | .03 |
| Age, median (IQR), y | 1.8 (0.8–5.0) | 1.9 (0.9–4.9) | 1.1 (0.2–2.9) | 1.9 (0.9–5.0) | 2.1 (1.0–5.3) | ||
| Race | |||||||
| White | 672 (61) | 1699 (59) | .05 | 159 (69) | 895 (54) | 645 (65) | <.001 |
| Black | 381 (34) | 976 (34) | 59 (26) | 644 (39) | 273 (27) | ||
| Other | 57 (5) | 210 (7) | 13 (6) | 118 (7) | 79 (8) | ||
| Hispanic/Latino ethnicity | 444 (40) | 1176 (41) | .66 | 73 (32) | 582 (35) | 521 (52) | <.001 |
| Insurancec | |||||||
| Public | 992/1107 (90) | 2507/2858 (88) | .17 | 165/229 (72) | 1409/1637 (86) | 933/992 (94) | <.001 |
| Private | 96/1107 (9) | 305/2858 (11) | 62/229 (27) | 192/1637 (12) | 51/992(5) | ||
| Premature birthd | 44/835 (5) | 190/2158 (9) | .001 | 21 (11) | 109/1230 (9) | 60/728 (8) | .60 |
| Breastfeedingd | 802/1107 (72) | 2053/2863 (72) | .64 | 151/199 (76) | 845/1233 (69) | 595/730 (82) | <.001 |
| Preschool/school attendance | 358 (32) | 1180/2881 (41) | <.001 | 57 (25) | 705/1653 (43) | 418 (42) | <.001 |
| AGE exposuree | 49 (4) | 873 (30) | <.001 | 47 (20) | 492 (30) | 334 (34) | <.001 |
| Duration of illness, mean (SD), df | NA | 2.93 (1.9) | 3.49 (2.0) | 2.64 (1.8) | 3.29 (1.9) | <.001g | |
| Use of Diapersd | NA | 1833/2171 (84) | 179/200 (90) | 1049/1238 (85) | 605/733 (83) | .050 | |
| Antibiotics administered during visit | NA | 135/2878 (5) | 63/230 (27) | 57/1654 (3) | 15/994 (2) | <.001 | |
| IRT during visit | NA | 371/2880 (13) | 186 (81) | 182/1654 (11) | 3/995 (0) | <.001 | |
Numbers are n (%), median (IQR) or mean (SD).
Abbreviations: AGE, acute gastroenteritis; ED, emergency department; HCs, healthy controls; IP, inpatient; IQR, interquartile range; IRT, intravenous rehydration therapy; NA, not applicable; OP, outpatient; SD, standard deviation.
aData represent no. (%) of children unless otherwise specified.
b P values based on Pearson × 2 test, unless otherwise specified.
cAs reported by parent or legal guardian.
dOnly in children <5 years old.
eExposure inside or outside household.
fDuration at presentation.
g P value based on linear regression with robust standard errors.
Comparisons by Enrollment Settings
Compared with AGE case patients from the ED and OP settings, hospitalized children with AGE were more likely to be white and male and less likely to attend preschool or school or to have had contact with someone with AGE (Table 1). Children with AGE who presented to the ED, compared with those from other settings, were more likely to be black, less likely to have been breastfed, and had shorter reported duration of illness before enrollment (Table 1). Children in the OP setting had a higher percentage of Hispanic ethnicity and were more likely to have public insurance (Table 1). No differences in prematurity were noted between the settings; however, hospitalized children were more likely to receive antibiotics and intravenous rehydration therapy (Table 1).
Virus Testing
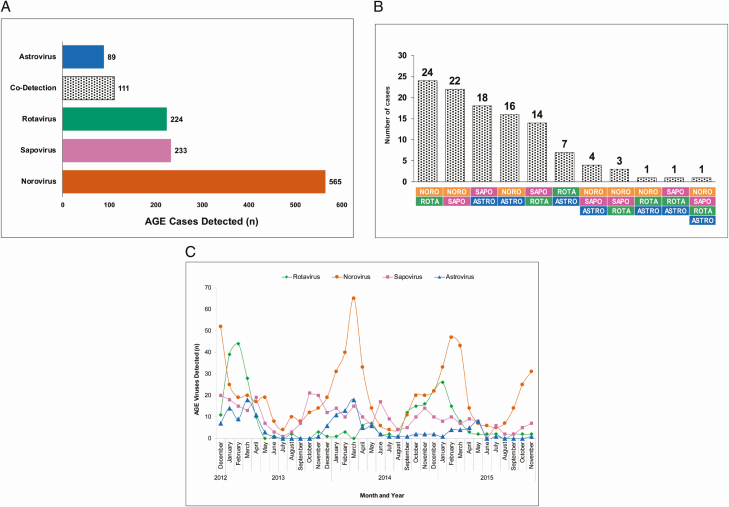
At least 1 virus was detected in 1222 (42%) of 2885 stool samples from case patients (Figure 1). Specifically, norovirus was detected in 636 AGE samples, sapovirus in 296, rotavirus in 275, and astrovirus in 137 (Figure 1); among these, 565 (89%), 233 (79%), 224 (81%), and 89 (65%), respectively, were single-virus detections. More than 1 virus was detected in 111 (9%) of virus-positive AGE case patients (Figure 2A and 2B). Of the HC samples, 164 (15%) tested positive for ≥1 virus: 93 with norovirus, 51 with sapovirus, 21 with astrovirus, and 15 with rotavirus (Figure 1); among these, 80 (86%), 39 (76%), 15 (71%), and 14 (93%), respectively, were single-virus detections. More than 1 virus was detected in 16 (10%) of virus-positive HC samples (Figure 1). All 4 viruses (includes total number detected) were more frequently detected in AGE case patients than in HCs (norovirus, 22% vs 8%, respectively; rotavirus, 10% vs 1%; sapovirus, 10% vs 5%; and astrovirus, 5% vs 2%; P < .001 for each virus, respectively). Of note, 89% of the rotavirus strains (244 of 275) were G12P[8] during this 3-year period.
Figure 2.
A, Viral pathogens detected in acute gastroenteritis (AGE) case patients. Total numbers of AGE case patients with astrovirus, rotavirus, sapovirus, norovirus, or codetections are shown. B, Viral codetections, with combinations represented by colors below bars. C, Seasonal monthly distribution of AGE viruses detected in case patients over the study period. Abbreviation: AGE, acute gastroenteritis.
Children with no viral detection were older and less likely to be white than those in all virus-positive groups (Table 2). Prior AGE exposure was reported at a higher frequency for children with rotavirus, norovirus, and >1 pathogen group (Table 2). Children with norovirus reported the shortest duration of symptoms before enrollment, compared with other groups (Table 2).
Table 2.
Demographics and Clinical Characteristics of Children with AGE by Viral Pathogens Detected
| Children with AGE, No. (%)a | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Rotavirus Only (n = 224) | Norovirus Only (n = 565) | Astrovirus Only (n = 89) | Sapovirus Only (n = 233) | >1 Viral Pathogen (n = 111) | No Viral Pathogen (n = 1663) | P Valueb |
| Surveillance yearc | |||||||
| 2012–2013 | 109 (49) | 148 (26) | 27 (30) | 89 (38) | 45 (41) | 600 (36) | <.001 |
| 2013–2014 | 55 (25) | 213 (38) | 46 (52) | 98 (42) | 33 (29) | 558 (34) | |
| 2014–2015 | 60 (27) | 204 (36) | 16 (18) | 46 (20) | 33 (29) | 505 (30) | |
| Male sex | 124 (55) | 278 (49) | 49 (55) | 126 (54) | 56 (50) | 877 (53) | .57 |
| Age, y | |||||||
| Mean (SD) | 3.29 (3.00) | 3.12 (3.45) | 2.99 (2.14) | 2.86 (2.92) | 2.52 (2.89) | 3.73 (4.03) | <.001d |
| Median (IQR) | 2.2 (1.2–4.7) | 1.6 (0.9–3.9) | 2.3 (1.4–4.3) | 1.8 (1.0–3.4) | 1.5 (0.9–2.7) | 2.0 (0.8–5.7) | |
| <1 | 43 (19) | 174 (31) | 12 (13) | 61 (26) | 35 (32) | 516 (31) | |
| 1 | 62 (28) | 148 (26) | 26 (29) | 65 (28) | 41 (37) | 324 (19) | |
| 2–4 | 67 (30) | 129 (23) | 37 (42) | 63 (27) | 18 (16) | 350 (21) | |
| 5–17 | 52 (23) | 114 (20) | 14 (16) | 44 (19) | 17 (15) | 473 (28) | |
| Race | |||||||
| White | 147 (66) | 364 (64) | 59 (66) | 139 (60) | 66 (59) | 924 (56) | .01 |
| Black | 66 (29) | 161 (29) | 24 (27) | 78 (33) | 38 (35) | 609 (37) | |
| Other | 11 (5) | 40 (7) | 6 (7) | 16 (7) | 7 (6) | 130 (8) | |
| Hispanic/Latino ethnicity | 92 (41) | 260 (46) | 36 (40) | 100 (43) | 44 (40) | 644 (39) | .08 |
| Premature birthe | 17/172 (10) | 29/446 (7) | 7/75(9) | 18/189 (10) | 9/94 (10) | 110/1182 (9) | .58 |
| Breastfeedinge | 119/171 (70) | 343/450 (76) | 57/75 (76) | 135/188 (72) | 64/93 (69) | 873/1185 (74) | .46 |
| Preschool or school attendance | 96/223 (43) | 196 (35) | 40 (45) | 100 (43) | 48 (43) | 700/1660 (42) | .04 |
| AGE exposuref | 81 (36) | 218 (39) | 25 (28) | 63 (27) | 40 (36) | 446 (27) | <.001 |
| Duration of illness, mean (SD), dg | 2.82 (1.6) | 2.57 (1.8) | 3.20 (2.0) | 3.26 (2.0) | 2.81 (2.0) | 3.02 (2.0) | <.001d |
| Use of Diaperse | 143/172 (83) | 387/451 (86) | 55/75 (73) | 166/189 (88) | 83/94 (74) | 1016 (61) | .06 |
| Antibiotics administered during visit | 12 (5) | 19/563 (3) | 6 (7) | 6 (3) | 3 (3) | 89/1658 (5) | .14 |
| IRT during visit | 38 (17.0) | 76/564 (13) | 9 (10) | 20 (9) | 15 (14) | 213/1659 (13) | .16 |
Abbreviations: AGE, acute gastroenteritis; IQR, interquartile range; IRT, intravenous rehydration therapy; SD, standard deviation.
aData represent no. (%) of children unless otherwise specified.
b P values based on Pearson × 2 test, unless otherwise noted.
cSurveillance year from December 1 to November 30.
d P value based on linear regression with robust standard errors.
eOnly in children <5 years old.
fExposure inside or outside household.
gDuration at presentation.
Seasonality
Seasonal variations in AGE viruses are presented in Figure 2C. Norovirus and astrovirus peaked during winter months. Rotavirus peaked in the 2012–2013 and 2014–2015 winter seasons, whereas in the 2013–2014 season the number of positive samples was very low. Sapovirus did not show a strong seasonal pattern; however, detections were lowest in summer months.
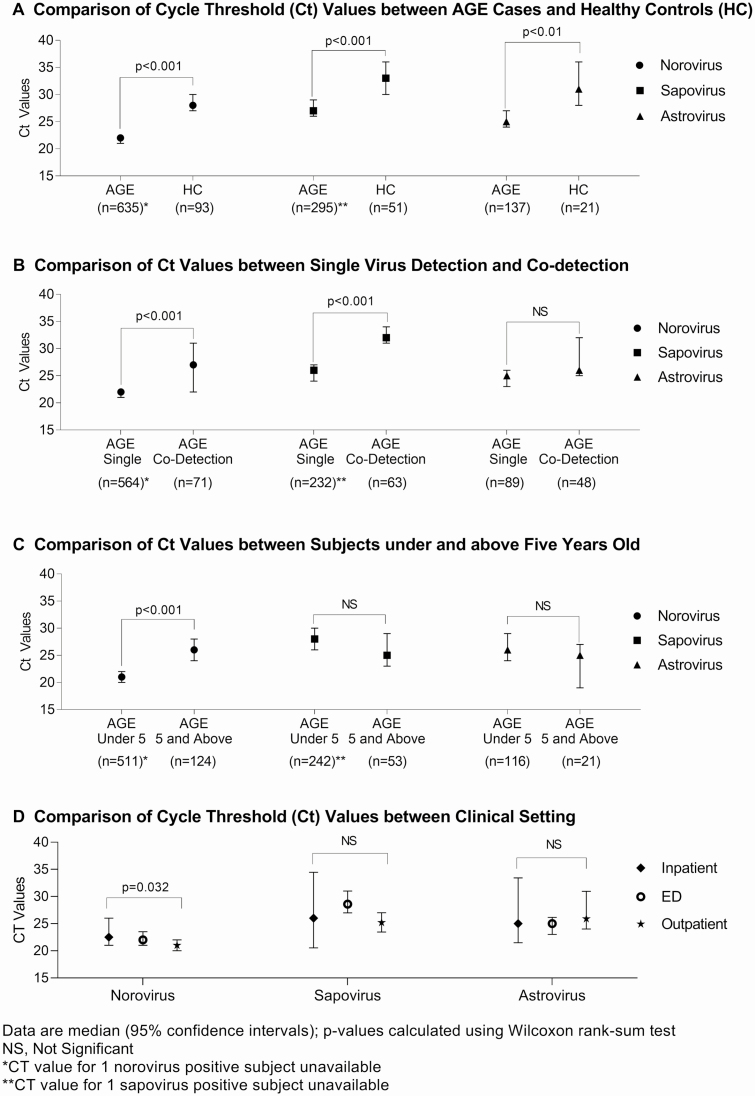
Estimated Viral Load of Norovirus, Astrovirus, and Sapovirus
The median Ct values of all 3 viruses were significantly lower in AGE case patients than in HCs, indicative of a higher viral load (Figure 3A). Specimens with single norovirus and sapovirus detections also had significantly lower Ct values than specimens with coviral detections (Figure 3B). Norovirus Ct values were statistically lower in AGE case patients <5 years old than in older children (P < .001) (Figure 3C). Norovirus Ct values were significantly lower for AGE specimens in the OP setting than for those in IP or ED settings, whereas there were no differences in sapovirus and astrovirus specimens (Figure 3D). A higher CT value (lower viral load) was associated with a longer duration between symptom onset and stool collection for both norovirus and astrovirus, a difference that was statistically significant (P < .001).
Figure 3.
A, Viral loads of norovirus, astrovirus, and sapovirus in stool specimens in acute gastroenteritis (AGE) case patients and healthy controls (HCs), shown as cycle threshold (Ct) values. B, Viral loads of norovirus, astrovirus, and sapovirus in stool specimens by singly detected and codetected viruses. C, Comparison of Ct values between subjects by age groups. D, Viral loads of norovirus, astrovirus, and sapovirus in stool specimens by clinical setting. Data are shown as medians with 95% confidence intervals, and P values were calculated using the Wilcoxon rank sum test (NS, not significant). Note that Ct values were missing for 1 norovirus-positive and 1 sapovirus–positive case patient (both <5 years old). Abbreviations: AGE, acute gastroenteritis; Ct, cycle threshold; ED, emergency department; HCs, healthy controls.
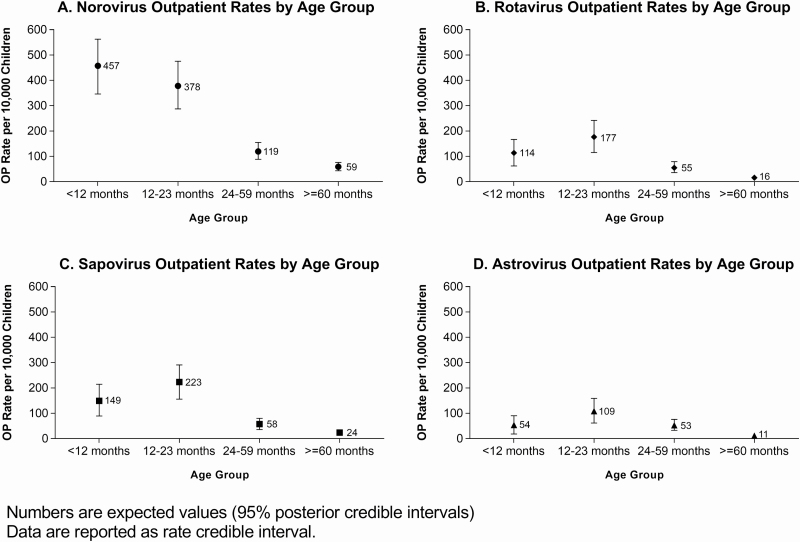
OP Virus Rates in AGE Case Patients
The rates of OP visits for AGE due to laboratory-confirmed norovirus, rotavirus, sapovirus, and astrovirus by age group are displayed in Figure 4A–4D, and the rates for all 4 viruses over a 3-year period are presented in Supplementary Figure 1A–1D. Details of the rates are described in the Supplementary Material. Rates in children <5 years old were higher than those in children ≥5 years old for all 4 viruses.
Figure 4.
Rates of outpatient (OP) visits by age group, for acute gastroenteritis due to laboratory-confirmed norovirus (A), rotavirus (B), sapovirus (C), and astrovirus (D). Rates are shown as expected values with 95% posterior credible intervals; data are reported as rate credible intervals. Abbreviations: mo, month; OP, outpatient.
Clinical Presentation of Infections With a Single Virus
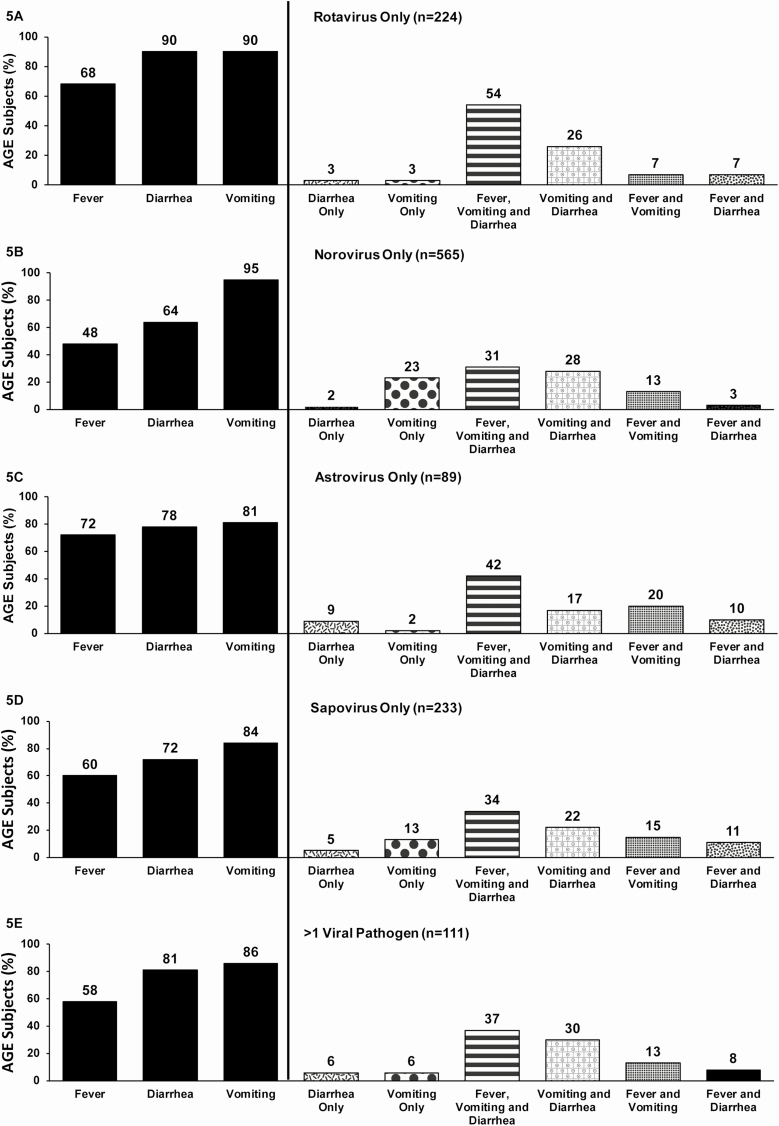
Overall, 2395 (83%) and 1992 (69%) of the children with a stool specimen presented with vomiting or diarrhea, respectively, with only 5% of the children presenting with 1 episode of vomiting only and no diarrhea. Specifically by individual single virus, the majority of children with rotavirus single infection presented with vomiting (90%) or diarrhea (90%), and 54% presented with fever, vomiting, and diarrhea (Figure 5A). The combination of fever, vomiting, and diarrhea was higher among children with rotavirus infection than for the other 3 viruses (Figure 5A–D). Nearly every child with a norovirus single infection (94%) presented with vomiting, and these children were more likely than those infected with other viruses to have a symptomatic profile of vomiting only (Figure 5B). One-third of children who were infected with norovirus only or sapovirus only presented with all 3 symptoms of fever, vomiting, and diarrhea (Figure 5B and 5D). Children with astrovirus-only infection had the highest percentage of diarrhea only (9%), compared with other 3 viruses (Figure 5D).
Figure 5.
Frequency of fever, diarrhea, and vomiting according to viral pathogen detection in acute gastroenteritis (AGE) case patients. A, Rotavirus only. B, Norovirus only. C, Astrovirus only. D, Sapovirus only. E, More than 1 pathogen. Abbreviation: AGE, acute gastroenteritis.
DISCUSSION
Our prospective, active viral AGE surveillance study of children in ED, IP, and OP settings over 3 consecutive years revealed that norovirus continued to be the leading cause of medically attended viral AGE in children in the post–rotavirus vaccine era, with nearly one-quarter of symptomatic children testing positive for norovirus. Our study supports findings of an earlier study conducted by the NVSN over 2 consecutive seasons (2008–2010) in 3 different cities, including Nashville [4]. In that study, children <5 years of age had norovirus OP rates of 368 and 260 per 10 000 persons and rotavirus OP rates of 119 and 0 per 10 000 persons in the 2 seasons, respectively. In comparison, we observed OP rates for children <5 years old ranging from 197 to 300 per 10 000 persons for norovirus and 62 to 135 per 10 000 for rotavirus during our 3-season study period. Our study also included sapovirus and astrovirus OP rates in children <5 years old, with the lowest OP rates associated with astrovirus, ranging from 32 to 87 per 10 000 persons. These results confirm that the burden of pediatric viral AGE due to these 4 pathogens is substantial.
Even in the post–rotavirus vaccine era, rotavirus remains an important cause of severe AGE disease, which may result in hospitalization [11, 12]. We documented that rotavirus is still an important pathogen in children who present with AGE, including children who were hospitalized. Rotavirus was still detected frequently in all 3 seasons (approximately 10% of AGE case patients), with peak activity seen in the 2012–2013 and 2014–2015 seasons. In addition, the AGE OP rates attributed to rotavirus decreased from the first to second season but then increased slightly in the third study season. This biannual pattern of higher rotavirus activity in seasons ending in odd years (ie, 2012–2013 and 2014–2015), and lower activity in seasons ending in even years (ie, 2013–2014) has been noted since the 2008–2009 season [5, 13].
In contrast to the earlier NSVN study, we included children ≥5 years old and noted that children with rotavirus were older than those with the other 3 viruses, suggesting that vaccine introduction may be shifting the age of children with diagnosed rotavirus illness. However, the AGE OP rates for rotavirus in children ≥5 years old still remained lower than those in younger children. This was also true for the other 3 viruses. Our study highlights the importance of continued active viral surveillance in the post–rotavirus vaccine era and of including all children who present with AGE to further document changes in pathogen detection, especially when the majority of our rotavirus cases were G12P[8].
Molecular testing for viral, bacterial, and parasitic causes of AGE has become widespread in clinical settings, often leading to uncertainty about the relationship between pathogen detection and disease causation. AGE pathogens have also been detected in the stool of asymptomatic children [4, 9]. Pathogen codetection has also been reported [9, 14], making it difficult to ascertain which pathogens are responsible for a child’s illness.
We compared virus detection in AGE case patients and HCs and found that all 4 viruses were statistically more frequently found in AGE case patients. Rotavirus had the lowest number of positive results in HCs; however, testing for rotavirus was performed with an enzyme immunoassay that was less sensitive than molecular assays used for other viruses in initial screening. Sapovirus, norovirus, and astrovirus all occurred with higher viral loads in samples from AGE case patients than in samples from virus-positive HCs. These findings suggest that higher viral loads are correlated with the presence of symptoms and likely contribute to viral transmission and outbreaks [15]. Moreover, we noted that a lower viral load was correlated with longer time to stool collection from the onset of symptoms for norovirus and sapovirus. Lower Ct values (higher viral load) have also previously been observed in children in whom norovirus viremia developed [16, 17]. Taken together, these findings indicate that detection of rotavirus, norovirus, astrovirus, or sapovirus in symptomatic children is causally associated with their AGE illness.
Identification of multiple viruses/pathogens in clinical settings has become more common as gastroenteritis multiplex molecular assays have been adopted for use [6, 7]. For example, in a study evaluating OP and IP stool specimens using the BioFire FilmArray system to detect viral, bacterial, and parasitic pathogens, 7.8% had multiple pathogens detected [7]. In the previous NVSN analysis, 13% of AGE case patients had viral coinfections [14]. Codetection with other viruses was also somewhat common in our cohort (4% of AGE case patients). If all specimens were tested for additional pathogens, the number of codetections would likely increase. Interestingly, we noted that for norovirus and astrovirus, the Ct values were lower (higher viral load) in single detections versus codetections. The significance of these differences is unclear, and further studies are warranted to define a clinically meaningful viral load and/or Ct value during coinfections. The key question remains whether it is possible to establish cutoffs that can assist clinicians in predicting disease severity and in the management of positive reverse-transcription polymerase chain reaction results for common AGE viruses; however, many of the molecular diagnostic gastrointestinal panels do not provide information on viral loads (Ct values).
Severe AGE illness was noted in our study. Hospitalization was associated with increased likelihood of receiving antibiotic and intravenous rehydration therapy and having a longer duration of symptoms at presentation compared with the other enrollment settings. Typically, young children are susceptible to dehydration and are at higher risk of severe AGE [18]. A longer duration of illness could be explained by delay in seeking medical care, which may have promoted dehydration requiring hospitalization.
The major strengths of this study are that we included ED, OP, and IP settings; collected data associated with severe disease; conducted viral surveillance over 3 seasons; and prospectively collected stool samples from children without relying on a clinician’s decision to submit a sample for testing. We also included HCs that were frequency matched for demographics and season. Even though we included children ≥5 years old, they were less likely than younger children to provide a stool sample, which slightly skewed our results and rates toward a younger age group. Our data are consistent with others demonstrating that rates of viral AGE disease in children <5 years old are higher than those in older children [19, 20]. However, our study was limited to 1 county in Tennessee and 1 facility and therefore may not be generalizable to other US cities or regions. Even though we tested for 4 leading causes of viral AGE, 58% of stool samples tested negative.
A prior study tested 216 stool samples from the Nashville NVSN study population collected between 2008 and 2011 for viruses, bacteria, and parasites and found that 22% of children with AGE had bacterial pathogens; however, the majority of these were Clostridioides difficile, the clinical significance of which remains unclear owing to frequent colonization in young children [21]. Thus, as additional funding resources become available, expanded testing of negative samples for other AGE pathogens—for example, enteric adenovirus, enterovirus, and bacteria—is warranted to help define the full etiologic spectrum of pediatric AGE. Moreover, we used an enzyme immunoassay for initial rotavirus testing, which is less sensitive than polymerase chain reaction [22]. Another factor that could have affected viral detection is the protocol-specified specimen collection window within 10 days of enrollment; however, the median time for specimen collection and testing for 2885 AGE case patients was 1 day (interquartile range, 0–3 days), and only 7% of the specimens were collected >5 days after enrollment. In addition, our definition for an AGE case included just 1 vomiting episode within 24 hours, which also could have contributed to lower viral detection; however, only 5% of the children with stool collection met this definition
In summary, AGE continues to cause substantial pediatric disease and is an important reason for children to receive medical care. Norovirus continues to be the most common virus detected in all settings. As expected, AGE case patients had a higher frequency of detection than HCs for all 4 pathogens. However, positivity in some HCs suggests that asymptomatic infection with these viruses may contribute to transmission and outbreaks. Higher viral loads were found in samples from children with AGE than in HCs and in children with a single virus detected than in those with coinfections. Our study highlights the importance of ongoing, active viral AGE surveillance and suggests that viral loads could inform the care of patients with AGE. Our results also confirm continued success of rotavirus vaccination as the number of rotavirus infections remains low in medically attended AGE visits. The burden of norovirus highlights the potential value of norovirus vaccines in development.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the New Vaccine Surveillance Network, also the clinical trial associates, and the families who participated in this study.
Financial support. This work was supported by the Centers for Disease Control and Prevention through a cooperative agreement with the National Center for Advancing Translational Sciences, National Institutes of Health (grants U01IP001063 and UL1 TR000445).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016; 388:3027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Troeger C, Khalil IA, Rao PC, et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr 2018; 172:958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim HS, Rotundo L, Nasereddin T, et al. Time trends and predictors of acute gastroenteritis in the United States: results from National Health and Nutrition Examination Survey 2005–2014. J Clin Gastroenterol 2017; 51:693–700. [DOI] [PubMed] [Google Scholar]
- 4. Payne DC, Vinjé J, Szilagyi PG, et al. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med 2013; 368:1121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sederdahl BK, Yi J, Jerris RC, et al. Trends in rotavirus from 2001 to 2015 in two paediatric hospitals in Atlanta, Georgia. Epidemiology and Infection 2018; 146:465–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang H, Morrison S, Tang YW. Multiplex polymerase chain reaction tests for detection of pathogens associated with gastroenteritis. Clin Lab Med 2015; 35:461–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu J, Gratz J, Amour C, et al. A laboratory-developed TaqMan Array Card for simultaneous detection of 19 enteropathogens. J Clin Microbiol 2013; 51:472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bowen MD, Mijatovic-Rustempasic S, Esona MD, et al. Rotavirus strain trends during the postlicensure vaccine era: United States, 2008–2013. J Infect Dis 2016; 214: 732–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hassan F, Kanwar N, Harrison CJ, et al. Viral Etiology of acute gastroenteritis in <2-year-old US children in the post-rotavirus vaccine era. J Pediatric Infect Dis Soc 2019; 8:414–21. [DOI] [PubMed] [Google Scholar]
- 10. Staat MA, Payne DC, Donauer S, et al. New Vaccine Surveillance Network (NVSN) Effectiveness of pentavalent rotavirus vaccine against severe disease. Pediatrics 2011; 128:e267–75. [DOI] [PubMed] [Google Scholar]
- 11. Ahmed SM, Hall AJ, Robinson AE, et al. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis 2014; 14:725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O’Ryan ML, Peña A, Vergara R, et al. Prospective characterization of norovirus compared with rotavirus acute diarrhea episodes in Chilean children. Pediatr Infect Dis J 2010; 29:855–9. [DOI] [PubMed] [Google Scholar]
- 13. Hallowell BD, Parashar UD, Curns A, DeGroote NP, Tate JE. Trends in the laboratory detection of rotavirus before and after implementation of routine rotavirus vaccination—United States, 2000–2018. MMWR Morb Mortal Wkly Rep 2019; 68:539–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chhabra P, Payne DC, Szilagy PG, et al. Etiology of viral gastroenteritis in children <5 years of age in the United States, 2008–2009. J Infect Dis 2013; 208:790–800. [DOI] [PubMed] [Google Scholar]
- 15. Shioda K, Barclay L, Becker-Dreps S, et al. Can use of viral load improve norovirus clinical diagnosis and disease attribution? Open Forum Infect Dis 2017; 4:ofx131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fumian TM, Justino MC, D’Arc Pereira Mascarenhas J, et al. Quantitative and molecular analysis of noroviruses RNA in blood from children hospitalized for acute gastroenteritis in Belém, Brazil. J Clin Virol 2013; 58:31–5. [DOI] [PubMed] [Google Scholar]
- 17. Reymão TKA, Fumian TM, Justino MCA, et al. Norovirus RNA in serum associated with increased fecal viral load in children: detection, quantification and molecular analysis. PLoS One 2018; 13:e0199763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vega RM, Avva U. Pediatric dehydration. StatPearls 2019. [PubMed] [Google Scholar]
- 19. Tate JE, Patel MM, Steele AD, et al. Global impact of rotavirus vaccines. Expert Rev Vaccines 2010; 9:395–407. [DOI] [PubMed] [Google Scholar]
- 20. Pinto JM, Petrova A. Detection of acute gastroenteritis etiology in hospitalized young children: associated factors and outcomes. Hosp Pediatr 2017; 7:536–41. [DOI] [PubMed] [Google Scholar]
- 21. Nicholson MR, Van Horn GT, Tang YW, et al. Using multiplex molecular testing to determine the etiology of acute gastroenteritis in children. J Pediatr 2016; 176:50–6 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mijatovic-Rustempasic S, Tam KI, Kerin TK, et al. Sensitive and specific quantitative detection of rotavirus A by one-step real-time reverse transcription-PCR assay without antecedent double-stranded-RNA denaturation. J Clin Microbiol 2013; 51:3047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.